- 1College of Animal Science and Technology, Northeast Agricultural University, Harbin, China
- 2Heilongjiang Wellhope Agri-Tech Co., Ltd., Harbin, China
- 3Harbin Wellhope Trading Co., Ltd., Harbin, China
This trial was designed to investigate the effects of industrial hemp ethanol extraction byproduct (IHEEB) and Chinese wildrye hay (CWH) replacement of alfalfa hay (AH) on digestibility, and lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein dairy cows. Nine healthy multiparous Holstein cows (parity = 3) with similar body weights (584 ± 12.3 kg), days in milk (108 ± 11.4), and milk yields (30 ± 1.93 kg; all mean ± standard deviation) were used in a replicated 3 × 3 Latin square design with 3 periods of 21 d. During each period, each group consumed 1 of 3 diets: (1) 0% IHEEB (0IHEEB); (2) 6.0% IHEEB and 1.7% Chinese wildrye hay (6IHEEB); (3) 10.8% IHEEB and 4.3% Chinese wildrye hay (11IHEEB). The diets in each group were isocaloric and isonitrogenous, with similar contents of concentrate and silage but different ratios of IHEEB and CWH to replace AH. The results showed that increasing the substitute did not affect the total-tract apparent nutrient digestibility. There was no difference in lactation performance of dairy cows fed the three diets, except for the cows' somatic cell count (SCC), which decreased with the increase in the amount of the substitute. Cannabidiol and tetrahydrocannabinol were not detected in milk samples of dairy cows in the different treatment groups. 6IHEEB and 11IHEEB-fed cows showed a linear decrease in total volatile fatty acids (VFA) and butyrate compared to the 0IHEEB cows. Plasma IL-1β content quadratically decreased with feeding IHEEB and CWH, and other blood parameters were unaffected. The rumen fluid's relative abundances of Bacteroidota, Fibrobacterota, and Prevotellaceae quadratically increased, while Firmicutes tended to decrease quadratically as the substitution increased. Feeding IHEEB and CWH linearly increased the relative abundances of Firmicutes, Lachnospiraceae, Monoglobaceae, and Butyricicoccaceae in the feces. As the substitution increased, the cost of dairy farming was reduced. In summary, substituting AH with IHEEB and CWH in diets did not affect the total-tract apparent nutrient digestibility, improved milk composition, and plasma immune indices. It changed the bacterial composition in rumen fluid and feces and improved dairy farming benefits.
Introduction
With the consumption of milk and dairy products increasing worldwide, the number of dairy cattle has increased, and the problem of increasing the demand of roughage resources for dairy cattle has become increasingly predominant (1, 2). Alfalfa hay (AH) is a widely used roughage for dairy cows, but it is in limited quantities and expensive. As a result, a large number of agricultural processing byproducts are produced each year and are increasingly favored by dairy farms because of their high-quality fiber, abundant protein, and low prices (3–5).
Industrial hemp is defined as an annual herb of the cannabis genus in the cannabis family, whose flowers, seeds, stems, leaves, and roots contain <0.3% tetrahydrocannabinol (THC) (6). Industrial hemp can provide ruminants with a rich source of crude protein (CP), crude fiber, and minerals, but residues of active ingredients may pose a risk to consumers when they consume these animal products (7, 8). Industrial hemp ethanol extraction byproducts (IHEEB) are the residue of hemp's flower and leaf parts after ultrasound-assisted extraction of cannabidiol (CBD) in ethanol. They are generally considered to have no other value or use. After extraction, most cannabinoids in industrial hemp are removed (9). Growing legalization and demand are anticipated to increase the global production of hemp and its byproducts (10). Currently, the research on industrial hemp and its processing byproducts mainly focus on the application of seed and hemp seed cake in livestock and poultry, which is considered to have a good application prospect (11–13). However, the application of industrial hemp ethanol extraction byproducts in dairy cows has not been studied.
Some studies have shown that CBD has neuroprotective, antioxidant, analgesic, anti-inflammatory, and anti-anxiety properties and can reduce proinflammatory factors in plasma (14). In fact, CBD has very low toxicity in humans and other species and has not shown teratogenic or mutagenic activity (15, 16). Even though IHEEB can be used as feed for livestock and poultry, due to its processing mode of grinding leading to small particle size, IHEEB should be used in combination with long fiber feed for dairy cows. Chinese wildrye hay (CWH) is one of the common forage grasses with long fiber in northeast China. Recent experimental reports have shown that agricultural byproduct replacement of conventional feed in ruminants has an effect on nutrient digestibility, rumen fermentation, milk composition, and bacterial communities (5, 17). We hypothesized that the combination of IHEEB and CWH could replace AH, provide nutrients required for daily production of dairy cows, improve rumen fluid and feces microbiota composition, improve production performance, promote rumen fermentation, and exert anti-inflammatory effects in dairy cows. Therefore, this study aimed to evaluate the effect of replacing AH with IHEEB and CWH on digestibility, lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein dairy cows.
Materials and methods
Industrial hemp ethanol extraction byproduct
Alfalfa hay (AH) and Chinese wildrye hay (CWH) were provided by Heilongjiang Wellhope Animal Husbandry Co., Ltd. (Harbin, China). Industrial hemp ethanol extraction byproduct (IHEEB) was provided by Heilongjiang Zhongsheng Biotechnology Co., Ltd. (Daqing, China). The specific processing is as follows: First, the flower spikes and hemp leaves at the top of harvested industrial hemp plants were dried until the water content was about 10%. Then they were crushed with a grinder to pass through a 5-mm mesh sieve. Finally, the crushed substance was continuously ultrasound-assisted extracted in ethanol solution several times until almost all cannabinoids were dissolved in the extract. Then, the residue after extraction was recycled by drying to obtain the IHEEB.
Animals, diets, and experimental design
This experiment was approved by Northeast Agricultural University and was carried out at Zhongwang Dairy Farm (Heihe, China) from December 2021 to February 2022. All animal care and handling procedures were done in accordance with the regulations of the Animal Welfare and Ethics Committee of Northeast Agricultural University (Protocol number: NEAUEC20210245). Nine healthy multiparous Holstein cows (parity = 3) with similar body conditions (BW = 584 ± 12.3 kg, DIM = 108 ± 11.4, milk yield = 30 ± 1.93 kg; mean ± SD) were assigned in a replicated 3 × 3 Latin square design with 3 periods of 21 d (initial 14 d of diet adaption and final 7 d of sample collection). During each period, groups consumed 1 of 3 treatment diets. The content of concentrate and silage in each treatment diet was similar, but the ratio of IHEEB and CWH to replace AH was different. The treatments were (1) 0% IHEEB (0IHEEB); (2) 6.0% IHEEB and 1.7% Chinese wildrye hay (6IHEEB); (3) 10.8% IHEEB and 4.3% Chinese wildrye hay (11IHEEB). According to the Cornell-Penn-Miner dairy model (18), the purpose of formulating an isocaloric isonitrogenous diet was to provide sufficient energy and protein for a cow producing 35 kg/d of milk containing 3.5% fat and 3.1% protein (18). During the experiment, each cow was housed in a separate enclosure with a concrete floor, dry manure and clean rice husk bedding, self-locking neck clamps in the feeding channel, smooth ceramic tiles on the feeding floor, and free drinking water. Cows were fed twice daily (0,530 and 1,730 h) at 105% ad libitum intake and milked thrice daily (0,500–0,530 h, 1,300–1,330 h, and 2,000–2,030 h). The feed was pushed up at least 10 times daily, especially after milking.
Feeds and feces
The total mixed ration (TMR) delivered and refused were weighed and sampled daily for individual cows for 7 consecutive days (d 15–21) each period to calculate dry matter intake (DMI), and the particle size of TMR was measured by using the Penn State Particle Separator (19, 20). Fecal samples (500 g) were collected from the cow rectum every 9 h for 3 consecutive days (d 15, 16, and 17) and composited for each cow each period (21). Also, a portion of the fecal sample (~5 g) collected at d 17 was stored instantly in liquid nitrogen until the determination of bacterial communities. During these 3 days, all TMR, orts, feed ingredients, and fecal samples were collected, stored at −20°C, and mixed per cow for each period. Indigestible neutral detergent fiber (iNDF) concentrations in the diet and feces were used as internal markers to estimate the total-tract apparent digestibility of nutrients (22). The iNDF content in the feces, TMR, and orts was determined by in situ incubation for 288 h, as described by Huhtanen et al. (23). All feeds and fecal samples were dried at 55°C for 48 h, ground through a 1-mm screen, then placed in sealed bags and stored at 4°C until used for subsequent chemical composition analysis. They were analyzed for the contents of DM (method 930.15), CP (method 976.05), ether extract (method 920.39), and ash (method 942.05) according to AOAC International (24). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were analyzed using Ankom 220 Fiber Analyzer (Ankom Technology Corp., Macedon, NY), according to the methods of Van Soest et al. (25). Starch content was measured using the Megazyme Total Starch Assay Kit (K-TSTA; Megazyme International Ireland Ltd.). CBD and THC were analyzed using a high-performance liquid chromatograph with an ultraviolet detector (LC-20A; Shimadzu Corp., Japan) at National Market Regulation Technology Innovation Center (Industrial Hemp) (Qiqihar, China) (26). The lower limit of detection (LOD) and lower limit of quantification (LOQ) of cannabinoids was 0.03 and 0.1 mg/kg.
Milk yield and composition analysis
The milk yield of each cow was recorded for 3 consecutive days (d18, 19, and 20) during the experimental period. Milk samples were collected from each cow while the cows were milked in a parallel milking parlor. According to the real milk yield of cows three times a day, milk samples (50 mL) were mixed with potassium dichromate and stored at 4°C until used for subsequent determination of milk components. The protein, fat, lactose, and milk urea nitrogen (MUN) concentrations and SCC of milk samples were analyzed by a 4-channel spectrophotometer (MilkoScan; Foss Electric, Hillerød, Denmark) at the Heilongjiang Academy of Agricultural Reclamation (Harbin, China). CBD and THC in milk were analyzed by liquid chromatography-mass spectrometry (Triple Quad 5500 + QTRAP Ready; AB Sciex Ltd., USA), according to the methods of Escrivá et al. (27). The LOD and LOQ of cannabinoids in milk were 1.5 ng/ml and 5 ng/ml.
Blood collection and analyses
Blood samples were collected from the coccygeal vein before morning feeding on 2 consecutive days (d 20 and 21 of each period) into sodium heparin tubes. They were separated at 3,000 × g for 15 min at 4°C to obtain plasma and stored in a microtube at −20°C until analysis. A fully automatic biochemical analyzer was used to analyze the total protein, albumin, globulin, triglyceride, total cholesterol, urea nitrogen, and glucose in plasma (Mindray BS-420; Shenzhen Mindray Bio-medical Electronics Co. LTD). The non-esterified fatty acid (NEFA), β-hydroxybutyric acid (BHBA), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), catalase, glutathione peroxidase (GSH-Px), malondialdehyde (MDA), immune globulin A (IgA), immune globulin G (IgG), immune globulin M (IgM), soluble CD3 and soluble CD4 levels in plasma were determined by following the manufacturer's instructions for commercial colorimetric analysis kits (Beijing sinouk institute of biological technology). Plasma interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), prolactin, triiodothyronine, and thyroid hormone concentrations were determined using commercial bovine ELISA kits (Beijing sinouk institute of biological technology).
Ruminal fluid collection and analyses
Rumen fluid (150–200 mL) was collected by gastric tube at 3 h after morning feeding on d 21 of each period. The first 100 mL of rumen fluid collected initially was discarded to prevent saliva contamination. After the rumen fluid was filtered with four layers of gauze, the pH of the filtrate was measured immediately with a pH meter (PHS-3C, Nanjing Nanda Analytical Instrument Application Research Institute). 10 mL filtrate was placed into centrifuge tubes, and 2 mL metaphosphoric acid solution (25%, wt/vol) was added immediately. After mixing, it was stored at −20°C until VFA concentration was analyzed by gas chromatography [GC-8A; Shimadzu Corp; (28)]. 0.2 mL sulfuric acid solution (50%, vol/vol) was added to another 10 ml filtrate, which was mixed and stored at −20°C until ammonia-N concentration was analyzed using the phenol-hypochlorite method (29). The microbial protein (MCP) in rumen fluid was separated by differential centrifugation according to the method of Cotta and Russell (30), and the concentration of MCP was determined by Coomassie brilliant blue method (31). Finally, 9 mL filtrate was divided into freezable tubes and immediately stored in liquid nitrogen for rumen bacterial community analysis.
Bacterial communities
The analysis of rumen fluid and fecal samples was carried out at Biomarker Technologies Co. Ltd. using high-throughput sequencing. The DNA was extracted from the samples using an MN NucleoSpin 96 Soi DNA kit (Gene Company Limited) according to the kit's instructions. The DNA obtained from each sample was subjected to 2-step PCR amplification to construct a small-fragment sequencing library described by Jiang et al. (32). The PCR products obtained in the first step of amplification were used as templates for the second step of Solexa PCR amplification (Applied Biosystems Inc.). The V3-V4 region of the 16S rRNA gene was amplified using primers 338F (50-ACTCCTRCGGGAGGCAGCAG-30) and 806R (50-GGACTACCVGGGTATCTAAT-30) (33). The Solexa PCR products from the Solexa PCR amplification were purified using an OMEGA DNA purification column (Gene Company Limited). The purified products were quantified using a Quant-iT PicoGreends DNA Assay Kit (Gene Company Limited) following the kit's instructions. Then, the amplicons were sequenced at Biomarker Technologies Co., Ltd. using Illumina HiSeq 2500 sequencing platform (Illumina Inc.; Novaseq 6000; paired-end; 250 bp). The resulting raw image data files were analyzed by base calling and converted to the original sequenced reads. The original tag data (1,439,607 and 1,433,006 raw reads for the ruminal and fecal samples, respectively) were obtained using FLASH software [version 1.2.11; (34)]. The raw tags obtained by sequencing were filtered by Trimmomatic software [version 0.33; (35)], and then, Cutadapt software [version 1.9.1; (36)] was used to identify and remove primer sequences, and clean reads (1,430,677 and 1,423,646 clean tags for the ruminal and fecal samples, respectively) without primer sequences were obtained. We then use Usearch software [version 10.0; (37)] to perform double-ended sequence stitching on Clean Reads of each sample through overlap and then conduct length filtering on the data after stitching according to the length range of different regions. UCHIME software [version 8.1; (38)] was used to identify and remove chimeric sequences to obtain the final effective tags (1,111,160 and 1,126,303 effective tags for the ruminal and fecal samples). The tags were binned into operational taxonomic units (OTU) using the clustering program USEARCH [version 10.0; (39)] based on a 97% sequence similarity level. The obtained OTU was eventually used for taxonomic assignment. The representative sequences for each OTU were compared with the Silva (Release 128; www.arb-silva.de) database to obtain taxonomic classification at the phylum, class, order, family, and genus levels. The relative abundances of taxa at the phylum, family, and genus level were determined using QIIME software [version 1.9.1; (40)] to compare the bacterial community composition in the rumen and feces among treatments. Alpha diversity indices, including Chao, Shannon, Ace, and Simpson, were determined using QIIME software [version 1.9.1; (40)].
Statistical analysis
Before analyses, all data were screened for normality using the UNIVARIATE procedure of SAS. All data from the experiment were analyzed in a 3 × 3 Latin square design using the Proc Mixed procedure of SAS (version 9.2; SAS Institute Inc., Cary, NC), according to the model Yijkm = μ + Ti + Pj + Ck + Sm + Eijkm, where Yijkm was the observation, μ was the overall mean, Ti was the fixed effect of the treatment, Pj was the fixed effect of the period, Ck was the random effect of the cows, Sm was the fixed effect of the square, and Eijkm was the residual error. No carryover effects were detected (P > 0.05) for any data. Orthogonal polynomial contrasts were also used to analyze the linear and quadratic effects of increasing IHEEB and CWH on each variable. For this experiment, Duncan's multiple range tests were used. Significant differences were declared at P ≤ 0.05, and trends were defined at 0.05 < P ≤ 0.10.
Results
Roughage and diet characteristics
The three roughages differ greatly in nutrient composition (Table 1). IHEEB had higher Ash, CP, EE, Ca, and P levels than others, while CWH had the highest content of DM and NDF. Most of the nutrient composition values of alfalfa were between IHEEB and CWH, except ADF. In addition, IHEEB contains 0.03% CBD. The contents of CP, pdNDF, ether extract, Ca, and P were slightly increased, while the contents of NDF, ADF, NFC, and starch were slightly decreased with increased IHEEB and CWH supplemental levels (Table 2). Although the proportion of particles retained on the third sieve (1.18–8.0 mm) of the Penn State Particle Separator did not differ among treatments, with the proportion of replacements increasing, the proportion of particles retained on the first and second sieves decreased, whereas particles retained on the bottom pan were increased. As a result, the physically effective neutral detergent fiber (peNDF) of the 6IHEEB and 11IHEEB groups were smaller than that of the 0IHEEB group, especially the 11IHEEB group.
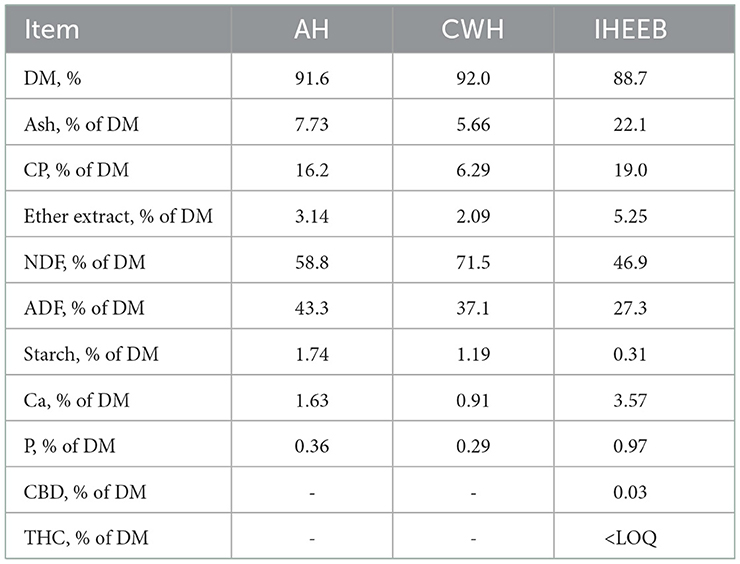
Table 1. Chemical composition of alfalfa hay (AH), Chinese wildrye hay (CWH), and industrial hemp ethanol extraction byproduct (IHEEB).

Table 2. Ingredients and chemical composition (% of DM) of the 3 dietsa.
Nutrient intake and digestibility
As presented in Table 3, cows fed 6IHEEB and 11IHEEB showed linearly decreased intake of CP (P = 0.03) and ADF (P = 0.01), but there was no significant difference in total-tract apparent digestibility among the three groups.

Table 3. Intake and total-tract apparent digestibility of nutrients in Holstein cows fed the 3 diets.
Milk production and components
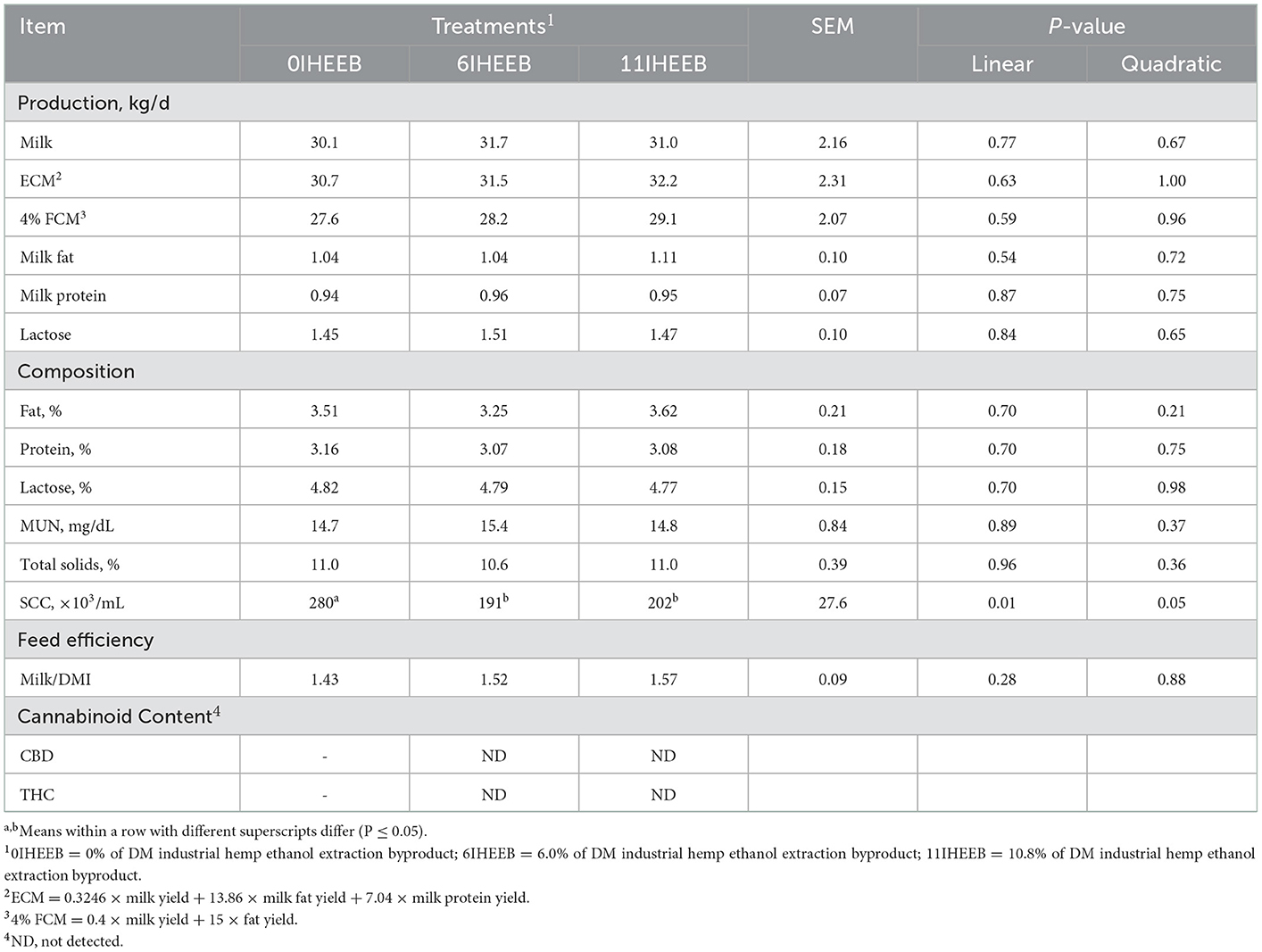
The milk production, composition, and feed efficiency in the three diets are shown in Table 4. There was no difference in lactation performance of dairy cows fed the three diets, except that the somatic cell count (SCC) showed a decrease, and the 6IHEEB group was the lowest (linear, P = 0.01; quadratic, P = 0.05). CBD and THC were not detected in milk samples of the different treatments.
Rumen fermentation
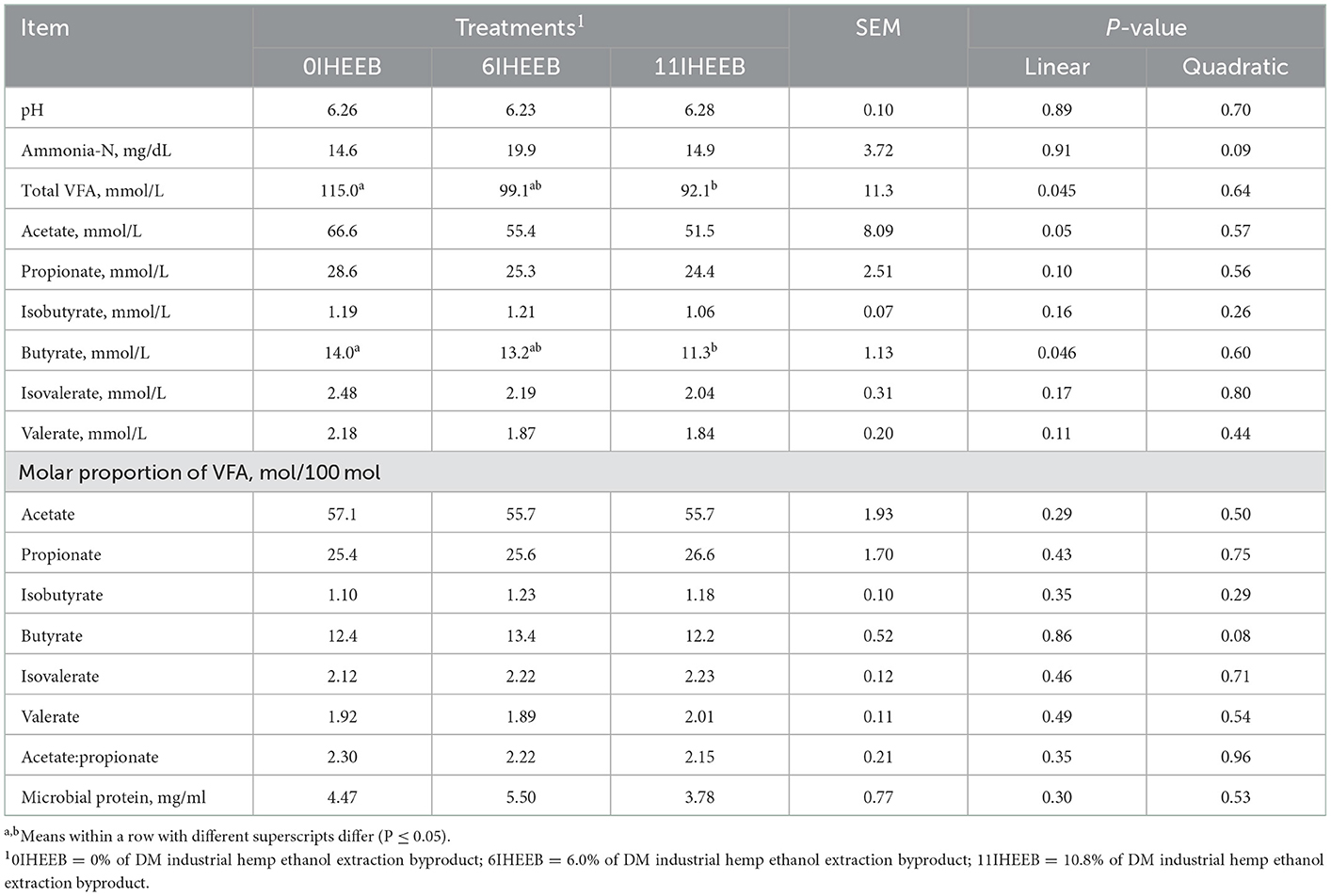
As shown in Table 5, there was a linear decrease in total VFA (P = 0.045), acetate (P = 0.05), and butyrate (P = 0.046) in cows fed 6IHEEB and 11IHEEB compared to cows fed 0IHEEB. With the addition of IHEEB, the content of Ammonia-N (P = 0.09) and the molar proportion of butyrate (P = 0.08) showed a quadratic increase trend.
Plasma metabolites
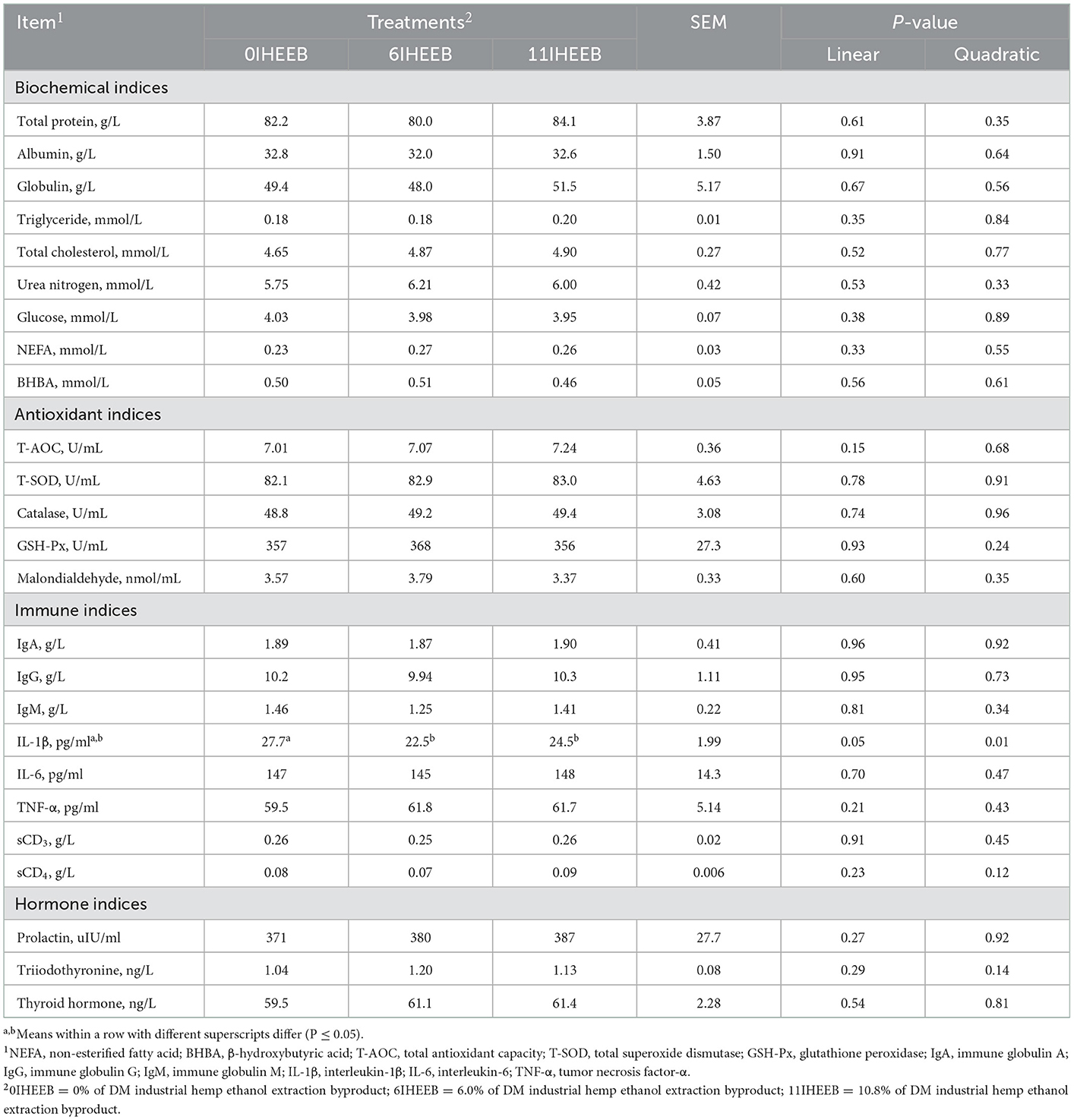
The plasma biochemical, antioxidant, immune, and hormone indices did not differ among treatments; however, plasma IL-1β content decreased (linear, P = 0.05; quadratic, P = 0.01) with the increase in the substitute (Table 6).
Bacterial communities
The treatments did not affect the ruminal fluid bacterial OTU, coverage, Shannon, Simpson, Ace, and Chao indices (P > 0.10), as indicated in Table 7. There was a quadratic decrease in fecal bacterial coverage (P = 0.03) with the increase in substitution. The relative abundances of Bacteroidota (P = 0.01), Fibrobacterota (P = 0.03), and Prevotellaceae (P = 0.01) quadratically increased, and Firmicutes (P = 0.06) tended to decrease quadratically with IHEEB and CWH supplementation in Table 8. There was no difference in the relative abundance of rumen fluid bacteria at the genus level among the different treatments (P > 0.10). The relative abundances of Firmicutes (P = 0.04), Lachnospiraceae (P = 0.047), Monoglobaceae (P = 0.04), Butyricicoccaceae (P = 0.04), unclassified_Clostridia (P = 0.01), UCG_009 (P = 0.04) and unclassified_Clostridia (P = 0.01) increased linearly, and unclassified_Clostridia_UCG_014 (P = 0.02) decreased linearly with the increase in the substitute (Table 9).
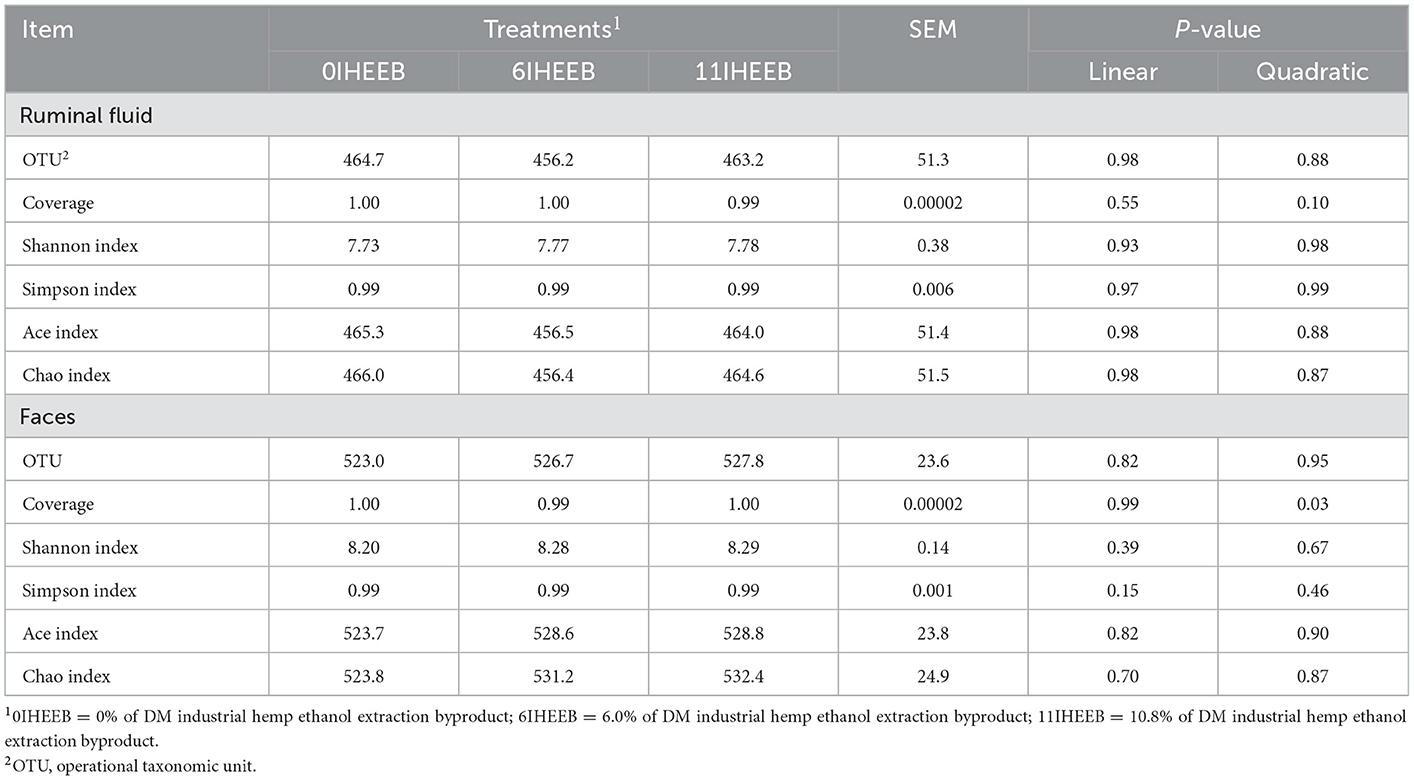
Table 7. Microbial diversity indices for the bacterial communities in the ruminal fluids and feces of Holstein cows fed the 3 diets.
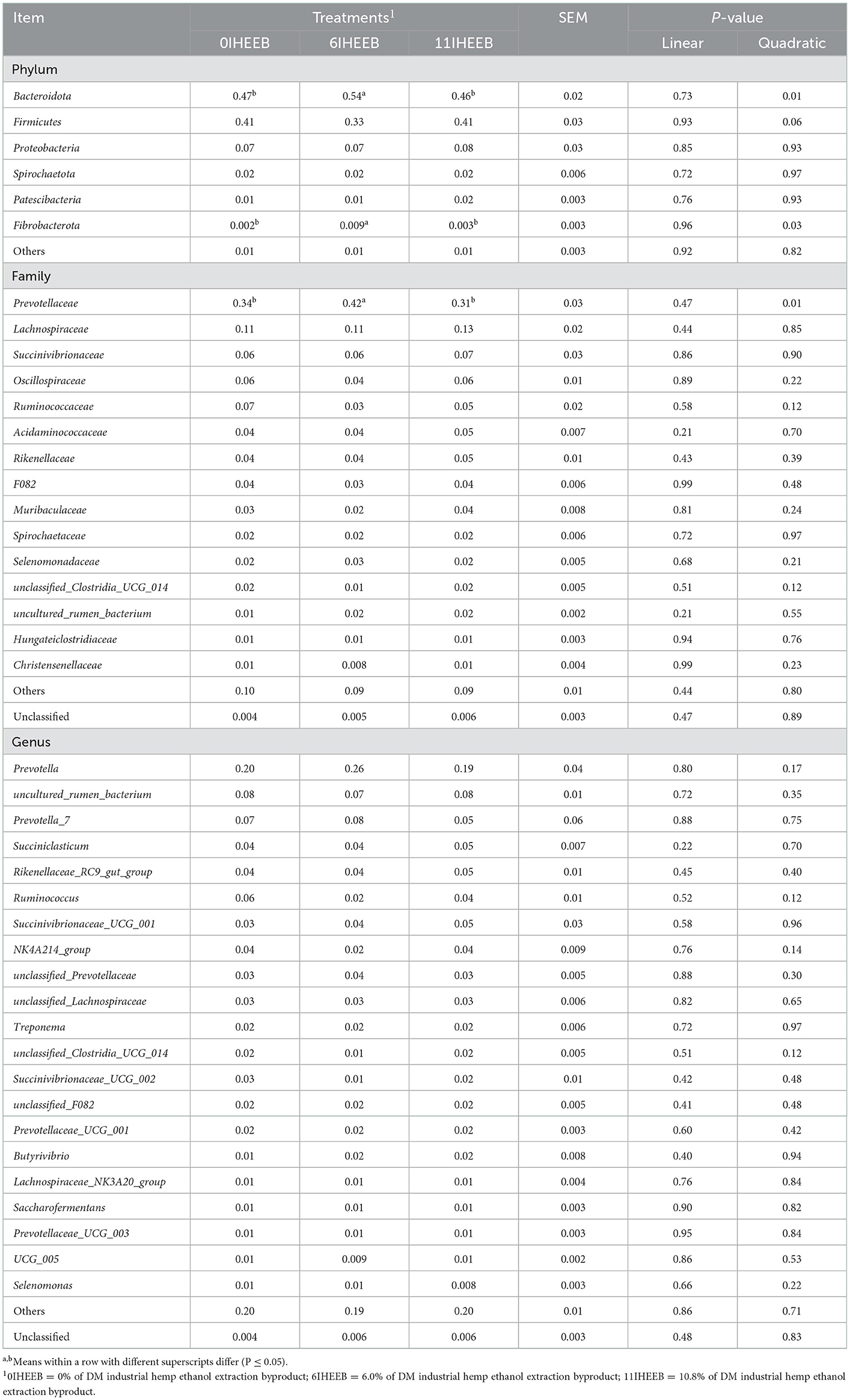
Table 8. Relative abundances (>1.0%) of the bacterial phyla, families, and genera in the ruminal fluids of Holstein cows fed the 3 diets.
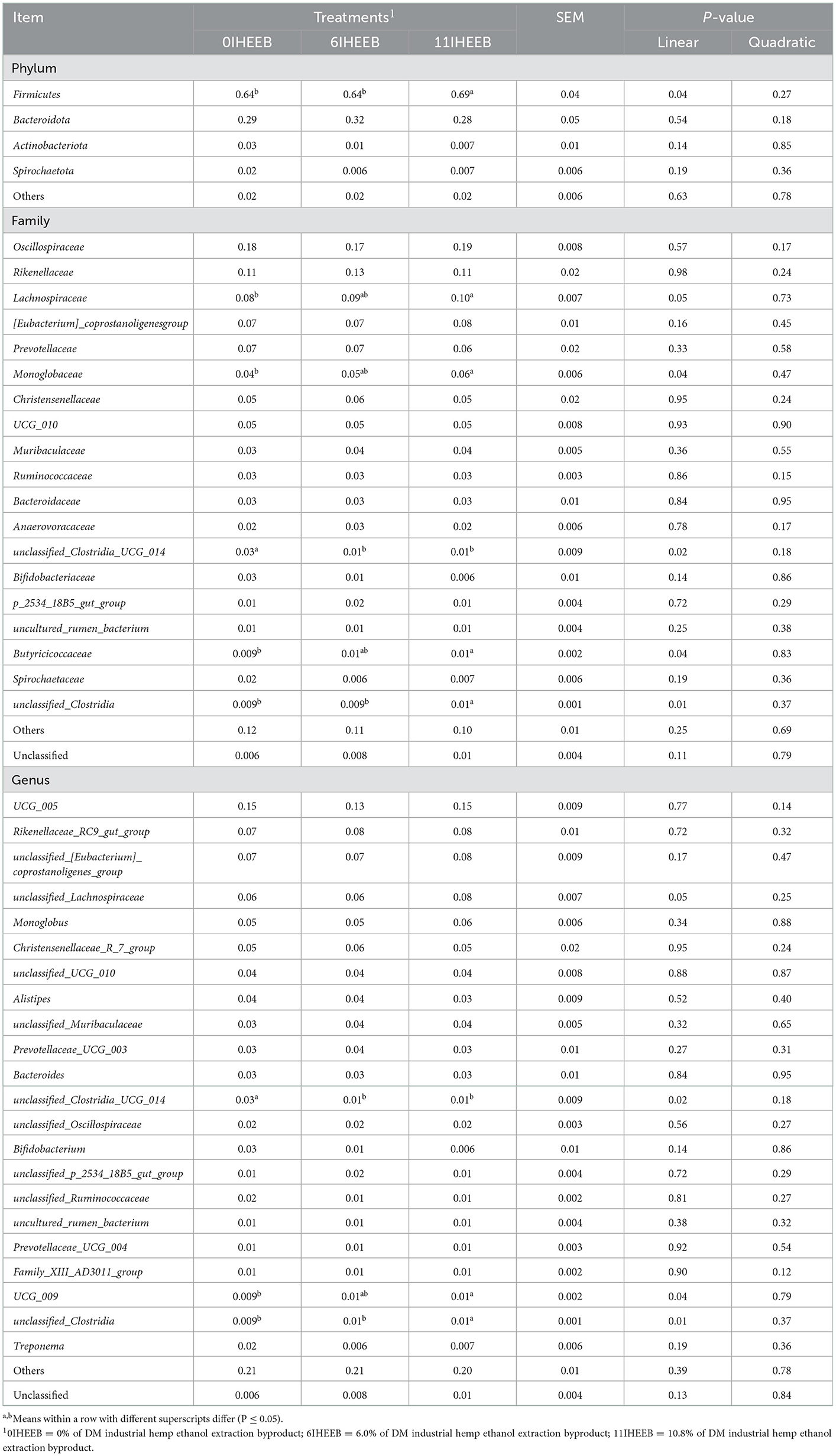
Table 9. Relative abundances (>1.0%) of the bacterial phyla, families, and genera in the feces of Holstein cows fed the 3 diets.
Economic benefits
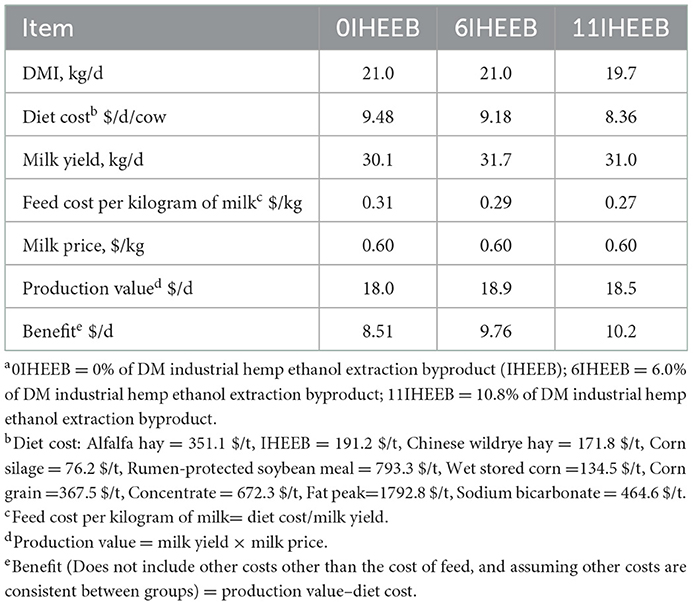
With the increase of IHEEB and CWH substitution, the cost of diet was reduced, resulting in lower feed cost per kilogram of milk, which greatly increased the benefit of each cow, especially 11IHEEB, which increased by $1.65 per day compared with 0IHEEB (Table 10).
Discussion
Diet characteristics
As an emerging and highly sought-after plant in recent years, industrial hemp produces a large number of processing byproducts yearly (10). IHEEB's development as ruminant animal feed can effectively alleviate the challenge of insufficient roughage resources for dairy cattle while making the rest worth of industrial hemp better utilized. By comparing the chemical composition of the three roughage groups, it is not difficult to find that the nutrient composition of AH, such as CP and NDF, is between IHEEB and CWH, so IHEEB and CWH can replace AH in the diet by combination. In this experiment, the content of CP in alfalfa hay was lower than that reported by Ghelichkhan et al. (41), while the content of NDF and ADF was higher, which may be caused by the late variety and harvesting period. Due to extraction and processing, IHEEB contains only a very small amount of cannabinoids, much less than the safe amount (42–44). Many factors affect peNDF in feed. The most important of these is the particle size of the feed, which promotes chewing activity (45). Therefore, in this study, CWH, a long fiber feed, was used to increase the peNDF of the diet to stimulate ruminant activity and prevent adverse effects on performance, digestibility, or rumen fermentation. The peNDF of the diets in this study all met the optimal range of Llonch et al. (46). Therefore, IHEEB can be considered an unconventional roughage resource with high safety and nutritional value, and it is feasible to substitute a part of AH with CWH.
Nutrient intake and digestibility, lactation performance and rumen fermentation
Dry matter intake did not differ significantly, and mixing with silage masked the small amount of plant aroma of IHEEB, successfully eliminating the concerns of early scholars about low palatability and showing that cows can quickly adapt and accept the new unconventional feed (13). Although there were individual differences in dietary intake among the three groups, there did not seem to be much effect on the digestive properties of the diet, indicating that according to the total-tract apparent digestibility, IHEEB can be well-digested and absorbed by the intestinal tract of dairy cows.
In this study, IHEEB and CWH substitution did not affect the milk yield or milk composition, except for the SCC, which was decreased in milk. There were no changes in milk yield and composition for the three treated cow groups, consistent with the same feed intake observed. The SCC usually refers to the total number of cells in each milliliter of milk, mostly white blood cells (macrophages, neutrophils, and lymphocytes), with a small percentage being epithelial cells shed by mammary tissue. It is an important indicator commonly used to reflect the health of the udder of dairy cows (47). In this study, the decrease in SCC may be attributed to its strong correlation with IL-1β in the blood (48). Interestingly, we found no THC or CBD in the milk; therefore, the milk can be circulated and commercialized.
In this study, rumen pH did not change with increasing IHEEB and CWH replacement which was similar to the results of Jiang et al. (32). Rumen fluid ammonia-N concentration is related to the degradation of dietary protein in the rumen, which is utilized by rumen microorganisms. Rumen ammonia-N levels indicate more unutilized ammonia in the rumen of cows receiving the 6IHEEB diet than those receiving the other diets (49). In this study, the decrease in total VFA may be attributed to the small TMR particle size with the substitution of IHEEB and CWH in the diet, and the potential NDF could have escaped rumen fermentation and entered the post-intestinal digestion, leading to the decrease in rumen VFA production (50). In addition, different diet compositions and ruminal degradation characteristics may also be responsible for the lower VFA in dairy cows after ingestion of alternative diets. It has been suggested that IHEEB contains lower ruminal effective degradation rates compared to AH (51). Acetate and butyrate are precursors of milk fat synthesis (52). In this experiment, their concentration decreased in rumen fluid with the increase in replacement amount, but the milk fat content was not affected, possibly because dietary carbohydrates can also be fermented into VFA in the posterior gut for milk composition synthesis (53). We also found that with the substitution of IHEEB and CWH in the diet, the molar ratio of butyrate showed a curve-increasing trend, which may be caused by the change in the abundance of bacteria producing butyrate in rumen fluid, such as Prevotellaceae (54).
Plasma metabolites
In this experiment, the three diets had little effect on the plasma parameters of dairy cows, except that with the increase of IHEEB and CWH substitution, IL-1β content showed a decrease, which could be associated with the slight CBD content of IHEEB. The CBD is the main non-psychoactive component of cannabinoids extracted from the industrial hemp plant (14). Numerous studies have shown that CBD treatment can reduce proinflammatory cytokines, reduce IL-1β levels in the blood, and alleviate disease or disability (55, 56). Studies have shown that CBD decreases the transmigration of blood leukocytes by downregulating the expression of IL-1β (57). The IL-1β activates a large number of neutrophils and monocytes into the mammary gland, at which time the number of somatic cells is greatly increased, resulting in mammary gland redness and inflammation caused by an immune response, so that mammary gland cells cannot work normally and have an inhibitory effect on lactation in dairy cattle (58). In addition, we did not observe any obvious effects of CBD on antioxidant indexes, and IHEEB and CWH replacement of AH did not change the plasma hormone and biochemical parameters of dairy cows.
Bacterial communities
In this experiment, there was no significant difference in species diversity index between rumen fluids and feces, indicating that the three treatments did not affect the overall microbial species number, species abundance, and species evenness. The changes we observed in the abundance of bacterial communities were related to the composition of the diet. As mentioned above, the change in the ratio of the three roughages, especially the smaller IHEEB particles, resulted in more digestion passing through the rumen, which resulted in a change in the bacterial community in rumen fluid and feces (50).
The clustering results showed that 14 phyla, 21 classes, 38 orders, 59 families, 135 genera, and 166 species belonging to bacteria were identified in the ruminal fluid samples. By comparing the microbial community composition of the rumen at the phylum level, it can be found that Bacteroidota, Firmicutes, and Proteobacteria occupy the absolute dominant position, which is consistent with the research results of other scholars (59). The only difference is that in this experiment, Bacteroidota accounted for a larger proportion and showed a quadratic increase, while Firmicutes showed a quadratic decrease trend, and there was a negative correlation between the abundance of Bacteroidota and Firmicutes (60). Usually, healthy cows had more Bacteroidota, fewer Firmicutes, and more Prevotellaceae in their rumen fluid than cows with mastitis (61). The enhancement of Prevotellaceae may increase the abundance of short-chain fatty acids, especially butyrate, which can act as an energy substrate for intestinal epithelial cells (54). This may explain the quadratic increase in the molar ratio of butyrate observed in this experiment. Our results showed no change in the abundance of Proteobacteria, mainly composed of Gram-negative bacteria, and played a role in rumen nitrogen metabolism, indicating that IHEEB and CWH supplementation did not affect substrate protein processing ability (62). Therefore, the MCP content was similar among the three groups in this experiment. Fibrobacterota is the main cellulose-decomposing bacteria in the rumen of dairy cows. The increase of substitution enhanced the abundance of Fibrobacterota and increased the ability of cellulose decomposing in the rumen fluid in this experiment (63). Furthermore, starch, protein, peptides, hemicellulose, and pectin can be metabolized by rumen Prevotellaceae (64). The increased relative abundance of Prevotellaceae in the rumen fluid is beneficial for improving the degradation rate of diets, especially the concentrate part (65). This is consistent with the results of this experimental study, in which the 6IHEEB group had a higher VFA than the other group of the replacement diet.
The clustering results showed that a total of 11 phyla, 17 classes, 35 orders, 66 families, 149 genera, and 157 species belonging to bacteria were identified in the fecal samples. As previously mentioned, with the increase of IHEEB and CWH substitution, more dietary nutrients could enter the posterior gut for digestion and thus have certain effects on intestinal microbes. In this study, with the increase of substitution, the abundance of Firmicutes in feces increased, and it was always the phylum with the largest abundance, which was consistent with the study of Liu et al. (66). At the family level, the abundance of Lachnospiraceae, Monoglobaceae, Butyricicoccaceae, and unclassified_Clostridia increased, and the abundance of unclassified_Clostridia_UCG_014 decreased as IHEEB and CWH were added. They all belong to the Firmicutes, and their increase is the main reason for the increase in Firmicutes (67). The increased Lachnospiraceae content may mean that more nutrients can be absorbed by producing more volatile fatty acids (68). Kim et al. (69) found that Monoglobaceae Monoglobus had a strong pectin degradation ability, which could decompose more pectin in the diet. Clostridia is associated with glycan degradation potential, and its increase can help animals avoid glycan loss in this study (70). All these results suggest that by increasing the substitution level, more nutrients can be efficiently digested and absorbed in the large intestines, which may explain the lack of difference in the total-tract apparent digestibility and milk components among the three groups of cows.
Economic benefits
As an important part of dairy farm income, milk yield, milk composition, and feed cost are critical. The profitability of dairy farms can be improved by increasing milk production and improving milk composition to obtain a better price and reduce the cost of farming, such as feed costs (71). This study showed that replacing AH in the diet with a lower-priced combination of IHEEB and CWH induced no change in milk composition, resulting in the same price of milk produced and no effect on milk volume, consequently giving rise to almost the same production benefit from milk sales. Sales revenue is constant, and feed costs decrease as replacement rates increase, ultimately reducing the cost of feeding dairy farms: the less investment and constant income, the more profitable the dairy farm. In this experiment, the daily income of each cow in the 6IHEEB and 11IHEEB groups was $1.25 and 1.65 higher than that in the 0IHEEN group, making more money for the dairy farm.
Conclusions
This study shows that IHEEB is a safe and valuable unconventional feed for dairy cows. Using IHEEB and CWH to replace 50% AH in a dairy diet can improve rumen fermentation, reduce plasma IL-1β content, reduce SCC in milk, improve rumen bacterial community and reduce feeding costs. Using IHEEB and CWH to replace 100% AH in a dairy diet can not affect the total-tract apparent nutrient digestibility, whereas it can improve the fecal bacterial community and maximize the benefit of dairy farms. In conclusion, this study not only solves the limitations of the IHEEB application but also provides data reference for a new unconventional feed for dairy cows and improves dairy farming benefits.
Data availability statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found at: NCBI; PRJNA887401.
Ethics statement
The animal study was reviewed and approved by Animal Welfare and Ethics Committee of Northeast Agricultural University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
YW, YL, JH, and YZ participated in the design of this study. YW, QY, XW, JS, and PH performed most of the experiments. ML revised the final version of the manuscript. YL and YZ supervised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the earmarked fund for China Agriculture Research System (CARS36).
Acknowledgments
The authors acknowledge the staff of Zhongwang Dairy Farm for their assistance in milking and sample collection.
Conflict of interest
JH was employed by Heilongjiang Wellhope Agri-Tech Co., Ltd. PH was employed by Harbin Wellhope Trading Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fernández-Rico S, Mondragón A, López-Santamarina A, Cardelle-Cobas A, Regal P, Lamas A, et al. A2 milk: new perspectives for food technology and human health. Foods. (2022) 11:2387. doi: 10.3390/foods11162387
2. Polsky L, von Keyserlingk M. Invited review: effects of heat stress on dairy cattle welfare. J Dairy Sci. (2017) 100:8645–57. doi: 10.3168/jds.2017-12651
3. Capanoglu E, Tomás-Barberán FA. Introduction to novel approaches in the valorization of agricultural wastes and their applications. J Agric Food Chem. (2022) 70:6785–6. doi: 10.1021/acs.jafc.2c03433
4. Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, Valéro JR. Bio-processing of agro-byproducts to animal feed. Crit Rev Biotechnol. (2012) 32:382–400. doi: 10.3109/07388551.2012.659172
5. Bradford BJ, Mullins CR. Invited review: strategies for promoting productivity and health of dairy cattle by feeding nonforage fiber sources. J Dairy Sci. (2012) 95:4735–46. doi: 10.3168/jds.2012-5393
6. USDA Agricultural Marketing Service. Establishment of a Domestic Hemp Production Program. (2021). Available online at: https://www.federalregister.gov/documents/2019/10/31/2019-23749/establishment-of-a-domestic-hemp-production-program (accessed November 1, 2021).
7. Kleinhenz MD, Magnin G, Ensley SM, Griffin JJ, Goeser J, Lynch E, et al. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl Anim Sci. (2020) 36:489–94. doi: 10.15232/aas.2020-02018
8. Kleinhenz MD, Magnin G, Lin Z, Griffin J, Kleinhenz KE, Montgomery S, et al. Plasma concentrations of eleven cannabinoids in cattle following oral administration of industrial hemp (Cannabis sativa). Sci Rep. (2020) 10:12753. doi: 10.1038/s41598-020-69768-4
9. Brighenti V, Pellati F, Steinbach M, Maran D, Benvenuti S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J Pharm Biomed Anal. (2017) 143:228–36. doi: 10.1016/j.jpba.2017.05.049
10. Semwogerere F, Katiyatiya C, Chikwanha OC, Marufu MC, Mapiye C. Bioavailability and bioefficacy of hemp by-products in ruminant meat production and preservation: a review. Front Vet Sci. (2020) 7:572906. doi: 10.3389/fvets.2020.572906
11. Eriksson M, Wall H. Hemp seed cake in organic broiler diets. Anim Feed Sci Technol. (2012) 171:205–13. doi: 10.1016/j.anifeedsci.2011.10.007
12. Karlsson L, Finell M, Martinsson K. Effects of increasing amounts of hempseed cake in the diet of dairy cows on the production and composition of milk. Animal. (2010) 4:1854–60. doi: 10.1017/S1751731110001254
13. Parker NB, Bionaz M, Ford HR, Irawan A, Trevisi E, Ates S. Assessment of spent hemp biomass as a potential ingredient in ruminant diet: nutritional quality and effect on performance, meat and carcass quality, and hematological parameters in finishing lambs. J Anim Sci. (2022) 100:1–21. doi: 10.1093/jas/skac263
14. Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. (2017) 175:133–50. doi: 10.1016/j.pharmthera.2017.02.041
15. Rosenkrantz H, Fleischman RW, Grant RJ. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol Appl Pharmacol. (1981) 58:118–31. doi: 10.1016/0041-008X(81)90122-8
16. Rosenkrantz H, Hayden DW. Acute and subacute inhalation toxicity of Turkish marihuana, cannabichromene, and cannabidiol in rats. Toxicol Appl Pharmacol. (1979) 48:375–86. doi: 10.1016/0041-008X(79)90421-6
17. Kobayashi Y, Oh S, Myint H, Koike S. Use of Asian selected agricultural byproducts to modulate rumen microbes and fermentation. J Anim Sci Biotechnol. (2016) 7:70. doi: 10.1186/s40104-016-0126-4
18. Tedeschi LO, Chalupa W, Janczewski E, Fox DG, Sniffen CJ, Munson R, et al. Evaluation and application of the CPM Dairy nutrition model. J Agric Sci. (2008) 146:171–82. doi: 10.1017/S.0021859607007587
19. Kononoff PJ, Heinrichs AJ, Buckmaster DR. Modification of the Penn State forage and total mixed ration particle separator and the effects of moisture content on its measurements. J Dairy Sci. (2003) 86:1858–63. doi: 10.3168/jds.S0022-0302(03)73773-4
20. Maulfair DD, Heinrichs AJ. REVIEW: methods to measure forage and diet particle size in the dairy cow. Prof Anim Sci. (2012) 28:489–93. doi: 10.15232/S1080-7446(15)30396-X
21. Bugoni M, Takiya CS, Grigoletto NS, Nunes AT, Vittorazzi PC, Chesini RG, et al. Dry malt extract from barley partially replacing ground corn in diets of dairy cows: Nutrient digestibility, ruminal fermentation, and milk composition. J Dairy Sci. (2022) 105:5714–22. doi: 10.3168/jds.2021-21682
22. Lee C, Hristov AN. Short communication: evaluation of acid-insoluble ash and indigestible neutral detergent fiber as total-tract digestibility markers in dairy cows fed corn silage-based diets. J Dairy Sci. (2013) 96:5295–9. doi: 10.3168/jds.2012-6442
23. Huhtanen P, Kaustell K, Jaakkola S. The use of internal markers to predict total digestibility and duodenal flow of nutrients in cattle given six different diets. Anim Feed Sci Technol. (1994) 48:211–27. doi: 10.1016/0377-8401(94)90173-2
24. AOAC International. Official Methods of Analysis. 17th ed. Arlington, VA: AOAC International (2000).
25. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
26. Hädener M, König S, Weinmann W. Quantitative determination of CBD and THC and their acid precursors in confiscated cannabis samples by HPLC-DAD. Forensic Sci Int. (2019) 299:142–50. doi: 10.1016/j.forsciint.2019.03.046
27. Escrivá Ú, Andrés-Costa MJ, Andreu V, Picó Y. Analysis of cannabinoids by liquid chromatography-mass spectrometry in milk, liver and hemp seed to ensure food safety. Food Chem. (2017) 228:177–85. doi: 10.1016/j.foodchem.2017.01.128
28. Stewart CS, Duncan SH. The effect of avoparcin on cellulolytic bacteria of the ovine rumen. J Gen Microbiol. (1985) 131:427–35. doi: 10.1099/00221287-131-3-427
29. Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. (1980) 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
30. Cotta Michael A, Russell James B. Effect of peptides and amino acids on efficiency of rumen bacterial protein synthesis in continuous culture. J Dairy Sci. (1982) 65:226–34. doi: 10.3168/jds.S0022-0302(82)82181-4
31. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. (1976) 72:248–54. doi: 10.1016/0003-2697(76)90527-3
32. Jiang X, Xu HJ, Ma GM, Sun YK, Li Y, Zhang YG. Digestibility, lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein cows fed a fermented corn gluten-wheat bran mixture as a substitute for soybean meal. J Dairy Sci. (2021) 104:2866–80. doi: 10.3168/jds.2020-19072
33. Mao S, Zhang M, Liu J, Zhu W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci Rep. (2015) 5:16116. doi: 10.1038/srep16116
34. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
35. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/bioinformatics/btu170
36. Marcel M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. (2011) 17:10–10. doi: 10.14806/ej.17.1.200
37. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. (2010) 26:2460–1. doi: 10.1093/bioinformatics/btq461
38. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. (2011) 27:2194–200. doi: 10.1093/bioinformatics/btr381
39. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
40. Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinf. (2011) 36:10.7.1–7.20. doi: 10.1002/0471250953.bi1007s36
41. Ghelichkhan M, Eun JS, Christensen RG, Stott RD, MacAdam JW. Urine volume and nitrogen excretion are altered by feeding birdsfoot trefoil compared with alfalfa in lactating dairy cows1. J Anim Sci. (2018) 96:3993–4001. doi: 10.1093/jas/sky259
42. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. (1980) 21:175–85. doi: 10.1159/000137430
43. Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the lennox-gastaut syndrome. N Engl J Med. (2018) 378:1888–97. doi: 10.1056/NEJMoa1714631
44. Suraev A, Lintzeris N, Stuart J, Kevin RC, Blackburn R, Richards E, et al. Composition and use of cannabis extracts for childhood epilepsy in the Australian community. Sci Rep. (2018) 8:1–14. doi: 10.1038/s41598-018-28127-0
45. Goulart RS, Vieira R, Daniel J, Amaral RC, Santos VP, Toledo Filho SG, et al. Effects of source and concentration of neutral detergent fiber from roughage in beef cattle diets: comparison of methods to measure the effectiveness of fiber. J Anim Sci. (2020) 98:1–9. doi: 10.1093/jas/skaa108
46. Llonch L, Castillejos L, Ferret A. Increasing the content of physically effective fiber in high-concentrate diets fed to beef heifers affects intake, sorting behavior, time spent ruminating, and rumen pH. J Anim Sci. (2020) 98:skaa192. doi: 10.1093/jas/skaa192
47. Rupp R, Beaudeau F, Boichard D. Relationship between milk somatic-cell counts in the first lactation and clinical mastitis occurrence in the second lactation of French Holstein cows. Prev Vete Medi. (2000) 46:99–111. doi: 10.1016/S0167-5877(00)00142-2
48. Shaheen T, Ahmad SB, Rehman MU, Muzamil S, Bhat RR, Hussain I, et al. Investigations on cytokines and proteins in lactating cows with and without naturally occurring mastitis. J King Saud Univ, Sci. (2020) 32:2863–7. doi: 10.1016/j.jksus.2020.07.009
49. Davidson S, Hopkins BA, Diaz DE, Bolt SM, Brownie C, Fellner V, et al. Effects of amounts and degradability of dietary protein on lactation, nitrogen utilization, and excretion in early lactation Holstein cows. J Dairy Sci. (2003) 86:1681–9. doi: 10.3168/jds.S0022-0302(03)73754-0
50. Firkins JL. Effects of feeding nonforage fiber sources on site of fiber digestion. J Dairy Sci. (1997) 80:1426–37. doi: 10.3168/jds.S0022-0302(97)76072-7
51. Wang Y, Gao J, Cheng C, Lv J, Lambo MT, Zhang G, et al. Nutritional values of industrial hemp byproducts for dairy cattle. Animals. (2022) 12:3488. doi: 10.3390/ani12243488
52. Storry JE, Rook JA. Effect in the cow of intraruminal infusions of volatile fatty acids and of lactic acid on the secretion of the component fatty acids of the milk fat and on the composition of blood. Biochem J. (1965) 96:210–7. doi: 10.1042/bj0960210
53. Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. (1990) 70:567–90. doi: 10.1152/physrev.1990.70.2.567
54. Esquivel-Elizondo S, Ilhan ZE, Garcia-Peña EI, Krajmalnik-Brown R. Insights into butyrate production in a controlled fermentation system via gene predictions. mSystems. (2017) 2:e00051–17. doi: 10.1128/mSystems.00051-17
55. Liu C, Li H, Xu F, Jiang X, Ma H, Seeram NP. Cannabidiol protects human skin keratinocytes from hydrogen-peroxide-induced oxidative stress via modulation of the caspase-1-IL-1β axis. J Nat Prod. (2021) 84:1563–72. doi: 10.1021/acs.jnatprod.1c00083
56. Henshaw FR, Dewsbury LS, Lim CK, Steiner GZ. The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies. Cannabis Cannabinoid Res. (2021) 6:177–95. doi: 10.1089/can.2020.0105
57. Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. (2013) 59:141–50. doi: 10.1016/j.nbd.2013.06.016
58. Wilson DJ, Gonzalez RN, Das HH. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J Dairy Sci. (1997) 80:2592–8. doi: 10.3168/jds.S0022-0302(97)76215-5
59. Ran T, Tang SX, Yu X, Hou ZP, Hou FJ, Beauchemin KA, et al. Diets varying in ratio of sweet sorghum silage to corn silage for lactating dairy cows: Feed intake, milk production, blood biochemistry, ruminal fermentation, and ruminal microbial community. J Dairy Sci. (2021) 104:12600–15. doi: 10.3168/jds.2021-20408
60. Chen S, Cheng H, Wyckoff KN, He Q. Linkages of firmicutes and bacteroidetes populations to methanogenic process performance. J Ind Microbiol Biotechnol. (2016) 43:771–81. doi: 10.1007/s10295-016-1760-8
61. Wang Y, Nan X, Zhao Y, Jiang L, Wang M, Wang H, et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J Anim Sci Biotechnol. (2021) 12:36. doi: 10.1186/s40104-020-00543-1
62. Yin YY, Liu YJ, Zhu WY, Mao SY. Effects of acarbose addition on ruminal bacterial microbiota, lipopolysaccharide levels and fermentation characteristics in vitro. Asian-Australas J Anim Sci. (2014) 27:1726–35. doi: 10.5713/ajas.2014.14292
63. Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. (2006) 58:572–82. doi: 10.1111/j.1574-6941.2006.00190.x
64. Carberry CA, Kenny DA, Han S, McCabe MS, Waters SM. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl Environ Microbiol. (2012) 78:4949–58. doi: 10.1128/AEM.07759-11
65. Zhang L, Jiang X, Liu X, Zhao X, Liu S, Li Y, et al. Growth, health, rumen fermentation, and bacterial community of Holstein calves fed Lactobacillus rhamnosus GG during the preweaning stage1. J Anim Sci. (2019) 97:2598–608. doi: 10.1093/jas/skz126
66. Liu JH, Zhang ML, Zhang RY, Zhu WY, Mao SY. Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb Biotechnol. (2016) 9:257–68. doi: 10.1111/1751-7915.12345
67. Ghyselinck J, Verstrepen L, Moens F, Van Den Abbeele P, Bruggeman A, Said J, et al. Influence of probiotic bacteria on gut microbiota composition and gut wall function in an in-vitro model in patients with Parkinson's disease. Int J Pharm X. (2021) 3:100087. doi: 10.1016/j.ijpx.2021.100087
68. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. (2017) 19:29–41. doi: 10.1111/1462-2920.13589
69. Kim CC, Healey GR, Kelly WJ, Patchett ML, Jordens Z, Tannock GW, et al. Genomic insights from Monoglobus pectinilyticus: a pectin-degrading specialist bacterium in the human colon. ISME J. (2019) 13:1437–56. doi: 10.1038/s41396-019-0363-6
70. Eilam O, Zarecki R, Oberhardt M, Ursell LK, Kupiec M, Knight R, et al. Glycan degradation (GlyDeR) analysis predicts mammalian gut microbiota abundance and host diet-specific adaptations. MBio. (2014) 5:e01526. doi: 10.1128/mBio.01526-14
Keywords: industrial hemp ethanol extraction byproduct, lactation performance, plasma metabolites, bacterial community, dairy cow
Citation: Wang Y, Yu Q, Wang X, Song J, Lambo MT, Huang J, He P, Li Y and Zhang Y (2023) Replacing alfalfa hay with industrial hemp ethanol extraction byproduct and Chinese wildrye hay: Effects on lactation performance, plasma metabolites, and bacterial communities in Holstein cows. Front. Vet. Sci. 10:1061219. doi: 10.3389/fvets.2023.1061219
Received: 05 October 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Kai Wang, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Yanliang Bi, Feed Research Institute (CAAS), ChinaZhiyong Hu, Shandong Agricultural University, China
Copyright © 2023 Wang, Yu, Wang, Song, Lambo, Huang, He, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Li,  bGl5YW5nMTQwNTA1M0BzaW5hLmNvbQ==; Yonggen Zhang,
bGl5YW5nMTQwNTA1M0BzaW5hLmNvbQ==; Yonggen Zhang,  emhhbmd5b25nZ2VuQHNpbmEuY29t
emhhbmd5b25nZ2VuQHNpbmEuY29t
 Yiqiang Wang
Yiqiang Wang Qingyuan Yu1
Qingyuan Yu1 Jiamei Song
Jiamei Song Modinat Tolani Lambo
Modinat Tolani Lambo Yang Li
Yang Li