- 1School of Biology and Food Engineering, Chuzhou University, Chuzhou, China
- 2Department of Animal Resources Science, Dankook University, Cheonan, Republic of Korea
- 3China Light Industry Hesheng Technology Co., Ltd, Chuzhou, China
- 4College of Life Science, Linyi University, Linyi, China
Introduction: This study evaluated the effects of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on growth performance, apparent ileal digestibility, cecal bacteria counts, small intestinal morphology and digestive enzymes activities, and jejunal nutrient transporters gene expression in broiler chicks.
Methods: A total of 768 one-day-old Ross 308 broiler chicks were randomly Q18 assigned into 3 groups based on the initial body weight (42.00 ± 0.08 g). The experimental periods were 35 days. There were 16 replicates per group and 16 birds per cage. Dietary treatments included a basal diet supplemented with 0, 0.1, or 0.2% BPM to form CON, BPM0.1 (consisting Bacillus subtilis with 1.0 × 107 viable spore and Pichia farinose with 1.0 × 107 viable spore per kg diet), and BPM0.2 (consisting Bacillus subtilis with 2.0 × 107 viable spore and Pichia farinose with 2.0 × 107 viable spore per kg diet) groups.
Results and discussion: Dietary supplementation of graded levels of BPM has positive effects on growth performance of broiler chicks, manifesting in the increase of body weight gain during days 1–35 as well as the decrease of feed conversion ratio during days 1–7, 21–35, and 1–35. Moreover, BPM supplementation positively improved ileal energy and crude protein digestibility, increased Lactobacillus counts, optimized intestinal morphology, enhanced intestinal digestive enzymes activities, and upregulated jejunal SGLT-1, GLUT-2, and PEPT-1 expression. Therefore, BPM supplementation improved growth performance of broiler chicks, which was partially related to the improvement in intestinal nutrient absorption capacity.
Introduction
Antibiotics have been widely used in poultry husbandry to improve their growth performance and feed efficiency for a long time. However, the abuse of antibiotics has been proved to lead to antibiotic resistance in microbial communities. People are trying to find alternatives to antibiotics in poultry husbandry (1). Probiotics have great potential to be used as a substitute for antibiotics in the diet of broiler chicks to improve their growth performance (2–4). They stimulate the development of beneficial microbiota and disturb the colonization of pathogens in the intestine, and further regulate gastrointestinal functions and enhance enteric immune system (5, 6).
Multiple probiotic strains such as Lactobacillus, Pediococcus, Bifidobacterium, and Bacillus spp., have been recorded to be used in the diet of animals and humans (7–10). The Bacillus spp. has been reported to be able to regulate the intestinal environment by producing extracellular enzymes, and further enhancing the digestibility and absorption of nutrients in the gut (11, 12). The Pichia spp. may also have the same action mode (10). Despite the benefits of Bacillus and Pichia spp. have been observed in the application with single-strain. However, no study has investigated the effects of Bacillus and Pichia spp. mixture on the production performance in broiler chicks. It is reported that multiple-strain probiotics appear to be more effective in improving the growth performance and health status of animals than single probiotic strains (13). The application of multiple-strain probiotics provides such thoughts as more strains imply a broader spectrum of efficacy, even additive and/or synergistic effects. Based on the results observed in our previous study, we found that feeding finishing pigs with Bacillus subtilis and Pichia farinose mixture (BPM) containing diet have positive effects on their growth performance and intestinal microbiota (13).
Therefore, we hypothesized that dietary supplementation of BPM positively improved growth performance of broiler chicks, which was achieved by regulating intestinal beneficial bacteria counts and improving intestinal nutrient absorption capacity. The objective of this study was to evaluate the effects of dietary supplementation of BPM on growth performance, apparent ileal digestibility, cecal bacteria counts, small intestinal morphology and digestive enzymes activities, and jejunal nutrient transporters gene expression in broiler chicks.
Materials and methods
The experimental protocol describing the management and care of animals were revised and approved by the Animal Care and Use Committee of Dankook University, Cheonen, South Korea.
Source of probiotic complex
The probiotic complex (BioPro-alpha) was obtained from a commercial company (Deaho, Seoul, Korea). This product contained a spray-dried mixture of Bacillus subtilis (1.0 × 107 viable spore/g of product) and Pichia farinose (1.0 × 107 viable spore/g of product) in the form of powder (http://www.daeho.com/eng/index.html; Supplementary material 1).
Experimental design, animals, and diets
A total of 768 one-day-old mixed-gender Ross 308 broiler chicks were purchased from a commercial hatchery (Yang Ji Company, Cheonan, South Korea) and raised in stainless steel cages (1.75 × 1.55 m) and fed experimental feed for 35 days. The house was provided with programmable lighting and ventilation. Feed and water were provided ad libitum and diets were prepared in pellet form. Broiler chicks were raised in a room (32°C) for the first 3 days and the temperature reduced by 2°C every week until 24°C maintaining humidity around 65%. Birds were randomly assigned to 3 groups based on the initial body weight (42.00 ± 0.08 g). There were 16 replicates per group and 16 birds per cage. Dietary conditions were based on a basal diet supplemented with 0, 0.1, or 0.2 % BPM to form CON, BPM0.1 (consisting Bacillus subtilis with 1.0 × 107 viable spore and Pichia farinose with 1.0 × 107 viable spore per kg diet), and BPM0.2 (consisting Bacillus subtilis with 2.0 × 107 viable spore and Pichia farinose with 2.0 × 107 viable spore per kg diet) groups. Experimental periods were divided into 3 phases (phase 1, 1–7; phase 2, 8–21; phase 3, 22–35). Diets (Table 1) were formulated according to the recommendation of NRC (14).
Sampling and measurements
Feed analysis
Diets were analyzed for crude protein (CP), calcium (Ca), phosphorus (P), and amino acids (AA) by AOAC (15) procedures. CP was determined by the Kjeldahl methodology (N × 6.25). Dietary Ca was analyzed by atomic absorption spectrophotometry after wet ash procedures, and P was determined by colorimetric procedure. For the determination of methionine (Met) and cysteine (Cys), the samples were oxidized with performic acid overnight at 0°C. Met and Cys composition was measured using an AA Analyzer (Beckman 6300, Beckman Coulter, Inc., Fullerton, CA) after 24-h hydrolysis in HCl.
Growth performance
Broiler chicks were weighed pen-basis to calculate the body weight gain (BWG). Feed intake (FI) was recorded on days 1, 7, 21, and 35. The information was then used to calculate FCR.
Apparent ileal digestibility
During days 28-35, feeding broiler chicks with 0.2% chromium oxide containing diet. On the final day, a portion of the small intestine from Meckel's diverticulum proximal to the ileocecal junction was removed in order to collect ileal digesta samples for apparent ileal digestibility (AID) measurements. According to the procedure established by the AOAC (15), the crude protein (nitrogen × 6.25; method 968.06) and dry matter (method 930.15) composition in the digesta samples were analyzed. The combustion heat was measured by a bomb calorimeter (Parr 6100; Parr Instrument Co., Moline, IL, USA) to determine the gross energy content of the feed and excreta samples. Chromium concentrations were determined by atomic absorption spectrophotometry (UV-1201, Shimadzu, Kyoto, Japan). The apparent digestibility values for ileal nutrients were calculated by a formula provided by Dang et al. (16).
Cecal bacteria counts
The digesta contents of the birds were collected from the caeca into micro-tubes. Samples were placed on ice and transported to the laboratory, where analysis was immediately carried out. One g of cecal digesta sample was blended with 9 ml sterile peptone water and mixed for 1 min on a vortex stirrer. Counts of viable bacteria in the cecal samples were determined by plating serial 10-fold dilutions (10−3 to 10−6) onto MRS agar (Difco Laboratories, Detroit, MI, USA) and MacConkey agar (Difco Laboratories, Detroit, MI, USA) plates to isolate Lactobacillus and Escherichia coli, respectively. The MRS agar plates were then incubated for 24 h at 37°C under anaerobic conditions. The MacConkey plates were incubated for 24 h at 37°C under aerobic conditions. After the incubation periods, colonies of the respective bacteria were counted and expressed as the logarithm of colony-forming units per g (log10 CFU/g).
Small intestinal samples preparation
The whole small intestine was removed and the adherent material of the small intestine was carefully removed under ice-cold saline, weighed, and separated into the duodenum, jejunum, and ileum. About the 1-cm long segment from the middle of intestinal part were taken in duplicate and placed in 2 separate tubes. One sample was fixed with 10% neutral-buffered formalin solution for histology and the other sample was frozen in liquid nitrogen, and then stored at −80°C for measuring digestive enzymes activities and nutrient transporter gene expression.
Small intestinal morphology
The preparation of the intestinal sample cutting block was the same as the above. A microtome was used to make five cuts that were 5 μm. The cuts were stained with hematoxylin-eosin. The values were measured using a light microscope. Measurements of villus height and depth were determined at a magnification of 10X. A minimum of five measurements per slide were made for each parameter and averaged into one value (17). Values presented means from 10 adjacent villi and only vertically oriented villi were measured.
Intestinal digestive enzymes activities
To extract the broiler digestive enzymes and quantify the protein concentration, the segment of duodenum, jejunum, and ileum were homogenized in 0.2 M phosphate buffer pH 7 (1:5 w/v), using a microhomogenizer (THP-220; Omni International, Kennesaw, GA, USA). The homogenate was centrifuged at 13,000 × g, at 4°C for 20 min, and aliquots prepared which were kept at −20°C until use. The protein concentration from a crude enzyme extract was compared to a standard curve of bovine serum albumin (BSA), within a linear range, according to the standard method of Lowry et al. (18).
Jejunal nutrient transporter gene expression
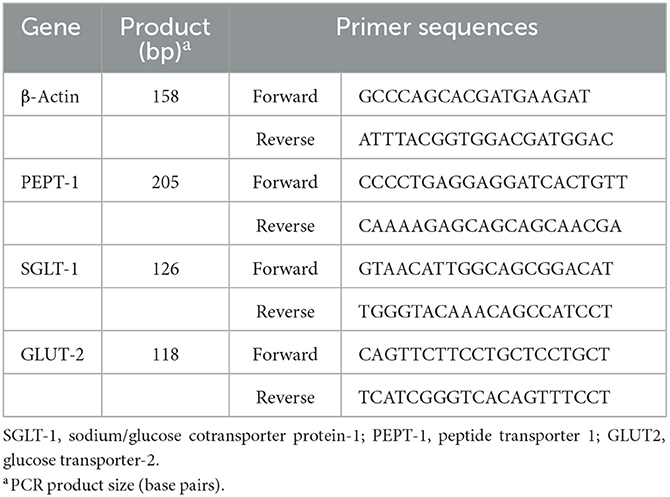
Total RNA was isolated from muscle samples using RNAiso Reagent (TaKaRa, Dalian, Liaoning, China). The RNA integrity was assessed by electrophoresis on a 1% agarose gel containing formaldehyde. The RNA concentration was measured using a Beckman DU-640 spectrophotometer (Beckman). The sequences of primers for the genes tested were specifically designed according to the sequences located in GenBank (Table 2). The total RNA samples were purified and subjected to reverse transcription using the Takara PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Dalian, China) and processed for cDNA synthesis as per Takara PrimeScript RT instructions (19). The relative expression levels of sodium/glucose cotransporter protein-1 (SGLT-1), glucose transporter-2 (GLUT-2), and peptide transporter 1 (PEPT-1) genes in pectoral muscle were analyzed by RT-PCR, which was performed in a 10 μL reaction mix containing 1 μL 2 × SYBR Premix Ex Taq II (TakaRa, Dalian, China), 3 μL dH2O, 0.5 μL of the upstream and downstream primers, and 1 μL cDNA using a Bio-Rad CFX-96 thermocycler (Bio-Rad, CA). The reaction conditions were as follows: initial denaturation at 95°C for 30 s and 44 cycles of amplification at 72°C for 30 s. The annealing was carried out for 40 s at temperatures specific to each target gene. At the end of the amplification, step-wise melting curves were performed to confirm the product specificity. The cytoskeletal protein, β-actin, was used as the internal reference. The gene expression levels of the samples were determined by the 2−ΔΔCt method (20).
Statistical analysis
All data were statistically analyzed using the General Linear Model procedure (SAS Inst. Inc., Cary, NC, USA) in a randomized completely block design. The replicate cage was used as the experimental unit. Orthogonal contrasts were used to examine the linear and quadratic effects in response to the increase of dietary BPM concentrations. Variability in the data was expressed as the standard error of means (SEM), P < 0.05 was considered statistically significant.
Results
Dietary supplementation of graded levels of BPM linearly increased BWG during days 1-35 (P = 0.031), whereas linearly decreased FCR during days 1-7 (P = 0.008), 21-35 (P = 0.016), and 1–35 (P = 0.001). However, BPM supplementation did not affect FI (Table 3).
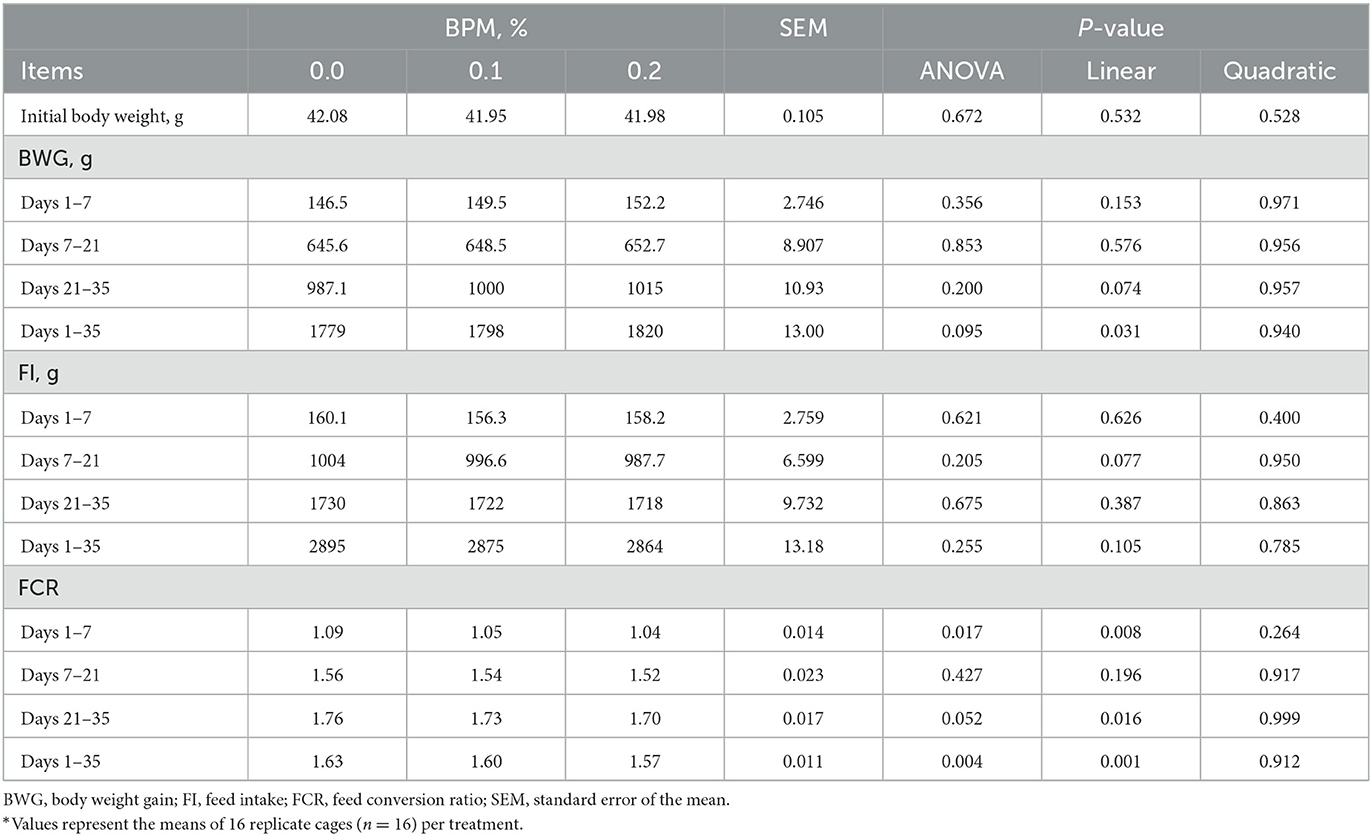
Table 3. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on growth performance of broiler chicks*.
Energy (P = 0.027) and crude protein (P = 0.025) digestibility increased linearly with the dose of BPM increased in the diet, but dry matter digestibility did not differ among groups (Table 4).
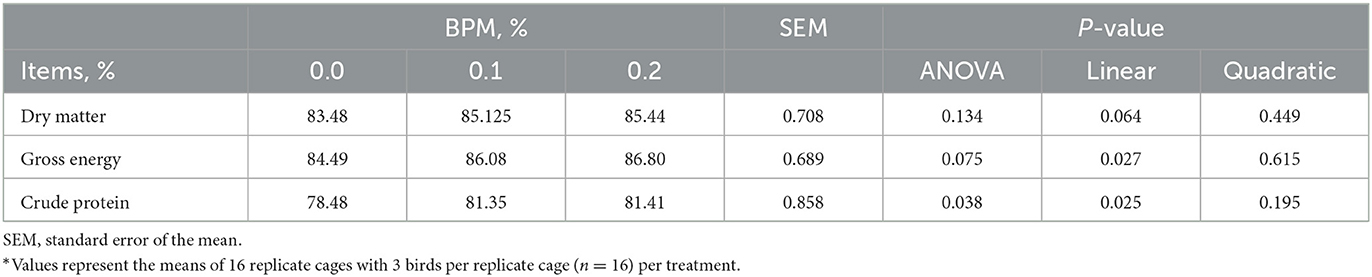
Table 4. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on apparent ileal digestibility of broiler chicks*.
Broiler chicks fed with BPM containing diet led to an increase in cecal Lactobacillus counts (P = 0.025) in a dose-dependent manner, but cecal Escherichia coli did not affect by BPM supplementation (Table 5).
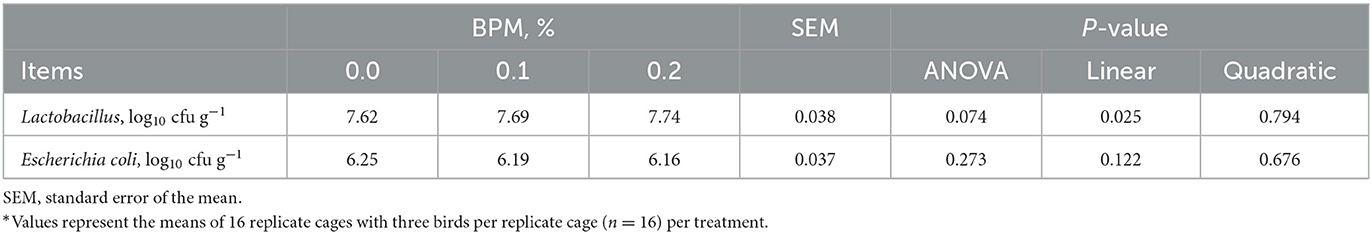
Table 5. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on cecal bacteria counts of broiler chicks*.
Effects of graded levels of BPM supplementation on small intestinal morphology of broiler chicks were shown in Table 6. As observed, the villus height and its ratio to crypt depth in duodenum and ileum were increased linearly with the dose of BPM increased in the diet (P < 0.05). Additionally, linear decreases in jejunal and ileal crypt depth were observed in broiler chicks fed with graded levels of BPM-containing diet (P < 0.05).
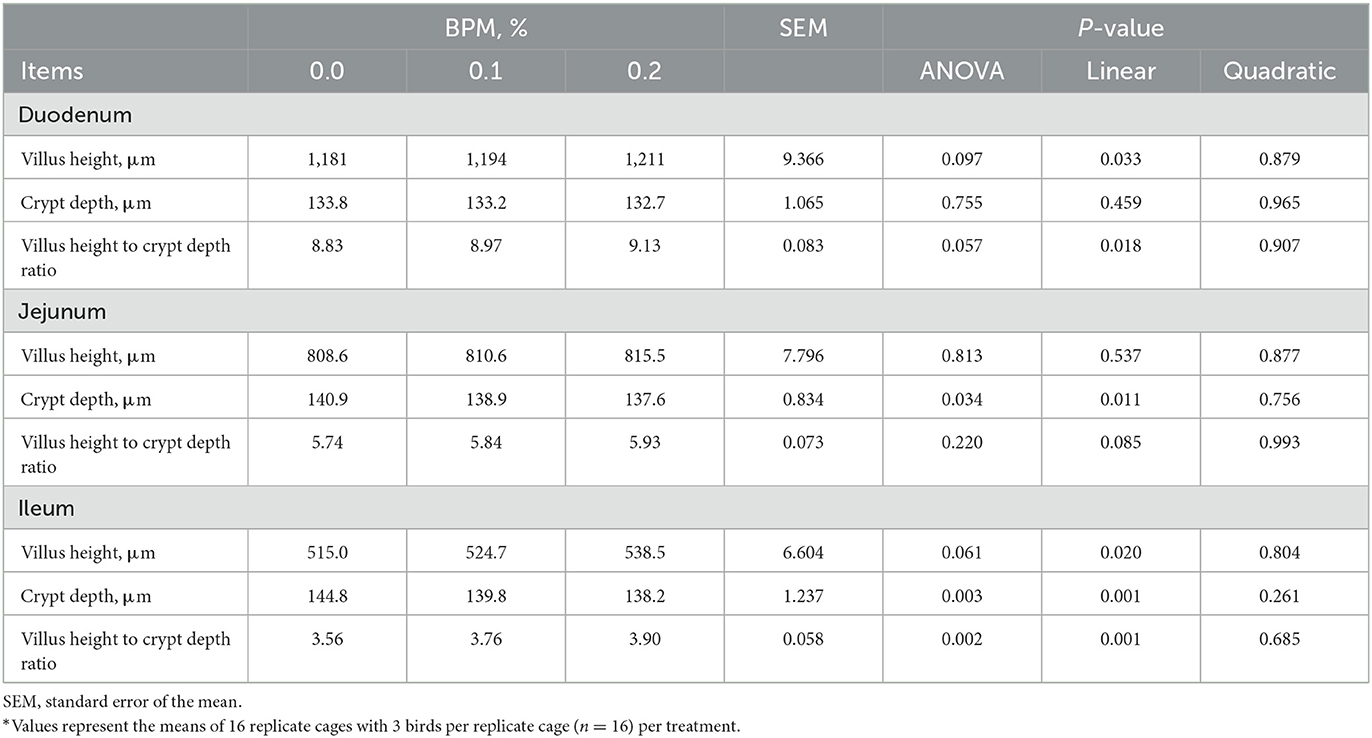
Table 6. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on duodenal, jejunal, and ileal morphology of broiler chicks*.
Lipase activity in duodenum (P = 0.027), amylase activity in jejunum (P = 0.002), cellulase activity in jejunum (P = 0.018), and cellulase activity in ileum (P = 0.003) increased linearly with the dose of BPM increased in the diet (Table 7).
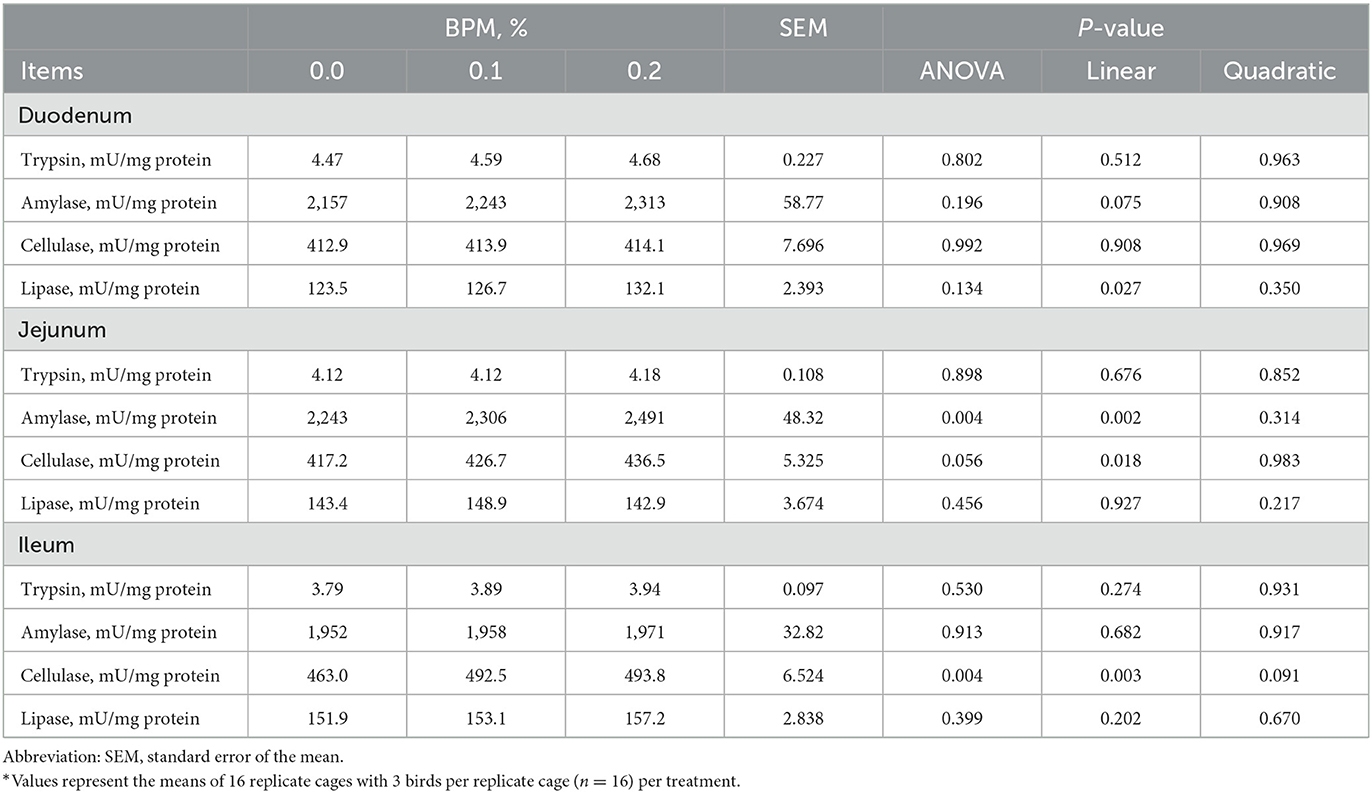
Table 7. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on duodenal, jejunal, and ileal digestive enzymes activities of broiler chicks*.
Moreover, we observed that the expression of SGLT-1 (P = 0.005), GLUT-2 (P = 0.012), and PEPT-1 (P = 0.009) in jejunum increased linearly with the dose of BPM increased in the diet (Table 8).

Table 8. Effect of dietary supplementation of Bacillus subtilis and Pichia farinose mixture (BPM) on jejunal nutrient transporter gene expression of broiler chicks*.
Discussion
Some studies have reported that dietary supplementation of probiotics is able to improve the growth performance of broiler chicks (21). In the present study, feeding broiler chicks with graded levels of BPM containing positively improved their growth performance. Tarabees et al. (22) reported that broiler chicks fed the diet supplemented with probiotic mixture improved the BWG and decreased the FCR. Fazelnia et al. (23) also observed an increase in BWG and a decrease in FCR caused by broiler chicks fed with probiotic mixture containing diet. Therefore, the BWG of broiler chicks increased by BPM supplementation was considered to be related to the reduction of FCR, which corresponded to the improvement of nutrient digestibility (24–26).
As expected, in this study, broiler chicks fed the diet supplemented with BPM linearly increased AID of crude protein and energy. Similarly, Zhang and Kim (27) noted that dietary supplementation of multi-strain probiotics decreased FCR and increased the AID of amino acids in broiler chicks. Giang et al. (28) observed an increase of apparent ileal crude protein, crude fiber, and organic matter digestibility as well as a decrease of FCR in broiler chicks consuming probiotic complexes containing diet. We considered that the growth performance positively affected by BPM supplementation was partially related to the reduction of FCR caused by nutrient digestibility improvement.
Microbiota presents in the intestine play a key role in improving feed efficiency and/or nutrient digestibility (29). The supplementation of probiotics is a suitable strategy to regulate intestinal microbiota. Probiotics are able to inhibit the colonization of pathogenic bacteria in the intestine (30). Tarabees et al. (22) reported that broiler chicks fed the diet supplemented with probiotic mixture increased the counts of beneficial bacteria while decreased the counts of harmful bacteria in the cecum. In the present study, we also observed an increase in cecal Lactobacillus counts caused by feeding broiler chicks with graded levels of BPM containing diet. Recently, a gut metagenomic analysis study in broiler chicks revealed the prominent roles of cecal Lactobacillus counts in the improvement of feed efficiency in broiler chicks. They reported that the feed efficiency was related to the abundance of Lactobacillus in the cecum (31). We considered that the supplementation of BPM was beneficial to regulate the intestinal microbiota, thus generate positive effects on the growth performance.
Additionally, the development of the gastrointestinal tract is critical to optimize the growth performance of poultry in the early growth stage (32). Digestion and absorption of nutrients occur in the small intestine of birds (33, 34). In the small intestine, abundant villi endow a large surface area for nutrient absorption and enzyme secretion (35). In general, the villus height, crypt depth, and their ratio are common parameters to be used to reflect the small intestine development (36). Giannenas et al. (37) reported that the supplementation of probiotics stimulated the development of villus height in broiler chicks. Sen et al. (38) demonstrated that feeding broiler chicks with probiotics containing diet increased villus height and the ratio of villus height to crypt depth in both duodenum and ileum in a dose-dependent manner. In the present study, we also observed that BPM supplementation improved intestinal morphology. We speculated that the supplementation of BPM was beneficial to enhance the nutrient absorption capacity of broiler chicks by modulating intestinal morphology.
Moreover, the digestive enzymes play an important role in digesting nutrients into smaller nutritional molecules so as to facilitate the absorption by the host (39). Abdel-Moneim et al. (40) noted that the activities of small intestinal proteases, lipase, and amylase increased in Japanese quail fed with probiotic containing diet. Gong et al. (41) also observed that the activities of trypsin, amylase, lipase, and protease in duodenum increased in feeding broiler chicks with probiotic containing diet. We also observed an increase in small intestinal digestive enzyme activities in the current study. We considered that the BPM supplementation was beneficial to enhance digestive enzyme activities, and therefore improved nutrient digestibility.
The transport mediators expressed in the apical and basal membranes of enterocytes are important to achieve nutrient digestion and absorption (42, 43). Nutrients are transported into enterocytes by special transporters (44). Glucose is the key fuel and important metabolic substrate in poultry, it is absorbed by intestinal epithelium via the apically located SGLT-1 and transported into the blood via GLUT-2 which is expressed on the basolateral membrane (45, 46). PEPT-1 is a solute carrier for oligopeptides. It acts as a cotransporter in intestine by a proton-dependent manner (47). In the present study, dietary supplementation of BPM upregulated the expression of SGLT-1, GLUT-2, and PEPT-1 in the jejunum. Similarly, Faseleh Jahromi et al. (48) reported that feeding broiler chicks with probiotic mixture containing diet had pronounced effects on the expression of GLUT-2 and SGLT-1 in the small intestine. Wang et al. (49) also observed the nutrient transporter genes of laying hens upregulated, and the production performance further improved, by probiotic supplementation. Therefore, the supplementation of BPM was beneficial to enhance the nutrient absorption capacity.
Conclusion
In this study, feeding broiler chicks with BPM containing improved growth performance of broiler chicks, which was partially attributed to the improvement in nutrient absorption capacity, manifesting in the increase of cecal beneficial bacteria counts, optimization of intestinal morphology, enhancement of intestinal digestive enzyme activities, and the upregulation of nutrient transporter gene expression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Dankook University, Cheonen, South Korea.
Author contributions
HW: writing—original draft, investigation, and writing—review and editing. LF and JZ: formal analysis and investigation. IK: conceptualization, methodology, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Scientific Research Starting Foundation of Chuzhou University, China (2022qd014).
Conflict of interest
HW was employed by the company China Light Industry Hesheng Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1086349/full#supplementary-material
References
1. Dang DX, Choi SY, Choi YJ, Lee JH, Castex M, Chevaux E, et al. Probiotic, paraprobiotic, and hydrolyzed yeast mixture supplementation has comparable effects to zinc oxide in improving growth performance and ameliorating post-weaning diarrhea in weaned piglets. Probiot Antimicrob Prot. (2023) 2023:1–10. doi: 10.1007/s12602-022-10008-8
2. Griggs JP, Jacob JP. Alternatives to antibiotics for organic poultry production. J Appl Poul Res. (2005) 14:750–6. doi: 10.1093/japr/14.4.750
3. Meng QW, Yan L, Ao X, Zhou TX, Wang JP, Lee JH, et al. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J Anim Sci. (2010) 88:3320–6. doi: 10.2527/jas.2009-2308
4. Dang DX, Zou Q, Xu Y, Cui Y, Li X, Xiao Y, et al. Feeding broiler chicks with bacillus subtilis, clostridium butyricum, and enterococcus faecalis mixture improves growth performance and regulates cecal microbiota. Probiot Antimicrob Prot. (2022) 2022:1–12. doi: 10.1007/s12602-022-10029-3
5. Salim HM, Kang HK, Akter N, Kim DW, Kim JH, Kim MJ, et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci. (2013) 92:2084–90. doi: 10.3382/ps.2012-02947
6. Mashayekhi H, Mazhari M, Esmaeilipour O. Eucalyptus leaves powder, antibiotic and probiotic addition to broiler diets: effect on growth performance, immune response, blood components and carcass traits. Animal. (2018) 12:2049–55. doi: 10.1017/S1751731117003731
7. Ouwehand A. C., Salminen S., and Isolauri E. Probiotics: an overview of beneficial effects. Lactic Acid Bact Gen Metabol Appl. (2002) 3:279–89. doi: 10.1007/978-94-017-2029-8_18
8. O'dea EE, Fasenko GM, Allison GE, Korver DR, Tannock GW, Guan LL. Investigating the effects of commercial probiotics on broiler chick quality and production efficiency. Poult Sci. (2006) 85:1855–63. doi: 10.1093/ps/85.10.1855
9. Cartman ST, La Ragione RM, Woodward MJ. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl Environ Microbiol. (2008) 74:5254–8. doi: 10.1128/AEM.00580-08
10. Intanoo M, Kongkeitkajorn MB, Suriyasathaporn W, Phasuk Y, Bernard JK, Pattarajinda V, et al. Effect of supplemental Kluyveromyces marxianus and Pichia kudriavzevii on aflatoxin M1 excretion in milk of lactating dairy cows. Animals. (2020) 10:709. doi: 10.3390/ani10040709
11. Samanya M, Yamauchi KE. Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var natto Comparative Biochemistry and Physiology Part A. Mol Integrative Physiol. (2002) 133:95–104. doi: 10.1016/S1095-6433(02)00121-6
12. Chen KL, Kho WL, You SH, Yeh RH, Tang SW, Hsieh CW, et al. Effects of Bacillus subtilis var natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poultry Sci. (2009) 88:309–15. doi: 10.3382/ps.2008-00224
13. Wang YC, Hu SY, Chiu CS, Liu CH. Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol. (2019) 84:1050–8. doi: 10.1016/j.fsi.2018.11.017
16. Dang DX, Chun SG, Kim IH. Phytase expressed from Saccharomyces pombe ameliorates footpad lesions in cage-reared broiler chicks. Vet Med Sci. (2022) 8:654–9. doi: 10.1002/vms3.745
17. Tako E, Ferket PR, Uni Z. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult Sci. (2004) 83:2023–8. doi: 10.1093/ps/83.12.2023
18. Lowry O, Rosebrough N, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. (1951) 193:265–75. doi: 10.1016/S0021-9258(19)52451-6
19. Zhang M, Liu YL, Fu CY, Wang J, Chen SY, Yao J, et al. Expression of MyHC genes, composition of muscle fiber type and their association with intramuscular fat, tenderness in skeletal muscle of Simmental hybrids. Mol Biol Rep. (2014) 41:833–40. doi: 10.1007/s11033-013-2923-6
20. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. (2008) 3:1101–8. doi: 10.1038/nprot.2008.73
21. Ateya AI, Arafat N, Saleh RM, Ghanem HM, Naguib D, Radwan HA, et al. Intestinal gene expressions in broiler chickens infected with Escherichia coli and dietary supplemented with probiotic, acidifier and synbiotic. Vet Res Commun. (2019) 43:131–42. doi: 10.1007/s11259-019-09753-z
22. Tarabees R, Gafar KM, El-Sayed MS, Shehata AA, Ahmed M. Effects of dietary supplementation of probiotic mix and prebiotic on growth performance, cecal microbiota composition, and protection against Escherichia coli O78 in broiler chickens. Probiotics Antimicrob Proteins. (2019) 11:981–9. doi: 10.1007/s12602-018-9459-y
23. Fazelnia K, Fakhraei J, Yarahmadi HM, Amini K. Dietary supplementation of potential probiotics Bacillus subtilis, Bacillus licheniformis, and Saccharomyces cerevisiae and synbiotic improves growth performance and immune responses by modulation in intestinal system in broiler chicks challenged with salmonella typhimurium. Probiotics Antimicrob Proteins. (2021) 13:1081–92. doi: 10.1007/s12602-020-09737-5
24. Prakash A, Saxena VK, Singh MK. Genetic analysis of residual feed intake, feed conversion ratio and related growth parameters in broiler chicken: a review. World's Poultry Sci J. (2020) 76:304–17. doi: 10.1080/00439339.2020.1735978
25. Zhai H, Adeola O, Liu J. Phosphorus nutrition of growing pigs. Animal Nutri. (2022) 9:127–37. doi: 10.1016/j.aninu.2022.01.010
26. Liu JB, Xue PC, Cao SC, Liu J, Chen L, Zhang HF, et al. Effects of dietary phosphorus concentration and body weight on postileal phosphorus digestion in pigs. Anim Feed Sci Technol. (2018) 242:86–94. doi: 10.1016/j.anifeedsci.2018.06.003
27. Zhang ZF, Kim IH. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. (2014) 93:364–70. doi: 10.3382/ps.2013-03314
28. Giang HH, Viet TQ, Ogle B, Lindberg JE. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest Sci. (2010) 129:95–103. doi: 10.1016/j.livsci.2010.01.010
29. Stanley D, Denman SE, Hughes RJ, Geier MS, Crowley TM, Chen H, et al. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl Microbiol Biotechnol. (2012) 96:1361–9. doi: 10.1007/s00253-011-3847-5
30. Wang X, Zhang P, Zhang X. probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. (2021) 26:6076. doi: 10.3390/molecules26196076
31. Yan W, Sun C, Yuan J, Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci Rep. (2017) 7:1–11. doi: 10.1038/srep45308
32. Das R, Mishra P, Jha R. In ovo feeding as a tool for improving performance and gut health of poultry: a Review. Front Vet Sci. (2021) 8:754246. doi: 10.3389/fvets.2021.754246
33. Vaezi G, Teshfam M, Bahadoran S, Farazyan H, Hosseini S. Effects of different levels of lysine on small intestinal villous morphology in starter diet of broiler chickens. Glob Veterin. (2011) 7:523–6.
34. Liu H, Zhang J, Zhang S, Yang F, Thacker PA, Zhang G, et al. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agricult Food Chem. (2014) 62:860–6. doi: 10.1021/jf403288r
35. Dibner JJ, Richards JD. The digestive system: challenges and opportunities. J Appl Poult Res. (2004) 13:86–93. doi: 10.1093/japr/13.1.86
36. Chen MJ, Zhou JY, Chen YJ, Wang XQ, Yan HC, Gao CQ, et al. The in ovo injection of methionine improves intestinal cell proliferation and differentiation in chick embryos by activating the JAK2/STAT3 signaling pathway. Animal Nutri. (2021) 7:1031–8. doi: 10.1016/j.aninu.2021.03.009
37. Giannenas I, Papadopoulos E, Tsalie E, Triantafillou EL, Henikl S, Teichmann K, et al. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol. (2012) 188:31–40. doi: 10.1016/j.vetpar.2012.02.017
38. Sen S, Ingale SL, Kim YW, Kim JS, Kim KH, Lohakare JD, et al. Effect of supplementation of Bacillus subtilis LS 1-2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res Vet Sci. (2012) 93:264–6. doi: 10.1016/j.rvsc.2011.05.021
39. Wang B, Zhou Y, Tang L, Zeng Z, Gong L, Wu Y, et al. Effects of Bacillus amyloliquefaciens Instead of antibiotics on growth performance, intestinal health, and intestinal microbiota of broilers. Front Vet Sci. (2021) 8:679368. doi: 10.3389/fvets.2021.679368
40. Abdel-Moneim AME, Selim DA, Basuony HA, Sabic EM, Saleh AA, Ebeid TA, et al. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod. (2020) 52:671–80. doi: 10.1007/s11250-019-02055-1
41. Gong L, Wang B, Mei X, Xu H, Qin Y, Li W, et al. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Animal Sci J. (2018) 89:1561–71. doi: 10.1111/asj.13089
42. Dong XY, Wang YM, Yuan C, Zou XT. The ontogeny of nutrient transporter and digestive enzyme gene expression in domestic pigeon (Columba livia) intestine and yolk sac membrane during pre-and posthatch development. Poult Sci. (2012) 91:1974–82. doi: 10.3382/ps.2012-02164
43. Yaghobfar A, Kalantar M. Effect of non-starch polysaccharide (NSP) of wheat and barley supplemented with exogenous enzyme blend on growth performance, gut microbial, pancreatic enzyme activities, expression of glucose transporter (SGLT1) and mucin producer (MUC2) genes of broiler chickens. Brazilian J Poultry Sci. (2017) 19:629–38. doi: 10.1590/1806-9061-2016-0441
44. Mott CR, Siegel PB, Webb Jr KE, Wong EA. Gene expression of nutrient transporters in the small intestine of chickens from lines divergently selected for high or low juvenile body weight. Poult Sci. (2008) 87:2215–24. doi: 10.3382/ps.2008-00101
45. Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. (2009) 587:195–210. doi: 10.1113/jphysiol.2008.159616
46. Wong EA, Gilbert ER, Miska KB. Nutrient transporter gen e expression in poultry, livestock, and fish. Biol Domes Animals. (2017) 3:319–44. doi: 10.1201/9781315152080-11
47. Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. (1997) 113:332–40. doi: 10.1016/S0016-5085(97)70112-4
48. Faseleh Jahromi M, Wesam Altaher Y, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, et al. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int J Biometeorol. (2016) 60:1099–110. doi: 10.1007/s00484-015-1103-x
Keywords: Pichia farinose, Bacillus subtilis, probiotic mixture, broiler chick, nutrient absorption
Citation: Wang H, Fu L, Zhang JY and Kim IH (2023) Bacillus subtilis and Pichia farinose mixture improves growth performance and nutrient absorption capacity in broiler chicks. Front. Vet. Sci. 10:1086349. doi: 10.3389/fvets.2023.1086349
Received: 01 November 2022; Accepted: 27 February 2023;
Published: 23 March 2023.
Edited by:
Muhammad Saeed, Cholistan University of Veterinary and Animal Sciences, PakistanReviewed by:
Shiping Bai, Sichuan Agricultural University, ChinaKai Qiu, Chinese Academy of Agricultural Sciences (CAAS), China
Copyright © 2023 Wang, Fu, Zhang and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Wang, d2gyMjAyMDNAMTYzLmNvbQ==; In Ho Kim, aW5ob2tpbUBkYW5rb29rLmFjLmty
 Huan Wang
Huan Wang Lu Fu1
Lu Fu1 Jian Ying Zhang
Jian Ying Zhang