- Department of Veterinary Medicine and Animal Production, Unit of Parasitology, University of Federico II, Naples, Italy
In southern Italy, the number of autochthonous cases of Dirofilaria immitis in dogs has increased considerably. This also occurs in the Campania region, particularly in coastal areas, where infections with D. immitis and Dirofilaria repens have been reported more frequently. Therefore the aim of the present study was to better investigate the occurrence of Dirofilaria spp. in a local dog shelter in Castel Volturno (Campania region, southern Italy). Briefly, a total of 260 blood samples were analysed for identification of microfilariae (mff) and detection of Dirofilaria immitis antigen. Dogs were classified according to their age (1–3 years; 4–6 years; 7–11 years; > 11 years) and length of stay in the shelter at the time of sampling (dogs that entered in the shelter in the last 4 months; dogs housed in the shelter for more than 4 months up to 2 years; dogs housed for more than 2 years). The modified Knott’s test revealed that 195 dogs (75.0%) were positive for circulating mff of Dirofilaria spp. Specifically, 104/260 (40.0%) dogs were positive for D. immitis and 91/260 (35.0%) were positive for D. repens. In addition, 72/260 (27.7%) dogs had both D. immitis and D. repens mff. Antigen testing revealed that 78/260 (30.0%) dogs were positive for D. immitis. However, 26/104 (25.0%) of the dogs with D. immitis mff were antigen-negative. The overall k concordance between the modified Knott’s test and the antigenic test was ≤0.2 (poor) (p = 0.000). The results of the logistic regression model showed a significant association between Dirofilaria exposure and the period of time the dogs had spent in the shelter at the time of sampling. Dogs housed in the shelter for 4 months (group 1) and between 4 months and 2 years (group 2) had higher Dirofilaria positivity than dogs in group 3 (housed for more than 2 years) (80.4% vs. 79.6% vs. 62.4%, respectively). Moreover, male dogs and older dogs (between 7 and 11 years of age) were more likely to be infected with Dirofilaria spp.
1. Introduction
Heartworm disease and subcutaneous dirofilariosis caused by Dirofilaria immitis and D. repens (Spirurida, Onchocercidae), respectively, are important vector-borne diseases, especially for dogs and cats (1–4). In addition, zoonotic infections, mostly due to D. repens, are a major public health problem (4). The epidemiological situation of canine dirofilariosis based on data reported in recent studies has shown that the prevalence of both D. immitis and D. repens is increasing in Europe and the southerneastern regions of Asia and Africa (1, 4, 5). However, there are studies that continuously report changes in the prevalence of both D. immitis and D. repens pathogens, identifying non-endemic areas with increased prevalence and previously endemic/hyper-endemic areas with decreasing prevalence (6–8). For example, the prevalence of D. immitis in northern Italy has decreased from >40% (9) to 8% in owned dogs over the last three decades (6, 7), due to the increased awareness of veterinary practitioners and better prevention strategies. In southern Italy, the number of autochthonous cases and foci of D. immitis in dogs has increased considerably (10, 11). This also occurs in the Campania region, particularly in coastal areas, where infections with D. immitis and D. repens have been reported more frequently and the prevalence of D. repens has increased from 2% (12) to 10% (13). As with D. immitis, hunting dogs, stray dogs, and dogs housed in kennels are more exposed, with seroprevalence ranging from 0.2 to 4.4% (14–16). The results of the questionnaire survey on heartworm and subcutaneous dirofilariosis addressed to veterinary practitioners throughout Italy (7) showed that at least one clinical case per year of cardiopulmonary dirofilariosis was diagnosed in dogs with frequent co-infestation by D. immitis and D. repens in Campania. Recent studies confirm the presence of autochthonous cases of D. immitis with a prevalence of approximately 0.1% (13, 17). Based on the last two reports of two cases of heartworm disease (HW) detected during post-mortem examination of two roaming dogs (17) and a screening study in which 10% of dogs tested positive for D. repens (13), both studies from the urban area of Castel Volturno in the Campania region of southern Italy, the aim of the present study was to describe an outbreak of infection in a dog shelter from the same area.
2. Materials and methods
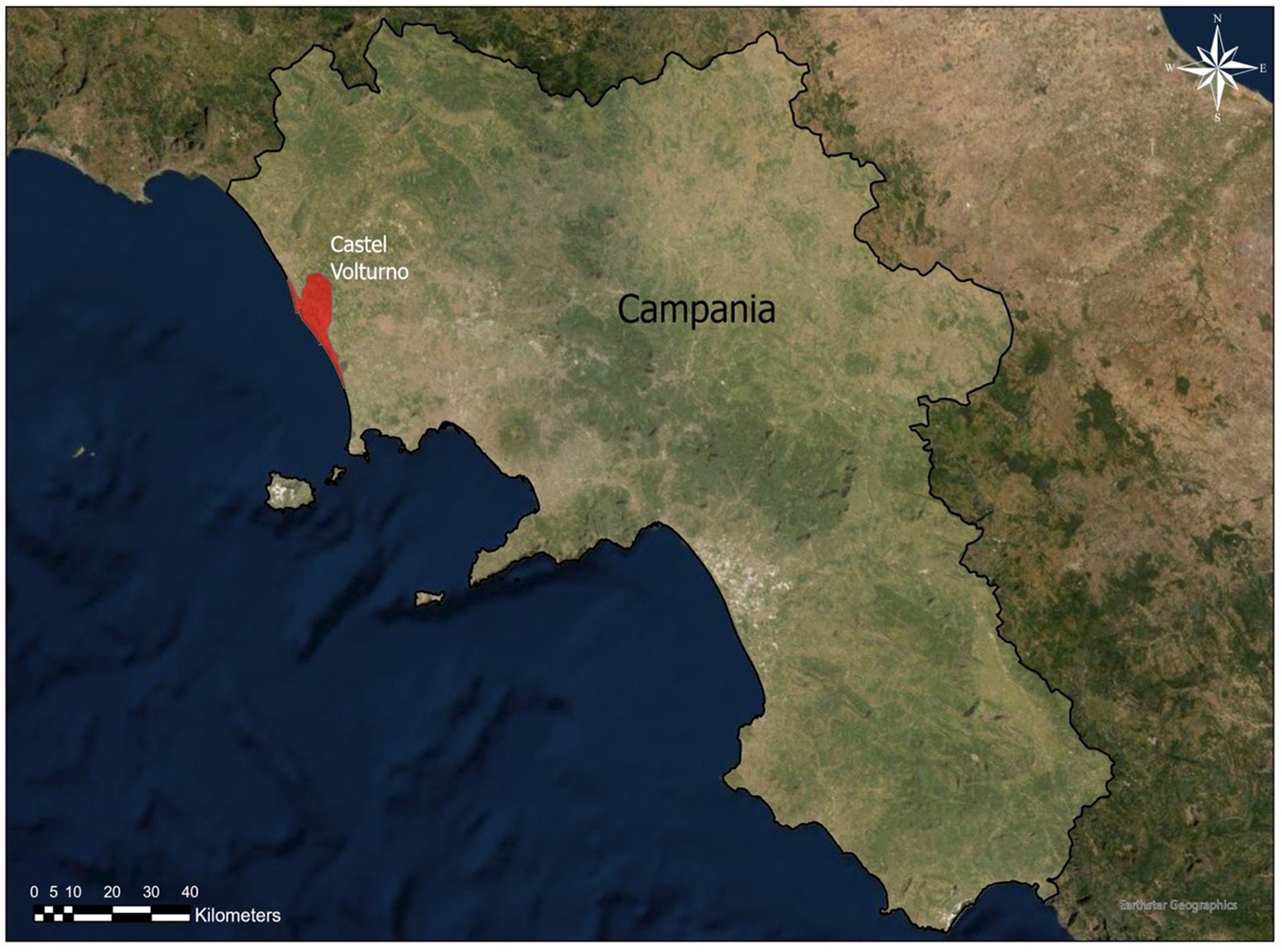
The study was conducted in a dog shelter located in Castel Volturno (Figure 1), a deltaic coastal plain of the Volturno River, an area where veterinary practitioners have reported a high incidence of D. repens infection (13).
2.1. Ethics aspects
The study started in July 2020 and ended July 2021. The protocol of this study was approved by the Ethical Committee for Animal Experiments of the Department of Veterinary Medicine and Animal Production, University of Federico II Naples, Italy (Approval Number 0093204/2022). Informed consent was obtained from the shelter owner before starting the study.
2.2. Animals and collection of blood samples
Screening for Dirofilaria spp. was performed on all shelter dogs, with a few exceptions, as follows. Dogs younger than 9 months of age and dogs that have received doxycycline in the last month at the time of sampling, were excluded from the study. The shelter housed a total of 285 dogs, and based on the above exclusion criteria, a total of 260 dogs (96 females, 164 males, age range: 2–14 years) were selected and sampled. The dogs were housed in outdoor boxes, each box hosting 3–4 dogs. The management of the shelter in terms of parasitic disease diagnosis and treatment strategy was as follows: i) all dogs (new arrivals and others with symptoms) were tested for Ehrlichia/Anaplasma and Leishmania infantum; ii) all dogs were treated with a topical formulation (based on fipronil) against ectoparasites. Moreover, no screening for Dirofilaria spp., was performed until June 2020 (the start of the study) and no prophylactic treatment against Dirofilaria spp. was perfomed in the shelter. Anamnestic data were collected for the dogs studied, including age, sex, health status (i.e., activity level, appetite, health problems, skin abnormalities). Data were also collected on the length of stay of the dogs in the shelter at the time of sampling.
2.3. Laboratory analyses
All samples (N = 260 EDTA blood and serum samples) were examined for identification of circulating microfilariae (mff) using the modified Knott’s test (18) and for detection of D. immitis antigen using the Petcheck Canine Heartworm test (IDEXX). Moreover, molecular analyses [PCR protocol as described by Rishniw et al. (19)] were used to confirm the diagnosis of dirofilariosis in case of co-infections with both D. immitis and D. repens.
2.3.1. Modified Knott test and molecular analysis
A modified Knott’s test was used for the detection of circulating mff of Dirofilaria spp. as follows. One mL of EDTA blood was mixed with 9 mL of distilled water and centrifuged at approximately 500 g for 3–5 min. The supernatant was removed from the tube and the content was stained with 1–2 drops of 1% methylene blue. One drop was placed on a microscope slide covered with a coverslip and observed under an optical microscope at 100X (18) to assess the level of microfilariaemia (mff/mL). The Dirofilaria negative/positive status of each dog included in the study was based on the examination of the entire contents of the sediment resulted from the modified Knott’s test. For molecular identification of microfilaria species, genomic DNA was extracted from 200 microliters of each blood sample using the DNeasy® Blood and Tissue kit (Qiagen, Germany), according to the manufacturer’s instructions. Molecular analyses were performed according to the multiplex PCR protocol for simultaneous detection of the different Dirofilaria species, described by Rishniw et al. (19). Amplification of the internal transcribed spacer 2 (ITS2) region was carried out using the primers DIDR-F1 and DIDR-R1 with expected amplification product sizes of 542, 484, 578 and 584 bp for D. immitis, D. repens, A. reconditum and A. dracunculoides, respectively. The PCR reactions were increased to 25 μL of total volume, and included 5 μL of genomic DNA for each sample amplification, respectively (3).
2.4. Statistical analysis
The data of the dogs included in the study – i.e. gender, age, length of stay in the shelter at the time of sampling – were analysed by univariate statistical analysis using the Pearson’s Chi-square test for independence, to test the association with positivity for D. immitis and D. repens. For this purpose, dogs were divided into the following age classes: class 1) 1–3 years (n = 76); class 2) 4–6 years (n = 75); class 3) 7–11 years (n = 81); class 4) > 11 (n = 28) years. In addition, the dogs were divided into three groups based on the length of stay in the shelter at the time of sampling as follows: group 1) dogs that entered in the shelter in the last 4 months (n = 51); group 2) dogs that were housed in the shelter for more than 4 months up to 2 years (n = 108); group 3) dogs that were housed for more than 2 years (n = 109). The Kappa (k) statistic was used to measure the concordance between the antigenic test and the modified Knott’s test using the following criteria (20): ≤0.2 = poor; 0.21–0.40 = fair; 0.41–0.60 = moderate, 0.61–0.80 = good and ≥ 0.80 = very good. Therefore, all the dogs were divided into four groups based on the number of microfilariae/ml (0 mff = negative (group 1); 1–100 mff (group 2); 101–500 mff (group 3); 501 > mff (group 4). Moreover, multivariable (logistic regression) statistical analyses were performed using the Dirofilaria spp. exposure (positive/negative) as the dependent variable. Only the independent variables that showed significance (p < 0.01) in the univariate test were used for the logistic regression model. When an interaction between variables was suspected, the logistic regression model was run with and without these variables to assess possible effect modification on their behalf (21). The significance level was set at a p value <0.05. Data analysis was performed using SPSS version 17 software, Chicago, IL, United States.
3. Results
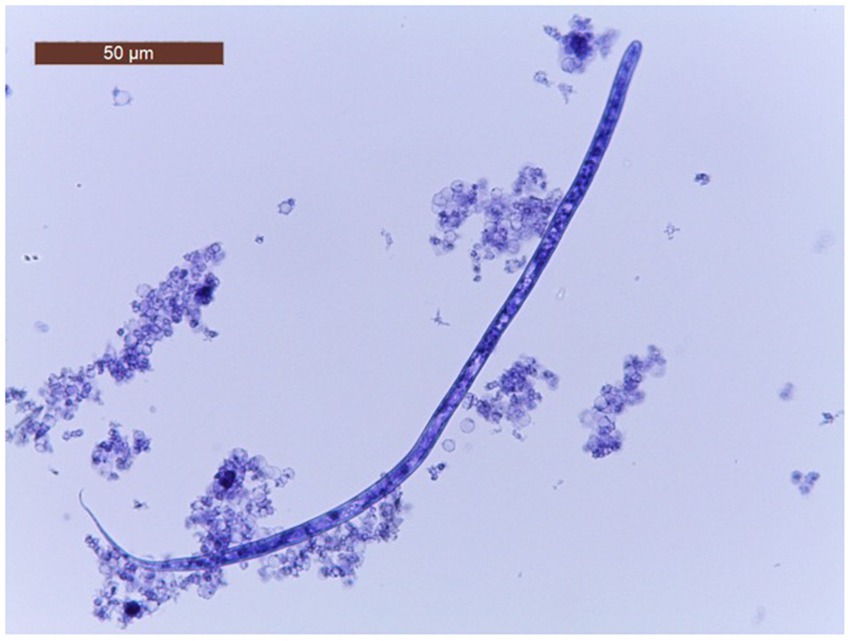
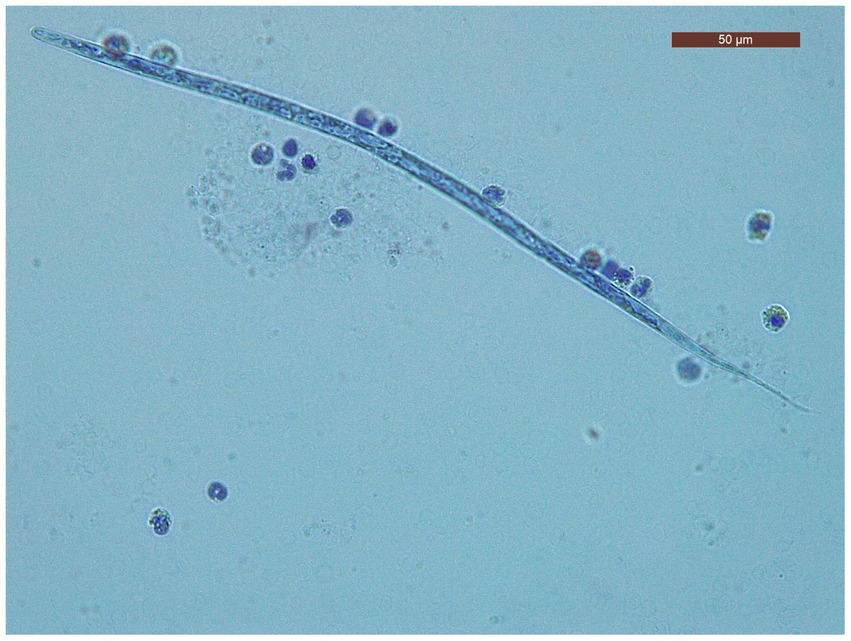
The characteristics of the dogs from the shelter are shown in Table 1. The mean age of dogs in the trial was 7.06 years (minimum 1 year; maximum14 years). The modified Knott’s test revealed that 195/260 dogs (75%; 95% CI = 69.2–80.05) were positive for circulating mff of Dirofilaria spp. Specifically, 104/260 (40.5.%; 95% CI = 34.05.-46.25) dogs were positive for D. immitis mff (Figure 2) and 91/260 (35%; 95% CI = 29.3–41.17) were positive to D. repens mff (Figure 3). In addition, 72/260 (27.7%; 95% CI = 22.43–33.63) dogs had both D. immitis and D. repens mff. All dogs in which the modified Knott test showed coinfection with both pathogens were subsequently confirmed with a PCR test. Antigen testing showed 78/260 (30%; 95%CI = 24.57–36.03) dogs positive to D. immitis (Table 2). However, 26/104 (25%; 95%CI = 17.26–34.62) of the dogs with D. immitis mff were antigen-negative. Moreover, none of the positive dogs with D. repens mff mono-infection gave positive results on the antigen test for D. immitis.
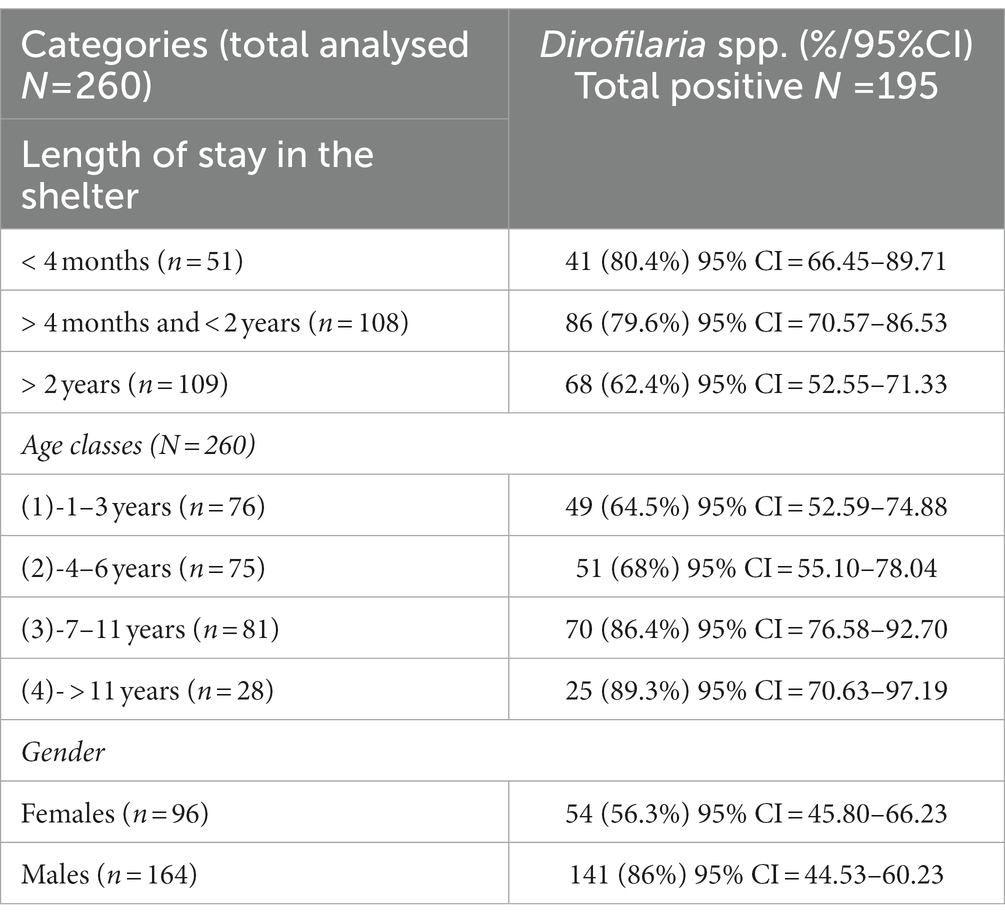
Table 1. Positivity of Dirofilaria spp. in the dogs included in the study by category (length of stay in the shelter, age, gender).
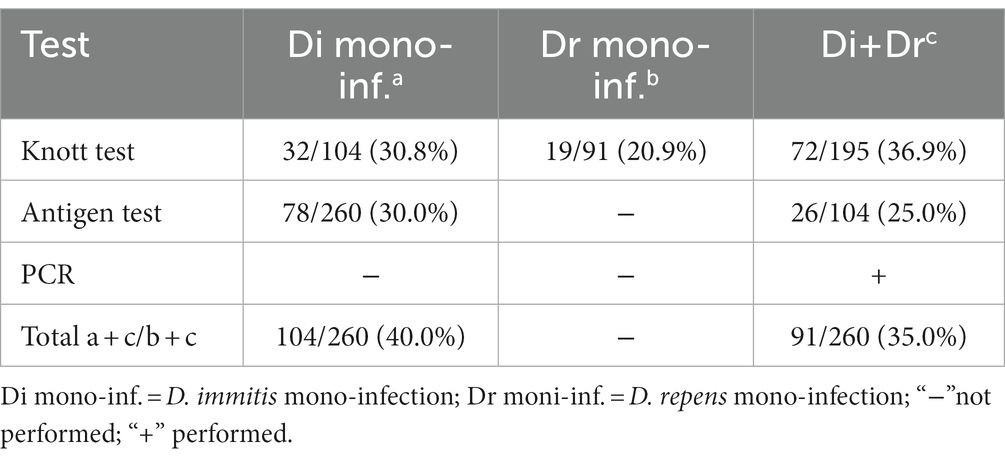
Table 2. Results of the modified Knott test, antigen test and PCR for all dogs included in the study.
The prevalence for both pathogens was almost twice as high in males (86%; 95%CI = 44.53–60.23) than in females (57%; 95% CI = 45.80–66.23) (Table 1). As expected, the prevalence was lowest in age class 1 (64.5%; 95% CI = 52 59–74.88) and highest in age classes 2 (68%; 95% CI = 55.10–78.04), 3 (86.4%; 95% CI = 76.58–92.70) and 4 (89.3%; 95% CI = 70.63–97.19), respectively. There was a significant difference with respect to the length of stay of the dogs in the shelter, reflecting mainly an increase in prevalence in group 1 (80.4%; 95% CI = 66.45–89.71 p = 0.012) and group 2 (86%; 95% CI = 70.57–86.53; p = 0.001) in which all dogs had newly arrived in the shelter for 4 months or between 4 months and 4 years, and came from different localities in the Campania region, including the city center of Naples. The results regarding the groups with the number of microfilariae were as follows: 0 mff/ml (group 1, n = 72 dogs); 1–100 mff/ml (group 2, n = 34 dogs); 101–500 mff/ml (group 3, n = 61 dogs); >501mff/ml (group 4, n = 93 dogs). The overall k concordance between the Knott’s test and the antigenic test was: ≤0.2 (=poor) (p = 0.000). The majority of the dogs had no health problems at the time of sampling. However, 13 dogs showed some symptoms: skin problems (skin lesions, poor quality of fur, itching), with five dogs showing small soft nodules in the subcutaneous tissues. All the dogs that presented nodules tested positive for D. repens in subsequent PCR testing. The results of the logistical regression model showed a significant association between Dirofilaria spp. exposure and length of stay in the shelter at the time of sampling. In this regard, the positivity to Dirofilaria was higher in dogs that had been housed in the shelter for 4 months (group 1) and in the dogs that had been housed between 4 months and 2 years (group 2), than in the other dogs of group 3 (dogs that had been housed for more than 2 years) (80.4%; OR = 1.93; 95% CI = 66.45–89.71; p = 0.012 and 79.6%; OR = 1.48; 95% CI = 70.57–86.53; p = 0.001) vs. (62.4%; 95% CI = 52.55–71.33; p = 0.089). In addition, male dogs (86%; OR = 2.02; value of p = 0.004) and older dogs (between 7 and 11 years: 86.4%; OR = 1.65; p = 0.003 and more than 11 years: 89.3%; OR = 3.83; p = 0.002) were more exposed to Dirofilaria infection. Moreover, all dogs were previously tested for Leishmania (before this study) and none of them resulted positive. However, to exclude possible cross-reactions with Angiostrongylus vasorum to the antigenic test of D. immitis, which has been reported (22), all dogs in this study were tested for A. vasorum antigen, but the result was negative.
4. Discussion and conclusions
The data presented in this manuscript on the occurrence of Dirofilaria spp. in a shelter dog from southern Italy (Campania region) are consistent with those endemic/hyperendemic areas in the Mediterranean region (4, 8, 10, 11, 23–25). The present study revealed a high prevalence of both D. immitis (40%) and D. repens (35%) in a dog shelter located in a semi-urban area of Castel Volturno, representing a high risk of infection not only for companion animals, but also for humans. Although the screening included only one dog shelter in the Campania region, the survey of dirofilariosis status was conducted for the entire dog population housed in the shelter. In addition, two previous studies conducted in the same area – one reporting two cases of HW disease at post-mortem examination of two roaming dogs, and the second showing the presence of both D. immitis and D. repens in owned dogs (13, 17) – had underscored the need for further evaluation of dirofilariosis risk in dogs in the coastal area of Castel Volturno, which provides suitable climatic and environmental conditions to sustain year-round populations of mosquito vectors and Dirofilaria pathogens (13, 26).
The overall prevalence of D. immitis and D. repens (microfilariae) in the studied dog population in the Campania region was 75%. This result may be indicative of an increased risk of dirofilariosis in the studied area, although further studies with larger dog populations (both stray and owned dogs), should be investigated for Dirofilaria spp. However, this study is consistent with other studies recently conducted in other regions of southern Italy, where a high prevalence of D. immitis has been reported [(e.g., 10, 11, 27–29)].
The apparent emerging risk of D. immitis infection in the Campania region raises important questions. There are many factors related to the vectors (presence of competent mosquito species) and the hosts (owner compliance with chemoprophylaxis, presence of wild canid reservoirs) that may influence the prevalence of HW disease in different areas of southern Italy. There are few studies in the literature on the prevalence of D. immitis in the Campania region that showed an increase in seroprevalence in dogs over time (11, 12, 14, 30).
Dirofilaria repens is considered one of the most widespread zoonotic pathogens in Europe, and the occurrence of zoonotic cases goes hand in hand with infection in dogs. Currently, D. repens infection occurs in southern Italy, especially in coastal areas. The results of the questionnaire survey on dirofilariosis addressed to freelance veterinarians throughout Italy (7) showed clinical cases of D. repens and/or D. immitis in dogs in the Campania region. The prevalence of D. repens ranges from 1.5 to 10% in Campania (13, 17) and 0.8% in the neighboring Molise region (31). Our study showed prevalence values of D. repens (35%) higher than those reported in the above studies. However, sheltered dogs are generally more exposed to canine vector-borne diseases (32, 33), and therefore future investigations will need to be conducted on owned dogs in the area to better understand the epidemiology of Dirofilaria spp.
Given the zoonotic potential of both D. repens and D. immitis, vector control and systematic treatment of domestic and stray dogs should be considered to reduce the risk of human infection. Humans are usually dead-end hosts, although there have been cases with fertile adults and/or microfilariaemia due to D. repens (34–37). In most patients, D. immitis locates in the lungs and causes pulmonary dirofilariosis, whereas D. repens appears as nodules in subcutaneous tissues or as free worms under the conjunctiva (ocular dirofilariosis). Pulmonary dirofilariosis may be confused with pulmonary neoplasms (benign, carcinoma, metastasis), tuberculosis, or mycotic infections (37) and presents a diagnostic challenge.
The lack of entomological surveys in the Campania region is an important gap in the knowledge of Dirofilaria transmission of in this coastal area of southern Italy, which has several favorable environmental characteristics for the survival and development of the vector and, consequently, for the spread of both D. immitis and D. repens (26).
In the present study, almost half of the positive dogs had been infected before their arrival at the shelter. In addition, statistical analysis showed that male dogs were more exposed to D. immitis than females. To date, there is no evidence of an actual predisposition of sex to these pathogens, although many studies have reported similar results (38–40). On the other hand, age is considered an important risk factor for D. immitis infection. In fact, the prevalence in older dogs is generally higher than in younger dogs, which is related to the increasing duration of exposure to mosquitoes (41, 42). Accordingly, our study showed the highest prevalence in dogs in the age classes >11 years (89.3%) and 7–11 years (86.4%) and the lowest in dogs in the age classes 1–3 years (64.5%) and 4–6 years (68%).
From a diagnostic point of view, our results showed that the antigen test alone (which is usually the most commonly used for the diagnosis of D. immitis) was not sufficient, as the modified Knott test detected a higher number of positive samples. This finding is not consistent with other studies (43, 44), in which the proportion of antigen-positive dogs was higher than that of Knott-positive dogs. However, one study showed the same pattern with dogs resulted positive with the modified Knott test but negative with the antigen test (45). The disagreement between the modified Knott test and the antigen test in the present study could be due to the low concentration of free antigen available for detection by serological tests. Also, it has been reported that the immune-complex formation can lead to false-negative antigen test results and that pre-heating serum samples can disrupt immune-complexes, leading to a positive test result (28, 46, 47). However, the authors chose not to pre-heat the serum samples because there were also dogs co-infected with D. repens. Moreover, it has been previously reported that mono-infection with D. repens can lead to false-positive antigen tests for D. immitis (48, 49). In addition, blocked antigens are more likely to occur in microfilaraemic dogs, in which a higher antibody response occurs compared to amicrofilaremic dogs (43, 50); this may also justify our results from the present study.
In this study, the antigen of D. immitis was detected using only one test (Petchek HW antigen test). However, as reported in a previous study by Constantinoiu et al. (43), double checking of antigen-negative samples from dogs with mff of D. immitis, using a different antigen test might be beneficial for accurate diagnosis of HW disease. Several studies have demonstrated that molecular methods can be a highly sensitive and specific analytical tool for simultaneous diagnosis and characterization of infections, and provide more reliable data compared to serological and parasitological methods (3, 51). In the present study, there were no differences between the modified Knott test and molecular tests. Similar results were obtained in studies in which the modified Knott test was found to be effective, sensitive, and compatible with PCR (3, 52).
In conclusion, HW disease is spreading over time for the reasons mentioned above, and southern Italy, once considered a low-risk area, is increasingly becoming the site of autochthonous outbreaks. We conclude that dog shelters in southern Italy are hotspots for Dirofilaria spp. transmission and strongly recommend education and veterinary advice for systematic treatment and the use of a diagnostic strategy with multiple tests to detect positive dogs in a given population (10, 11, 33). Proper management of these infections should be based on an effective and correct approach to diagnosis and up-to-date therapy, as well as practical prophylactic measures to protect animals and humans.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the ethical committee of animal experiments of the Department of Veterinary Medicine and Animal Production, University of Federico II Naples, Italy (Approval Number 0093204/2022). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
LC, VC, AB, and LR: conceptualization. LC, VC, SI, and AB: data curation. LC, VC, and SI: formal analysis. LC and VC: investigation. LC and AB: methodology. LC, LR, and MM: project administration. LR and MM: supervision. LC, VC, and AB: writing – original draft. LR and MM: writing – review & editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
The content of this manuscript has been presented [IN PART] at the 75° conference of Italian Society of Veterinary Sciences (SISVET): Lavinia Ciuca, Valeria Caruso, Sergio Illiano, Maria Paola Maurelli, Giuseppe Cringoli, Laura Rinaldi. EMERGING RISK OF DIROFILARIA SPP. INFECTION IN SHELTER DOGS IN SOUTHERN ITALY, 2022, Pp. 333.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Capelli, G , Genchi, C , Baneth, G , Bourdeau, P , Brianti, E , Cardoso, L, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasites &Vectors. (2018) 11:663. doi: 10.1186/s13071-018-3205-x
2. Ciuca, L , Roman, C , Prisco, F , Miron, L , Acatrinei, D , Paciello, O, et al. First report of Dirofilaria repens infection in a microfilaraemic cat from Romania. Vet Parasitol Reg Stud. (2020) 22:100497. doi: 10.1016/j.vprsr.2020.100497
3. Ciuca, L , Vismarra, A , Lebon, W , Beugnet, F , Morchon, R , Rinaldi, L, et al. New insights into the biology, diagnosis and immune response to Dirofilaria repens in the canine host. Vet Parasitol X. (2020) 4:100029. doi: 10.1016/j.vpoa.2020.100029
4. Genchi, C , and Kramer, LH . The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet Parasitol. (2020) 280:108995. doi: 10.1016/j.vetpar.2019.108995
5. McCall, JV , Genchi, C , Kramer, LH , Guerrero, J , and Venco, L . Heartworm disease in animals and humans. Adv Parasitol. (2008) 66:193–285. doi: 10.1016/S0065-308X(08)00204-2
6. Genchi, C , Rinaldi, L , Mortarino, M , Genchi, M , and Cringoli, G . Climate and dirofilaria infection in Europe. Vet Parasitol. (2009) 163:286–92. doi: 10.1016/j.vetpar.2009.03.026
7. Genchi, M , Rinaldi, L , Venco, L , Cringoli, G , Vismarra, A , and Kramer, L . Dirofilaria immitis and D. repens in dog and cat: a questionnaire study in Italy. Vet Parasitol. (2019) 267:26–31. doi: 10.1016/j.vetpar.2019.01.014
8. Montoya-Alonso, JA , Morchon, R , Falcon-Cordon, Y , Falcon-Cordon, S , Simon, F , and Carreton, E . Prevalence of heartworm in dogs and cats of Madrid, Spain. Parasites Vectors. (2017) 10:354. doi: 10.1186/s13071-017-2299-x
9. Guerrero, J , Genchi, C , Vezzoni, A , Ducos De Lahitte, J , Bussieras, J , Rojo, FA, et al. (1989). Distribution of Dirofilaria immitis in selected areas of Europe and South America. GF Otto (Ed.), Proceedings of the Heartworm Symposium’ in Southern Italy. Animals (Basel) 11
10. Brianti, E , Panarese, R , Napoli, E , De Benedetto, G , Gaglio, G , Bezerra-Santos, MA, et al. Dirofilaria immitis infection in the Pelagie archipelago: the southernmost hyperendemic focus in Europe. Transbound Emerg Dis. (2022) 69:1274–80. doi: 10.1111/tbed.14089
11. Napoli, E , De Benedetto, G , Ciuca, L , Bosco, A , Lia, RP , Veneziano, V, et al. New distribution patterns of Dirofilaria immitis in Italy. Front Vet Sci. (2023) 10:1162403. doi: 10.3389/fvets.2023.1162403
12. Cringoli, G , Rinaldi, L , Veneziano, V , and Capelli, G . A prevalence survey and risk analysis of filariosis in dogs from the Mt. Vesuvius area of southern Italy. Vet Parasitol. (2001) 102:243–52. doi: 10.1016/S0304-4017(01)00529-5
13. Ferrara, M , Maglione, R , Ciccarelli, D , Mundis, SJ , Di Loria, A , Pisaroglo de Carvalho, M, et al. Prevalence of Dirofilaria repens in dogs living in deltaic coastal plain of the Volturno River (Italy): a geographical risk model of infection. J Helminthol. (2022) 96:e12. doi: 10.1017/S0022149X22000062
14. Del Prete, L , Maurelli, MP , Pennacchio, S , Bosco, A , Musella, V , Cringoli, CL, et al. Dirofilaria immitis and Angiostrongylus vasorum: the contemporaneous detection in kennels. BMC Vet Res. (2015) 11:305. doi: 10.1186/s12917-015-0619-y
15. Petruccelli, A , Ferrara, G , Iovane, G , Schettini, R , Ciarcia, R , Caputo, V, et al. Seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals (Basel). (2020) 11:9. doi: 10.3390/ani11010009
16. Piantedosi, D , Neola, B , D'Alessio, N , Di Prisco, F , Santoro, M , Pacifico, L, et al. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitol Res. (2017) 116:2651–60. doi: 10.1007/s00436-017-5574-z
17. Santoro, M , Miletti, G , Vangone, L , Spadari, L , Reccia, S , and Fusco, G . Heartworm disease (Dirofilaria immitis) in two roaming dogs from the urban area of Castel Volturno Southern Italy. Front Vet Sci. (2019) 28:270. doi: 10.3389/fvets.2019.00270
18. Genchi, M , Ciuca, L , Vismarra, A , Ciccone, E , Cringoli, G , Kramer, L, et al. Evaluation of alternative reagents on the performance of the modified Knott's test. Vet Parasitol. (2021) 298:109555. doi: 10.1016/j.vetpar.2021.109555
19. Rishniw, M , Barr, SC , Simpson, KW , Frongillo, MF , Franz, M , and Dominguez Alpizar, JL . Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol. (2006) 135:303–14. doi: 10.1016/j.vetpar.2005.10.013
20. Altman, DG , (1991) Practical Statistics for Medical Research. Chapman & Hall; Boca Raton (1991). pp. 277–321.
21. Hosmer, DW , and Lemeshow, S . Applied Logistic Regression. 2nd ed. New York: John Wiley & Sons, Inc. (2000).
22. Schnyder, M , and Deplazes, P . Cross-reactions of sera from dogs infected with Angiostrongylus vasorum in commercially available Dirofilaria immitis test kits. Parasit Vectors. (2012) 5:258. doi: 10.1186/1756-3305-5-258
23. Alho, AM , Meireles, J , Schnyder, M , Cardoso, L , Belo, S , Deplazes, P, et al. Dirofilaria immitis and Angiostrongylus vasorum: the current situation of two major canine heartworms in Portugal. Vet Parasitol. (2018) 252:120–6. doi: 10.1016/j.vetpar.2018.01.008
24. Genchi, C , Bowman, D , and Drake, J . Canine heartworm disease (Dirofilaria immitis) in Western Europe: survey of veterinary awareness and perceptions. Parasit Vectors. (2014) 7:206. doi: 10.1186/1756-3305-7-206
25. Vrhovec, MG , Pantchev, N , Failing, K , Bauer, C , Travers-Martin, N , and Zahner, H . Retrospective analysis of aanine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol Res. (2017) 116:131–44. doi: 10.1007/s00436-017-5499-6
26. Genchi, C , Rinaldi, L , Cascone, C , Mortarino, M , and Cringoli, G . Is heartworm disease really spreading in Europe? Vet Parasitol. (2005) 133:137–48. doi: 10.1016/j.vetpar.2005.04.009
27. Mendoza-Roldan, J , Benelli, G , Panarese, R , Iatta, R , Furlanello, T , Beugnet, F, et al. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009-2019: changing distribution patterns. Parasit Vectors. (2020) 13:193. doi: 10.1186/s13071-020-04063-9
28. Panarese, R , Iatta, R , Mendoza-Roldan, JA , Szlosek, D , Braff, J , Liu, J, et al. Comparison of diagnostic tools for the detection of Dirofilaria immitis infection in dogs. Pathogens. (2020) 9:499. doi: 10.3390/pathogens9060499
29. Panarese, R , Iatta, R , Beugnet, F , and Otranto, D . Incidence of Dirofilaria immitis and Leishmania infantum infections in sheltered dogs from southern Italy. Transbound Emerg Dis. (2022) 69:891–4. doi: 10.1111/tbed.14025
30. Rinaldi, L , Del Prete, L , Noviello, E , Musella, V , and Cringoli, G . (2015). Dirofilaria infection in dogs from the Campania region of southern Italy. Second conference on neglected vectors and vector-borne diseases (EurNegVec) with management and working group meetings on the COST ACTION TD1303, Izmir, Turkey, March 31- April 2, 2015, 80.
31. Gizzarelli, M , Foglia Manzillo, V , Ciuca, L , Morgoglione, ME , Fayala, NEIHB , Cringoli, G, et al. Simultaneous detection of parasitic vector borne diseases: a robust cross-sectional survey in hunting, stray and sheep dogs in a Mediterranean area. Front Vet Sci. (2019) 6:288. doi: 10.3389/fvets.2019.00288
32. Lane, JN , Litster, A , Little, SE , Rodriguez, JY , Mwacalimba, KK , Sundstrom, KD, et al. Optimizing heartworm diagnosis in dogs using multiple test combinations. Parasit Vectors. (2021, 224) 14. doi: 10.1186/s13071-021-04715-4
33. Otranto, D , Dantas-Torres, F , Mihalca, AD , Traub, RJ , Lappin, M , and Baneth, G . Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. (2017) 33:813–25. doi: 10.1016/j.pt.2017.05.013
34. Bronshteyn, AM , Malyshev, NA , Jarov, SN , Fedianina, LV , Frolova, AA , Supriaga, VG, et al. A first autochthonous human case of the long standing microfilaraemia due to Dirofilaria repens in Russia and a first experience of combined therapy of dirofilariasis repens. Epidemiol Infect Dis. (2013) 3:47–52. doi: 10.17816/EID40741
35. Grandi, G , Morchon, R , Kramer, L , Kartashev, V , and Simon, F . Wolbachia in Dirofilaria repens, an agent causing human subcutaneous Dirofilariasis. J Parasitol. (2008) 94:1421–3. doi: 10.1645/GE-1575.1
36. Poppert, S , Hodapp, M , Krueger, A , Hegasy, G , Niesen, WD , Kern, WV, et al. Dirofilaria repens infection and concomitant meningoencephalitis. Emerg Infect Dis. (2009) 15:1844–6. doi: 10.3201/eid1511.090936
37. Simón, F , González-Miguel, J , Diosdado, A , Gómez, PJ , Morchón, R , and Kartashev, V . The complexity of zoonotic Filariasis Episystem and its consequences: a multidisciplinary view. Biomed Res Int. (2017) 2017:6436130. doi: 10.1155/2017/6436130
38. Mircean, V , Dumitrache, MO , Gyorke, A , Pantchev, N , Jodies, R , Mihalca, AD, et al. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Disease. (2012) 12:595–604. doi: 10.1089/vbz.2011.0915
39. Traversa, D , Aste, G , Milillo, P , Capelli, G , Pampurini, F , Tunesi, C, et al. Autochthonus foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in Central Italy. Vet Parasitol. (2010) 169:128–32. doi: 10.1016/j.vetpar.2009.12.034
40. Yildirim, A , Ica, A , Atalay, O , Duzlu, O , and Inci, A . Prevalence and epidemiological aspects of Dirofilaria immitis in dogs from Kayseri Province, Turkey. Res Vet Science. (2007) 82:358–63. doi: 10.1016/j.rvsc.2006.08.006
41. Vieira, AL , Vieira, MJ , Oliveira, JM , Simões, AR , Diez-Baños, P , and Gestal, J . Prevalence of canine heartworm (Dirofilaria immitis) disease in dogs of Central Portugal. Parasite. (2014) 21:5. doi: 10.1051/parasite/2014003
42. Yuasa, Y , Hsu, TH , Chou, CC , Huang, CC , Huang, WC , and Chang, CC . The comparison of spatial variation and risk factors between mosquito-borne and tick-borne diseases: seroepidemiology of Ehrlichia canis, Anaplasma spp. and Dirofilaria immitis in dogs. Comp Immunol Microbiol Infect Dis. (2012) 35:599–606. doi: 10.1016/j.cimid.2012.08.001
43. Constantinoiu, C , Croton, C , Paterson, MBA , Knott, L , Henning, J , Mallyon, J, et al. Prevalence of canine heartworm infection in Queensland, Australia: comparison of diagnostic methods and investigation of factors associated with reduction in antigen detection. Parasit Vectors 10. (2023) 16:63. doi: 10.1186/s13071-022-05633-9
44. Panarese, R , Iatta, R , Latrofa, MS , Zatelli, A , Ćupina, AI , Montarsi, F, et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: an expanding threat in the Mediterranean region. Int J Parasitol. (2020) 50:555–9. doi: 10.1016/j.ijpara.2020.04.002
45. Ciuca, L , Vismarra, A , Constanza, D , Di Loria, A , Meomartino, L , Ciaramella, P, et al. Efficacy of oral, topical and extended-release injectable formulations of moxidectin combined with doxycycline in Dirofilaria immitis naturally infected dogs. Parasit Vectors 6. (2023) 16:54. doi: 10.1186/s13071-023-05673-9
46. Ciuca, L , Genchi, M , Kramer, L , Mangia, C , Miron, LD , Prete, LD, et al. Heat treatment of serum samples from stray dogs naturally exposed to Dirofilaria immitis and Dirofilaria repens in Romania. Vet Parasitol. (2016) 225:81–5. doi: 10.1016/j.vetpar.2016.05.032
47. Drake, J , Gruntmeir, J , Merritt, H , Allen, L , and Little, SE . False negative antigen tests in dogs infected with heartworm and placed on macrocyclic lactone preventives. Parasit Vectors. (2015) 8:68. doi: 10.1186/s13071-015-0698-4
48. Sobotyk, C , Savadelis, MD , and Verocai, GG . Detection and cross-reaction of Dirofilaria repens using a commercial heartworm antigen test kit. Vet Parasitol. (2021) 289:109302. doi: 10.1016/j.vetpar.2020.109302
49. Venco, L , Manzocchi, S , Genchi, M , and Kramer, LH . Heat treatment and false-positive heartworm antigen testing in ex vivo parasites and dogs naturally infected by Dirofilaria repens and Angiostrongylus vasorum. Parasit Vectors Nov. (2017) 9:476. doi: 10.1186/s13071-017-2444-6
50. Morchon, R , Lopez-Belmonte, J , Bazzocchi, C , Grandi, G , Kramer, L , and Simon, F . Dogs with patent Dirofilaria immitis infection have higher expression of circulating IL-4, IL-10 and iNOS mRNA than those with occult infection. Vet Immunol Immunopathol. (2007) 115:184–8. doi: 10.1016/j.vetimm.2006.10.004
51. Little, S , Saleh, M , Wohltjen, M , and Nagamori, Y . Prime detection of Dirofilaria immitis: understanding the influence of blocked antigen on heartworm test performance Parasit. Vectors. (2018) 11:186. doi: 10.1186/s13071-018-2736-5
52. Gomes-de-Sá, S , Santos-Silva, S , Moreira, AS , Barradas, PF , Amorim, I , Cardoso, L, et al. Assessment of the circulation of Dirofilaria immitis in dogs from northern Portugal through combined analysis of antigens, DNA and parasite forms in blood. Acta Trop. (2023) 239:106799. doi: 10.1016/j.actatropica.2022.106799
Keywords: Dirofilaria immitis , Dirofilaria repens , southern Italy, shelter dogs, zoonotic risk
Citation: Ciuca L, Caruso V, Illiano S, Bosco A, Maurelli MP and Rinaldi L (2023) Emerging risk of Dirofilaria spp. infection in shelter dogs in southern Italy. Front. Vet. Sci. 10:1112036. doi: 10.3389/fvets.2023.1112036
Edited by:
Ettore Napoli, University of Messina, ItalyReviewed by:
Rodrigo Morchón García, University of Salamanca, SpainGianluca D’Amico, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2023 Ciuca, Caruso, Illiano, Bosco, Maurelli and Rinaldi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lavinia Ciuca, bGF2aW5pYS5jaXVjYUB1bmluYS5pdA==
 Lavinia Ciuca
Lavinia Ciuca Valeria Caruso
Valeria Caruso Antonio Bosco
Antonio Bosco Maria Paola Maurelli
Maria Paola Maurelli