
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 17 April 2023
Sec. Parasitology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1094554
This article is part of the Research Topic Wildlife Parasitology: Emerging Diseases and Neglected Parasites View all 20 articles
Species of genus Crenosoma have a wide distribution and are reported in Europe, the Americas, and Asia. Currently, the genus includes 14 nominal species, out of which 9 are parasitic in mustelids. Two species are mostly reported in mustelids from Europe, namely C. melesi and C. petrowi. Up to now, no genetic sequences are deposited in GenBank for any of the two. The aims of this study were to investigate the distribution, prevalence, and diversity of Crenosoma spp. infecting mustelids in Romania and to genetically characterize the species. Mustelids (n = 247) were collected over a period of 7 years from different locations in Romania and the respiratory tract was removed and examined for nematodes. Detected nematodes were morphologically identified and fragments of two genes were sequenced. Sampled mustelids included Eurasian badger, Meles meles (n = 102), Eurasian otter, Lutra lutra (n = 20), beech marten, Martes foina (n = 36), European pine marten, Martes martes (n = 5), steppe polecat, Mustela eversmanii (n = 1), European mink, Mustela lutreola (n = 1), least weasel, Mustela nivalis (n = 2), European polecat, Mustela putorius (n = 78), and marbled polecat, Vormela peregusna (n = 1). Nematodes from Eurasian badgers were morphologically identified as C. melesi (n = 13, 12.74%) and C. petrowi (n = 3, 2.94%). Nematodes from the beech martens were identified as C. petrowi (n = 6, 16.66%), C. vulpis (n = 1, 2.78%) and Crenosoma spp. (n = 3, 8.33%). Co-infections with two Crenosoma species were detected in one beech marten (C. petrowi + C. vulpis, n = 1, 2.77%) and in one European pine marten [C. petrowi + C. vulpis (n = 1, 20%)]. Two genes of Crenosoma melesi and C. petrowi were partly sequenced for the first time. We report new host-parasite associations for M. martes and C. vulpis. However, further studies are needed in order to determine the host-parasite associations and to improve the understanding of the epidemiology of Crenosoma nematodes.
Nematodes of the family Crenosomatidae are found in the respiratory tract and sinuses of various mammals (1). The family includes five genera: Paracrenosoma Yun and Kontrimavichus, 1936 (in the respiratory system of insectivores), Troglostrongylus Vevers, 1923 (in the respiratory system of felids), Prestwoodia Anderson, 1978 (in the sinuses of opossums of genus Didelphis), Otostrongylus de Bruyn, 1933 (in the respiratory tract of seals), and Crenosoma Molin, 1861 (in the trachea, bronchi, and bronchioles of carnivores and insectivores) (2). Molinofilaria Vuylsteke 1956 (in the bronchi and veins of pinnipeds) is considered similar to Otostrongylus, but the classification of this genus remains unclear (Anderson, 1978).
The genus Crenosoma is morphologically distinguishable by the presence of a striated and folded cuticle (3). Species belonging to the genus are distributed in Europe, the Americas, and Asia (2, 4–8, 10) (Table 1). The latest review of the genus Crenosoma lists 14 nominal species (43), out of which 9 are found in mustelids. Out of these, three species are found in the New World (C. brasiliense Vieira et al. 2012, C. goblei Dougherty, 1945, C. hermani Anderson, 1962) and six in the Old World [C. melesi Jančev and Genov, 1988, C. petrowi Morozov, 1939, C. schachmatovae Kontrimavichus, 1969, C. schulzi Gagarin, 1958, C. taiga Skrjabin and Petrov, 1928, C. vulpis (Dujardin, 1844)].
There is a relatively large body of literature, often confusing, with reports of various Crenosoma species in mustelids. For instance, Stunženas and Binkiene (43) list C. petrowi as a species distributed in Eurasia but among the hosts, they list two American mustelids. Such a wide distribution over two biogeographical regions is often related to a poor species definition and the absence of genetic data, as most reports are based on morphological identifications. The two most common species of Crenosoma reported in mustelids in Europe are C. melesi and C. petrowi. Surprisingly, prior to the present study, no gene sequences were known for any of the two.
In Romania, Crenosoma vulpis infection in carnivores was documented only in foxes (51, 52), and Crenosoma spp. in bears (53, 54) with limited knowledge regarding the species diversity and distribution range among other carnivores. Wild carnivores are important reservoirs for parasites that can infect domestic animals and humans (55–58). At the same time, badgers are the least studied group in this direction, even though they are reservoirs for Mycobacterium bovis (Infantes-Lorenzo et al., 2019). Romania has a remarkable diversity and abundance of mustelids, with nine extant species recorded in the country (59).
Considering the very limited knowledge and the existence of a wide variety of Crenosoma spp. parasites in mustelids, the present paper aimed to investigate the distribution, prevalence, and diversity of these species among mustelids in Romania. Additionally, identified species were also characterized by partial sequencing of two genes, and the risk factors related to sex, age, and geographical localization were analyzed simultaneously.
Between March 2014 and March 2021, 247 carcasses of mustelids were collected by hunters or found as roadkills in different regions of Romania (Supplementary material 1). Details regarding the sex, age, date and locality of collection were recorded and the carcasses were sent to the Department of Parasitology and Parasitic Diseases of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca where they were kept individually in labeled sealed plastic bags at −20°C until examination. The entire respiratory tract of each animal was removed, and the trachea, the large bronchi, and the bronchioles were thoroughly checked for the presence of nematodes under a stereomicroscope. The lungs were immersed in tap water for a few hours and manually compressed to extract the remaining nematodes. The water was then filtered through sieves and scrutinized for parasites. All nematodes were collected using fine entomological tweezers and washed in physiological saline solution. Nematodes were placed in 4% formalin (for further morphological identification) and 70% ethanol (for molecular analysis). When only one specimen was detected, it was placed in 70% ethanol. A coproscopic and larvoscopic examination was not done due to the freezing and decomposition of the carcasses.
Each nematode was temporary mounted on a glass slide in mineral oil and identified based on the morphological descriptions (8). Photographs and measurements of the specimens were taken using an optical microscope (Olympus BX61) connected to a digital camera (DP72 with Cell∧F imaging software Olympus Corporation, Tokyo, Japan). The following morphometric features were evaluated in males: body length, body width, number of anterior rings, length and maximum width of the esophagus, lengths of spicules, and length and width of gubernaculum. In females, the following morphological features were evaluated: body length, body width, number of anterior rings, length and maximum width of the esophagus, tail length, and egg size. All sizes are given in micrometers (μm).
Genomic DNA was isolated from one or more specimens preserved in ethanol, using a commercial kit (Isolate II Genomic DNA Kit, meridian Bioscience, London, UK), according to the manufacturer's instructions. The samples were processed by means of PCR amplification and bidirectional sequencing of three genetic markers, as previously described (Table 2). Only the samples that yielded high quality sequences of all three markers were further analyzed using MEGA X software (63). The pairwise distances were evaluated, and the evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model (64).
The statistical analysis was performed using EpiInfo 7 software (CDC, USA). The prevalence of infection and its 95% Confidence Interval (95% CI) were established and the differences among various categories (age, sex, bioregions) were evaluated by chi-square test, and considered significant at p ≤ 0.05.
The distribution map was generated using ArcMap 10.6.1 software.
The examined mustelids were morphologically identified as Eurasian badger, Meles meles (n = 102), Eurasian otter, Lutra lutra (n = 20), beech marten, Martes foina (n = 36), European pine marten, Martes martes (n = 5), steppe polecat, Mustela eversmanii (n = 1), European mink, Mustela lutreola (n = 1), least weasel, Mustela nivalis (n = 2), European polecat, Mustela putorius (n = 78), and marbled polecat, Vormela peregusna (n = 1). Crenosoma spp. nematodes were detected in the trachea, bronchi and bronchioles (Figure 1) of 26 mustelids (10.6%; 95% CI 7.0–15.1), namely: 16 Eurasian badgers (15.7%; 95% CI 9.2–24.2), 9 beech martens (25%; 95% CI 12.1–42.2), and one European pine marten (20%; 95% CI 0.5–71.6). The nematodes collected from the Eurasian badgers were morphologically identified as C. melesi (n = 13, 12.7%) and C. petrowi (n = 3, 2.9%). In beech martens C. petrowi (n = 6, 16.7%), C. vulpis (n = 1, 2.8%) and Crenosoma spp. (n = 3, 8.3%) were identified, the latter being in a very bad condition, which rendered them unidentifiable to species level. None of the Eurasian badgers, was co-infected with C. melesi and C. petrowi. Co-infections with two Crenosoma species were detected in one beech marten (CJ007577) [C. petrowi + C. vulpis (2.8%)] and the only positive European pine marten (CJ007578) [C. petrowi + C. vulpis (20%)].

Figure 1. Adult nematodes of C. petrowi in the trachea of a badger collected in Borod locality. Arrow head: Female nematodes with a thin, black tube inside; Black arrow: Male nematodes, smaller and white.
Adult C. melesi presented specific circular folds visible along the length of the body (Figure 2a), starting from the middle of the esophagus until after the middle of the body length, with a region in which they were less obvious, followed by their reach back in the region of the anal opening (Figure 2b). The females' body length was between 4.6 and 14.9 mm and their width from 249.9 to 971 μm. From a lateral view, the vulva protruded significantly above the cuticle. Inside the two uteri, eggs in different development stages were observed. The tail has a conic shape with two evident subterminal papillae. The adult males were 4.1 to 5.9 mm in length and 149 to 434.3 μm in width. The cuticular folds were visible until almost half of the body length, after which they disappeared. The copulatory bursa had three lobes, with the lateral ones more developed than the median one. The bursa was sustained by rays which ended in large globular papillae (Figure 2c). The two spicules were almost equal in length, and each was split in two branches in their middle and distal part (Figure 2d).
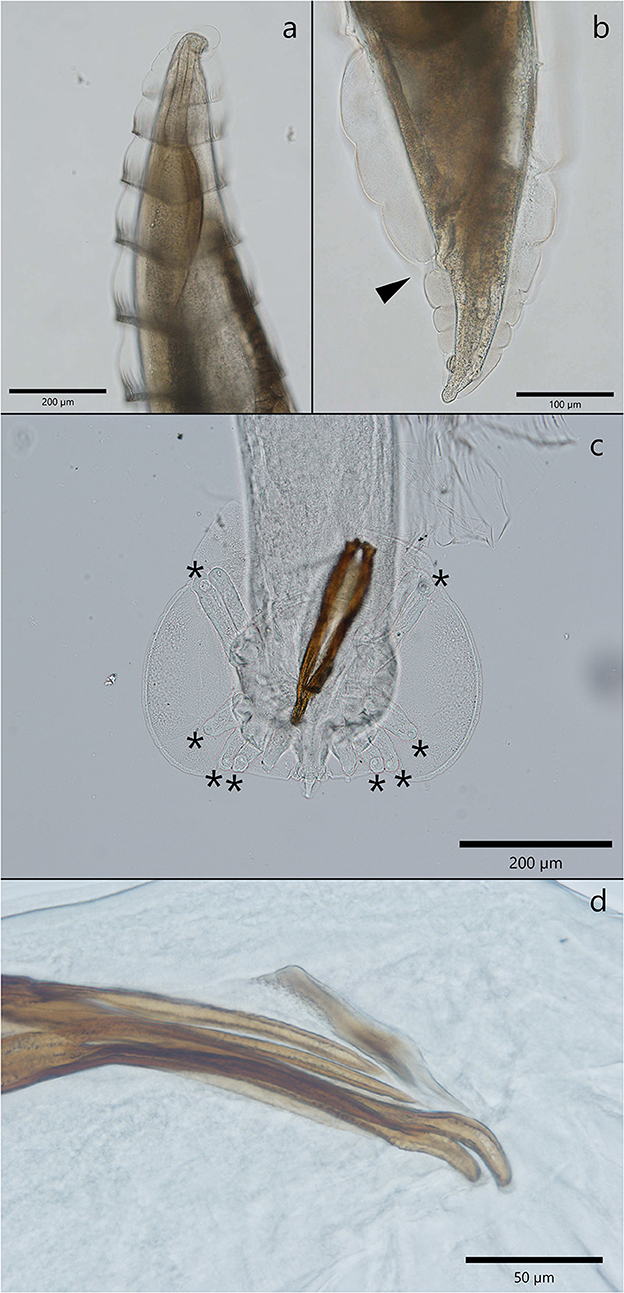
Figure 2. Morphological characteristics of C. melesi. (a) The specific circular folds. (b) Caudal extremity of a female with the cuticular folds visible and the anal opening marked with an arrow head. (c, d) Morphological characteristics of a male C. melesi. (c) The trilobated copulatory bursa with the presence of terminal globular papilla in each ray (asterix); (d) note the spicules splited in two branches.
Crenosoma petrowi adults had a transparent cuticle that formed evident folds that protrude in their posterior part (Figure 3e). The rings formed by the cuticle were visible until half of the body length for female worms and only in the anterior third in males, where the folds stretch and become unapparent. The females detected in Eurasian badger hosts were 6.7–9.2 mm in length and in 402.2–406 μm in width. The ones detected in the two Martes species were 3.6 to 5.8 mm in length and 283.3–384.1 μm in width. The vulva was lacking the appendage above the cuticle, and it was localized in the anterior third of the worm. Eggs and larvae were visible inside the uteri. The anal opening was very close to the posterior extremity of the nematode (110–210 μm) (Figure 3d). Male nematodes collected from badgers were 3.4–4 mm in length and 220.3–262.3 μm in width. The male specimens collected from both species of martens were slightly smaller, 2.4–3.2 mm in length and 154.8–224.8 μm in width. The copulatory bursa had three well distinctive parts, and it is sustained by rays. The spicules were almost equal in length and slightly curved at their caudal end (Figures 3a, b). On the dorsal side of the spicules there was a thin protrusion visible in the second third of the length (Figures 3a, c). The gubernaculum had the shape of a barque from a lateral view (Figure 3b).
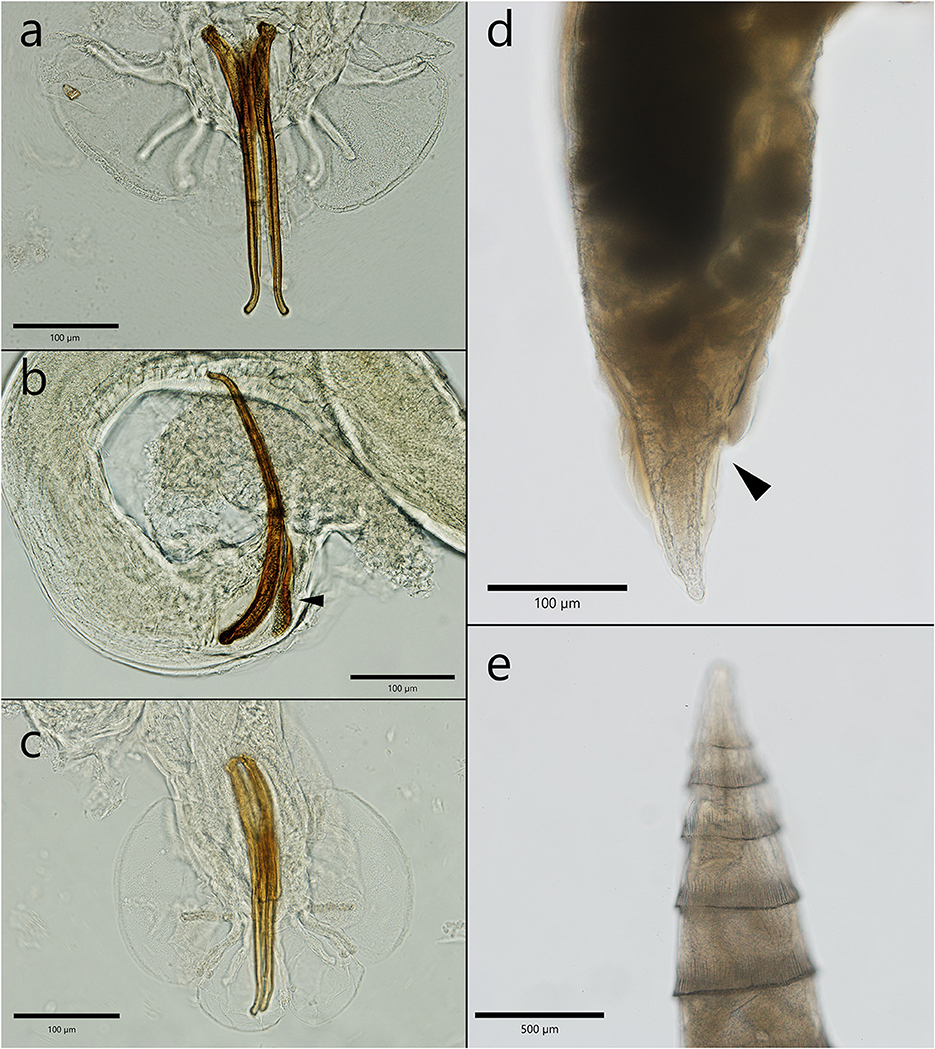
Figure 3. The morphological characteristics of male and female C. petrowi. (a) Posterior extremity of a male with the two spicules—note the thin protrusion; (b) lateral view of a male caudal bursa—the characteristic shape of the gubernaculum (arrow head); (c) the posterior extremity of a male with the typical caudal bursa; (d) posterior extremity of a female nematode; (e) specific folds in the anterior extremity.
Crenosoma vulpis adults have a cuticular sheath that formed evident folds visible in the anterior part and were stretched in the posterior extremity. The females detected in Martes foina were 9.4–10.3 mm in length and 348.6–427 μm in width. The ones collected from Martes martes were 4.6 to 6.7 mm in length and 357.3–455.4 μm in width. The vulva was positioned almost in the middle of the body, closer to the posterior extremity and the anus was at 117.3–189.3 μm from the caudal extremity (Figure 4a). Two papillae (phasmids) are visible on the lateral parts of the tail. The eggs containing a larva were visible and measured only in one specimen collected from Martes foina and had a size of 75.2–76 × 39.7–42.7 μm (Figure 4b). No male specimens of C. vulpis were found.
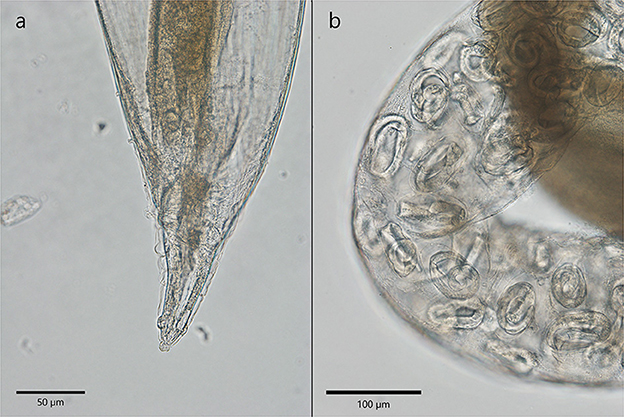
Figure 4. The morphological characteristics of C. vulpis. (a) The posterior extremity of a female with stretched cuticular folds. Anal opening is visible; (b) Note the presence of eggs containing larva in the uterus of a female.
The measurements of all the nematodes that were morphologically characterized are available in the Supplementary material 2.
High-quality sequences were obtained for all of the three markers for a total of 21 Crenosoma specimens, belonging to 14 hosts: ten Eurasian badgers, three beech martens, and one pine marten. The LSU sequences were highly conserved and insufficient for a clear differentiation among species (Supplementary material 3). However, the differences and distances between the cox 1 isolates were in agreement with the morphological identification of the species. The phylogenetic analysis revealed that C. melesi formed a separate clade, while C. petrowi clustered with C. vulpis and is more closely related to C. goblei (Figure 5).
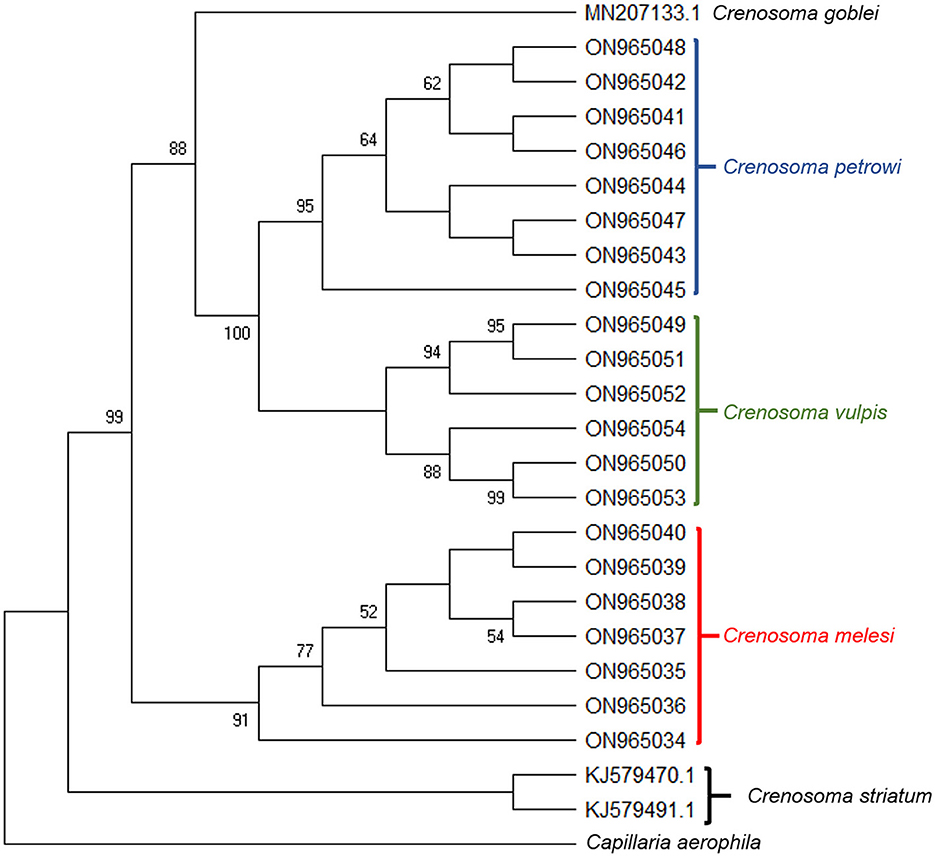
Figure 5. Bootstrap consensus tree inferred from 1000 replicates, taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The analysis involved 24 Crenosoma cox 1 nucleotide sequences obtained during the present study (21) or retrieved from the GenBank database (3), and one sequence of Capillaria aerophila, used as outgroup.
According to the bioregion, overall, the differences in the prevalence of infection were significant (X2 = 13.18; d.f. = 4; p = 0.01).
In the Eurasian badgers, juveniles were significantly more frequently infected as compared to adults (X2 = 8.95; d.f. = 1; p = 0.002), while the differences between sex and bioregion were not significant. For the beech martens, no significant differences were identified.
Thirteen (12.75%) of the Eurasian badgers were infected with C. melesi, while the other three (2.94%) harbored C. petrowi. Among beech martens, C. petrowi was identified in six (16.67%) individuals, of which one (2.78%) was co-infected with C. vulpis. The remaining three (8.33%) were positive for Crenosoma spp. The statistical data is available in Supplementary material 4. The distribution map of the Crenosoma species and the positive hosts is shown in Figure 6.
The genus Crenosoma is widely distributed and its species infect a wide variety of mammal hosts. Crenosoma vulpis, infecting mainly canid hosts, is the most studied species of the genus, followed by C. striatum, parasitic in hedgehogs (Table 1). Although there are numerous studies which report the infection with Crenosoma, the identification was based only on the morphological characteristics, correlated with the assumed host specificity. The morphological identification of species is mainly based on the number and aspect of the anterior cuticular folds, the aspect of the female tales and the dimensions and aspects of the copulatory bursa and rays in male nematodes. Nowadays, when genetic tools are largely available, the lack of molecular data could be considered a limitation of the published studies, which could have affected the knowledge on parasite-host associations. Interestingly, up to now, there is very little information about the genetic sequences of Crenosoma species. Partial cox1 gene sequences are available for only three species in the GenBank (C. striatum, C. vulpis, and C. goblei), while SSU and/or LSU sequences are known for four species (C. striatum, C. vulpis, C. goblei, and C. mephitidis).
The present study brings important details regarding the diversity of Crenosoma species in mustelids and host-parasite association. Moreover, two species, namely C. melesi and C. petrowi were reported for the first time in Romania. Crenosoma melesi is a respiratory strongyle typically infecting Eurasian badgers, which was initially described in Bulgaria, followed by few reports in other European countries. Crenosoma melesi was identified also in other mustelid species (Table 1). Although in our study we found a higher prevalence of infection in juvenile Eurasian badgers, as no data is available from other studies across the range of this host, we cannot conclude that there is an age risk. Generally, higher prevalence in young animals has been documented for other nematode species and it is believed that this could be due to their curious behavior, increased immunity gained with the age, or differences in their food habits (52, 65).
Crenosoma petrowi was identified in three mustelid species: Meles meles, Martes foina, and Martes martes. Additionally, this species is much more similar to C. vulpis in regard to its wide range of parasitized animals, including canids and Ursus americanus, and also to its wide distribution range (Table 1). Moreover, the present results are in accordance with this statement as based on the phylogenetic analysis, C. petrowi is closely related to C. vulpis (Supplementary material 3), and genetically distant to C. melesi. However, the reports of C. petrowi in canid hosts are questionable, as artificial infections of red foxes were unsuccessful (17).
Previously, only C. vulpis was morphologically identified in red foxes from Romania (52), and is now morphologically and molecularly confirmed in two mustelid species, M. martes and M. foina. In red foxes, the abundance of C. vulpis has a strong positive relationship with the presence of wetlands, and environmental factors mostly act on the intermediate hosts, regulating the distribution pattern (52). However, animals examined in the present study were grouped based on bioregions, and both animals infected with C. vulpis originated in a continental bioregion, located in the same county (Maramureş). This infection could be associated with an endemic area of C. vulpis rather than a specific parasite-host association, with mustelids being only accidental hosts.
The absence of C. vulpis in Eurasian badgers could be related to a previous misidentification of this species in badgers' hosts, or to a negative parasite-host association. Until more information is available on this topic, we can hypothesize that badgers are unsuitable hosts that may accidentally get infected in endemic areas. The only positive M. martes was found to be co-infected with C. vulpis and C. petrowi, which to the best of our knowledge is the first report of a co-infection with two species of the Crenosoma genus in the same host. Similarly, in one M. foina we detected the same species association. These findings underline the importance of complementary identification methods, such as molecular typing of specimens, especially for genera that are known to have more than one species parasitic in a particular host. Ideally, morphological identification should be followed by molecular confirmation.
In addition, C. vulpis was detected for the first time in a European pine marten. The morphological descriptions of Crenosoma specimens from the present study are in accordance with data previously reported (8, 16), with slight differences in morphometrics.
Besides the two species of martens and the badgers, all the other mustelids were negative for infection with Crenosoma spp. For M. nivalis, M. lutreola, M. eversmanii and V. peregusna the lack of infection could be attributed to the low number of examined animals. However, 31 L. lutra and 78 M. putorius were examined with no Crenosoma nematodes detected. Both mustelids are suitable hosts and were previously found to be infected with Crenosoma spp. (Petrov, 1940; Kontrimaviciius et al., 1976; Nugaraitė et al., 2014; Kretschmar, 2016). There is only one report of infection with C. vulpis in one otter, in the Asian part of the former USSR (16). Most likely, due to its habitat and food preferences consisting mainly in crustaceans and fish (66), this host is not ecologically exposed to infective Crenosoma larvae. Infection with C. taiga was identified only once in one European polecat from a zoo in Moscow in 1940 (15) and since then, no other reports are available. More recently, infection with C. schachmatovae was reported in polecats from Lithuania (18, 19). In the present study, all the examined polecats were negative, and we could speculate that C. schachmatovae is absent in Romania (Table 1).
The present paper presents the first complex study on Crenosoma parasitic in mustelids and points up the need of respecting specific identification protocols when dealing with multiple-species parasites. Moreover, the study highlights the need for further studies in order to elucidate the host-parasite associations, pathological implications on mustelid hosts, as well as the exploration of other gene sequences and their suitability for phylogenetic taxonomy.
There are three species of the genus Crenosoma infecting Romanian mustelids. Co-infections with two species of the genus in the same animal host are possible. We report a new host-parasite association for M. martes and C. vulpis. Sequences of C. melesi and C. petrowi were described for the first time. The cox1 difference between the identified species is in accordance with the morphological identification. Further studies are needed in order to determine the host-parasite associations and to improve the understanding of the epidemiology of Crenosoma nematodes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The animal study was reviewed and approved by the ethical decision nr. 232 23.11.2020, approved by Bioethics Committee of USAMV Cluj-Napoca.
GD helped with the collection of the carcasses, performed the necropsies, morphologically identified the nematodes, analyzed the data, and wrote the manuscript. AI performed necropsies, the molecular work, and the statistical analysis. CG performed the necropsies and revised the manuscript. AM coordinated the study, financially supported the molecular work, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The molecular work performed in this study was financially supported by an internal grant of USAMVCN nr. 24843 of the AM, and the fieldwork was supported by an internal Grant of USAMVCN nr. 21659/1.10.2021 of the GD.
We would like to thank all of our collaborators that contributed to the collection of carcasses over the last 6 years and to all our students that were actively involved in the necropsies, especially Andreea Iani, Alexandru Felea, and Alexandru Sarmaşi. Also, special thanks go to our collaborators from the Grigore Antipa National History Museum for their long-term help with collecting study material. A great appreciation for our very talented colleague, Carla Andreea Culda, who draw the graphical abstract for this paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1094554/full#supplementary-material
1. Anderson RC. Nematode parasites of vertebrates: their development and transmission. Cabi. (2000) 153–6. doi: 10.1079/9780851994215.0000
2. Vieira FM, Pereira LC, Lima SD, Moraes Neto AH, Gonçalves PR, Luque JL. Crenosoma brasiliense sp. n. (Nematoda: Metastrongyloidea) parasitic in lesser grison, Galictis cuja (Molina, 1782) (Carnivora, Mustelidae) from Brazil, with a key to species of Crenosoma Molin, 1861. Folia Parasitol. (2012) 59:187–94. doi: 10.14411/fp.2012.026
3. Anderson RC. “Keys to genera of the Superfamily Metastrongyloidea,” In Anderson RC, Chabaud AG, Willmott S, eds. CI H Keys to the Nematode Parasites of Vertebrates. Commonwealth Agricultural Bureaux, Farnham Royal, UK (1978). p. 1–40.
4. Skrjabin KI, Petrow AM. A description of the genus Crenosoma Molin, 1861 (Metastrongylidae, Nematoda). Parasitol. (1928) 20:329–35. doi: 10.1017/S0031182000011732
5. Dougherty EC. A review of the genus Crenosoma Molin, 1861 (Nematoda: Trichostrongylidae)—its history, taxonomy, adult morphology and distribution. Proc Helminthol Soc Wash. (1945) 12:45–62.
6. Craig RE, Anderson RC. The genus Crenosoma (Nematoda: Metastrongyloidea) in New World mammals. Can J Zool. (1972) 50:1555–61. doi: 10.1139/z72-204
7. Addison EM, Pybus MJ, Rietveld HJ. Helminth and arthropod parasites of black bear, Ursus americanus, in central Ontario. Can J Zool. (1978) 56:2122–6. doi: 10.1139/z78-288
8. Jančev J, Genov T. On the morphology and taxonomy of species from the genus Crenosoma Molin, 1861 (Nematoda: Crenosomatidae) in Bulgaria. Helminthology. (1988) 25:45–63.
9. Torres J, Miquel J, Feliu C, Motje M, Casanova JC. Helminthological investigation of Mustela nivalis Linnaeus, 1766 in Spain—a mustelid broadly spread all over Western Europe and hardly studied from a parasitic viewpoint. Parasitol Hung. (1997) 29:55–65.
10. Torres J, Miquel J, Fournier P, Fournier-Chambrillon C, Liberge M, Fons R, et al. Helminth communities of the autochthonous mustelids Mustela lutreola and M. putorius and the introduced Mustela vison in South-Western France. J Helminthol. (2008) 82:349–55. doi: 10.1017/S0022149X08046920
11. Yamaguti, S. (1961). Systema Helminthum. Vol. III. The Nematodes of Vertebrates. New York: Interscience Publishers.
12. Miller GC, Harkema R. Helminths of some wild mammals in the southeastern United States. Proc Helminthol Soc Wash. (1968) 35:119–25.
13. Forrester DJ. “Mustelidae,” In: Forrester DJ, ed Parasites and Diseases of Wild Mammals. Gainesville, Florida: Florida Univ Press of Florida (1992). p. 151–62.
14. Groves BA, Yabsley MJ, Swanepoel L, Garner MM. Lungworm (Crenosoma goblei) infection in unweaned free-ranging raccoons (Procyon lotor) in Washington State, USA. J Wild Dis. (2020) 56:419–23. doi: 10.7589/2019-03-060
15. Petrov AM. Paraziticheskie chervi kun'ikh Moskovskogo Zooparka. Trudy Moskov Zooparka. (1940) 1:202–31.
16. Kontrimaviciius VL, Delyamure SL, Boev SN. Metastrongyloidei domašnich I dikich zivotnych. Osnovy Nematodologii vol. 26 (Ed.K.M.Ryzhikov). Izdatielstvo Nauka, Moskva (1976).
17. Addison EM, Fraser GA. Life cycle of Crenosoma petrowi (Nematoda: Metastrongyloidea) from black bears (Ursus Americanus). Can J Zool. (1994) 72:300–2. doi: 10.1139/z94-041
18. Nugaraite D, Mažeika V, Paulauskas A. Helminths of mustelids (Mustelidae) in Lithuania. Biologija. (2014) 60. doi: 10.6001/biologija.v60i3.2970
19. Nugaraite D, Mažeika V, Paulauskas A. Helminths of mustelids with overlapping ecological niches: Eurasian otter Lutra lutra (Linnaeus, 1758), American mink Neovison vison Schreber, 1777, and European polecat Mustela putorius Linnaeus, 1758. Helminthologia. (2019) 56:66–74. doi: 10.2478/helm-2018-0035
20. Anderson RC. The systematics and transmission of new and previously described metastrongyles (Nematoda: Metastrongylidae) from Mustela vison. Can J Zool. (1962) 40:893–920. doi: 10.1139/z62-081
21. Morozov FN. The parasitic worms of Mustelidae in Gorkiy 's region. Tr Gor'k Gos Pedagog Inst. (1939) 4:3–44.
22. Shakhmatova VI. Helminths of mustelidae in the karelian SSR. Tr Gel'mintol Lab Akad Nauk SSSR. (1966) 17:277–89.
23. Kontrimaviciius VL. Helminths of Mustelids and Trends in Their Evolution. Moscow, Russia, Nauka, (1969). p. 432.
24. Tazieva ZK, Lobachev YS. Helminths of Martes foina and Mustela erminea in the Dzhungarsk and Zailisk Alatau. Probl Parazitol. (1969) 246–8.
25. Dakova V, Panayotova-Pencheva M. Lung parasites in stone martens (Martes foina L.) from Bulgaria. Int. J. Biol. Biomed. Eng. (2018) 12:247–50.
26. Seville RS, Addison EM. Nongastrointestinal helminths in marten (Martes americana) from Ontario, Canada. J Wild Dis. (1995) 31:529–33. doi: 10.7589/0090-3558-31.4.529
27. Ribas A, Milazzo C, Foronda P, Casanova JC. Research Note New data on helminths of stone marten, Martes foina (Carnivora, Mustelidae), in Italy. Helminthologia. (2004) 41:59–61.
28. Segovia JM, Torres J, Miquel J, Sospedra E, Guerrero R, Feliu C, et al. Analysis of helminth communities of the pine marten, Martes martes, in Spain: Mainland and insular data. Acta Parasitol. (2007) 52:156–64. doi: 10.2478/s11686-007-0012-5
29. Shakhbiev HH. Sezonnaja i vozrastnaja dinamika ankilostomoza i uncinarioza sobak v Chechenskoj. Materials. (2010) 2:81–2.
30. Kokolova LM, Illarionov AI. Helminth fauna in sable (Martes zibellina Linnaeus, 1758) from Yakutia. Rossiiskii Parazitologicheskii Zhurnal. (2017).
31. Itin GS, Kravchenko VM. Ecological and faunistic characteristics of helminth communities in American mink (Mustela vison) in biocenoses of the North–West Caucasus. Materialy dokladov mezhdunarodnoi nauchnoi konferentsii, Teoriya i praktika bor'by s parazitarnymi boleznyami, Vypusk 18, Moscow, Russia. (2017). p. 188–190.
32. Mahjoub HA, Murphy N, Mather PM, Greenwood SJ, Conboy GA. Clinical crenosomosis in a black bear (Ursus americanus). Vet Parasitol RegStud Rep. (2020) 20:100380. doi: 10.1016/j.vprsr.2020.100380
33. Panayotova-Pancheva M, Dakova V. New data on helminth parasites of the stone marten Martes foina (Erxleben, 1777) (Carnivora: Mustelidae) in Bulgaria. Acta Zool Bulg. (2021) 73:113–8.
34. Magi M, Banchi C, Barchetti A, Guberti V. The parasites of the badger (Meles meles) in the north of Mugello (Florence, Italy). Parassitologia. (1999) 41:533–6.
35. Torres J, Miquel J, Motjé M. Helminth parasites of the Eurasian badger (Meles meles L) in Spain: a biogeographic approach. Parasitol Res. (2001) 87:259–63. doi: 10.1007/s004360000316
36. Davidson RK, Handeland K, Gjerde B. The first report of Aelurostrongylus falciformis in Norwegian badgers (Meles meles). Acta Vet Scandinav. (2006) 48:1–4. doi: 10.1186/1751-0147-48-6
37. Kretschmar F. Die Parasiten des Europäischen Iltisses Mustela putorius Linnaeus, 1758 in Deutschland (Doctoral dissertation, lmu) (2016).
38. Martínez-Rondán FJ, De Ybáñez Tizzani MR, López-Beceiro P, Fidalgo AM, Martínez-Carrasco L, et al. The American mink (Neovison vison) is a competent host for native European parasites. Vet Parasitol. (2017) 247:93–9. doi: 10.1016/j.vetpar.2017.10.004
39. Byrne RL, Fogarty U, Mooney A, Harris E, Good M, Marples NM, et al. The helminth parasite community of European badgers (Meles meles) in Ireland. J Helminthol. (2019) 94:7. doi: 10.1017/S0022149X19000051
40. Gagarin VG. “Materials on the helminth fauna of wild carnivores in southern Kirgiz SSR,” In: Collected Papers on Helminthology Presented to Prof. R. S. Shults on his 60th Birthday. Alma-Ata: Kazakhskoe Gosudarstvennoe Izdatelstvo. (1958). p. 116–121.
41. Andreiko OF, Pinchuk LM. Parasites of mustelids and felines in the Moldavian SSR. Parasites Mustelids Felines Moldavian SSR. (1966) 101–10.
43. Stunženas V, Binkiene R. Description of Crenosoma vismani n. sp., parasitic in the lungs of Lynx lynx (L.) (Carnivora: Felidae), with identification key to the species of the genus Crenosoma Molin, 1861 (Nematoda: Crenosomatidae). Syst Parasitol. (2021) 98:73–83. doi: 10.1007/s11230-020-09961-1
44. Oyarzún-Cadagán JA. Pesquisa de nematodos pulmonares en perros y gatos de las ciudades de Río Bueno y La Unión, Provincia del Ranco, Chile. M.Sc. Thesis, Universidad Austral De Chile Valdivia. (2013). Available online at: http://cybertesis.uach.cl/tesis/uach/2013/fvo.98p/doc/fvo.98p.pdf
46. Pfeiffer AS, Böckeler W, Lucius R. Parasiten der haus-, nutz- und wildtiere schleswig-holsteins: parasiten der inneren organe des steinmarders (Martes foina). Zeitschrift für Jagdwissenschaft. (1989) 35:100–12. doi: 10.1007/BF02242095
47. Popiołek M, Jarnecki H, Łuczyński TA. Record of Crenosoma vulpis (Rudolphi, 1819) (Nematoda, Crenosomatidae) from the Eurasian badger (Meles meles L.) from Poland. Wiad Parazytol. (2009) 55, 437–439.
48. Latrofa MS, Lia RP, Giannelli A, Colella V, Santoro M, D'Alessio N, et al. Crenosoma vulpis in wild and domestic carnivores from Italy: a morphological and molecular study. Parasitol Res. (2015) 114:3611–7. doi: 10.1007/s00436-015-4583-z
49. Figueiredo A, Oliveira L, De Madeira Carvalho L, Fonseca C, Torres RT. Helminth parasites of stone marten (Martes foina) in central Portugal. Ann Parasitol. (2018) 64. doi: 10.17420/ap6401.134
50. Lemming L, Jørgensen AC, Nielsen LB, Nielsen ST, Mejer H, Chriél M, et al. Cardiopulmonary nematodes of wild carnivores from Denmark: Do they serve as reservoir hosts for infections in domestic animals? Int J Paras: Parasite Wildl. (2020) 13:90–7. doi: 10.1016/j.ijppaw.2020.08.001
51. Onac D, Oltean M, Mircean V, Jarca A, Cozma V. Red foxes, an important source of zoonotic parasites in Romania. Sci Parasitol. (2015) 16:112–7.
52. Deak G, Gherman CM, Ionică AM, Péter Á, Sándor DA, Mihalca AD, et al. Biotic and abiotic factors influencing the prevalence, intensity and distribution of Eucoleus aerophilus and Crenosoma vulpis in red foxes, Vulpes vulpes from Romania. Int J Parasitol Parasites Wildl. (2020) 12:121–5. doi: 10.1016/j.ijppaw.2020.05.009
53. Mircean V, Chivu R, Jurj R, Dumitrache MO, Cozma V. Prevalence of endoparasites in brown bears (Ursus Arctos) From Natural Habitats In Romania. Poster presented during EMOP XI, European Multicolloquium of Parasitology, Cluj-Napoca, Romania. SY20.P.07. (2012).
54. Borka-Vitális L, Domokos C, Földvári G, Majoros G. Endoparasites of brown bears in Eastern Transylvania, Romania. Ursus. (2017) 28:20–30. doi: 10.2192/URSU-D-16-00015.1
55. Di Cerbo AR, Manfredi MT, Bregoli M, Milone NF, Cova M. Wild carnivores as source of zoonotic helminths in north-eastern Italy. Helminthol. (2008) 45:13–9. doi: 10.2478/s11687-008-0002-7
56. Carmena D, Cardona GA. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet Parasitol. (2014) 202:69–94. doi: 10.1016/j.vetpar.2014.03.009
57. Deak G, Gherman CM, Ionică AM, Vezendan AD, D'Amico G, Matei IA, et al. Angiostrongylus vasorum in Romania: an extensive survey in red foxes, Vulpes vulpes. Parasit Vectors. (2017) 10:330. doi: 10.1186/s13071-017-2270-x
58. Cybulska A, Kornacka A, Bień J, Gozdzik K, Kalisińska E, Łanocha-Arendarczyk N, et al. The occurrence of Trichinella spp. in red foxes (Vulpes vulpes) in different regions of Poland: current data. Vector-Borne Zoonotic Dis. (2016) 16:717–21. doi: 10.1089/vbz.2016.1996
59. Murariu D, Munteanu D. Fauna României. Mammalia, vol. XVI, Fascicula 5—Carnivora. Editura Academiei Române, Bucureşti (2005). p. 223.
60. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. (1994) 3:294–9.
61. Nadler SA, D'Amelio S, Fagerholm HP, Berland B, Paggi L. Phylogenetic relationships among species of Contracaecum Railliet and Henry, 1912 and Phocascaris Høst, 1932 (Nematoda: Ascaridoidea) based on nuclear rDNA sequence data. Parasitol. (2000) 121:455–63. doi: 10.1017/S0031182099006423
62. Carreno RA, Nadler SA. Phylogenetic analysis of the Metastrongyloidea (Nematoda: Strongylida) inferred from ribosomal RNA gene sequences. J Parasitol. (2003) 89:965–73. doi: 10.1645/GE-76R
63. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9. doi: 10.1093/molbev/msy096
64. Hasegawa M, Kishino H, Yano TA. Dating the human-ape split by a molecular clock of mitochondrial DNA. J Mol E22. (1985) 160–74. doi: 10.1007/BF02101694
65. Helm JR, Morgan ER, Jackson MW, Wotton P, Bell R. Canine angiostrongylosis: an emerging disease in Europe. J Vet Emerg Crit Care. (2010) 20:98–109. doi: 10.1111/j.1476-4431.2009.00494.x
66. Dettori EE, Balestrieri A, Zapata-Perez VM, Bruno D, Rubio-Saura N, Robledano-Aymerich F, et al. Distribution and diet of recovering Eurasian otter (Lutra lutra) along the natural-to-urban habitat gradient (river Segura, SE Spain). Urban Ecosyst. (2021) 24:1221–30. doi: 10.1007/s11252-021-01109-3
Keywords: animal-host association, Crenosoma spp., Meles meles, mustelids, Romania
Citation: Deak G, Ionică AM, Gherman CM and Mihalca AD (2023) Diversity of Crenosoma species in mustelids with the first molecular characterization of C. melesi and C. petrowi. Front. Vet. Sci. 10:1094554. doi: 10.3389/fvets.2023.1094554
Received: 10 November 2022; Accepted: 28 February 2023;
Published: 17 April 2023.
Edited by:
Vito Colella, The University of Melbourne, AustraliaCopyright © 2023 Deak, Ionică, Gherman and Mihalca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgiana Deak, Z2VvcmdpYW5hLmRlYWtAdXNhbXZjbHVqLnJv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.