- 1School of Life Sciences, Zhengzhou University, Zhengzhou, China
- 2Henan Zhongze Biological Engineering Co., Ltd., Zhengzhou, China
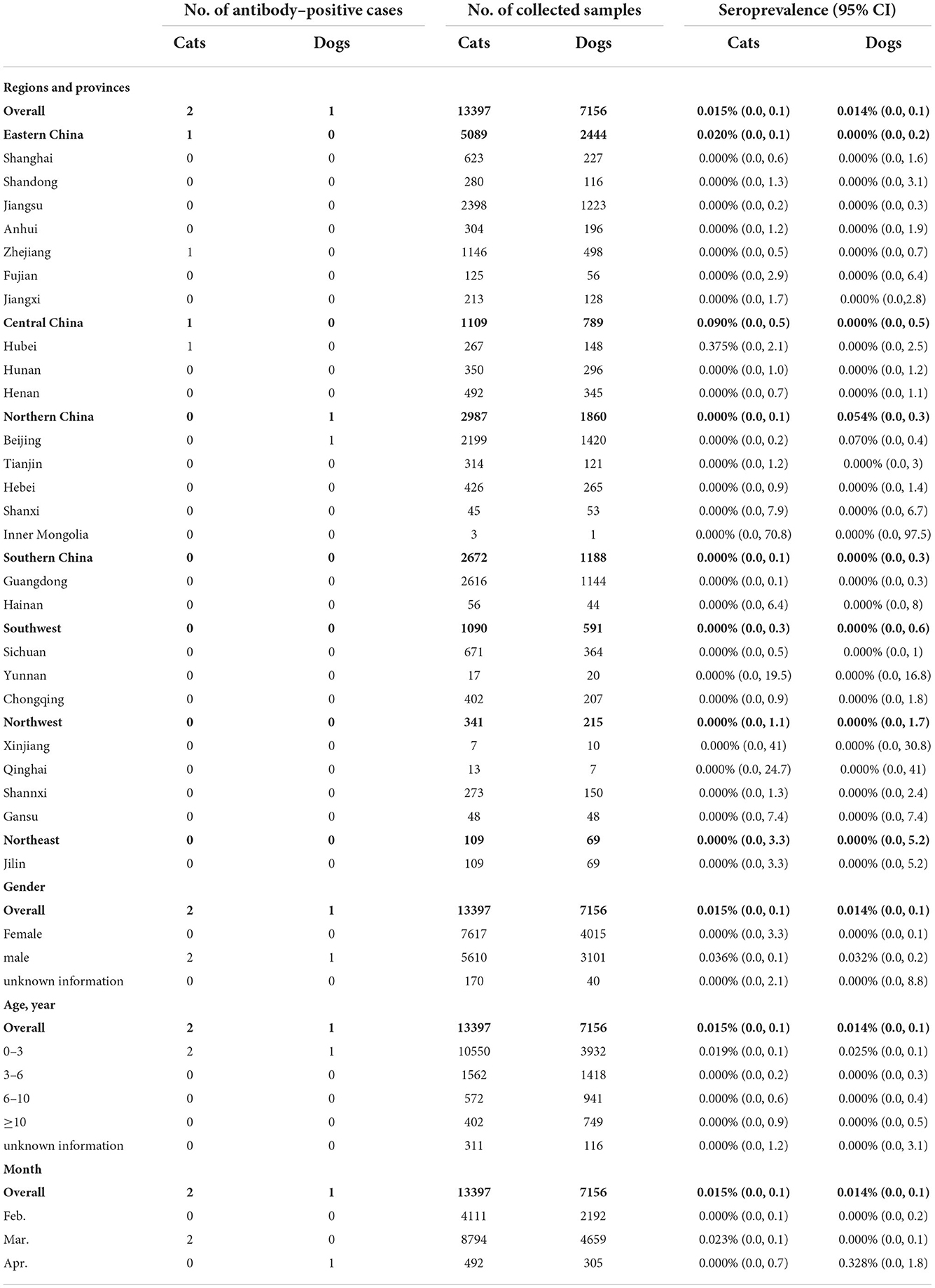
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) can be transmitted from human to companion animals. The national wide serological surveillance against SARS-CoV-2 was conducted among pet animals, mainly in cats and dogs, 1 year after the first outbreak of COVID-19 in China. All sera were tested for SARS-CoV-2 IgG antibodies using an indirect enzyme linked immunosorbent assay (ELISA) based on the receptor binding domain (RBD) of spike protein. This late survey takes advantage of the short duration of the serological response in these animals to track recent episode of transmission. A total of 20,592 blood samples were obtained from 25 provinces across 7 geographical regions. The overall seroprevalence of SARS-CoV-2 infections in cats was 0.015% (2/13397; 95% confidence intervals (CI): 0.0, 0.1). The virus infections in cats were only detected in Central (Hubei, 0.375%) and Eastern China (Zhejiang, 0.087%) with a seroprevalence estimated at 0.090 and 0.020%, respectively. In dogs, the seroprevalence of SARS-CoV-2 infections was 0.014% (1/7159; 95% CI: 0.0, 0.1) in the entire nation, seropositive samples were limited to Beijing (0.070%) of Northern China with a prevalence of 0.054%. No seropositive cases were discovered in other geographic regions, nor in other companion animals analyzed in this study. These data reveal the circulation of SARS-CoV-2 in companion animals, although transmission of the virus to domestic cats and dogs is low in China, continuous monitoring is helpful for the better understand of the virus transmission status and the effect on animals.
Introduction
The COVID-19 (Coronavirus Disease 2019) pandemic seriously threaten the global public health. The typical symptoms of this disease are fever, cough, difficulty breathing, severe pneumonia, and even death (1). According to the coronavirus dashboard of World Health Organization (WHO), the confirmed cases and deaths reached 542,188,789 and 6,329,275, respectively, while the confirmed cases and death in China were 126,384 and 5,696, respectively until June 29, 2022 (2). The main cause of severe symptomatic cases and deaths is attributed to cytokines storm and the subsequent leading of acute respiratory distress syndrome (ARDS) (3).
Angiotensin-converting enzyme II (ACE2) is the receptor of spike (S) glycoprotein during SARS-CoV-2 infection (4, 5). Protein sequences and structural modeling analysis revealed that ACE2 is widely distributed in animal species, representing a potential risk of cross-species transmission for this virus (6, 7). SARS-CoV-2 infection has been reported in companion animals including cats, dogs, rabbits, ferrets and minks (8, 9). Animal experimental infections indicate that cats can infect SARS-CoV-2. Virus inoculated cats seroconverted against S, nucleocapsid (N) and RBD antigens within 5–7 days, and the specific antibody titer reached the peak at day 14. Moreover, the virus can be efficiently transmitting between cats, animals exposed to inoculated cats can produce specific antibodies against S and N proteins within 2 weeks (10, 11). However, the duration of the serological response in companion animals is still poorly documented, antibodies titer against RBD protein reached the peak on day 10 in natural infected cats and decreased to the detection limitation within 110 days (12). The susceptibility to SARS-CoV-2 are relatively lower in dogs. Experimental infections showed IgG antibodies against S and RBD proteins were detectable at day 14, and reached plateau or start to decrease by day 42 (11). In Europe, the seroprevalence of SARS-CoV-2 infection in companion animals was found to range from 0.4 to 3.5% in cats and 0.2 to 3.3% in dogs (13–17). In minks infected farms, SARS-CoV-2 transmitted to cats and dogs were analyzed by PCR and whole genome sequencing, revealing a mink-to-cat transmitting rate of 12%, but no mink-to-dog spread happened (18). Experimental infections confirmed the ferret-to-ferret spread of SARS-CoV-2. The virus RNA could be detected from nasal washes and fecal in both directly contact group and indirectly contact group within 4 days, while the viral RNA in saliva and urine was detected only in the directly contact group (19). Recent studies have documented infection cases in pet ferrets and ferrets back to human propagation events (20, 21).
Several serological test methods have been used to detect IgG and/or IgM antibodies against SARS-CoV-2 infection in humans (22–25). Recently, an indirect ELISA and a multi-species ELISA have been developed to detect antibodies against SARS-CoV-2 in several animal species by using RBD as antigen (17, 26–29).
The objective of this study was to investigate the SARS-CoV-2 seroprevalence in companion animals 1 year after the first outbreak in China. This “late” serological survey can provide useful information on the current rate of transmission between human and companion animals.
Materials and methods
Sample collections
An intensive sampling campaign was organized in seven geographic regions of China to collect blood samples from companion animals (Figure 1, Table 1). Animal samples were collected over a 3-month period (February 1 to April 18, 2021), mainly from cats and dogs but also from rabbits, hedgehogs, and guinea pigs. Animal hospitals were involved in the sampling campaign. Information on participating animals, including morphological parameters and clinical symptoms were recorded. Serum samples were stored at −20°C. Sera collected from SARS-CoV-2 vaccine immunized animals and unvaccinated animals were stored in this laboratory.
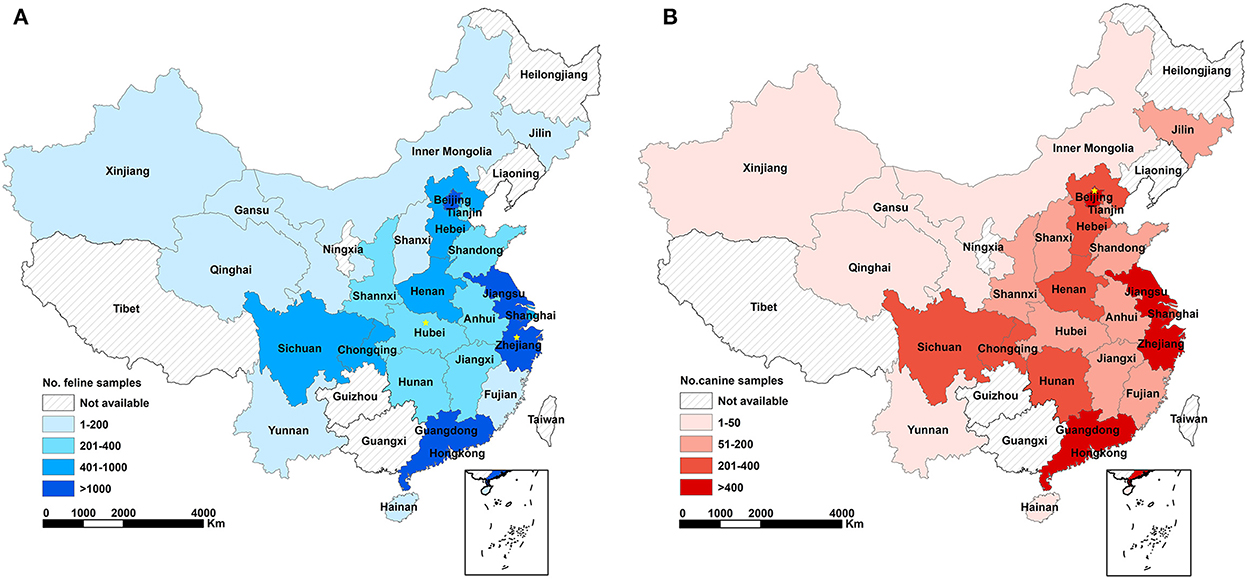
Figure 1. Geographical coverage of serological survey against SARS-CoV-2 infections in cats (A) and dogs (B) and the amount of analyzed samples in China. Different colors represent corresponding range of sampling numbers in each province. Yellow star represents seropositive case (n = 1) determined by ELISA and colloidal gold testing strips.
Detection of SARS-CoV-2 antibodies by indirect enzyme-linked immunosorbent assay (ELISA)
SARS-CoV-2 antibodies were tested by indirect ELISA. Briefly, ELISA plates were coated with 200 ng/well of recombinant SARS-CoV-2 RBD protein. After saturation with 5% skimmed milk at 37 °C for 2 h, ELISA plates were washed 3 times with PBST and incubated with 100 μL of serum samples (1:100) at 37 °C for 1 h. Horseradish peroxidase-conjugated SAP (1:5000) (Bersee, Beijing, China) was used as secondary antibody. After revealing the assay with TMB substrate/H2SO4, the optical densities (ODs) were measured at 450 nm to compare the level of anti- RBD antibodies in each sample. The cut-off values were determined as the mean value of seronegative samples against SARS-CoV-2 plus 3 times of the Standard Deviations.
Preparation of colloidal gold-based immunochromatographic strip
The colloidal was prepared using trisodium citrate method (30, 31). Briefly, 1 mL 1% chloroauric acid (Sigma–Aldrich Corporation, St. Louis, MO, USA) and 1.6 mL 1% trisodium citrate solution (Sigma–Aldrich Corporation, St. Louis, MO, USA) were added into 100 mL boiling water, kept on boiling for 5 min until the solution color changed to red, then cooled down to room temperature (RT). The quality of prepared colloidal gold was tested by UV–vis absorption spectra (Thermo Fisher Scientific, Rockford, IL, USA) and transmission electron microscope (TEM) H-600 (Hitachi High-Tech Corporation, Tokyo, Japan). S protein, purchased from Sino Biological Inc. (Beijing, China), was incubated with the colloidal gold solution at RT for 30 min, followed by centrifugation at 4°C 12,000 x g for 30 min; discarded the supernatant, and resuspended the pellet with boric acid buffer containing 1% BSA (Sigma–Aldrich Corporation, St. Louis, MO, USA) to obtain the colloidal gold conjugated S protein. The immunochromatographic strips were prepared as described previously (30) with the modifications as follows. Colloidal gold conjugated S protein was dispensed on fiberglass pad, staphylococcal protein A (SPA, Bersee, Beijing, China) and mouse-anti-S protein monoclonal antibody were coated on test and control area of nitrocellulose membrane, respectively.
Detection of SARS-CoV-2 antibodies by using colloidal gold test strips
Serum samples were used at 1:100 dilutions in PBS. Sera (1:1000) collected from SARS-CoV-2 vaccine immunized mice was used as positive control. Test strips were loaded with 100 μL of sample and the results were recorded within 5 min incubation at RT. The test was considered as positive when the control line (C line) and the IgG test line (T line) appeared, and as negative when only the C line was visible. The result was invalid when no signal was observed in the C line.
Date management and analysis
Microsoft Excel 2016 (Microsoft Corporation of Redmond, Washington, USA) was used to aggregate the information including age, medical time, species, temperature, and clinical symptoms of animals, and were used to calculate the confidence intervals (CI) of collected data. ArcGIS software (Environmental Systems Research Institute, Redlands, CA) was used to combine the collected data with geography distributions. The sensitivity, specificity, the positive predictive value and the negative predictive value of tested methods were calculated as described (32, 33). Statistical analyze was performed by SPSS software (SPSS, Inc., Chicago, IL, USA), a chi-squared test was used for comparing the difference between male and female.
Results and discussion
One year after the first COVID-19 outbreak, a cross-sectional study was carried out to investigate the seroprevalence SARS-CoV-2 infections among domestic cats and dogs in China (Figure 1, Table 1). The minimum number of serum samples was calculated using Epitools (34) with the expected prevalence as 50 %, confidence level at 95 %, and the desired precision of 5 %. Besides, the sensitivity and specificity of ELISA method was 93.75 and 92.00%, respectively. The negative predictive value was 93.24 %, while the positive predictive value was 92.59 %. The result showed that sample size was required at least for 520. A total of 20,533 serum samples, collected from 25 provinces across 7 geographical regions, were tested for IgG antibodies against SARS-CoV-2 RBD protein using ELISA method. The colloidal gold-based immunochromatographic strip, of which the sensitivity and specificity was 89.87 and 94.67 %, respectively, while the negative and positive predictive values were 89.87 and 94.67 %, respectively, was further used to confirm the presence of SARS-CoV-2 antibodies of the positive samples screened by ELISA.
The overall seroprevalence of SARS-CoV-2 infections in the feline population was 0.015% (2/13397; 95% CI: 0.0, 0.1). Seropositive animals were only found in two of the seven regions included in the study, with a seroprevalence in Central and Eastern China estimated at 0.090% (1/1109; 95% CI: 0.0, 0.5) and 0.020% (1/5089; 95% CI: 0.0, 0.1), respectively. Narrow down to the province level, the seroprevalence was 0.375% (1/267; 95% CI: 0.0, 2.1) in Hubei (Central China) and 0.087% (1/1146; 95% CI: 0.0, 0.5) in Zhejiang (Eastern China). The seroprevalence of SARS-CoV-2 infections in the canine population was 0.014% (1/7159; 95% CI: 0.0, 0.1) in the entire nation. The seropositive sample originated from the Beijing (seroprevalence 0.070%; 1/1420; 95% CI: 0.0, 0.4) in Northern China (seroprevalence 0.054%; 1/1860; 95% CI: 0.0, 0.3) (Figure 1, Table 1).
The SARS-CoV-2 seroprevalence in the feline and canine populations in China was lower than the seroprevalence reported in several European countries, which ranged from 0.4 to 3.5% in cats and 0.2 to 3.3% in dogs (13–17). These results are expected given the long period distance from the peak outbreak of COVID-19 and the short duration of the serological response in cats. Furthermore, the human infection rate for SARS-CoV-2 was estimated at only 0.03% in China, a value 880- to 1200-fold lower than those documented in Europe. This low level of human infections in China may represent a low risk of exposure for companion animals, since SARS-CoV-2 infections in cats and dogs are considered to be favored by close contact with people with COVID-19 (35–37).
The seropositive animals were located in Wuhan, the most severely affected area of China, and Beijing, where sporadic cases were detected in the first quarter of 2021. Seropositive animals were also detected in Hangzhou, despite no reported indigenous cases of SARS-CoV-2 infections. Only one of the seropositive animals exhibited SARS-CoV-2 symptoms as diarrhea at the sampling date (Table 1, Supplementary Table S1). Frequent contact with COVID-19 patients or asymptomatic infected people is the leading cause of SARS-CoV-2 transmission from humans to animals (38–41). Until the beginning of April, 2022, the infection rate of COVID-19 was estimated at 0.65% (941,545/1,443,497,378) among humans in China. Under this condition, pet cats and dogs are in a low-risk state for a long time, these seropositive cases could be the outcome of an unusual long-standing serological response, a recent contamination, or a persistent infection. Despite a risk of “reservoir” for the virus, it was not possible to test the infectious status of these animals.
Although not statistically relevant (p = 0.079), it is noteworthy that only males (3/20533) were found seropositive in our serum collection that included samples of both genders (male: 42.4%, 8711/20553; female: 56.7%, 11632/20553), raising the question of a possible difference in sensitivity between sexes. Such a difference has already been documented in humans due to natural genetic differences between men and women (42, 43). Currently, the only animal species where a difference in sensitivity between males and females have been documented is the hamster (44).
Despite the limited number of seropositive samples, our data confirmed that SARS-CoV-2 can infect companion animals. In addition to the phenomenon that the coronavirus can be transmitted from humans to animals. Several studies have confirmed that human can also get infection acquired from infected animals, for example minks and pet hamster (45–47). Indicating the potential threatens of virus transmission from animals to humans. This led us to investigate the SARS-CoV-2 seroprevalence in other companion animals including rabbits and guinea pigs, two species known to be susceptible to SARS-CoV-2 (48–50). Hedgehog sera were also tested. The susceptibility of this species to SARS-CoV-2 is unknown, but hedgehog is considered a companion animal in China. As expected by the limited number of samples available, the 39 sera tested were all found negative for SARS-CoV-2 antibodies (Supplementary Table S2).
In conclusion, the transmission of SARS-CoV-2 to companion animals was limited in China, likely associated with a faster and better control of the disease epidemics. However, companion animals should be regarded as potential risk of back-transmission to humans, given the high potential for adaptation of RNA viruses. Continuous surveillance of antibody prevalence against SARS-CoV-2 in companion animals is helpful for better understanding the circulation of the virus in pet populations, and the effects of the virus on companion animals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Zhengzhou University.
Author contributions
AW and GZ contributed to conception and design of the study. XZ, YC, YS, HL, PD, and JY performed the investigation and statistical analysis. AW and XZ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was funded by the National Nature Science Foundation of China (NSFC) (grant number 32072944), Consulting Research Project of Chinese Academy of Engineering [grant numbers 2020-XY-76 (2020-KYGG-03-02)], 1125 Talent Gathering Plan Project of Zhengzhou, Key Projects of Zhengzhou University, and Key Science and Technology Projects of Henan Province (grant number 201100310100).
Conflict of interest
Authors YC, YS, HL, and PD were employed by Henan Zhongze Biological Engineering Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.986619/full#supplementary-material
References
1. Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (Covid-19)–anatomic pathology perspective on current knowledge. Diagn Pathol. (2020) 15:103. doi: 10.1186/s13000-020-01017-8
2. WHO. Who Coronavirus (Covid-19) Dashboard. (2022). Available online at: https://covid19.who.int/ (accessed 29, Jun 2022).
3. Fricke-Galindo I, Falfan-Valencia R. Genetics insight for Covid-19 susceptibility and severity: a review. Front Immunol. (2021) 12:622176. doi: 10.3389/fimmu.2021.622176
4. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. (2020) 117:11727–34. doi: 10.1073/pnas.2003138117
5. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on Ace2 and Tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80 e8. doi: 10.1016/j.cell.2020.02.052
6. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
7. Rodrigues J, Barrera-Vilarmau S. J MCT, Sorokina M, Seckel E, Kastritis PL, et al. Insights on cross-species transmission of SARS-CoV-2 from structural modeling. PLoS Comput Biol. (2020) 16:e1008449. doi: 10.1371/journal.pcbi.1008449
8. Stout AE, Andre NM, Jaimes JA, Millet JK, Whittaker GR. Coronaviruses in cats and other companion animals: where does SARS-CoV-2/Covid-19 Fit? Vet Microbiol. (2020) 247:108777. doi: 10.1016/j.vetmic.2020.108777
9. Fritz M, de Riols de Fonclare D, Garcia D, Beurlet S, Becquart P, Rosolen SG, et al. First evidence of natural SARS-CoV-2 infection in domestic rabbits. Vet Sci. (2022) 9:49. doi: 10.3390/vetsci9020049
10. Gaudreault NN, Trujillo JD, Carossino M, Meekins DA, Morozov I, Madden DW, et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg Microbes Infect. (2020) 9:2322–32. doi: 10.1080/22221751.2020.1833687
11. Bosco-Lauth AM, Hartwig AE, Porter SM, Gordy PW, Nehring M, Byas AD, et al. Experimental infection of domestic dogs and cats with SARS-CoV-2: pathogenesis, transmission, and response to reexposure in cats. Proc Natl Acad Sci U S A. (2020) 117:26382–8. doi: 10.1073/pnas.2013102117
12. Zhang Q, Zhang H, Gao J, Huang K, Yang Y, Hui X, et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg Microbes Infect. (2020) 9:2013–9. doi: 10.1080/22221751.2020.1817796
13. Michelitsch A, Hoffmann D, Wernike K, Beer M. Occurrence of antibodies against SARS-CoV-2 in the domestic cat population of Germany. Vaccines (Basel). (2020) 8:772. doi: 10.3390/vaccines8040772
14. Stevanovic V, Vilibic-Cavlek T, Tabain I, Benvin I, Kovac S, Hruskar Z, et al. Seroprevalence of SARS-CoV-2 infection among pet animals in croatia and potential public health impact. Transbound Emerg Dis. (2020). doi: 10.1111/tbed.13924
15. Patterson EI, Elia G, Grassi A, Giordano A, Desario C, Medardo M, et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat Commun. (2020) 11:6231. doi: 10.1038/s41467-020-20097-0
16. Villanueva-Saz S, Giner J, Tobajas AP, Perez MD, Gonzalez-Ramirez AM, Macias-Leon J, et al. Serological evidence of SARS-CoV-2 and co-infections in stray cats in Spain. Transbound Emerg Dis. (2021). doi: 10.1111/tbed.14062
17. Zhao S, Schuurman N, Li W, Wang C, Smit LAM, Broens EM, et al. Serologic screening of severe acute respiratory syndrome coronavirus 2 infection in cats and dogs during first coronavirus disease wave, the Netherlands. Emerg Infect Dis. (2021) 27:1362–70. doi: 10.3201/eid2705.204055
18. van Aart AE, Velkers FC, Fischer EAJ, Broens EM, Egberink H, Zhao S, et al. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound Emerg Dis. (2021). doi: 10.22541/au.161821264.49927405/v1
19. Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, et al. Infection and rapid transmission of SARS-CoV-2 in Ferrets. Cell Host Microbe. (2020) 27:704–9 e2. doi: 10.1016/j.chom.2020.03.023
20. Giner J, Villanueva-Saz S, Tobajas AP, Perez MD, Gonzalez A, Verde M, et al. SARS-CoV-2 seroprevalence in household domestic ferrets (Mustela Putorius Furo). Animals (Basel). (2021) 11:667. doi: 10.3390/ani11030667
21. Gortazar C, Barroso-Arevalo S, Ferreras-Colino E, Isla J, de la Fuente G, Rivera B, et al. Natural SARS-CoV-2 infection in Kept Ferrets, Spain. Emerg Infect Dis. (2021) 27:1994–6. doi: 10.3201/eid2707.210096
22. Fotis C, Meimetis N, Tsolakos N, Politou M, Akinosoglou K, Pliaka V, et al. Accurate SARS-CoV-2 seroprevalence surveys require robust multi-antigen assays. Sci Rep. (2021) 11:6614. doi: 10.1038/s41598-021-86035-2
23. Afzal N, Tariq N, Raza S, Shakeel D. Diagnostic accuracy of electro-chemiluminescence immunoassay anti-SARS-CoV-2 serological test. Cureus. (2021) 13:e12588. doi: 10.7759/cureus.12588
24. Jalkanen P, Pasternack A, Maljanen S, Melen K, Kolehmainen P, Huttunen M, et al. A combination of N and S antigens with iga and igg measurement strengthens the accuracy of SARS-CoV-2 Serodiagnostics. J Infect Dis. (2021) 224:jiab222. doi: 10.1093/infdis/jiab222
25. Chen R, Ren C, Liu M, Ge X, Qu M, Zhou X, et al. Early detection of SARS-CoV-2 Seroconversion in humans with aggregation-induced near-infrared emission nanoparticle-labeled lateral flow immunoassay. ACS Nano. (2021) 15:acsnano.1c01932. doi: 10.1021/acsnano.1c01932
26. Kaczorek-Lukowska E, Wernike K, Beer M, Wrobel M, Malaczewska J, Mikulska-Skupien E, et al. High seroprevalence against SARS-CoV-2 among dogs and cats, Poland, 2021/2022. Animals (Basel). (2022) 12:2016. doi: 10.3390/ani12162016
27. Wernike K, Aebischer A, Michelitsch A, Hoffmann D, Freuling C, Balkema-Buschmann A, et al. Multi-species elisa for the detection of antibodies against SARS-CoV-2 in animals. Transbound Emerg Dis. (2021) 68:1779–85. doi: 10.1111/tbed.13926
28. Schulz C, Martina B, Mirolo M, Muller E, Klein R, Volk H, et al. SARS-CoV-2-Specific antibodies in domestic cats during first Covid-19 wave, Europe. Emerg Infect Dis. (2021) 27:3115–8. doi: 10.3201/eid2712.211252
29. Zhao S, Li W, Schuurman N, van Kuppeveld F, Bosch BJ, Egberink H. Serological screening for Coronavirus infections in cats. Viruses. (2019) 11:743. doi: 10.3390/v11080743
30. Li G, Wang A, Chen Y, Sun Y, Du Y, Wang X, et al. Development of a colloidal gold-based immunochromatographic strip for rapid detection of severe acute respiratory syndrome coronavirus 2 spike protein. Front Immunol. (2021) 12:635677. doi: 10.3389/fimmu.2021.635677
31. Liao M, Yan J, Wang X, Qian H, Wang C, Xu D, et al. Development and clinical application of a rapid SARS-CoV-2 antibody test strip: a multi-center assessment across China. J Clin Lab Anal. (2021) 35:e23619. doi: 10.1002/jcla.23619
32. Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. Field Evaluation of the Ict malaria PF/pv immunochromatographic test for detection of plasmodium falciparum and plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in Eastern Indonesia. J Clin Microbiol. (1999) 37:2412–7. doi: 10.1128/JCM.37.8.2412-2417.1999
33. Jameie F, Dalimi A, Pirestani M, Mohebali M. Development of a multi-epitope recombinant protein for the diagnosis of human visceral leishmaniasis. Iran J Parasitol. (2021) 16:1–10. doi: 10.18502/ijpa.v16i1.5506
34. Sergeant E. Epitools Epidemiological Calculators. (2018). Available online at: http://Epitools.Ausvet.Com.Au (accessed October 21, 2022).
35. Segales J, Puig M, Rodon J, Avila-Nieto C, Carrillo J, Cantero G, et al. Detection of SARS-CoV-2 in a cat owned by a covid-19-affected patient in Spain. Proc Natl Acad Sci U S A. (2020) 117:24790–3. doi: 10.1073/pnas.2010817117
36. Garigliany M, Van Laere AS, Clercx C, Giet D, Escriou N, Huon C, et al. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg Infect Dis. (2020) 26:3069–71. doi: 10.3201/eid2612.202223
37. Barrs VR, Peiris M, Tam KWS, Law PYT, Brackman CJ, To EMW, et al. SARS-CoV-2 in quarantined domestic cats from Covid-19 households or close contacts, Hong Kong, China. Emerg Infect Dis. (2020) 26:3071–4. doi: 10.3201/eid2612.202786
38. Bienzle D, Rousseau J, Marom D, MacNicol J, Jacobson L, Sparling S, et al. Risk factors for SARS-CoV-2 infection and illness in cats and dogs(1). Emerg Infect Dis. (2022) 28:1154–62. doi: 10.3201/eid2806.220423
39. Stanojevic S, Radojicic S, Misic D, Srejic D, Vasiljevic DV, Prokic K, et al. Frequency of SARS-CoV-2 infection in dogs and cats: results of a retrospective serological survey in Sumadija District, Serbia. Prev Vet Med. (2022) 208:105755. doi: 10.1016/j.prevetmed.2022.105755
40. Calvet GA, Pereira SA, Ogrzewalska M, Pauvolid-Correa A, Resende PC, Tassinari WS, et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with Covid-19 in Rio De Janeiro, Brazil. PLoS ONE. (2021) 16:e0250853. doi: 10.1371/journal.pone.0250853
41. Goryoka GW, Cossaboom CM, Gharpure R, Dawson P, Tansey C, Rossow J, et al. One health investigation of SARS-CoV-2 infection and seropositivity among pets in households with confirmed human Covid-19 cases-Utah and Wisconsin, 2020. Viruses. (2021) 13:813. doi: 10.3390/v13091813
42. Raza HA, Sen P, Bhatti OA, Gupta L. Sex hormones, autoimmunity and gender disparity in Covid-19. Rheumatol Int. (2021). doi: 10.1007/s00296-021-04873-9
43. Pan Y, Li X, Yang G, Fan J, Tang Y, Hong X, et al. Seroprevalence of SARS-CoV-2 immunoglobulin antibodies in Wuhan, China: part of the city-wide massive testing campaign. Clin Microbiol Infect. (2021) 27:253–7. doi: 10.1016/j.cmi.2020.09.044
44. Yuan L, Zhu H, Zhou M, Ma J, Chen R, Chen Y, et al. Gender associates with both susceptibility to infection and pathogenesis of SARS-CoV-2 in Syrian Hamster. Signal Transduct Target Ther. (2021) 6:136. doi: 10.1038/s41392-021-00552-0
45. Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. (2021) 371:172–7. doi: 10.1126/science.abe5901
46. Hammer AS, Quaade ML, Rasmussen TB, Fonager J, Rasmussen M, Mundbjerg K, et al. SARS-CoV-2 transmission between mink (Neovison Vison) and Humans, Denmark. Emerg Infect Dis. (2021) 27:547–51. doi: 10.3201/eid2702.203794
47. Yen HL, Sit THC, Brackman CJ, Chuk SSY, Gu H, Tam KWS, et al. Transmission of SARS-CoV-2 Delta Variant (Ay127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet. (2022) 399:1070–8. doi: 10.1016/S0140-6736(22)00326-9
48. Mykytyn AZ, Lamers MM, Okba NMA, Breugem TI, Schipper D, van den Doel PB, et al. Susceptibility of rabbits to SARS-CoV-2. Emerg Microbes Infect. (2021) 10:1–7. doi: 10.1080/22221751.2020.1868951
49. Smith TRF, Patel A, Ramos S, Elwood D, Zhu X, Yan J, et al. Immunogenicity of a DNA vaccine candidate for Covid-19. Nat Commun. (2020) 11:2601. doi: 10.1038/s41467-020-16505-0
Keywords: SARS-CoV-2, seroprevalence, companion animals, ELISA, colloidal gold test strips
Citation: Wang A, Zhu X, Chen Y, Sun Y, Liu H, Ding P, Zhou J, Liu Y, Liang C, Yin J and Zhang G (2022) Serological survey of SARS-CoV-2 in companion animals in China. Front. Vet. Sci. 9:986619. doi: 10.3389/fvets.2022.986619
Received: 05 July 2022; Accepted: 14 November 2022;
Published: 30 November 2022.
Edited by:
Sonja Hartnack, University of Zurich, SwitzerlandReviewed by:
Elisabetta Razzuoli, Liguria and Valle d'Aosta (IZSTO), ItalyJuan Manuel Carreño Qurioz, Icahn School of Medicine at Mount Sinai, United States
Waleed Mahallawi, Taibah University, Saudi Arabia
Copyright © 2022 Wang, Zhu, Chen, Sun, Liu, Ding, Zhou, Liu, Liang, Yin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaiping Zhang, emhhbmdnYWlwQDEyNi5jb20=
†These authors have contributed equally to this work
 Aiping Wang
Aiping Wang Xifang Zhu
Xifang Zhu Yumei Chen1,2
Yumei Chen1,2 Hongliang Liu
Hongliang Liu Peiyang Ding
Peiyang Ding Gaiping Zhang
Gaiping Zhang