- 1Centro de Investigación Veterinaria de Tandil (CIVETAN), UNCPBA-CICPBA-CONICET, Tandil, Buenos Aires, Argentina
- 2CONICET, Buenos Aires, Argentina
- 3Departamento Zoonosis Rurales Azul, Ministerio de Salud de la Provincia de Buenos Aires, Azul, Buenos Aires, Argentina
- 4Facultad de Agronomía, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Azul, Buenos Aires, Argentina
- 5Universidad Nacional del Centro de la Provincia de Buenos Aires, Facultad de Ciencias Veterinarias, SAMP, Tandil, Buenos Aires, Argentina
Leptospirosis is an infectious disease caused by pathogenic Leptospira that affect humans and animals. This disease is complex and non-eradicable in nature. Therefore, the understanding of it is epidemiology in different environments is crucial to implement prevention and control measures. The prevalence of Leptospira infection in beef cattle farms is affected by multiple environmental, management and individual factors. In this study, a cross-sectional serological survey was carried on to estimate the prevalence of Leptospira antibodies in beef cattle in Tandil and Ayacucho Departments (Buenos Aires Province) and to identify risk factors and spatial clusters associated with seropositivity. Using a probabilistic two-stage sampling, 25 farms and 15 animals per farm were selected. The Microagglutination Test was used to analize all serum samples. Bivariate and multivariate analyses were performed. Seventy-three out of 375 cows were seropositive, representing a positivity rate of 19.47% (95% CI: 10.51–28.42), with Sejroe and Pomona being the most reactive serogroups: 9.33% (95% CI: 6.26–12.41) and 8.27% (95% CI: 5.35–11.19), respectively. The prevalence in Ayacucho was 23.11% (95% CI: 10.05–36.17), and in Tandil, 14% (95% CI: 3.25–24.75). The animals from Ayacucho presented 2.01 (1.16–3.49) more chances of being positive compared with those from Tandil (p < 0.01). After the Generalized Linear Mixed Model (GLMM) with random effect of farm-level risk, the presence of lagoons (OR: 7.32, 95% CI: 1.68–31.8, p < 0.05) and undulating terrain (OR: 0.24, 95% CI: 0.07–0.74, p < 0.05) were associated with bovine leptospirosis. Four spatial clusters with higher rates of seropositivity were detected. A new GLMM was performed with the significant variables detected in the first GLMM and a new variable, “being inside the spatial cluster,” being the only one that remained significant (OR: 9.58, 95% CI: 3.39–27.08, p < 0.0001). The animals inside the clusters belonged to farms with a greater presence of creeks (OR: 9.03, 95% CI: 3.37–24.18, p < 0.0001), higher accumulated rainfall (OR: 1.01, 95% CI: 1–1.01, p < 0.0001) and less undulating terrain (OR: 0.18, 95% CI: 0.10–0.35, p < 0.0001). We conclude that Leptospira is seroprevalent in beef cattle in Tandil and Ayacucho Departments, especially in the latter, where the largest cattle farms are located. Prevalence of seropositivity animals was associated with selected environmental risk factors.
Introduction
Leptospirosis is a zoonotic disease with a worldwide distribution. It is caused by pathogenic helical spirochetes of the Leptospira genus (family Leptospiraceae, order Spirochaetales), which may affect humans, domestic and wild animals (1). In livestock, leptospirosis can cause economic losses, particularly in developing countries (2). Most infections are caused by either Leptospira borgpetersenii or Leptospira interrogans. Although clinical signs of the disease caused by these two species are similar, spatial distribution and ways of transmission varies according to the species. L. interrogans is commonly acquired from contaminated surface water, while L. borgpetersenii is mainly transmitted host-to-host (3).
When an animal that has been infected (at an early age) becomes a carrier, its urine contaminates the moist soil, and foraging areas within the perimeter of the animal. Young, healthy animals of the same species within the same area become infected by the oldest sick animals; the contamitation of surface waters leads to the risk of infection of other animals, whether wild or domestic (4). It is widely known that Leptospira can survive for months in the environment under favorable conditions and that alkaline urine in cattle promotes its perpetuation (5). Animals may be maintenance hosts for some serovars but incidental hosts for others. Bovine leptospirosis occurs worldwide and results from infection by several serovars. Leptospira borgpetersenii serovar Hardjo (Hardjobovis) is the common strain of this serovar maintained by cattle, but Leptospira interrogans serovar Hardjo (Hardjoprajitno) also occurs in cattle in some parts of the world. Pomona, Grippotyphosa and Icterohaemorrhagiae are the serogroups most frequently identified in incidental infections in cattle, and their transmission is related to pigs, rodents and wildlife. The acute and severe forms of leptospirosis (fever, icterus, mortality) are uncommon and frequently associated with sporadic outbreaks in calves caused by incidental serovars. In adult cattle, infection often results in high abortion rates among the infected herds a few weeks after the acute phase of the disease (4). Common signs of leptospirosis include reproductive failure, abortion, stillbirths, fetal mummification, weak calves and agalactia (5). In Argentina, to date, L. interrogans serovar Pomona (6) has been isolated as an incidental serovar and L. borgpetersenii as a maintenance serovar in Hardjo type Hardjo Bovis (7).
It is important to study the serovars that can infect cattle in a region and to know the ecoepidemiology of the disease for the correct implementation of control and prevention measures in the production system. The prevalence of Leptospira is affected by environmental factors and cattle management (8). This study was carried on to estimate the prevalence of Leptospira antibodies in beef cattle in Tandil and Ayacucho Departments and to assess the associations between seropositivity and risk factors.
Materials and methods
Study area
Ayacucho and Tandil Departments are located in the central-east of Buenos Aires Province, Argentina. This province is part of the Pampas Region of the country, constituting the area with the best conditions for agricultural activities. However, the productive characteristics differ throughout the region. Remarkable climatic, edaphic and physiographic differences have determined various uses and production systems (9). Ayacucho is located within the sub-region called Pampa Deprimida (defined by the Salado river basin) at an average of 74 meters above sea level (37° 09′ S and 58° 28′ W). The climate is temperate, with an average annual temperature of 14.25°C and average rainfall of 870 mm. Livestock, mainly breeding beef cattle, is the mainstay of the economy. Tandil is located in the mountains of the Tandilia system, at an average altitude of 284 meters above sea level (37° 04′ S and 59° 08′ W). The climate is temperate, with an average annual temperature of 13.5°C and average rainfall of 879 mm. The local economy is based on agriculture, livestock and other productions. Ayacucho Department has a cattle population of 683,004 distributed in 1,721 farms, whereas Tandil has 248,696 cattle distributed in 793 farms (10). Ayacucho Department neighbors Tandil. The geographical contiguity within Ayacucho and Tandil districtis leads to flooding occurrence in Ayacucho. This is due to the geological characteristics of Ayacucho district (low altitude and slight slope) and its proximity to the Tandilia System (higher altitude subregion). This system works as a centre of rainwater dispersion toward the Rio de la Plata and the Atlantic Ocean. All the creeks crossing the department, such as Tandileofú, Chelforó, Perdido and Langueyú, have their source in the Tandilia system (9, 11).
Study design, sampling, and data collection
A cross-sectional serological survey was carried on from September 2017 to December 2020. Using a probabilistic two-stage sampling, 25 farms and fifteen animals per farm were selected. SENASA (Servicio Nacional de Sanidad y Calidad Agroalimentaria) and Fundación Aftosa provided the region's database. In the first stage, the number of farms to be selected was calculated using the ProMesa 1.3 programme (12) using the following formula:
Where p is the expected seroprevalence of cows reactive to Leptospira, z is the confidence level, ROH is the homogeneity rate, b is the number of animals selected per farm and e is the acceptable (allowable) absolute error. Our assumptions were as follows: a prevalence of 20.33% (13), a relative error of 33%, a low homogeneity rate (0.06–0.12) (14) and 15 animals sampled per farm. The minimum sample size estimated was 25 farms (375 animals). More farms were included from Ayacucho (14) because this department has the largest number of cattle farms. The second stage was conducted on each farm through systematic randomization of the animals. The animals included in the study were bulls and breeding females ≥15 months of age, that were not vaccinated against Leptospira spp. or had been vaccinated more than 6 months before sampling. In addition, a questionnaire was administered to collect epidemiological information about the potential risk factors associated with seropositivity to Leptospira spp. The survey was based on general production and environmental characteristics, feeding practices, health and reproduction status. All farms were georeferenced using Global Positioning Systems (GPS) to perform spatial analysis and subsequent search for meteorological and satellite information such as the presence of water bodies, potential evapotranspiration, temperature and solar radiation. Water bodies were detected using information from the Landsat 8 satellite (spatial resolution of 30 meters) and calculated with MNDWI (Modified Normalized Difference Water Index) (15) with the QGIS software. The potential evapotranspiration was obtained through the EOS-Terra satellite and estimated using the ENVI software. The temperature was calculated monthly through data from the National Meteorological Service and solar radiation, using information from the sensor CERES (Clouds and the Earth's Radiant Energy System) (16). On the farms, blood samples were collected from the animals by venipuncture. The blood was allowed to clot at room temperature and centrifuged at 1,500 rpm for 15 min. The sera were separated and stored at −20°C.
Serological testing
The Microagglutination Test (MAT), the reference serological test, was used for processing samples, considering a titer of ≥1:200 as a criterion of positivity. This is the cut-off point used in cattle in Argentina since leptospirosis is an endemic disease and because cattle are vaccinated in areas where this agent is present (17). Antibodies against pathogenic Leptospira were detected by the MAT in the Leptospirosis Laboratory, Department of Rural Zoonosis (Ministry of Health of Buenos Aires Province), according to WOAH (18) protocols.
A panel of live antigens of ten Leptospira spp. reference strains were used: L. interrogans serogroup Canicola serovar Canicola strain H. Utrecht IV, serogroup Hebdomadis serovar Hebdomadis strain Hebdomadis, serogroup Icterohaemorrhagiae serovar Copenhageni strain M20, serogroup Pomona serovar Pomona strain Pomona, serogroup Pyrogenes serovar Pyrogenes strain Salinem, serogroup Sejroe serovar Wolfii strain 3,705 and serogroup Sejroe serovar Hardjo strain Hardjoprajitno, L. borgpeterseni serogroup Ballum serovar Castellonis strain Castellon 3 and serogroup Tarassovi serovar Tarassovi strain Perepelitsin and L. kirschneri serogroup Grippotyphosa serovar Grippotyphosa strain Castellon 3. This panel was developed at 28–30°C in the Ellinghausen-McCullough-Johnson-Harris (EMJH) medium with no more than 15 days of growth. Serial serum dilutions were performed with phosphate-buffered saline (PBS, pH 7.2) starting from 1:100 dilution. The plates were incubated at 37°C for 90 min. After incubation, the serum-antigen mixtures were checked for agglutination under a dark field microscope. Tests were interpreted as positive when agglutination at ≥ 1:200 of at least 50% of the leptospires for any serogroup was observed. The highest serum dilution with >50% agglutination or ≤50% free leptospires, compared to the negative control, was considered the endpoint titer of quantitative MAT.
Data analysis
The data about each animal, the characteristics of each farm and laboratory results were entered into an Excel database (Microsoft, Redmond, WA, USA). Prevalence of anti-Leptospira spp. antibodies with the 95% Confidence Interval (CI) was estimated. Also, farms positivity was determined. The association between the outcome seropositivity to Leptospira spp., to Leptospira serogroup Sejroe (adapted serogroup) and to Leptospira serogroup Pomona (incidental serogroup) and the variables under analysis was assessed by a bivariate analysis using a Chi-squared test. Fisher's exact test was used if one or more cells expected value was <5. Odds ratios (OR) and 95% CI were also estimated and calculated for each variable. For quantitative variables, parametric or non-parametric tests were used. The null hypothesis was that there were no differences between groups. All the statistical tests were carried out at α=0.05. Quantitative and qualitative variables at p-value < 0.2 in the bivariate analysis were analyzed by a Generalized Linear Mixed Model (GLMM) with random effect of farm-level risk. All statistical analyses were performed with software R v. 4.0.2 (10).
Potential spatial clusters were investigated in the study area with space scan statistics using SaTScan software, v10.0.2. Poisson model for high rates was performed for detecting spatial patterns of the number of MAT positive events in a geographical location, taking each farm as a unit for analysis according to a known population at risk (19).
Another qualitative variable called “being inside the spatial clusters” was generated, and an animal was considered to be exposed to the variable when it belonged to a farm inside a significant cluster. The animal was not exposed to the variable when it belonged to a farm outside the significant clusters. GLMM was performed with the significant variables detected in the first GLMM with a new variable, “being inside the spatial clusters.”
Ethical considerations
This work has been approved by the Ethics Committee according to the Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine (U.N.C.P.B.A, Tandil, Argentina) http://www.vet.unicen.edu.ar.
Results
Twenty-five farms were sampled: 14 from Ayacucho and 11 from Tandil. The spatial distribution of the farms is shown in Figure 1. The size of the farms used for livestock ranged from 45 to 3,500 hectares. The largest farms were those located in Ayacucho. These farms had 100 to 1,800 females' cattle (cows and heifers), and the hectares devoted to livestock varied from 88 to 3,500. In Tandil, the hectares used for livestock ranged from 45 to 770, and the number of females varied from 50 to 550. In both departments, the pregnancy rate varied from 86 to 100% (22 farms), with an average of 92%. The average calving rate was 91% (14 fields), varying from 81 to 100%. These herds had no history of diagnosis of leptospirosis (Figure 1).
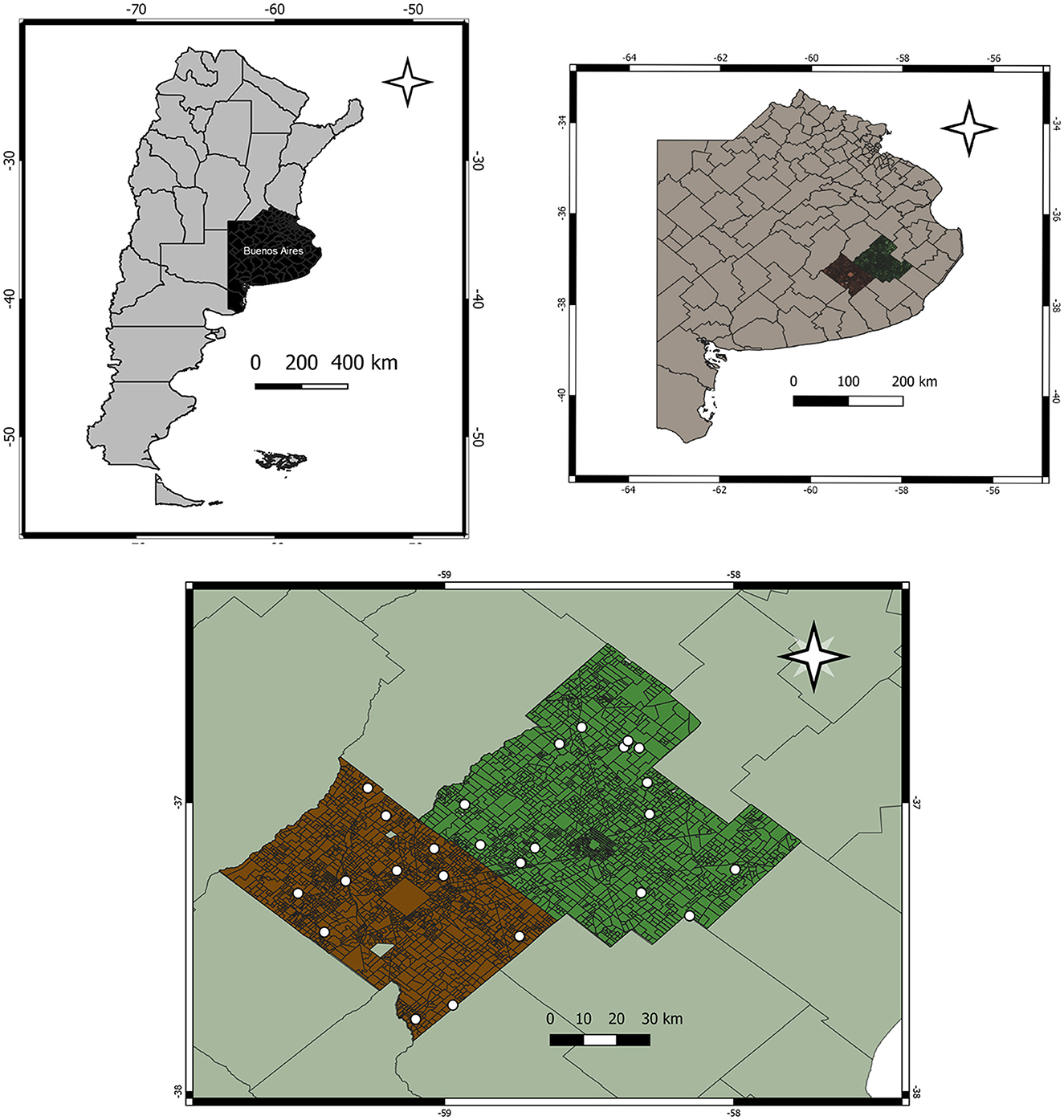
Figure 1. Map of Argentina. Map of Buenos Aires Province. Geographic boundaries of the Departments (Ayacucho and Tandil) and sampling sites.
Seventy-three out of 375 animals (19.47%; 95% CI: 10.51–28.42) were seropositive, and 72% (95% CI: 52.4–91.6) of the farms had at least one positive animal. Sejroe was the most prevalent serogroup (35/375), 9.33% (95% CI: 6.26–12.41), followed by Pomona (31/375), 8.27% (95% CI: 5.35–11.19), Hebdomadis (30/375), 8% (95% CI: 5.12–10.9), Tarassovi (4/375), 1.1% (95% CI: 0.29–2.71) and Canicola (2/375), 0.53% (95% CI: 0.07–1.91). Cross-reactions occurred between 2 and 3 or more serogroups in 26.03% (19/73) and 6.85% (5/73) of the positive samples, respectively.
Seroprevalence in Ayacucho was 23.11% (95% CI: 10.05–36.17), and in Tandil 14% (95% CI: 3.25–24.75). The animals from Ayacucho presented 2.01 (1.16–3.49) more chances to be positive as those from Tandil (p < 0.01). Besides, the farm seroprevalence in Ayacucho 80% (95% CI: 59.05–100.95) was not significaly differente from Tandil 60% (95% CI: 28–92.01(p > 0.05).
Tables 1, 2 show epidemiological data, individual factors, structural and management conditions on the farms and other environmental and geographical exposures as well as the relation with the seropositivity to the infection with Leptospira spp.
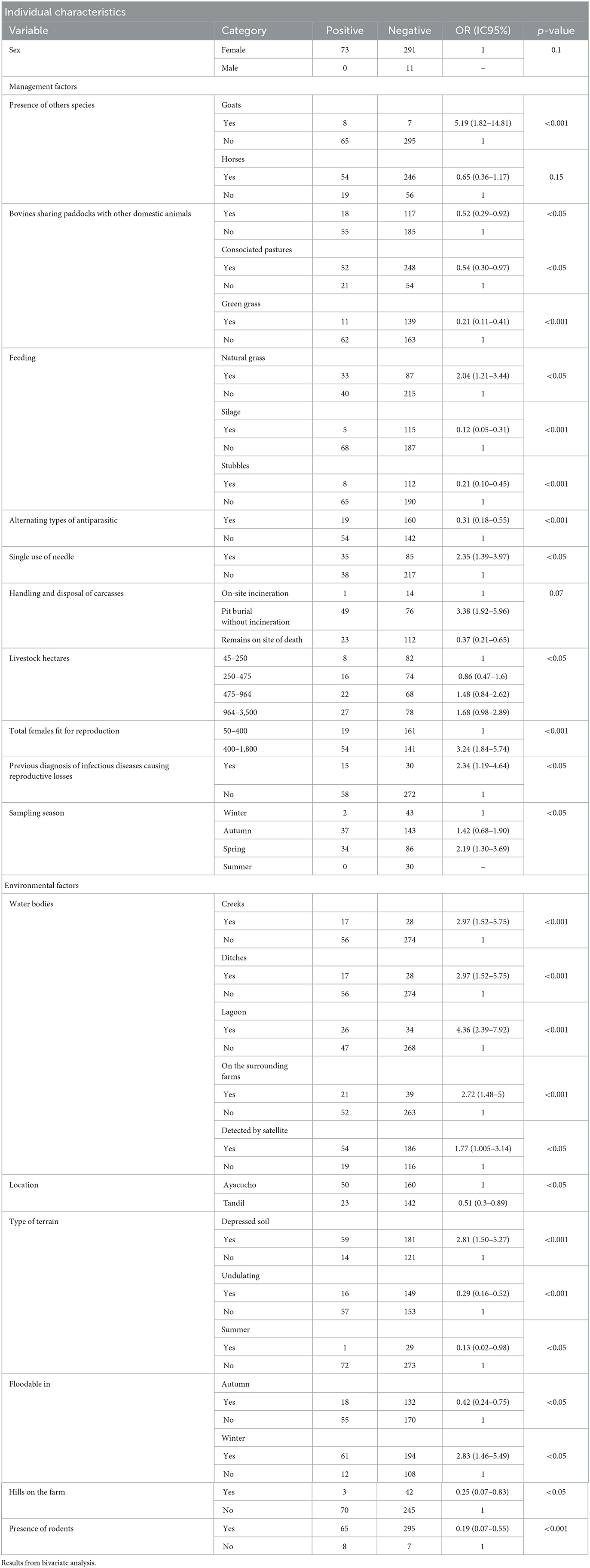
Table 1. Qualitative risk factors associated with leptospirosis seropositivity in beef cattle from Tandil and Ayacucho Departments, Buenos Aires Province, Argentina.
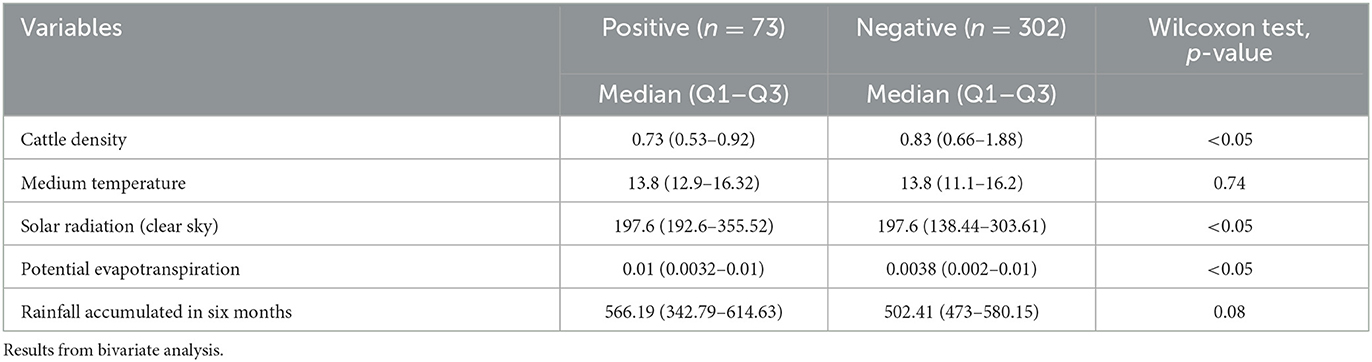
Table 2. Quantitative risk factors associated with leptospirosis seropositivity in beef cattle from Tandil and Ayacucho Departments, Buenos Aires Province, Argentina.
The GLMM with the smallest Akaike's information criterion (AIC = 310.35) was selected as the best one. The significant predictors that best explained seropositivity to Leptospira spp. were the predominance of undulating terrain (OR: 0.24, 95% CI: 0.07–0.74, p < 0.05) and presence of lagoons in the fields (OR: 7.32, 95% CI: 1.68–31.81, p < 0.05).
Concerning the spatial analysis, four high-rate clusters of seropositive cattles were found. Using the Poisson model, a spatial cluster (1) was detected in Ayacucho (37° 13′ 48″ S, 57° 59′ 24″ W; radius: 30.10 km; p < 0.001), where the risk of infection was nearly 28 (RR = 28.29) times higher on farms located inside the cluster than on those located elsewhere. A second cluster was detected (2) (36°44′ 24″ S, 58° 31′ 12″ W; radius 9.38 km, p < 0.001), where the risk of infection was 8 (RR = 8.47) times higher than on the farms located inside the cluster. Two other clusters (3 and 4) were detected in Ayacucho (36°55′48″ S, 58°17′24″ W; radius 0 km; p < 0.001), where the risk of infection was 22 (RR = 22.27) and in Tandil (37° 15′ 12.34″ S, 59° 0′ 13.28″ W, radius: 0 km; p < 0.001), where the risk of infection was 9.16 (Figure 2).

Figure 2. Spatial clusters of higher risk of leptospirosis found in Ayacucho and Tandil Departments.
When a new GLMM was performed including the significant variables detected in the first GLMM and the new variable “being inside the spatial clusters,” the only variable that remained significant was the new one (OR: 9.58, 95% CI:3.39–27.08, p < 0.001) (Table 3). This model was the best Akaike had (AIC: 297.61).
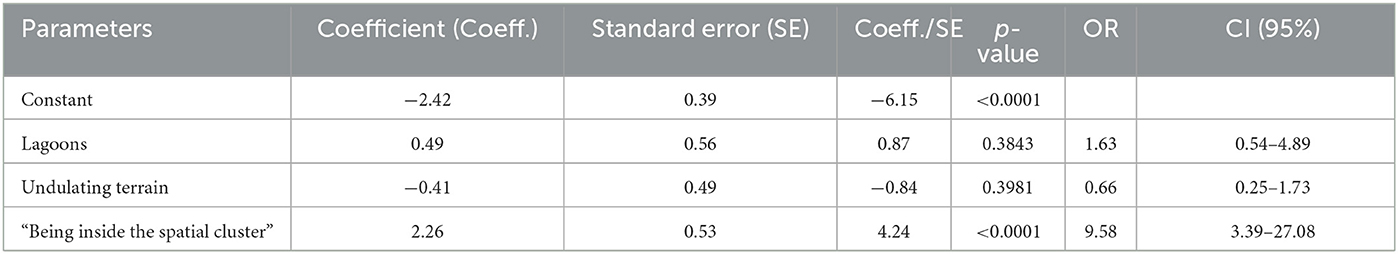
Table 3. Leptospirosis seropositivity predictors in beef cattle from Tandil and Ayacucho Departments as determined by the Generalized Linear Mixed Model (GLMM) with random effect of farm-level risk.
Considering the last analysis, factors associated with the variable “being inside the spatial clusters” were assessed through bivariate and multivariate analyses. Results from the bivariate analysis of qualitative and quantitative variables are presented in Tables 4, 5, respectively.
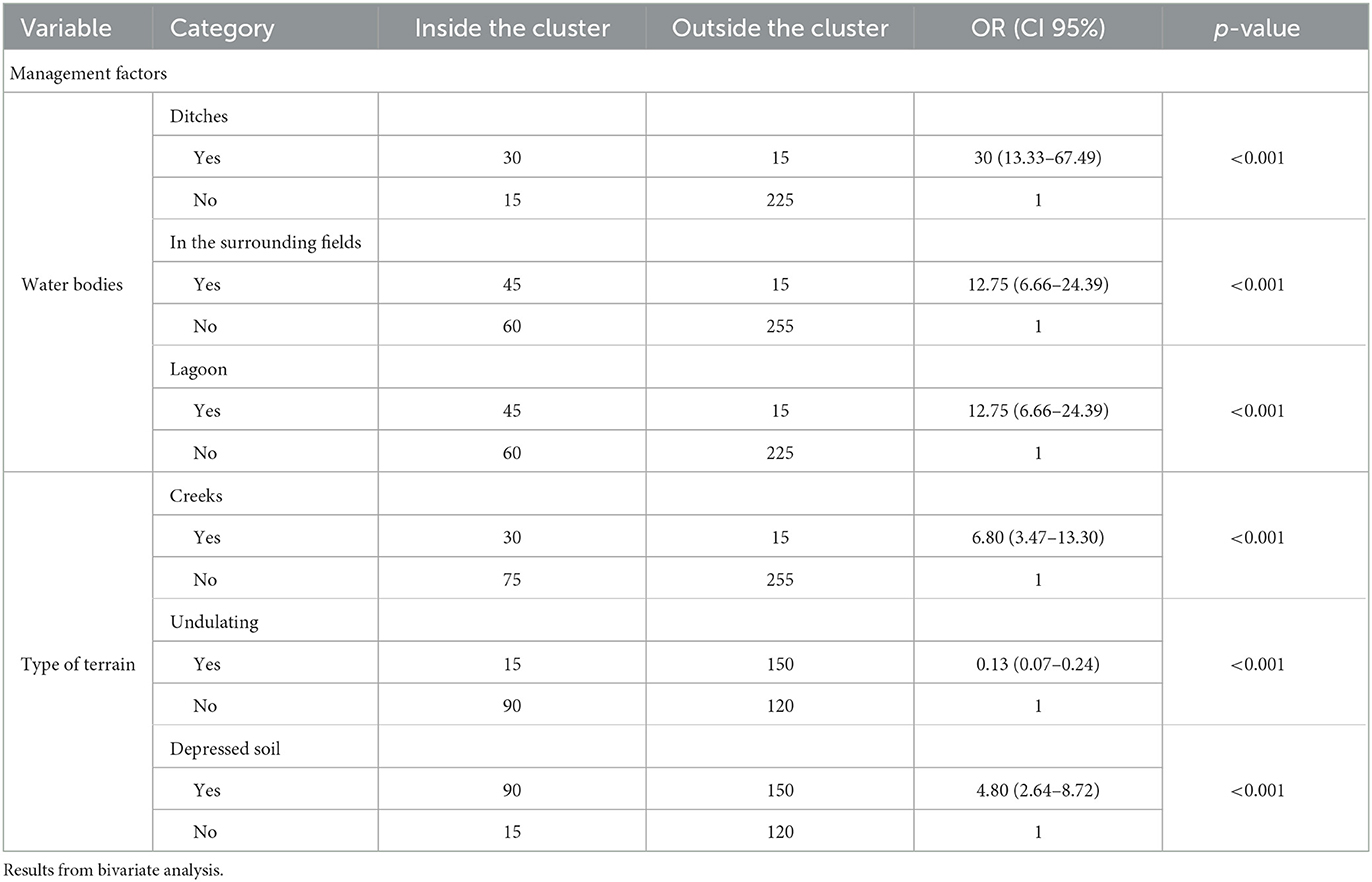
Table 4. Qualitative variables associated with the variable “being within the cluster” in beef cattle from Tandil and Ayacucho Departments, Buenos Aires Province, Argentina.
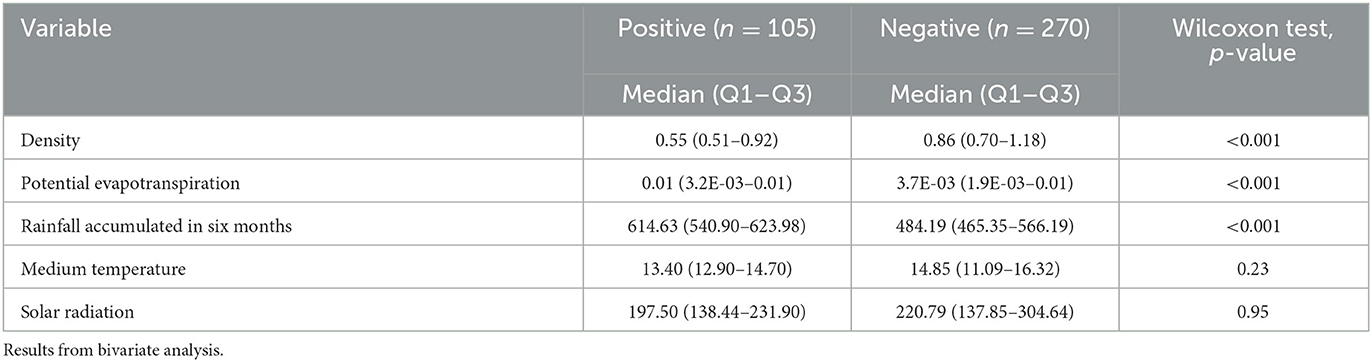
Table 5. Quantitative variables associated with the variable “being within the cluster” in beef cattle from Tandil and Ayacucho Departments, Buenos Aires Province, Argentina.
In the logistic regression model, the variables that best explained the variable “being inside the spatial clusters” were the presence of creeks on the farms (OR:9.03, 95% CI:3.37–24.18, p < 0.0001), the accumulated rainfall (OR:1.01, 95% CI:1–1.01, p < 0.0001), and the predominance of undulating terrain (OR:0.18, 95% CI:0.10–0.35, p < 0.0001) (Deviance:290, p-value:1 and df: 371).
Finally, a multivariate study was carried out to determine factors associated with the seroprevalence of Leptospira serogroup Sejroe and the seroprevalence of Leptospira serogroup Pomona. The significant variables found in the previous models and the presence of sheeps, goats and swines and the density of bovines were included in the model. In both cases, the presence of lagoons in the fields were associated with the infection (OR: 4.53, 95% CI: 1.19–17.16) (AIC:218.59) and (OR: 44.7, 95% CI:1.01–1,964) (AIC:146.86), respectively.
Discussion
Seroprevalence of leptospirosis found in the departments of Tandil and Ayacucho (19.47%) was similar to that reported by Linzzito et al. (20), with 20.33% of serologically positive cattle in the Cuenca del Salado region. However, these results differ from a study carried out in the insular region of the Paraná River Delta (Campana, Buenos Aires Province), where the prevalence found was 33.67% (out of a total of 199 animals studied) (21). The differences could be explained by the edaphoclimatic conditions that are different from those in the present study since insularity characteristics are favoirs the maintenance of Leptospira spp. (22). Also, the few records of diagnosis due to losses of Leptospira spp. on the farms studied in this research and the high prevalence recorded are evidence that the agent is probably underdiagnosed in our region.
The most reactive serogroups were Sejroe 9.33% (95% CI: 6.26–12.41), Pomona 8.27% (95% CI: 5.35–11.19) and Hebdomadis 8% (95% CI: 5.12–10.9). These results are in agreement with clinical reports associated with abortions, stillbirths and calf mortality in Argentina (6). Recently, episodes of reproductive losses associated with high titres of antibodies have been detected against serovars adapted to cattle, such as L. Hardjo and L. Wolffi (23). The most frequent serovars found by Gamietea et al. (21) were Pomona (32.66%), Wolffi (27.64%), Castellonis serogroup Ballum and Icterohaemorrhagiae. The differences between the two studies (such as the detection of L. Icterohaemorrhagiae) may be due to the important role that wildlife, particularly rodents, may be playing in the Paraná River Delta, a habitat of several wild species (21).
From the begining of the initialy, leptospirosis was considered an occupational or environmental disease: “Harvest Fever,” “Cane Cutter's Disease” (24). For this reason, many studies have focused on sources and environmental risk factors to understand its epidemiology (22, 25). Leptospira has been found in water and soil environments in rural and urban areas (26, 27). Several factors are asociated for perpetuation of the bacteria, such as warm temperature, humid environments, neutral or slightly alkaline pH, and the presence of organic material (22, 28). Consequently, surfe of water and animal overcrowding are likely to be important factors associated to leptospirosis outbreaks (27, 28). Likewise, in this work, the models that explained Leptospira seropositivity were the presence of lagoons and upper soil environments—probably due to the effect of water drainage—and the being inside the spatial clusters. Furthermore, the spatial clusters were determined by the presence of creeks, millimeters of rainfall accumulated in the last 6 months, and undulating terrain. This was also demonstrated in other studies where leptospirosis notifications were asocciated after rainfall (25, 29). Also, in a previous study, isolated Leptospira spp. from soil and water bodies (30). Contaminated water is one of the primary sources of leptospirosis for humans and animals (25, 26, 31). Some reports describe the successful isolation of pathogenic and virulent leptospires from freshwater or soil (31, 32). Also, the survival ability of leptospires for long periods has been reported (31).
The results of this study showed the high presence of the adapted-to-cattle serogroup and the incidental Pomona serogroup. The presence of small ruminants and swines did not explain Leptospira seropositivity in either adapted or incidental serogroups. The bovine density was not relevant either to explain the seropositivity to Leptospira since these farms continue to be mostly extensive production and do not concentrate many animals per land area. For this reason, it is necessary to consider which wildlife species and niches are found in the area because they are also carriers of several serovars (33, 34).
In the final model, the variable that best explained Leptospira seropositivity was to belong to spatial cluster. In other words, the geographic area was associated to high prevalence of infection. The fact that this factor has been found can only make us consider the need for new explorations to determine potential risk factors that are more relevant. Knowledge about environmental factors and determinants for the survival of pathogenic leptospires in the environment remains scarce, and contributes to the inadequate understanding of the basic features of leptospirosis epidemiology (35). More precisely, the association of the survival ability of different strains and the environmental conditions remains largely uninvestigated (22).
The animals in farms located at higher altitudes had a lower risk of infection, probably because, in these sites, the formation of water bodies is less likely, since the water drains down slope. According to Bierque et al. (35), leptospires would be resuspended by rain and existing water particles in the soil, accompanied by the effect of soil washing. Therefore, less positivity will be expected in the high areas because water drains to lower areas.
The spatial clusters found in Ayacucho were expected due to the detection of adverse environmental factors. At the same time, given the better agriculture aptitudes, Tandil has less herds than Ayacucho. Consecutively, there is a management practice that involves sending cows in the last third of pregnancy to pastures with lower yields and forage quality (low soils) so that they do not increase their body condition and thus prevent possible dystocia (36). This must be considered because it can increase the risk of diseases that cause pregnancy loss, so it is imperative to use preventive measures to avoid outbreaks of abortions.
A preventive measure to implement is to vaccinate against Leptospira spp. especially when moving animals, in environments where the infection is endemic and the land's characteristics favor the agent's survival. Particularly in Departments such as Ayacucho, where more water bodies are expected to appear due to its territorial conditions that do not favor water drainage. If the animals have not received a vaccination, especially in young categories, heifers or pregnant cows, these animals should not be sent to low pastures where the ground is flooded or has these potential characteristics. Likewise, it is recommended to avoid large extensions of water bodies, such as lagoons, because it is a determining factor for the concentration of fauna that comes to the water supply, in addition to the fact that they are lands less traveled by man. Cows, despite having waters troughs, often use these water sources for consumption and to reduce their body temperature. For this reason, drinkers are suggested to ensure water circulation and reduce the possibility of contamination.
It is worth mentioning that although this study had a two-stage and randomized sampling, only the farms whose owners agreed to participate in the study were accessed, which may have generated a sample selection bias. This could be associated with the fact that they were farms where the workers suspected they had a problem with this disease or it seemed relevant to them to have more knowledge about the health status of their herds.
Conclusions
Our findings suggest that leptospirosis is endemic in the departments of Tandil and Ayacucho. In addition, the predominance of undulating terrain may decrease the seroprevalence of leptospirosis. Also, the presence of lagoons on the farms would increase the seroprevalence. Ayacucho exhibited a higher seroprevalence of Leptospira, which could generate potential productive and reproductive losses. Therefore, it is essential to consider these factors to implement prevention measures that reduce the risk of animal and human infection. The more preventive measures implemented in animals, the lower the risk of exposure to humans (One World, One Health).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by the Ethical Committee of Animal Welfare (Facultad de Ciencias Veterinarias, UNCPBA) ResCA 087/02.
Author contributions
Design of the study, critical revisions, supervision, and administration project: JP and MR. Sample collection and writing original draft preparation: MM. Laboratory analysis: ES and MM. Data analysis and interpretation: JP, MM, and MR. Writing of the revised manuscript: MM and MR. All authors have read and agreed to the final published version of the manuscript.
Funding
This work was supported financially by the National Scientific and Technical Research Council, Argentina through the Project Scientific Development Integrated in Animal Health. MM holds a fellowship from the National Scientific and Technical Research Council, Argentina.
Acknowledgments
We would like to acknowledge all the veterinarians, producers and owners who collaborated with sampling of the fields. We also thank SENASA and Fundación Aftosa for providing us with the information to conduct the sampling, and UE-IHLLA institution for providing us with the tools to analyze the environment. We are also grateful for the projects FONARSEC 19 and the Project with China. The authors would also like to thank Héctor Tarabla for the manuscript review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grippi F, Giudice E, Pietro SD, Sciacca C, Santangelo F, Galluzzo P, et al. Leptospira interrogans serogroup sejroe serovar hardjo in aborting cows: two herd cases in Sicily (Italy). J Vet Res. (2020) 64:73–8. doi: 10.2478/jvetres-2020-0021
2. Suwancharoen D, Chaisakdanugull Y, Thanapongtharm W, Yoshida S. Serological survey of leptospirosis in livestock in Thailand. Epidemiol Infect. (2013) 141:2269–77. doi: 10.1017/S0950268812002981
3. Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, Cullen PA, et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc Natl Acad Sci USA. (2006) 103:14560–5. doi: 10.1073/pnas.0603979103
4. Ellis WA. Animal leptospirosis. Curr Top Microbiol Immunol. (2015) 387:99–137. doi: 10.1007/978-3-662-45059-8_6
5. Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. (2010) 140:287–96. doi: 10.1016/j.vetmic.2009.03.012
6. Draghi MG, Brihuega B, Benítez D, Sala JM, Biotti GM, Pereyra M, et al. Brote de leptospirosis en terneros en recría en la provincia de Corrientes, Argentina. Rev Argentina Microbiol. (2011) 43:42–4.
7. Koval AA, Brihuega BF, Grune Loffler S, López S, Saint Martin M, Lagioia GG, et al. Primer aislamiento de Leptospira borgpetersenii serovar Hardjo tipo Hardjo Bovis a partir de un caso clínico en Argentina. Rev Argentina Microbiol. (2020) 52:198–201. doi: 10.1016/j.ram.2019.10.002
8. Levett PN. Leptospirosis: a forgotten zoonosis? Clin Appl Immunol Rev. (2004) 4:435–48. doi: 10.1016/j.cair.2004.08.001
9. PROSAP. Estrategia provincial para el sector agroalimentario de la provincia de Buenos Aires. Resolución del Ministerio de Asuntos Agrarios, N.° 84/10 (2010).
10. R Core Team,. R: A Language Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2020). Available online at: http://www.R-project.org (accessed 20 July, 2022).
11. Giuseppucci J, Ortino J. La geografía. Zubiaurre, Pablo. Ayacucho una historia Tomo 1. Libros del Espinillo [Historia Regional]. 1ra. Ayacucho, 2009. 326 pág. 15x23 (2009).
12. León E, Duffy S, ProMESA (Programa de Muestreo Estadístico en Sanidad Animal). Anales 39° Jornadas Argentinas de Informática y Congreso Argentino de Agroinformática. (2010). p. 807–817.
13. Linzitto OR, Passaro D, Soncini A, Gatti EMM, Gómez MF, Bautista LE, et al. Prevalencia de leptospirosis emergentes en bovinos. Rev Enferm Infec Emer. (2014) 9.
14. Otte MJ, Gumm ID. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev Med Vet. (1997) 31:47–150. doi: 10.1016/S0167-5877(96)01108-7
15. Xu H. Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. Int J Remote Sens. (2006) 27:3025–33. doi: 10.1080/01431160600589179
16. Smith GL, Priestley KJ, Loeb NG, Wielicki BA, Charlock TP, Minnis P, et al. Clouds and Earth Radiant Energy System (CERES), a review: PAST, present and future. Adv Space Res. (2011) 48:254–63. doi: 10.1016/j.asr.2011.03.009
17. Brihuega B, Draghi MG, Farace MI, Francois S, Koval A, Petrakovsky J, et al. (2017). Informe Sobre Leptospirosis. Comisión Científica de Leptospirosis. Asociación Argentina de Veterinarios de Laboratorio de Diagnóstico.
18. World Organization for Animal Health. Terrestrial Manual online access Chapter 3.1.12. Leptospirosis. (2021). Available online at: https://www.oie.int/standardsetting/terrestrial-manual/access-online/ (accessed May 2021).
19. Pfeiffer DU, Robinson TP, Stevenson M, Stevens KB, Rogers DJ, Clements ACA. Spatial Analysis in Epidemiology. New York, NY: Oxford University Press (2008). doi: 10.1093/acprof:oso/9780198509882.001.0001
20. Linzitto OR, Passaro D, Soncini A, Gatti EMM, Gómez MF, Bautista LE, et al. Prevalencia de leptospirosis emergentes en bovinos. Rev Enferm Infec Emer. (2014) 39:32.
21. Gamietea IJ, Martínez M, Grune Loffler S, Romero G, Brihuega B. Datos preliminares sobre serovares de Leptospira spp. predominantes en bovinos de la región insular del partido de Campana, delta inferior bonaerense del río Paraná, Argentina. III Congreso Panamericano – VIII Congreso Argentino de Zoonosis (2014).
22. Barragan V, Olivas S, Keim P, Pearson T. Critical knowledge gaps in our understanding about environmental cycling and transmission of Leptospira. Appl Environ Microbiol. (2017) 83:e01190-17. doi: 10.1128/AEM.01190-17
23. Cantón G, Fiorentino A, Moreira A, Hecker Y, Verna A, Moore P, et al. Pérdidas reproductivas en bovinos de Argentina asociados a seropositividad a Leptospira interrogans sejroe wolffi y hardjo. XXI Asociación Argentina de Veterinarios de Laboratorios de Diagnóstico (AAVLD) (2016).
24. Tarantola A, Goarant C. Leptospirosis in French historical medical literature–Weil's disease or Kelsch's disease? Am J Trop Med Hyg. (2018) 99:1366–8. doi: 10.4269/ajtmh.18-0629
25. Vanasco NB, Schmeling MF, Lottersberger J, Costa F, Ko AI, Tarabla HD. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999-2005). Acta Tropics. (2018) 107:255–8. doi: 10.1016/j.actatropica.2008.06.007
26. Romero-Vivas CM, Thiry D, Rodríguez V, Calderón A, Arrieta G, Máttar S, et al. Molecular serovar characterization of Leptospira isolates from animals and water in Colombia. Biomédica. (2013) 33:179–84. doi: 10.7705/biomedica.v33i0.731
27. Zamir L, Baum M, Bardenstein S, Blum SE, Moran-Gilad J, Markovich MP, et al. The association between natural drinking water sources and the emergence of zoonotic leptospirosis among grazing beef cattle herds during a human outbreak. One Health. (2022) 14:100372. doi: 10.1016/j.onehlt.2022.100372
28. Astuti NT, Adi MS, Martini M, Setyaningsih Y, Saraswati LD. The presence of pathogenic Leptospira sp. in water bodies in Klaten district. Indian J Public Health Res Dev. (2019) 10:439–43. doi: 10.5958/0976-5506.2019.00087.1
29. Dhewantara PW, Hu W, Zhang W, Yin WW, Ding F, Mamun AA, et al. Climate variability, satellite-derived physical environmental data and human leptospirosis: a retrospective ecological study in China. Environ Res. (2019) 176:108523. doi: 10.1016/j.envres.2019.06.004
30. Barbagelata SF, Brihuega B, Loffler SG, Marcos VG, Pérez DC, Melillo JP, et al. Isolation of Leptospira borgpetersenii in water sources in Argentina. Rev Cubana Med Trop. (2013) 65:177–84.
31. Andre-Fontaine G, Aviat F, Thorin C. Waterborne leptospirosis: survival and preservation of the virulence of pathogenic Leptospira spp. in fresh water. Curr Microbiol. (2015) 71:136–42. doi: 10.1007/s00284-015-0836-4
32. Thibeaux R, Iraola G, Ferrés I, Bierque E, Girault D, Soupé-Gilbert ME, et al. Deciphering the unexplored Leptospira diversity from soils uncovers genomic evolution to virulence. Microbial Genomics. (2018) 4:000144. doi: 10.1099/mgen.0.000144
33. Loffler SG, Pavan ME, Vanasco B, Samartino L, Suarez O, Auteri C, et al. Genotypes of pathogenic Leptospira spp isolated from rodents in Argentina. Memórias Do Instituto Oswaldo Cruz. (2014) 109:163–7. doi: 10.1590/0074-0276140295
34. Saraullo VR, Grune Loffler S, Pastorino FL, Watanabe O, Alonso ML, Hamer M, et al. First report of pathogenic Leptospira spp. in Tadaridabrasiliensis bats (family Molossidae) and Eptesicus furinalis (family Vespertilionidae) of Argentina: New host species in this country? Asoc Argentina Microbiol Rev Argentina Microbiol. (2021) 53:210–5. doi: 10.1016/j.ram.2020.09.007
35. Bierque E, Thibeaux R, Girault D, Soupé-Gilbert ME, Goarant C. A systematic review of Leptospira in water and soil environments. PLoS ONE. (2020) 15:e0227055. doi: 10.1371/journal.pone.0227055
Keywords: Leptospira, seroprevalence, risk factors, beef cattle, Argentina, spatial analysis
Citation: Mazzanti M, Scialfa E, Rivero M and Passucci J (2023) Epidemiology of Leptospira spp. infection in a beef cattle area of Argentina. Front. Vet. Sci. 10:1083024. doi: 10.3389/fvets.2023.1083024
Received: 28 October 2022; Accepted: 01 February 2023;
Published: 21 February 2023.
Edited by:
Demelash Areda, Ottawa University, United StatesReviewed by:
Gabriel Mendes De Souza Martins, Fluminense Federal University, BrazilHassan Ismail Musa, Putra Malaysia University, Malaysia
Copyright © 2023 Mazzanti, Scialfa, Rivero and Passucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariana Mazzanti,  bWFyaWFuYW1henphbnRpQGhvdG1haWwuY29t
bWFyaWFuYW1henphbnRpQGhvdG1haWwuY29t
†These authors have contributed equally to this work
‡ORCID: Mariana Mazzanti orcid.org/0000-0002-7214-5757
 Mariana Mazzanti
Mariana Mazzanti Exequiel Scialfa3,4
Exequiel Scialfa3,4 Mariana Rivero
Mariana Rivero Juan Passucci
Juan Passucci