- 1Department of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon, Republic of Korea
- 2Research Institute for New Drug Development, Incheon National University, Incheon, Republic of Korea
- 3Convergence Research Center for Insect Vectors, Incheon National University, Incheon, Republic of Korea
- 4KU Center for Animal Blood Medical Science, College of Veterinary Medicine, Konkuk University, Seoul, Republic of Korea
Lawsonia intracellularis is the etiological agent of proliferative enteropathy, which is globally considered an important enteric disease in pigs and horses. Experimental studies suggest that the organism spreads by subclinical infection of many animals, including rabbits. Despite the importance of rabbits in the epidemiology of L. intracellularis, the extent of exposure to L. intracellularis in the rabbit population is poorly defined and remains unclear. The objective of this cross-sectional study was to investigate the seroprevalence and shedding of L. intracellularis in farmed rabbits. Furthermore, we aimed to identify risk factors associated with seropositivity. Sera from the rabbits were used to measure L. intracellularis-specific antibodies by immunoperoxidase monolayer assay, and rectal swabs were used to detect L. intracellularis DNA using a real-time PCR assay. Antibodies against L. intracellularis were detected in 12.3% of farms (20/163) and 6.3% of rabbits (49/774). Lawsonia intracellularis DNA in rectal swabs was detected in 3.8% of farms (6/156) and 1.2% of rabbits (8/667). The risk factor analysis showed that the presence of pigs or horses on the farm or the neighboring farm was associated with an increase in the risk of seropositivity (p < 0.05). We observed significantly increased odds of positivity for L. intracellularis in rabbits with a history of digestive trouble (diarrhea) on the farm during the 3 months before the samples were obtained (p < 0.05). Collectively, these findings demonstrated that L. intracellularis infection was evident among farmed rabbits and that rabbits might serve as an important reservoir for L. intracellularis epidemiology.
1. Introduction
Lawsonia intracellularis is a microaerophilic intracellular bacterium that infects the small and also large intestine in pigs and other animals, including hamsters and horses (1–5). The disease caused by L. intracellularis is characterized by cell proliferation, hemorrhage, necrosis, or any combination commonly referred to as “ileitis” or “proliferative enteropathy.” The infection is globally considered one of the important enteric diseases in pigs and horses and is known as porcine proliferative enteropathy and as equine proliferative enteropathy based on the corresponding host (6–9).
Lawsonia intracellularis has been associated with the colonization of enterocytes in a wide range of hosts, including rabbits, hamsters, rats, guinea pigs, swine, sheep, horses, white-tailed deer (Odocoileus virginianus), dogs, artic foxes (Alopex logopus), ferrets, ostriches (Struthio camelus), and rhesus macaques (10–13). The epidemiology of L. intracellularis is not fully known, but experimental studies suggest that the organism is spread by subclinical infection of a wide variety of animal species, including rabbits (14–17).
It is now well-known that L. intracellularis can infect rabbits and induce conditions referred to as acute typhlitis, histiocytic enteritis, enterocolitis, and proliferative enteropathy (11, 18–21). Despite the importance of rabbit species in L. intracellularis epidemiology, the extent of exposure to L. intracellularis in the rabbit population is poorly defined and remains unclear. The lack of baseline data on the prevalence of L. intracellularis in rabbits has resulted in a poor overall understanding of the epidemiology of proliferative enteropathy.
The objective of this cross-sectional study was to investigate the prevalence of antibodies to and the shedding of L. intracellularis in farmed rabbits in South Korea. Furthermore, we aimed to identify risk factors associated with seropositivity, including breed, sex, age, rearing type, type of rabbitries, feed type, presence of pigs or horses on the farm or the neighboring farm, and history of digestive trouble (diarrhea) on the farm.
2. Method
2.1. Rabbit farms and data collection
To obtain the serum and rectal swab samples for this study, farm selection and blood sampling were conducted in close collaboration with local veterinary practitioners and/or government veterinary officers. The study was carried out between March 2020 and February 2022, and we obtained information by carrying out farm visits as a part of veterinary practice, routine health appraisal, and consulting activities on rabbit farms. Informed consent was requested from individuals on every participating farm. Individual information about the breed, sex, age, rearing type (enclosed, outdoor, or mixed enclosed and outdoor), type of rabbitries [cage, fence (or free range)], or mixed [cage and fence (or free range)], feed type, presence of pigs or horses within the farm or the neighboring farm (within a 0.5 km radius), and history of digestive trouble (diarrhea) in the farm during the 3 months before the samplings was gathered through a detailed survey.
A true prevalence of 50% was assumed due to the absence of available previous data on prevalence of L. intracellularis infection among rabbits. The determined sample size needed was calculated as 28 for an unknown population size with a desired precision of 20%, a 95% confidence level and a test assumed with 95% sensitivity and 99% specificity (22, 23). A sample size of animals per rabbit farm was determined using USDA animal sample calculator (24).
2.2. Serum samples and rectal swabs
At each farm, blood samples and rectal swabs were taken randomly. Whole blood samples were collected from auricular veins and centrifuged to obtain sera for serological tests. The sera separated from the blood samples were stored at −80°C prior to use. Rectal swabs were collected using sterile cotton-tipped swabs inserted ~1 cm into the rectum and gently rotated to collect fecal material from the rectal wall. Swabs were retracted and placed in sterile conical tubes, the shaft was cut, and the tubes were closed and frozen at −20°C until shipped to the laboratory for DNA extraction. Rectal swabs that arrived at the laboratory were kept refrigerated at 4 °C prior to processing for nucleic acid purification within 48 h of collection. Samples with insufficient volume or poor quality were excluded from the analysis. Samples that were missing information needed for the risk analysis of this study were also excluded. A total of 774 serum and 667 rectal swab samples collected from 163 rabbit herds nationwide inland in South Korea were used in this study.
2.3. Serology
Sera from the rabbits were used to detect L. intracellularis-specific antibodies by immunoperoxidase monolayer assay (IPMA). The cultivation of L. intracellularis and serology using the IPMA technique were performed as described previously (25–27). The pathogenic isolate PHE/KK421 (Korean Collection for Type Cultures 10686BP, Daejeon, South Korea) was used to infect murine fibroblast-like McCoy cells (American Type Culture Collection CRL 1696, VA, USA). Briefly, a L. intracellularis culture plate was incubated with sera diluted at 1:60 in phosphate-buffered saline (PBS) for 30 min at 37°C and washed five times with PBS (pH 7.2). Sera collected from experimentally infected rabbits served as positive controls. Peroxidase-labeled anti-rabbit IgG antibody (Bethyl Laboratories, Montgomery, TX, USA) was diluted 1:500 in 2% bovine serum albumin and 0.08% Tween 80 in PBS and then applied at a concentration of 50 μl/well. The plate was incubated for 45 min at 37°C. The plate was washed again, and chromogen (3-amino-9-ethyl-carbazole, Dako Corporation, CA, USA) solution was added to each well. Then, the plate was incubated at room temperature for 20 min. The plate was washed with distilled water three times, allowed to dry, and examined using a BX50 microscope (Olympus, Tokyo, Japan). Positive samples exhibited red-labeled bacteria in both the cytoplasm of the infected McCoy cells and the extracellular space (28–31).
2.4. PCR
DNA purification was performed using a BioRobot M48 workstation apparatus (Qiagen, GmBH, Hilden, Germany) with a MagAttract DNA Mini M48 Kit (Qiagen) according to the manufacturer's recommendations. One negative extraction sample containing other bacterial cells (Escherichia coli) and one positive extraction sample containing L. intracellularis were included in each experiment to assess for contamination during the DNA extraction process. Nucleic acids were eluted in 50 μl of buffer and stored at −80°C. All purified DNA samples were assayed for the presence of the aspartate ammonia lyase (aspA) gene of L. intracellularis by real-time PCR. All purified DNA samples from the rectal swabs were assayed in triplicate for the presence of the L. intracellularis aspA gene by real-time PCR as described previously (22). This real-time TaqMan PCR assay enables the detection of a specific 104-base pair product of the aspA gene from L. intracellularis (GenBank accession no. AM180252).
Precautions were taken to minimize contamination during the precipitation, preamplification, and amplification steps, including performing all pipetting steps in a laminar flow cabinet and including positive (DNA from cell-grown L. intracellularis) and negative (L. intracellularis-free DNA from rectal swab samples) DNA controls. Furthermore, swabs were taken from centrifuges, laminar flow cabinets, and countertops and assayed for the L. intracellularis aspA gene by real-time PCR to assess potential contamination. A real-time PCR assay that targeted a universal sequence of the bacterial 16S rRNA gene was used as a quality control (i.e., efficiency of DNA purification and amplification) and as an indicator of fecal inhibition as described previously (32, 33).
2.5. Statistical analysis
Prevalence and Wilson's 95% confidence intervals (CIs) (34) were calculated using Epitools-Epidemiological Calculators (35). A logistic regression model was used to identify potential risk factors that were correlated with animal seropositivity. The variables in the univariable analysis were evaluated for pairwise collinearity or associations using Pearson's correlation coefficient or the chi-squared test for continuous or categorical variables, respectively. The strength of association was analyzed using odds ratios and 95% CIs. Results with a p-value < 0.05 were considered statistically significant. Statistical analyses in this study were performed using commercially available statistical software (SPSS Statistics for Windows, Version 25.0, IBM Corp., Armonk, NY, USA).
3. Results
Antibodies specific to L. intracellularis were detected in 6.3% of farmed rabbits (49/774), as shown in Table 1 and Figure 1. Lawsonia intracellularis DNA in the rectal swabs was detected by PCR in 1.2% farmed rabbits (8/667). Seropositive rabbits were observed in all the provinces surveyed, and the highest seroprevalence values for L. intracellularis were observed in Gyeonggi Province, with rates of 11.9% (28/235, 95% CI = 8.3–17.6). Rabbits shedding L. intracellularis were observed in most provinces surveyed except Gangwon Province.
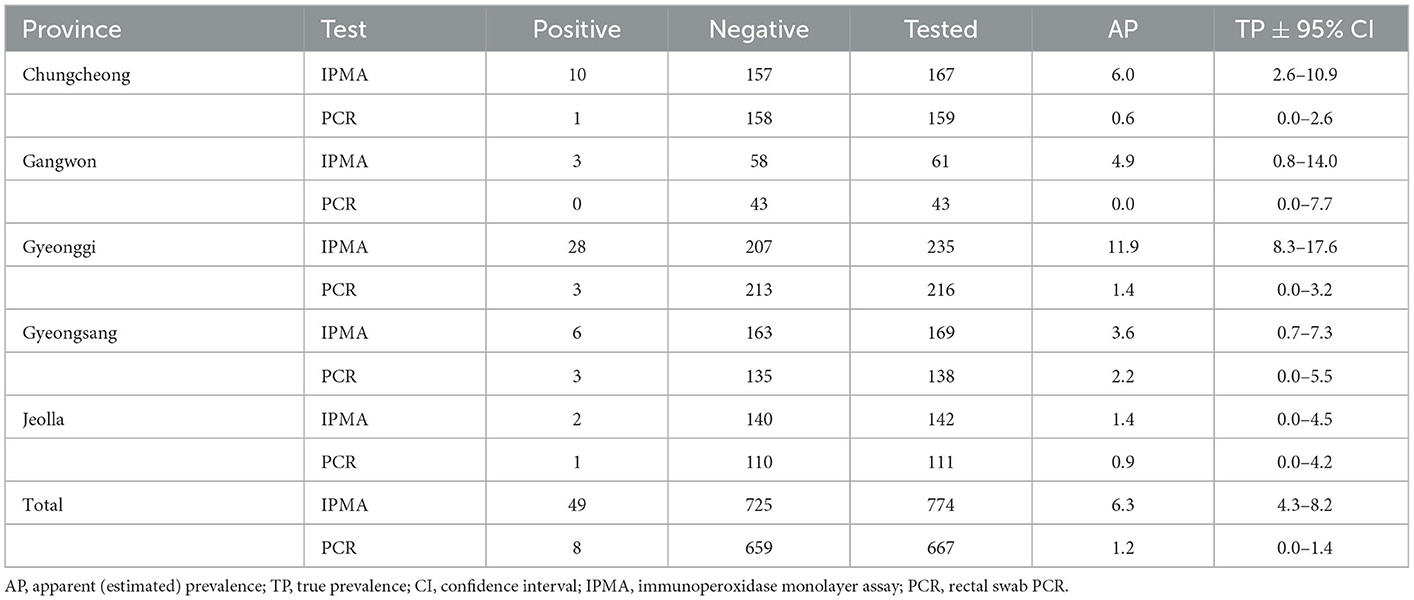
Table 1. Prevalence of antibodies to and shedding of Lawsonia intracellularis in farmed rabbits in South Korea.
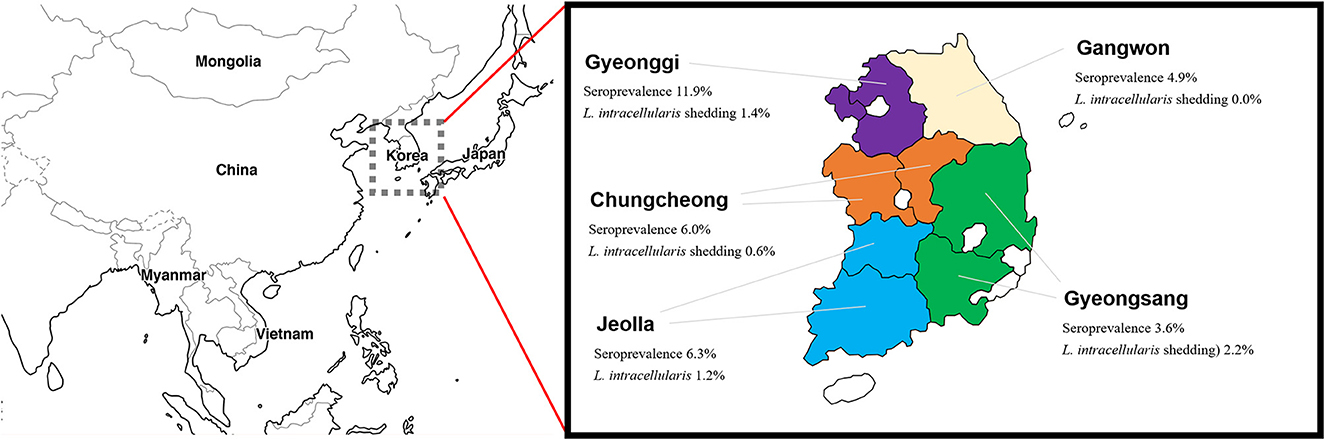
Figure 1. Geographical location of the provinces of South Korea and prevalence of Lawsonia intracellularis in farmed rabbits.
In the univariate analysis (Table 2), the risk factor analysis revealed that the presence of pigs or horses on the farm or the neighboring farm (within a 0.5 km radius) was associated with an increase in the risk of seropositivity (OR = 1.480, 95% CI = 1.117–1.959, p < 0.05). We observed significantly increased odds of positivity for L. intracellularis in the rabbits with a history of digestive trouble (diarrhea) on the farm during the 3 months before the samples were acquired (OR = 1.909, 95% CI = 1.127–3.234, p < 0.05). Although there was slightly higher seropositivity in crossbred animals, females, animals younger than 6 months old, animals that were reared outdoors only, animals in rabbitries with a fence or that were free range, or animals that were fed concentrated feed and grass, these associations were not statistically significant (p > 0.05).
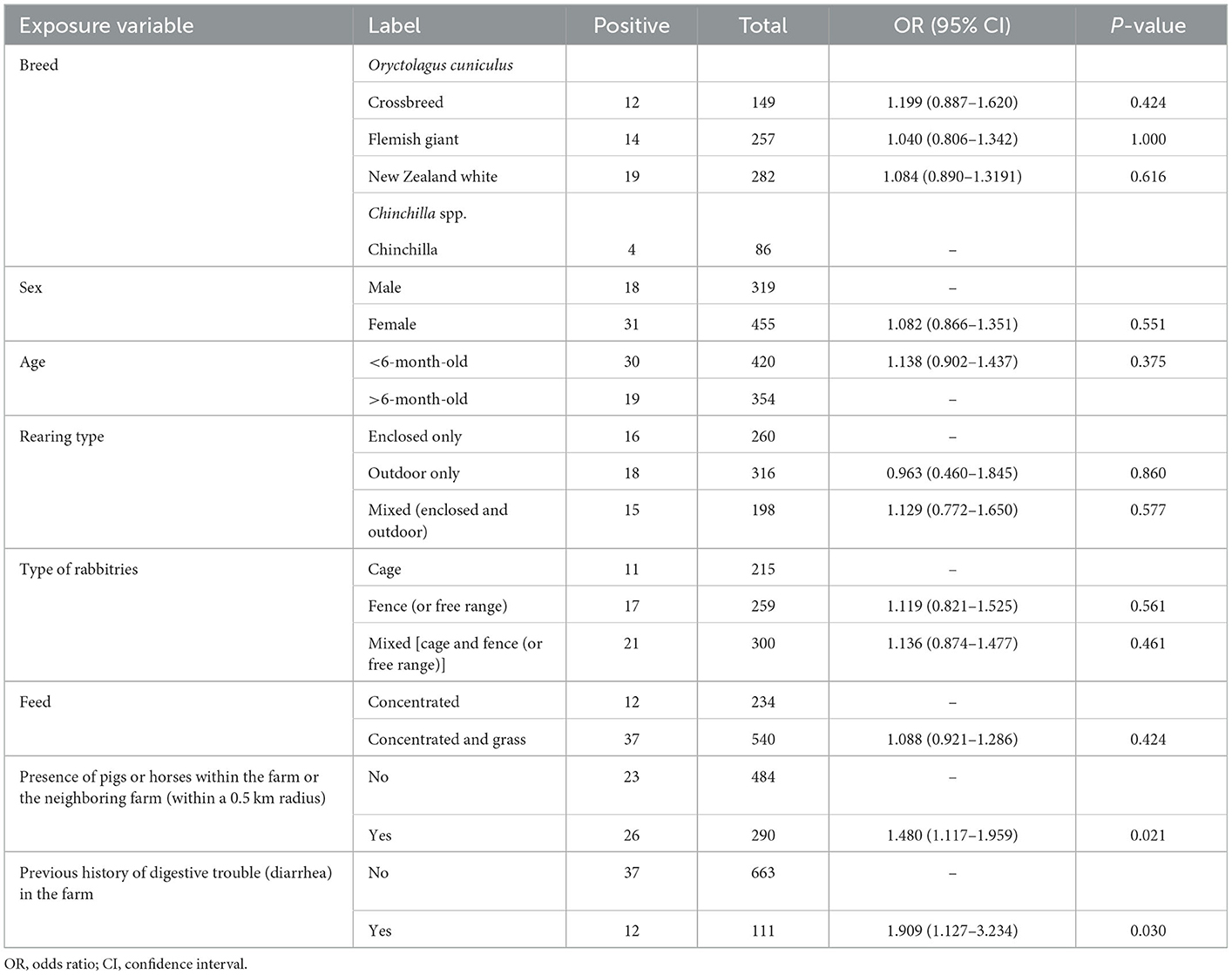
Table 2. Univariable analysis of Lawsonia intracellularis exposure variables relative to seropositivity outcomes in farmed rabbits in South Korea.
4. Discussion
Rabbits have been described as a susceptible host of L. intracellularis and as an animal model in the field of proliferative enteropathy research (15–21, 36–38), but the prevalence and associated risk factors in the rabbit population have not been reported thus far. The results of this study showed that exposure rates to L. intracellularis in farmed rabbits were not as high as those in pigs or horses.
In pigs, the seroprevalence of antibodies to L. intracellularis was reported to be 31.6% in European countries (39), 34.7% in Brazil (40), 57% in China (41), 84.2% within pig herds in Australia (42), 26%−59% among slaughter-age pigs in the Netherlands (43), and 44%−69% at the animal level and 100% at the farm level in South Korea (44). The prevalence of L. intracellularis shedding in pig feces was found to be 26.5% in European countries (39), 19.9% in South Korea (45), 65.7% at the herd level in Poland (46), and 46%−57% among slaughter-age pigs in the Netherlands (43). In horses, the seroprevalence of antibodies to L. intracellularis was reported to be 98.8% among adult horses in Belgium (47), and Kranenburg et al. previously demonstrated that the prevalence of L. intracellulari antibodies in foals increased significantly from 15% before weaning to 23% after weaning; it was 89% in yearlings and 99% in horses older than 2 years (48).
It has been reported that L. intracellularis infections in rabbits generally progress subclinically (14). However, the routes of L. intracellularis infection in rabbits are unclear, and the source of L. intracellularis infection in rabbits remains speculative. In this study, the presence of pigs or horses on the farm or the neighboring farm (within a 0.5 km radius) was found to be one of the main factors affecting the prevalence of L. intracellularis infection (p < 0.05). Another main factor identified in the present study was a history of digestive trouble (diarrhea) on the farm during the 3 months before the samples were acquired (p < 0.05). We observed no significant differences regarding the prevalence of L. intracellularis infection when rabbits were stratified by breed, sex, age, rearing type, type of rabbitries, or feed type (p > 0.05).
This study may serve as a basis for future epidemiological studies on L. intracellularis infections. The results of the present study demonstrate that the possibility that the range of susceptible species may broaden further and incorporate more domestic animals by interspecies transmission (spillover) cannot be discounted. In view of the host range of L. intracellularis, lagomorphs may represent a reservoir, amplifying host, or important biological vector of L. intracellularis due to their large population, their short reproductive cycle, and their close contact with pigs or horses.
Collectively, the results of the present study demonstrated that L. intracellularis infection was evident among the farmed rabbits analyzed, in which ~one in 10 farms exhibited exposure to L. intracellularis. The results of this study indicated that susceptible rabbits are at risk of becoming infected with L. intracellularis, although an outbreak of L. intracellularis among farmed rabbits in South Korea has yet to be reported. These results suggest that rabbit species might serve as an important reservoir for the transmission of L. intracellularis, highlighting the need for closer epidemiological investigation of L. intracellularis infections in rabbits.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Research Ethics Committee of Incheon National University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
J-YY conceived and designed the study, conducted the laboratory experiments, wrote the first draft, and revised the manuscript.
Funding
This work was supported by National Research Foundation of Korea (NRF) from the Ministry of Science and ICT (grant number: NRF-2019R1A2C108940414).
Acknowledgments
The author sincerely thanks all of the farmers for their cooperation in the collection of blood samples. The author would also like to acknowledge the individuals who helped to recruit farms to the study and the farmers who allowed us to collect serum samples at their farms. The author is very grateful to the local government veterinary officers and veterinary practitioners for their help in collecting blood samples from rabbits throughout the country.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jacobson M, Fellstrom C, Jensen-Waern M. Porcine proliferative enteropathy: an important disease with questions remaining to be solved. Vet J. (2010) 184:264–8. doi: 10.1016/j.tvjl.2009.05.010
2. Kroll JJ, Roof MB, Hoffman LJ, Dickson JS, Harris DL. Proliferative enteropathy: a global enteric disease of pigs caused by Lawsonia intracellularis. Anim Health Res Rev. (2005) 6:173–7. doi: 10.1079/AHR2005109
3. Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. (2000) 122:77–100. doi: 10.1053/jcpa.1999.0347
4. Lawson GH, McOrist S. The enigma of the proliferative enteropathies: a review. J Comp Pathol. (1993) 108:41–6. doi: 10.1016/S0021-9975(08)80225-3
5. Page AE, Slovis NM, Horohov DW. Lawsonia intracellularis and equine proliferative enteropathy. Vet Clin North Am Equine Pract. (2014) 30:641–58. doi: 10.1016/j.cveq.2014.08.001
6. Karuppannan AK, Opriessnig T. Lawsonia intracellularis: revisiting the disease ecology and control of this fastidious pathogen in pigs. Front Vet Sci. (2018) 5:181. doi: 10.3389/fvets.2018.00181
7. Pusterla N, Gebhart C. Equine proliferative enteropathy caused by Lawsonia intracellularis. Equine Vet Educ. (2009) 21:415–9. doi: 10.2746/095777309X453119
8. Pusterla N, Gebhart CJ. Equine proliferative enteropathy–a review of recent developments. Equine Vet J. (2013) 45:403–9. doi: 10.1111/evj.12075
9. Vannucci FA, Gebhart CJ. Recent advances in understanding the pathogenesis of Lawsonia intracellularis infections. Vet Pathol. (2014) 51:465–77. doi: 10.1177/0300985813520249
10. Cooper DM, Swanson DL, Gebhart CJ. Diagnosis of proliferative enteritis in frozen and formalin-fixed, paraffin-embedded tissues from a hamster, horse, deer and ostrich using a Lawsonia intracellularis-specific multiplex PCR assay. Vet Microbiol. (1997) 54:47–62. doi: 10.1016/S0378-1135(96)01264-3
11. Hotchkiss CE, Shames B, Perkins SE, Fox JG. Proliferative enteropathy of rabbits: the intracellular Campylobacter-like organism is closely related to Lawsonia intracellularis. Lab Anim Sci. (1996) 46:623–7.
12. Williams NM, Harrison LR, Gebhart CJ. Proliferative enteropathy in a foal caused by Lawsonia intracellularis-like bacterium. J Vet Diagn Invest. (1996) 8:254–6. doi: 10.1177/104063879600800220
13. Klein EC, Gebhart CJ, Duhamel GE. Fatal outbreaks of proliferative enteritis caused by Lawsonia intracellularis in young colony-raised rhesus macaques. J Med Primatol. (1999) 28:11–8. doi: 10.1111/j.1600-0684.1999.tb00084.x
14. Duhamel GE, Klein EC, Elder RO, Gebhart CJ. Subclinical proliferative enteropathy in sentinel rabbits associated with Lawsonia intracellularis. Vet Pathol. (1998) 35:300–3. doi: 10.1177/030098589803500410
15. Murakata K, Sato A, Yoshiya M, Kim S, Watarai M, Omata Y, et al. Infection of different strains of mice with Lawsonia intracellularis derived from rabbit or porcine proliferative enteropathy. J Comp Pathol. (2008) 139:8–15. doi: 10.1016/j.jcpa.2008.03.001
16. Pusterla N, Sanchez-Migallon Guzman D, Vannucci FA, Mapes S, White A, DiFrancesco M, et al. Transmission of Lawsonia intracellularis to weanling foals using feces from experimentally infected rabbits. Vet J. (2013) 195:241–3. doi: 10.1016/j.tvjl.2012.05.028
17. Sampieri F, Allen AL, Pusterla N, Vannucci FA, Antonopoulos AJ, Ball KR, et al. The rabbit as an infection model for equine proliferative enteropathy. Can J Vet Res. (2013) 77:110–9.
18. Horiuchi N, Watarai M, Kobayashi Y, Omata Y, Furuoka H. Proliferative enteropathy involving Lawsonia intracellularis infection in rabbits (Oryctlagus cuniculus). J Vet Med Sci. (2008) 70:382–92. doi: 10.1292/jvms.70.389
19. Lim JJ, Kim DH, Lee JJ, Kim DG, Kim SH, Min W, et al. Prevalence of Lawsonia intracellularis, Salmonella spp. and Eimeria spp in healthy and diarrheic pet rabbits J Vet Med Sci. (2012) 74:263–5. doi: 10.1292/jvms.11-0389
20. Schauer DB, McCathey SN, Daft BM, Jha SS, Tatterson LE, Taylor NS, et al. Proliferative enterocolitis associated with dual infection with enteropathogenic Escherichia coli and Lawsonia intracellularis in rabbits. J Clin Microbiol. (1998) 36:1700–3. doi: 10.1128/JCM.36.6.1700-1703.1998
21. Schoeb TR, Fox JG. Enterocecocolitis associated with intraepithelial Campylobacter-like bacteria in rabbits (Oryctolagus cuniculus). Vet Pathol. (1990) 27:73–80. doi: 10.1177/030098589002700201
22. Pusterla N, Mapes S, Rejmanek D, Gebhart C. Detection of Lawsonia intracellularis by real-time PCR in the feces of free-living animals from equine farms with documented occurrence of equine proliferative enteropathy. J Wildl Dis. (2008) 44:992–8. doi: 10.7589/0090-3558-44.4.992
23. Humphry RW, Cameron A, Gunn GJ. A practical approach to calculate sample size for herd prevalence surveys. Prev Vet Med. (2004) 65:173–88. doi: 10.1016/j.prevetmed.2004.07.003
24. United States Department of Agriculture Animal Sample Size Calculator. Available online at: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/ceah-toolbox/animal-sample-size-calculator (accessed 1 December 1, 2019).
25. Yeh JY, Kim TJ, Park SY, Song CS, Yoon YD, Kim SK, et al. Isolation of Lawsonia intracellularis in Korea and reproduction of proliferative enteropathy in pigs and hamsters. J Vet Med Sci. (2006) 68:499. doi: 10.1292/jvms.68.499
26. Guedes RM, Gebhart CJ. Comparison of intestinal mucosa homogenate and pure culture of the homologous Lawsonia intracellularis isolate in reproducing proliferative enteropathy in swine. Vet Microbiol. (2003) 93:159–66. doi: 10.1016/S0378-1135(03)00013-0
27. Guedes RM, Gebhart CJ, Deen J, Winkelman NL. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J Vet Diagn Invest. (2002) 14:528–530. doi: 10.1177/104063870201400618
28. Page AE, Slovis NM, Gebhart CJ, Wolfsdorf K, Mapes SM, Pusterla N. Serial use of serologic assays and fecal PCR assays to aid in identification of subclinical Lawsonia intracellularis infection for targeted treatment of Thoroughbred foals and weanlings. J Am Vet Med Assoc. (2011) 238:1482–9. doi: 10.2460/javma.238.11.1482
29. Pusterla N, Jackson R, Wilson R, Collier J, Mapes S, Gebhart C. Temporal detection of Lawsonia intracellularis using serology and real-time PCR in Thoroughbred horses residing on a farm endemic for equine proliferative enteropathy. Vet Microbiol. (2009) 136:173–6. doi: 10.1016/j.vetmic.2008.10.004
30. Frazer ML. Lawsonia intracellularis infection in horses: 2005-2007. J Vet Intern Med. (2008) 22:1243–8. doi: 10.1111/j.1939-1676.2008.0160.x
31. Guimaraes-Ladeira CV, Palhares MS, Oliveira JS, Ramirez MA, Guedes RM. Faecal shedding and serological cross-sectional study of Lawsonia intracellularis in horses in the state of Minas Gerais, Brazil. Equine Vet J. (2009) 41:593–596. doi: 10.2746/042516409X407639
32. Mapes S, Rhodes DM, Wilson WD, Leutenegger CM, Pusterla N. Comparison of five real-time PCR assays for detecting virulence genes in isolates of Escherichia coli from septicaemic neonatal foals. Vet Rec. (2007) 161:716–8.doi: 10.1136/vr.161.21.716
33. Windsor RC, Johnson LR, Sykes JE, Drazenovich TL, Leutenegger CM, De Cock HE. Molecular detection of microbes in nasal tissue of dogs with idiopathic lymphoplasmacytic rhinitis. J Vet Intern Med. (2006) 20:250–256.doi: 10.1111/j.1939-1676.2006.tb02854.x
34. Reiczigel J, Foldi J, Ozsvari L. Exact confidence limits for prevalence of a disease with an imperfect diagnostic test. Epidemiol Infect. (2010) 138:1674–8. doi: 10.1017/S0950268810000385
36. Sampieri F, Allen AL, Alcorn J, Clark CR, Vannucci FA, Pusterla N, et al. Efficacy of gallium maltolate against Lawsonia intracellularis infection in a rabbit model. J Vet Pharmacol Ther. (2014) 37:571–8.doi: 10.1111/jvp.12132
37. Watarai M, Yamato Y, Horiuchi N, Kim S, Omata Y, Shirahata T, et al. Enzyme-linked immunosorbent assay to detect Lawsonia intracellularis in rabbits with proliferative enteropathy. J Vet Med Sci. (2004) 66:735–7.doi: 10.1292/jvms.66.735
38. Watarai M, Yoshiya M, Sato A, Furuoka H. Cultivation and characterization of Lawsonia intracellularis isolated from rabbit and pig. J Vet Med Sci. (2008) 70:731–3. doi: 10.1292/jvms.70.731
39. Arnold M, Crienen A, Swam H, von Berg S, Jolie R, Nathues H. Prevalence of Lawsonia intracellularis in pig herds in different European countries. Porcine Health Manag. (2019) 5:31. doi: 10.1186/s40813-019-0137-6
40. Resende TP, Pereira CE, Gabardo Mde P, Haddad JP, Lobato ZI, Guedes RM. Serological profile, seroprevalence and risk factors related to Lawsonia intracellularis infection in swine herds from Minas Gerais State, Brazil. BMC Vet Res. (2015) 11:306. doi: 10.1186/s12917-015-0618-z
41. Wu Z, Ling Y, Tian D, Pan Q, Heegaard PM, He C. Seroprevalence of Lawsonia intracellularis antibodies in intensive pig farms in China. BMC Vet Res. (2014) 10:100. doi: 10.1186/1746-6148-10-100
42. Holyoake PK, Emery D, Gonsalves J, Donahoo M, Collins A. Prevalence of antibodies to Lawsonia intracellularis in pig herds in Australia. Aust Vet J. (2010) 88:186–8. doi: 10.1111/j.1751-0813.2010.00558.x
43. van der Heijden HM, Bakker J, Elbers AR, Vos JH, Weyns A, de Smet M, et al. Prevalence of exposure and infection of Lawsonia intracellularis among slaughter-age pigs. Res Vet Sci. (2004) 77:197–202. doi: 10.1016/j.rvsc.2004.04.007
44. Lee SW, Kim TJ, Park SY, Song CS, Chang HK, Yeh JK, et al. Prevalence of porcine proliferative enteropathy and its control with tylosin in Korea. J Vet Sci. (2001) 2:209–212. doi: 10.4142/jvs.2001.2.3.209
45. Suh DK, Song JC. Prevalence of Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella in swine herds. J Vet Sci. (2005) 6:289–93. doi: 10.4142/jvs.2005.6.4.289
46. Dors A, Pomorska-Mol M, Czyzewska E, Wasyl D, Pejsak Z. Prevalence and risk factors for Lawsonia intracellularis, Brachyspira hyodysenteriae and Salmonella spp. in finishing pigs in Polish farrow-to-finish swine herds. Pol J Vet Sci. (2015) 18:825–31. doi: 10.1515/pjvs-2015-0107
47. Loublier C, Cerri S, Gryspeerdt A, Amory H, Bauwens C, Cesarini C. High seroprevalence against Lawsonia intracellularis among adult horses in Belgium. J Equine Vet Sci. (2020) 95:103304. doi: 10.1016/j.jevs.2020.103304
Keywords: antibody, epidemiology, IPMA, proliferative enteropathy, qPCR, shedding
Citation: Yeh J-Y (2023) Prevalence and associated risk factors for Lawsonia intracellularis infection in farmed rabbits: A serological and molecular cross-sectional study in South Korea. Front. Vet. Sci. 10:1058113. doi: 10.3389/fvets.2023.1058113
Received: 30 September 2022; Accepted: 20 January 2023;
Published: 09 February 2023.
Edited by:
Anbu K. Karuppannan, Tamil Nadu Veterinary and Animal Sciences University, IndiaReviewed by:
Francisco Ruben Carvallo Chaigneau, Virginia Tech, United StatesRoberto Guedes, Federal University of Minas Gerais, Brazil
Copyright © 2023 Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung-Yong Yeh,  eWVoanlAaW51LmFjLmty
eWVoanlAaW51LmFjLmty
 Jung-Yong Yeh
Jung-Yong Yeh