- Small Animal Clinic, Veterinary Faculty, University of Ljubljana, Ljubljana, Slovenia
General anesthesia increases the production of reactive oxygen species (ROS), which can exacerbate or increase oxidative stress and thus affect the prognosis of surgical procedures. Oxidative stress has been implicated in the development of cardiovascular, dermatologic, oncologic, and other diseases in dogs, as well as ischemia and reperfusion injury. Some anesthetics, such as halogenated anesthetics, have been shown to stimulate the production of ROS, while others, such as propofol, have antioxidant properties. However, the antioxidant effects of these anesthetics may not be sufficient to counteract oxidative damage at the doses used clinically. Nevertheless, the effects of anesthetics should be considered to minimize oxidative damage during anesthesia in dogs to improve the outcome of procedures requiring general anesthesia. This mini-review addresses the current knowledge on oxidative stress during inhalational and intravenous anesthesia in dogs. There is still a lack of information on the management of anesthesia in dogs with respect to oxidative stress. Further research, including comprehensive clinical studies is needed to better understand oxidative injury mechanisms and improve perioperative protocols during anesthesia in dogs.
Introduction
Anesthetic metabolism, changes in tissue perfusion and oxygenation during anesthesia, and surgical trauma increase the generation of reactive oxygen species (ROS), which can aggravate oxidative stress in humans (1–5) and dogs (6–9). Oxidative stress has been associated with ischemia and reperfusion injury, as well as cardiovascular, dermatologic, oncologic, and other diseases in dogs (10). In the perioperative setting, oxidative stress may be a determining factor in the outcome of surgical procedures (1, 3, 5). Therefore, the choice of an anesthetic protocol is important because some anesthetics may stimulate the production of ROS, whereas others have antioxidant properties (3, 4).
The purpose of this mini-review is to summarize the current literature on the effects of inhalational and intravenous anesthetics on oxidative stress in dogs. Relevant literature was selected using the Pubmed database and the Google Scholar search engine. The search terms were “oxidative stress anesthesia dogs.” In the section on individual anesthetics, the search terms were the anesthetic sought with the words: “oxidative stress”; “antioxidant”; “oxidative dogs” (i.e., ketamine oxidative stress; ketamine antioxidant; ketamine oxidative dogs).
Oxidative stress, reactive oxygen species and antioxidants
The most recent definition of oxidative stress states that oxidative stress is “an imbalance between oxidants and antioxidants in favor of oxidants, resulting in disruption of redox signaling and control and/or molecular damage” (11).
Reactive oxygen species include oxygen free radicals [superoxide (), hydroxyl radical (•OH), peroxyl radical (ROO•)] and reactive nonradical species [singlet oxygen (1O2), hydrogen peroxide (H2O2), hypochlorous acid (HOCl)] (12, 13). Endogenous sources of ROS include metabolic processes in mitochondria and peroxisomes, inflammatory cellular reactions, and the catalytic action of cytochrome P450. External sources of ROS include radiation (ultraviolet, X-ray, and gamma rays), cigarette smoke, ultraviolet light, drugs, chemical reagents, industrial solvents, and other environmental pollutants (14, 15). Reactive oxygen species are involved in biological processes, such as cellular signal transduction, adaptation to stress (16), and cellular defense (17). Under pathological conditions, excessive amounts of ROS damage biologically important molecules such as lipids, proteins, and DNA and trigger reactions that can destroy the cell membrane, block the action of important enzymes, prevent normal cell division, destroy DNA, and block energy production (12, 18).
An antioxidant is any substance that delays, prevents, or eliminates oxidative damage to a target molecule (19). Antioxidants are endogenous or exogenous molecules that mitigate any form of oxidative/nitrosative stress or its consequences. The endogenous antioxidant system consists of enzymatic and non–enzymatic antioxidants. Antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) accelerate the transition from ROS to more stable products. Non-enzymatic antioxidants such as melatonin, thiol antioxidants (glutathione, thioredoxin, and lipoic acid), ubiquinone (coenzyme Q10), and uric acid act as free radical scavengers (12, 18, 20). Plasma albumin is a very abundant circulating antioxidant that acts either as a radical scavenger or a chelator of metal ions, heme, and other molecules (21, 22). Exogenous antioxidants supplied to the body through diet and supplements include ROS scavengers such as alpha-tocopherol (vitamin E), ascorbic acid (vitamin C), and carotenoids, as well as dietary components that enhance endogenous antioxidant activity (12, 14, 18).
The antioxidant capacity (TAC) of plasma or other body fluids represents the redox status of the organism, which is influenced by environmental and metabolic factors, physiological or pathological conditions, and dietary antioxidant intake (23–26). However, the TAC provides only a general insight into antioxidant status (27). Water-soluble antioxidants such as uric acid, ascorbic acid, proteins, and other low-molecular-weight antioxidants are retained in aqueous plasma compartments, whereas fat-soluble antioxidants (e.g., vitamin E, coenzyme Q10 and carotenoids) are hidden in lipoproteins (24). Determination of the antioxidant capacity of water- (ACW) and lipid-soluble (ACL) antioxidants is an accurate method for assessing the antioxidant capacity of plasma (28, 29).
Studies on the effects of anesthesia on oxidative stress in humans (3, 4, 30–33) and dogs (9, 34–46) are based on the determination of changes in antioxidant (and oxidative) capacity, activities of antioxidant enzymes, and markers of oxidative damage to DNA, proteins, and lipids.
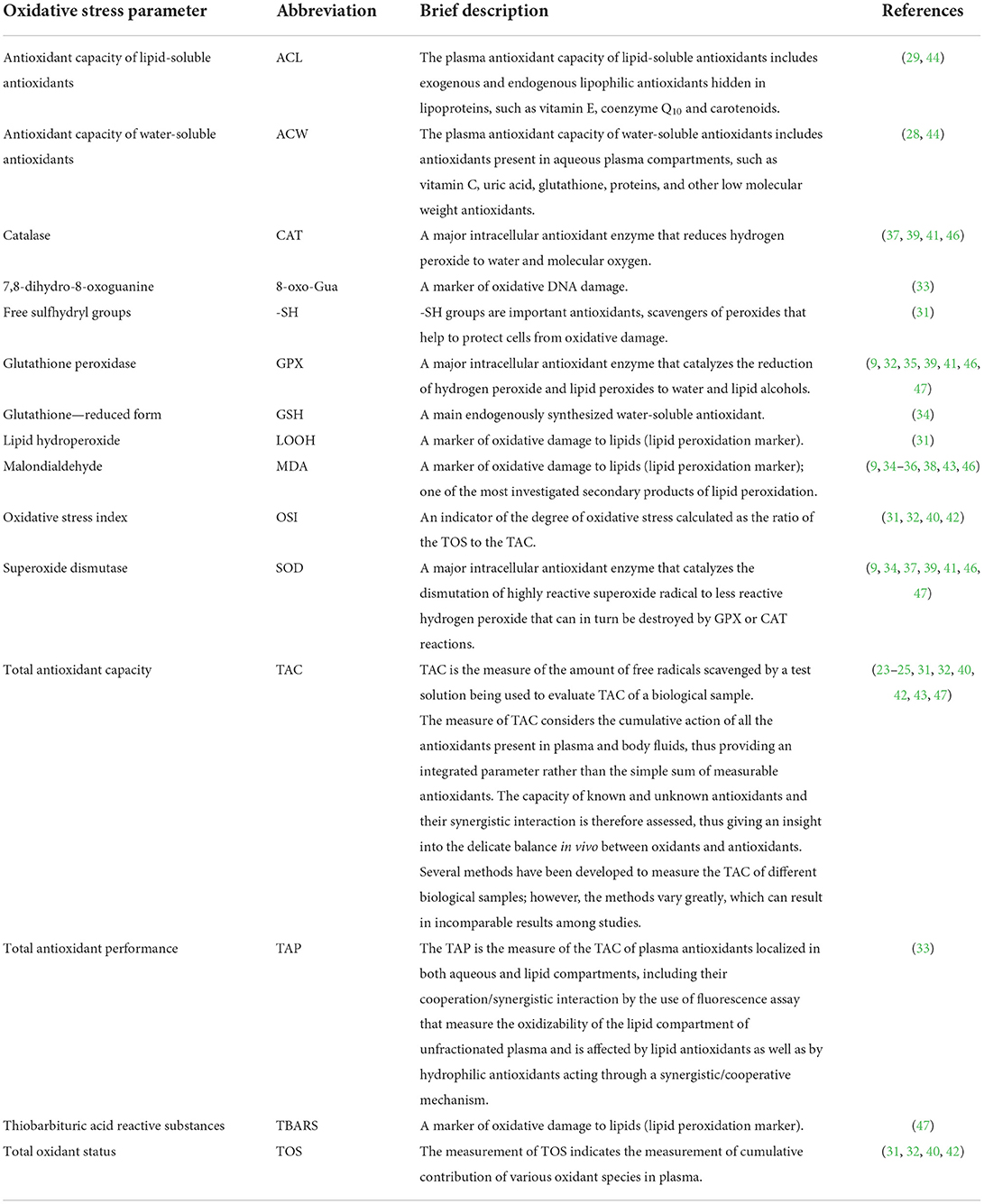
Studies on oxidative stress during anesthesia in dogs are summarized in Table 1. A brief presentation of some of the oxidative stress parameters mentioned in the review is summarized in Table 2.
Intravenous anesthetics
Thiopental
Thiopental is a barbiturate which exert antioxidant properties in vitro, either by scavenging ROS (48, 49) or by inhibiting the oxidative function of neutrophils (50, 51). However, these properties have been shown to be poor compared with propofol in vitro (49, 52) and in human (53) and animal (3, 40, 54) studies. Lee (40) compared the effect of propofol and thiopental at induction doses on oxidative stress parameters in dogs anesthetized with isoflurane during laparotomy and gastrotomy. Oxidative stress was assessed by total plasma oxidation status (TOS), TAC and oxidative stress index (OSI). The change ratio of each value was calculated and compared between the two groups. The TOS and OSI change ratio was lower in the propofol group. Propofol was more effective in maintaining TAC compared with thiopental at induction doses, although there was a time-dependent increase in TOS and OSI and a decrease in TAC during isoflurane anesthesia in both groups (40).
Ketamine
Ketamine, a non–competitive antagonist of N-methyl-D-aspartate receptors, has been shown to promote the formation of ROS in rats (5, 55, 56). However, according to other studies, ketamine inhibits the oxidative burst of canine peripheral blood phagocytes in vitro (57), and exhibits neuroprotective effects by its anti-inflammatory, antioxidant, and anti-apoptotic effects in animal models of cognitive disfunction (58). These properties were partially confirmed in a study on the effects of ketamine and propofol on cytokines, oxidative status and neutrophil functions in dogs (46). In a study on Wistar rats ketamine showed a higher antioxidant potential compared to etomidate (59). In human patients after the administration of ketamine and propofol at induction doses, the extent of lipid peroxidation (assessed by measurement of thiobarbituric acid reactive substances (TBARS) concentration) and the activity of GPX and SOD were lower in the propofol group, and plasma TAC was higher than in the ketamine group (47). Altug et al. (37) evaluated oxidative stress during desflurane anesthesia after induction with a combination of thiopental and midazolam or ketamine and midazolam. Thiopental increased SOD activity and acted as a better free radical scavenger by reducing high oxidation states of hemoglobin, whereas ketamine increased CAT activity and hemoglobin concentration. In conclusion, the authors emphasized the importance of induction agents for the antioxidant effect of desflurane anesthesia (37).
Propofol
Propofol, a short-acting hypnotic, is an alkylphenol (2,6-diisopropylphenol) that, like vitamin E, contains a phenolic hydroxyl group (OH). It reacts with free radicals to form a phenoxyl radical (60, 61). Like vitamin E, propofol acts synergistically with ascorbic acid, a water-soluble antioxidant that converts the propofol radical to the phenolic form (62). Due to its lipophilic nature, propofol may increase fluidity and strengthen the cell membrane against physical and hemodynamic stressors (62–64). In addition, propofol could substitute for vitamin E, especially when vitamin E stores are acutely depleted and need to be replaced rapidly (65, 66). The protective antioxidant effect of propofol has been demonstrated in experimental models of brain, liver, and heart injury (52, 67–71), isolated microsomes (65), neuroblastoma cell lines (72), and human clinical trials (47, 52, 73–77). It is manifested either by reaction with peroxyl radicals and the formation of less harmful phenoxyl radicals or by reaction with peroxynitrite (78, 79). Acquaviva et al. (80) demonstrated the neuroprotective effect of propofol on astroglial cells in vitro through its anti-apoptotic effect, reduction of cytotoxicity, and prevention of DNA damage by peroxynitrite (80).
Braz et al. (33) investigated the effects of anesthesia maintained with isoflurane or propofol on antioxidant status in healthy adults undergoing minor surgery. The study design assessed the antioxidant capacity of the aqueous plasma compartment (hydrophilic antioxidant capacity), total antioxidant performance (TAP) and individual antioxidants. Lipid-soluble antioxidants were represented by carotenoids, retinol (vitamin A), tocopherols, lycopenes, and others, whereas uric acid represented the hydrophilic antioxidants. The oxidized purine form, 7,8-dihydro-8-oxoguanine (8-oxo-Gua), in lymphocytes was examined to assess genetic oxidative damage. The results showed a decrease in alpha-tocopherol in both groups, whereas there was an increase in gamma-tocopherol in the propofol group compared with baseline values and compared with the isoflurane group after 2 h of anesthesia. The hydrophilic antioxidant capacity and TAP were increased in both groups compared with baseline, with no differences between the two groups. The antioxidant effect was dose dependent. The authors concluded that both anesthetic regimens increased hydrophilic antioxidant capacity and TAP and did not cause oxidative DNA damage (33).
Lee and Kim (41) demonstrated the effect of propofol at induction doses and as total intravenous anesthesia (TIVA) on the activity of SOD, GPX, and CAT in dogs. The three treatments were: Group 1, 2% isoflurane; Group 2, anesthesia induced with propofol and maintained with 1.5–2% isoflurane; Group 3, TIVA with propofol. Anesthesia was maintained for 60 min. The activity of SOD decreased from baseline to the end of anesthesia in the isoflurane groups. Catalase activity decreased from baseline to the end of anesthesia and 24 h after anesthesia in the isoflurane groups. In the propofol-TIVA group, CAT activity increased at the end and 24 h after anesthesia and was higher than in the group of dogs in which isoflurane was used to induce and maintain anesthesia (41).
Tomsič et al. (9, 44) examined the effects of propofol on oxidative stress in dogs with early-stage myxomatous mitral valve degeneration (MMVD) undergoing periodontal treatment. In one study, the authors compared the effects of TIVA with propofol to anesthesia induced with propofol and maintained with sevoflurane on vitamin E, SOD, GPX, and lipid peroxidation marker malondialdehyde (MDA) (9). The authors found no significant differences between the two anesthetic protocols for any of the oxidative status parameters measured. Compared with baseline values, vitamin E concentration decreased during anesthesia in both groups, and GPX activity increased 60 min after induction of anesthesia in the sevoflurane group. In another study, the authors examined the effect of TIVA with propofol and sevoflurane anesthesia after induction with propofol on plasma ACL and ACW levels in dogs with early-stage MMVD. Dogs without signs of MMVD (control group) were induced to anesthesia with propofol and maintained with sevoflurane. Anesthesia increased ACW values in all groups, although they were higher than baseline only in the propofol group after anesthesia. Additionally, 60 min after induction to anesthesia, ACW was higher in the MMVD/Propofol group compared to the MMVD/Propofol + Sevoflurane group. Furthermore, only propofol anesthesia increased ACL levels in dogs with MMVD compared with basal levels. This could be attributed to the antioxidant properties of propofol. The authors concluded that propofol may be more suitable than sevoflurane for anesthesia of dogs with early-stage MMVD in terms of antioxidant capacity (44).
Alipour et al. (43) investigated the effects of anesthesia with propofol and isoflurane on endocrine and oxidative variables during pneumoperitoneum in dogs. The authors found that TIVA with propofol, either with or without mechanical ventilation, can increase MDA production at the end of pneumoperitoneum, whereas none of the anesthetic techniques affected thyroid and cortisol levels (43).
Inhalational anesthetics
Inhalational anesthetics include the halogenated ethers (isoflurane, sevoflurane, desflurane, and enflurane), the alkane halothane, and the inorganic gaseous anesthetics (nitrous oxide and xenon) (3). Sevoflurane and isoflurane are the most used volatile anesthetics in veterinary practice.
Halogenated anesthetics trigger the phenomenon of ischemic preconditioning (81), an adaptive response to brief episodes of ischemia that allows protection of the myocardium from subsequent life-threatening ischemia and acts through the cellular signaling pathway. The volatile anesthetics cause the formation of ROS, initiating the signaling cascade that reduces the production of ROS in mitochondria during ischemia. Reactive oxygen species activate mitochondrial potassium-dependent ATP channels, presumably by activating a protein kinase C isoform. This results in less depolarization of the mitochondrial membrane potential and a lower electrochemical gradient for calcium ions. The reduced accumulation of calcium ions in mitochondria and the opening of mitochondrial pores protect mitochondria from damage (81). In addition, the bioavailability of ATP increases and the formation of ROS in the ischemic phase decreases (82).
Isoflurane
Isoflurane is a commonly used halogenated ether with a low metabolization rate and solubility (83). The effects of isoflurane on oxidative stress have not been fully elucidated and remain controversial. In an experimental model using human neuroglioma cells and mouse brain tissue, Ni et al. (84) sought to elucidate the mechanisms of oxidative damage to DNA by isoflurane. The phosphorylated form of histone protein H2A variant X at Ser139 (γH2A.X) was selected as a marker of oxidative DNA damage. The results of the study showed that isoflurane induced DNA damage at clinically relevant concentrations as determined by the increase in γH2A.X in human neuroglioma cells (84). In contrast, in the clinical study by Braz et al. (33) isoflurane anesthesia did not induce DNA damage assessed by determination of 8-oxo-Gua in healthy adults (33).
In dogs, isoflurane increases oxidative stress in a dose- and time-dependent manner, as shown by Lee (42). Beagle dogs were anesthetized with different minimum alveolar concentrations (MAC) of isoflurane. Dogs in group 1 received 1.28% (1 x MAC) isoflurane, whereas dogs in group 2 received 2 × MAC. The oxidant and antioxidant status of the dogs was determined by TOS, TAC, and OSI. The levels of TOS and OSI increased significantly, while the levels of TAC decreased in both groups after anesthesia. Changes were observed in group 1 60 min, and in group 2 30 and 60 min after induction of anesthesia. The levels of TOS were higher in group 2 than in group 1 at 30 and 60 min after induction of anesthesia, while TAC and OSI were significantly higher in group 2 than in group 1 at 60 min after induction to anesthesia (42). Yarsan et al. (38) compared the effects of halothane and isoflurane on plasma MDA concentrations in dogs. Changes were not significant; however, the MDA concentration was lower under halothane anesthesia, and levels returned to baseline within 24 hours (38).
Sevoflurane
Sevoflurane is metabolized in the liver by the isoform of the cytochrome P450 enzyme CYP2E1 (85, 86), and its metabolism has been shown to accelerate the formation of ROS (85) and impair energy metabolism in mitochondria (87, 88). Yalcin et al. (31) investigated the effects of desflurane and sevoflurane on selected oxidative stress parameters [TOS, TAC, lipid hydroperoxide (LOOH), total free sulfhydryl groups (-SH), and OSI] in mothers and neonates after elective cesarean section. Compared with baseline values, TOS and OSI were decreased in both groups. However, LOOH, TOS, and OSI were higher in maternal serum and umbilical artery blood in the desflurane group compared with the sevoflurane group. In addition, LOOH and -SH values were lower in the sevoflurane group compared with preoperative values, and there was no difference between groups in umbilical artery -SH and TAC values. The authors concluded that anesthetics could alter oxidative stress indices, and sevoflurane showed more favorable effects compared with desflurane (31). Similarly, Erbas et al. (32) compared the effects of sevoflurane, desflurane, and propofol on the oxidant and antioxidant systems of patients undergoing laparoscopic cholecystectomy. The oxidative stress parameters selected were TOS, TAC, and GPX. Compared with preoperative levels, there was an increase in postoperative TAC levels in the propofol and sevoflurane groups, and in postoperative TOS levels in the desflurane group. Glutathione peroxidase activity remained unchanged in both groups (32). These results are in general agreement with clinical studies investigating oxidative stress in dogs with early-stage MMVD during propofol and sevoflurane anesthesia (9, 44).
Other halogenated anesthetics
Naziroglu and Günay (35) investigated the effect of enflurane on serum concentrations of vitamins A, E, beta-carotene, GPX, lipid peroxidation, and biochemical and hematological parameters in healthy dogs. The results showed a decrease in serum vitamin E and beta-carotene concentrations, while serum MDA and vitamin A concentrations were increased during enflurane anesthesia. The authors pointed out that administration of antioxidant compounds such as vitamin C, vitamin E, and selenium may be beneficial in anesthetic complications (35).
El-Bassiouni et al. (34) studied the involvement of ROS and antioxidant defense mechanisms in liver tissue and plasma under different hypoxic conditions during halothane anesthesia. In liver tissue and plasma, there was an increase in MDA and a decrease in the free radical scavengers reduced glutathione (GSH), ascorbic acid, and especially vitamin E, with hypoxia being a major contributing factor. In addition, hypoxia and halothane inhibited hepatic SOD activity (34). Simeonova et al. (36) compared the effects of three anesthetic protocols on lipid peroxidation in dogs. Halothane anesthesia increased plasma MDA concentrations in dogs compared with the fentanyl and halothane groups and with dogs treated with lumbosacral epidural anesthesia (36).
Discussion
Intravenous and inhalational anesthetics can cause dose- and time-dependent cardiovascular and respiratory depression (59, 60) that may lead to hypoperfusion and hypoxia. In contrast, ketamine increases myocardial work and cardiac output, maintaining arterial pressure and heart rate (59). It has a mild effect on the respiratory system but can cause respiratory depression when used with other central nervous depressants (46). Anesthetic procedures in clinical trials include various agents for sedation, gentle induction of anesthesia, and analgesia. The metabolism of these agents and the stress response to pain during surgical procedures may aggravate the oxidative status of animals under anesthesia (7). The variability of anesthetic protocols and procedures may explain the conflicting results of the studies included in the present review. The preanalytical procedures and the wide variability in the methods used to measure oxidative stress parameters render some of the results incomparable between studies and may also be the reason for the conflicting results.
Conclusion
The effect of general anesthetics on oxidative stress is variable and not yet fully understood. Comprehensive studies are needed to investigate the effect of an anesthetic on oxidative status in dogs. These studies should include larger numbers of animals and measurement of a broader range of oxidative status parameters, including markers of oxidative damage to all biologically important molecules (lipids, proteins, and DNA), concentrations of antioxidants and activity of antioxidant enzymes, and measurement of the oxidative and/or reductive potency of a biological fluid.
Author contributions
KT and ANS conceptualized the manuscript. KT conducted the literature search and review and drafted the manuscript. ANS reviewed and improved the manuscript. Both authors approved the final version of the manuscript.
Funding
The publication of this review was funded by the Slovenian Research Agency, Grant No. P4-0053. The funder was not involved in the manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsuchiya M, Shiomoto K, Mizutani K, Fujioka K, Suehiro K, Yamada T, et al. Reduction of oxidative stress a key for enhanced postoperative recovery with fewer complications in esophageal surgery patients: randomized control trial to investigate therapeutic impact of anesthesia management and usefulness of simple blood test for prediction of high-risk patients. Medicine. (2018) 97:e12845. doi: 10.1097/MD.0000000000012845
2. Bogra J, Gangoo R, Pandey VC, Srivastava P. Effect on free radical generation with different anesthesia. J Indian Med Assoc. (2007) 105:128–32.
3. Senoner T, Velik-Salchner C, Luckner G, Tauber H. Anesthesia-induced oxidative stress: Are there differences between intravenous and inhaled anesthetics? Oxid Med Cell Longev. (2021) 2021:8782387. doi: 10.1155/2021/8782387
4. Kundović SA, Rašić D, Popović L, Peraica M, Crnjar K. Oxidative stress under general intravenous and inhalation anesthesia. Arh Hig Rada Toksikol. (2020) 71:169–77. doi: 10.2478/aiht-2020-71-3437
5. Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. (2005) 101:1275–87. doi: 10.1213/01.ANE.0000180999.81013.D0
6. Lee JY, Won HS, Hwang HK, Jeong SM, Kim MC. Evaluation of the systemic oxidative stress status during major orthopedic surgery in dogs: a clinical study. J Vet Clin. (2013) 30:1–4.
7. Sakundech K, Chompoosan C, Tuchpramuk P, Boonsorn T, Aengwanich W. The influence of duration on pain stress, oxidative stress, and total antioxidant power status in female dogs undergoing ovariohysterectomy. Vet World. (2020) 13:160–4. doi: 10.14202/vetworld.2020.160-164
8. Szczubial M, Kankofer M, Bochniarz M, Dabrowski R. Effects of ovariohysterectomy on oxidative stress markers in female dogs. Reprod Domest Anim. (2015) 50:393–9. doi: 10.1111/rda.12501
9. Tomsič K, Nemec Svete A, Nemec A, Domanjko Petrič A, Vovk T, Seliškar A. Influence of sevoflurane or propofol anesthesia on oxidative stress parameters in dogs with early stage myxomatous mitral valve degeneration. A preliminary study. Acta Vet-Beograd. (2018) 68:32–42. doi: 10.2478/acve-2018-0003
10. Russo C, Bracarense APF. Oxidative stress in dogs. Semin Cienc Agrar. (2016) 37:1431–40. doi: 10.5433/1679-0359.2016v37n3p1431
11. Sies H. On the history of oxidative stress: concept and some aspects of current development. Curr Opin Toxicol. (2018) 7:122–6. doi: 10.1016/j.cotox.2018.01.002
12. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. l Int J Biochem Cell Biol. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
13. Halliwell B, Gutteridge JMC. Oxygen: Boon yet Bane - introducing oxygen toxicity and reactive species. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Oxford: University Press (2015). p. 1–29.
14. Zadák Z, Hyspler R, Tichá A, Hronek M, Fikrová P, Rathouská J, et al. Antioxidants and vitamins in clinical conditions. Physiol Res. (2009) 58:S13–17. doi: 10.33549/physiolres.931861
15. Sharifi-Rad M, Anil Kumar NV, Zucca P, Varoni EM, Dini L, Panzarini E, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. (2020) 11:694. doi: 10.3389/fphys.2020.00694
16. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. (2012) 24:981–90. doi: 10.1016/j.cellsig.2012.01.008
17. Kohchi C, Inagawa H, Nishizawa T, Soma GI. ROS and innate immunity. Anticancer Res. (2009) 29:817–21.
18. Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. (2016) 15:71. doi: 10.1186/s12937-016-0186-5
19. Halliwell B, Gutteridge JMC. Antioxidant defences synthesized in vivo. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Oxford: University Press (2015). p. 77–151.
20. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. (2010) 4:118–26. doi: 10.4103/0973-7847.70902
21. Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. (2005) 41:1211–9. doi: 10.1002/hep.20720
22. Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. (2008) 582:1783–7. doi: 10.1016/j.febslet.2008.04.057
23. Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. (2000) 29:1106–14. doi: 10.1016/S0891-5849(00)00394-4
24. Yeum KJ, Russell RM, Krinsky NI, Aldini G. Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys. (2004) 430:97–103. doi: 10.1016/j.abb.2004.03.006
25. Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. (2010) 49:503–15. doi: 10.1016/j.freeradbiomed.2010.04.016
26. Tomsič K, Seliškar A, Lukanc B, Nemec Svete A. Plasma total antioxidant capacity and activities of blood glutathione peroxidase and superoxide dismutase determined in healthy dogs by using commercially available kits. Acta Vet-Beograd. (2016) 66:534–48. doi: 10.1515/acve-2016-0046
27. Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res. (2016) 12:166. doi: 10.1186/s12917-016-0792-7
28. Popov IN, Lewin G. Photochemiluminescent detection of antiradical activity: II. Testing of nonenzymic water-soluble antioxidants. Free Radic Biol Med. (1994) 17:267–71. doi: 10.1016/0891-5849(94)90082-5
29. Popov IN, Lewin G. Photochemiluminescent detection of antiradical activity; IV: testing of lipid-soluble antioxidants. J Biochem Biophys Methods. (1996) 31:1–8. doi: 10.1016/0165-022X(95)00021-I
30. Baysal Z, Togrul T, Aksoy N, Cengiz M, Celik H, Boleken ME, et al. Evaluation of total oxidative and antioxidative status in pediatric patients undergoing laparoscopic surgery. J Pediatr Surg. (2009) 44:1367–70. doi: 10.1016/j.jpedsurg.2008.11.031
31. Yalcin S, Aydogan H, Yuce HH, Kucuk A, Karahan MA, Vural M, et al. Effects of sevoflurane and desflurane on oxidative stress during general anesthesia for elective cesarean section. Wien Klin Wochenschr. (2013) 125:467–73. doi: 10.1007/s00508-013-0397-0
32. Erbas M, Demiraran Y, Yildirim HA, Sezen G, Iskender A, Karagoz I, et al. Comparison of effects on the oxidant/antioxidant system of sevoflurane, desflurane and propofol infusion during general anesthesia. Rev Bras Anestesiol. (2015) 65:68–72. doi: 10.1016/j.bjane.2014.05.004
33. Braz MG, Braz LG, Freire CMM, Lucio LMC, Braz JRC, Tang G, et al. Isoflurane and propofol contribute to increasing the antioxidant status of patients during minor elective surgery: a randomized clinical study. Medicine. (2015) 94:e1266. doi: 10.1097/MD.0000000000001266
34. El-Bassiouni EA, Abo-Ollo MM, Helmy MH, Ismail S, Ramadan MI. Changes in the defense against free radicals in the liver and plasma of the dog during hypoxia and/or halothane anesthesia. Toxicology. (1998) 128:25–34. doi: 10.1016/S0300-483X(98)00045-6
35. Naziroglu M, Günay C. The levels of some antioxidant vitamins, glutathione peroxidase and lipoperoxidase during the anesthesia of dogs. Cell Biochem Funct. (1999) 17:207–12. doi: 10.1002/(SICI)1099–0844(199909)17:3<207::AID–CBF830>3.0.CO;2-3
36. Simeonova GP, Todorova II, Gadjeva V, Dinev DN. Evaluation of lipid peroxidation associated with three anesthetic protocols in dogs. Rev Med Vet. (2004) 155:602–5.
37. Altug ME, Gönenci R, Yarsan E, Özturk A. Effect of induction agents on the antioxidative activity of desflurane in dogs. Yyü Vet Fak Derg. (2008) 19:29–33.
38. Yarsan E, Gurkan M, Pekcan Z, Ince S, Kumandas A. Effects of halothane and isoflurane anesthesia on antioxidant enzymes in dogs. J Anim Vet Adv. (2010) 9:2513–6. doi: 10.3923/javaa.2010.2513.2516
39. Choi KH, Lee J-Y, Jeong S-M, Kim M-C. Oxidative effects of isoflurane and medetomidine - Tiletamine / Zolazepam combination in beagle dogs. J Vet Clin. (2012) 29:119–23.
40. Lee JY. Oxidative stress due to anesthesia and surgical trauma and comparison of the effects of propofol and thiopental in dogs. J Vet Med Sci. (2012) 74:663–5. doi: 10.1292/jvms.11-0221
41. Lee JY, Kim MC. Effect of propofol on oxidative stress status in erythrocytes from dogs under general anesthesia. Acta Vet Scand. (2012) 54:76. doi: 10.1186/1751-0147-54-76
42. Lee JY. Evaluation of the total oxidant and antioxidant status of the plasma of dogs anesthetised with isoflurane. Vet Rec. (2013) 173:96. doi: 10.1136/vr.101299
43. Alipour F, Emami MR, Mohri M. Endocrine and oxidative stress characteristics in different anesthetic methods during pneumoperitoneum in dogs. Comp Clin Path. (2018) 27:1667–73. doi: 10.1007/s00580-018-2792-4
44. Tomsič K, Nemec Svete A, Nemec A, Domanjko Petrič A, Pirman T, Rezar V, et al. Antioxidant capacity of lipid- and water-soluble antioxidants in dogs with subclinical myxomatous mitral valve degeneration anesthetised with propofol or sevoflurane. BMC Vet Res. (2020) 16:305. doi: 10.1186/s12917-020-02529-7
45. Chongphaibulpatana P, Kumagai Y, Fukui D, Katayama M, Uzuka Y. The effect of inspired oxygen concentration on oxidative stress biomarkers in dogs under inhalation anesthesia. Can J Vet Res. (2020) 84:91–5.
46. Guzel O, Sevim G, Aydin Kaya D, Sezer D, Erek M, Esen Gursel F, et al. Ketamine or propofol anesthesia in dogs: how do they affect cytokines, antioxidants and neutrophil functions? J Hellenic Vet Med Soc. (2022) 73:3783–92. doi: 10.12681/jhvms.25780
47. Khoshraftar E, Ranjbar A, Kharkhane B, Tavakol Heidary S, Gharebaghi Z, Zadkhosh N, et al. Antioxidative effects of propofol vs. ketamin in individuals undergoing surgery. Arch Iran Med. (2014) 17:486–9.
48. Kim IK, Suh JK, Kim JH. Antioxidant effects and mechanism of thiopental and propofol on the rabbit abdominal aortic endothelial dependent vasorelaxation against reactive oxygen species. Korean J Anesthesiol. (2013) 65:S16–8. doi: 10.4097/kjae.2013.65.6S.S16
49. Murphy PG, Davies MJ, Columb MO, Stratford N. Effect of propofol and thiopentone on free radical mediated oxidative stress of the erythrocyte. Br J Anesth. (1996) 76:536–43. doi: 10.1093/bja/76.4.536
50. Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. (1998) 86:159–65. doi: 10.1213/00000539-199801000-00032
51. Wittmann S, Daniels S, Ittner KP, Fröhlich D. Thiopentone and methohexitone enantiomers do not act stereoselectively on the oxidative response in human neutrophils in vitro. Pharmacology. (2004) 72:12–9. doi: 10.1159/000078627
52. De la Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg. (1999) 89:1050–5. doi: 10.1213/00000539-199910000-00043
53. Yagmurdur H, Cakan T, Bayrak A, Arslan M, Baltaci B, Inan N, et al. The effects of etomidate, thiopental, and propofol in induction on hypoperfusion-reperfusion phenomenon during laparoscopic cholecystectomy. Acta Anesthesiol Scand. (2004) 48:772–7. doi: 10.1111/j.0001-5172.2004.00417.x
54. Yagmurdur H, Ayyildiz A, Karaguzel E, Ogus E, Surer H, Caydere M, et al. The preventive effects of thiopental and propofol on testicular ischemia-reperfusion injury. Acta Anesthesiol Scand. (2006) 50:1238–43. doi: 10.1111/j.1399-6576.2006.01145.x
55. De Oliveira L, Spiazzi CM, Bortolin T, Canever L, Petronilho F, Mina FG, et al. Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33:1003–8. doi: 10.1016/j.pnpbp.2009.05.010
56. Venâncio C, Félix L, Almeida V, Coutinho J, Antunes L, Peixoto F, et al. Acute ketamine impairs mitochondrial function and promotes superoxide dismutase activity in the rat brain. Anesth Analg. (2015) 120:320–8. doi: 10.1213/ANE.0000000000000539
57. Son KA, Kang JH, Yang MP. Ketamine inhibits the phagocytic responses of canine peripheral blood polymorphonuclear cells through the upregulation of prostaglandin E2 in peripheral blood mononuclear cells in vitro. Res Vet Sci. (2009) 87:41–6. doi: 10.1016/j.rvsc.2008.12.004
58. Wang R, Zhang Z, Kumar M, Xu G, Zhang M. Neuroprotective potential of ketamine prevents developing brain structure impairment and alteration of neurocognitive function induced via isoflurane through the PI3K/AKT/GSK-3β pathway. Drug Des Devel Ther. (2019) 13:501–12. doi: 10.2147/DDDT.S188636
59. Djuric M, Kostic S, Nikolic Turnic T, Stankovic S, Skrbic R, et al. The comparison of the effects of ketamine and etomidate on cardiodynamics, biochemical and oxidative stress parameters in Wistar male rats. Mol Cell Biochem. (2020) 474:125–34. doi: 10.1007/s11010-020-03838-z
60. Marik PE. Propofol: therapeutic indications and side-effects. Curr Pharm Des. (2004)10:3639–49. doi: 10.2174/1381612043382846
61. Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anesthetic effects. Eur J Pharmacol. (2009) 605:1–8. doi: 10.1016/j.ejphar.2009.01.007
62. Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Antioxidant protection of propofol and its recycling in erythrocyte membranes. Am J Respir Crit Care Med. (2002) 165:54–60. doi: 10.1164/ajrccm.165.1.2010134
63. Bahri MA, Seret A, Hans P, Piette J, Deby-Dupont G, Hoebeke M. Does propofol alter membrane fluidity at clinically relevant concentrations? An ESR spin label study. Biophys Chem. (2007) 129:82–91. doi: 10.1016/j.bpc.2007.05.011
64. Tsuchiya H, Ueno T, Tanaka T, Matsuura N, Mizogami M. Comparative study on determination of antioxidant and membrane activities of propofol and its related compounds. Eur J Pharm Sci. (2010) 39:97–102. doi: 10.1016/j.ejps.2009.11.001
65. Aarts L, Van der Hee R, Dekker I, De Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace α-tocopherol as an antioxidant. FEBS Lett. (1995) 357:83–5. doi: 10.1016/0014-5793(94)01337-Z
66. Hans P, Deby C, Deby-Dupont G, Vrijens B, Albert A, Lamy M. Effect of propofol on in vitro lipid peroxidation induced by different free radical generating systems: a comparison with vitamin E. J Neurosurg Anesthesiol. (1996) 8:154–8. doi: 10.1097/00008506-199604000-00010
67. Kokita N, Hara A. Propofol attenuates hydrogen peroxide-induced mechanical and metabolic derangements in the isolated rat heart. Anesthesiology. (1996) 84:117–27. doi: 10.1097/00000542-199601000-00014
68. Young Y, Menon DK, Tisavipat N, Matta BF, Jones JG. Propofol neuroprotection in a rat model of ischaemia reperfusion injury. Eur J Anesthesiol. (1997) 14:320–6. doi: 10.1046/j.1365-2346.1997.00130.x
69. Navapurkar VU, Skepper JN, Jones JG, Menon DK. Propofol preserves the viability of isolated rat hepatocyte suspensions under an oxidant stress. Anesth Analg. (1998) 87:1152–7. doi: 10.1213/00000539-199811000-00033
70. Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res. (2000) 45:360–9. doi: 10.1016/S0008-6363(99)00365-X
71. Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. (2008) 14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x
72. Zhang Y, Zuo Y, Li B, Xie J, Ma Z, Thirupathi A, et al. Propofol prevents oxidative stress and apoptosis by regulating iron homeostasis and targeting JAK/STAT3 signaling in SH-SY5Y cells. Brain Res Bull. (2019) 153:191–201. doi: 10.1016/j.brainresbull.2019.08.018
73. Xia WF, Liu Y, Zhou QS, Tang QZ, Zou HD. Comparison of the effects of propofol and midazolam on inflammation and oxidase stress in children with congenital heart disease undergoing cardiac surgery. Yonsei Med J. (2011) 52:0326–32. doi: 10.3349/ymj.2011.52.2.326
74. Hans P, Canivet JL, Pincemail J, Muller JP, Byttebier G, Lamy M. Plasma vitamin E, total lipids and myeloperoxidase levels during spinal surgery. A comparison between two anesthetic agents: propofol and isoflurane. Acta Anesthesiol Scand. (1991) 35:302–5. doi: 10.1111/j.1399-6576.1991.tb03294.x
75. Stratford N, Murphy P. Antioxidant activity of propofol in blood from anesthetized patients. Eur J Anesthesiol. (1998) 15:158–60. doi: 10.1111/j.0265-0215.1998.00261.x
76. Cinnella G, Vendemiale G, Dambrosio M, Serviddio G, Pugliese PL, Aspromonte G, et al. Effect of propofol, sevoflurane and desflurane on systemic redox balance. Int J Immunopathol Pharmacol. (2007) 20:585–93. doi: 10.1177/039463200702000316
77. Guo D, Li Y, Wang H, Wang X, Hua W, Tang Q, et al. Propofol post-conditioning after temporary clipping reverses oxidative stress in aneurysm surgery. Int J Neurosci. (2019) 129:155–64. doi: 10.1080/00207454.2018.1483920
78. Kahraman S, Demiryürek AT. Propofol is a peroxynitrite scavenger. Anesth Analg. (1997) 84:1127–9. doi: 10.1213/00000539-199705000-00032
79. Mouithys-Mickalad A, Hans P, Deby-Dupont G, Hoebeke M, Deby C, Lamy M. Propofol reacts with peroxynitrite to form a phenoxyl radical: demonstration by electron spin resonance. Biochem Biophys Res Commun. (1998) 249:833–7. doi: 10.1006/bbrc.1998.9235
80. Acquaviva R, Campisi A, Murabito P, Raciti G, Avola R, Mangiameli S, et al. Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: an alternative protective mechanism. Anesthesiology. (2004) 101:1363–71. doi: 10.1097/00000542-200412000-00017
81. Erturk E. Ischemia-reperfusion injury and volatile anesthetics. Biomed Res Int. (2014) 2014:526301. doi: 10.1155/2014/526301
82. Kunst G, Klein AA. Peri-operative anesthetic myocardial preconditioning and protection - cellular mechanisms and clinical relevance in cardiac anesthesia. Anesthesia. (2015) 70:467–82. doi: 10.1111/anae.12975
83. Dohoo SE. Isoflurane as an inhalational anesthetic agent in clinical practice. Can Vet J. (1990) 31:847–50.
84. Ni C, Li C, Dong Y, Guo X, Zhang Y, Xie Z. Anesthetic Isoflurane Induces DNA damage through oxidative stress and p53 pathway. Mol Neurobiol. (2017) 54:3591–605. doi: 10.1007/s12035-016-9937-8
85. Kharasch ED. Biotransformation of sevoflurane. Anesth Analg. (1995) 81:S27–38. doi: 10.1097/00000539-199512001-00005
86. Smith I, Nathanson M, White PF. Sevoflurane-a long-awaited volatile anesthetic. Br J Anesth. (1996) 76:435–45. doi: 10.1093/bja/76.3.435
87. Kevin LG, Novalija E, Riess ML, Camara AKS, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. (2003) 96:949–55. doi: 10.1213/01.ANE.0000052515.25465.35
88. Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg. (2009) 109:405–11. doi: 10.1213/ane.0b013e3181a93ad9
Keywords: anesthesia, dogs, inhalational anesthetics, intravenous anesthetics, reactive oxygen species, oxidative stress
Citation: Tomsič K and Nemec Svete A (2022) A mini-review of the effects of inhalational and intravenous anesthetics on oxidative stress in dogs. Front. Vet. Sci. 9:987536. doi: 10.3389/fvets.2022.987536
Received: 06 July 2022; Accepted: 22 August 2022;
Published: 12 September 2022.
Edited by:
Abdurrahman Aksoy, Ondokuz Mayis University, TurkeyReviewed by:
Luca Bellini, University of Padua, ItalyCopyright © 2022 Tomsič and Nemec Svete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alenka Nemec Svete, QWxlbmthLm5lbWVjc3ZldGVAdmYudW5pLWxqLnNp
 Katerina Tomsič
Katerina Tomsič Alenka Nemec Svete
Alenka Nemec Svete