
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci. , 22 December 2022
Sec. Veterinary Epidemiology and Economics
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.976215
 Saeed Khoshnood1,2†
Saeed Khoshnood1,2† Reza Pakzad2,3†
Reza Pakzad2,3† Maryam Koupaei4
Maryam Koupaei4 Maryam Shirani5
Maryam Shirani5 Almas Araghi6
Almas Araghi6 Golnaz Mokhtari Irani7
Golnaz Mokhtari Irani7 Melika Moradi8
Melika Moradi8 Iraj Pakzad1,9
Iraj Pakzad1,9 Nourkhoda Sadeghifard1
Nourkhoda Sadeghifard1 Mohsen Heidary10,11*
Mohsen Heidary10,11*Objectives: Brucellosis is one of the most prevalent zoonotic diseases common between humans and animals. Despite eradication efforts, the burden of the disease is well-known in endemic countries and in countries where brucellosis has not been an important health issue until recently. The aim of this study was to evaluate the prevalence, diagnosis, and manifestations of brucellosis.
Methods: In this study, PubMed, Web of Science, Scopus, Embase, and Google scholar databases were systematically searched to find studies published from 2011 to 2021. The search was conducted using text words and Medical Subject Headings (MeSH) Terms on the prevalence of brucellosis. Stata software 14.0 was used for all analyses.
Results: Based on the results, the pooled prevalence of brucellosis was 15.27% (95% CI: 9.68–21.86; heterogeneity I2 index: 97.43; p < 0.001) for man and 15.33% (95% CI: 7.19–25.75; heterogeneity I2 index: 98.19; p < 0.001) for woman. Age (coefficient: 0.240; p = 0.480), gender (coefficient: −0.017; p = 0.800), and publication year (coefficient: 0.114; p = 0.861) showed no significant effect on heterogeneity among studies. Egger's test indicated a significant publication bias for the prevalence of brucellosis (coefficient 3.894; p < 0.001). Moreover, the trim-and-fill method exhibited that the adjusted prevalence of brucellosis (18.30%, 95% CI: 14.10–22.52) was not significantly different from the original prevalence of brucellosis.
Conclusion: The pooled estimate for brucellosis prevalence was estimated as 15.53%. To better understand the epidemiology of brucellosis globally, more extensive studies are needed to be conducted throughout the world, especially in developing and low-income countries.
Brucellosis is one the most common zoonotic diseases affecting 500,000 cases annually (1). This disease was formerly known by names such as Malta fever, Mediterranean fever, Gibraltar fever, Cyprus fever, and Undulant fever (2). Among several species of Brucella identified, B. melitensis, B. abortus, and B. suis are the most and B. canis is the least important causative factor of human diseases (1, 2). To date, no disease has been reported to be caused by B. ovis and B. neotomae (1). Brucellosis has a wide range of clinical manifestations that frequently lasts from a few days to several years (3). In humans, the disease is rarely fatal but generally debilitating (4). It has various routes of transmission, including the gastrointestinal tract, respiratory tract, skin, and mucous membranes, as well as contact with body fluids. Disease transmission from animal to human occurs mainly through the consumption of raw meat and dairy products. Brucellosis is often misdiagnosed, which, in turn, leads to prolonged illness and inadequate treatment (3). Moreover, the symptoms of the disease are not specific and make it difficult to diagnose. The prevailing epidemiological situation of brucellosis in susceptible animals (livestock and wildlife) in a country or region plays a significant role in choosing a particular diagnostic test strategy. Diagnostic tests can be used for a variety of purposes, including confirmatory diagnosis, screening or prevalence studies, and confirmation of disease. In countries where brucellosis is being eradicated, surveillance is needed to prevent the reintroduction of brucellosis through importing infected animals or animal products. The validity of such diagnostic tests, especially in wildlife, is still an issue (5).
Diagnosis of brucellosis is conducted directly (bacteriological and molecular methods) and indirectly (in vitro serological methods and allergic methods in vivo). The “gold standard” for diagnosing this disease is direct bacteriological testing, that is, cultivation of Brucella isolated from body fluids or tissues. However, to circumvent the problems of bacteriological testing, molecular biological techniques, which are often based on polymerase chain reaction (PCR) amplification, are promisingly utilized to identify different types of Brucella species (6). Serological tests are very important in diagnosis but are often difficult to interpret (7). These methods are used for the initial screening of human brucellosis and also during the subsequent treatment. Serial serological tests are commonly recommended because of the frequent false-negative serological tests in the early days of infection and due to their capability of correct diagnosis and monitoring for response to therapy (8). Rose Bengal test (RBT), real-time PCR, serum agglutination test (SAT), and complement fixation test (CFT) are diagnostic methods for brucellosis (9). However, none of these tests can alone diagnose all different stages of brucellosis. Therefore, combined tests can be applied for definitive diagnosis.
Serum agglutination test is used for screening brucellosis and real-time PCR for the identification of Brucella DNA in serum samples (10). Complement fixation tests and RBT are used in combination to confirm brucellosis in many countries. The former test is used for its higher specificity, whereas the latter is used for its higher sensitivity. The primary binding assays include indirect fluorescent polarization assay (FPA), indirect enzyme-linked immunosorbent assay (iELISA), and the competitive ELISA (cELISA) that employ O-antigen or purified lipopolysaccharide (LPS) as the diagnostic reagent. Enzyme-linked immunosorbent assay tests have been developed to be more sensitive and specific alternatives to conventional tests (11). However, the gold standard in diagnosing brucellosis is culture (3). Data on risk factors for brucellosis among humans and animals are rare. However, risk factors associated with socio-demographic variables, animal contact practices, and dairy product consumption (milk and cheese) are attributed to human brucellosis. Regional traditional beliefs, as an indirect risk factor, may also affect the attitude toward the consumption of dairy products. These risk factors vary in different regions within and between countries, which contributes to the variation in the prevalence of brucellosis in varied geographical areas (12). Many occupations, such as butchers, ranchers/breeders, milkers, veterinarians, inoculators, and laboratory workers, as well as people involved in the packaging and sale of dairy products and raw meat, are at a high risk of brucellosis (10). Some people believe that treatments for brucellosis are very diverse and sufficient, but considering the high prevalence of this disease, it is a long way from eradication (13).
The prevalence of brucellosis is lower in developed countries than that in low-income countries (14). One of the most essential strategies to eradicate the disease is to use vaccinations. There is no human vaccine to prevent brucellosis because of multiple reasons, including infection after vaccination (15, 16). The best way to hinder the disease in humans is its prevention in animals (17). There is scant or no information on brucellosis in some developing countries (16). Veterinary complications and deaths from the infection cause substantial economic damage in these countries (4). In Kenya, Yemen, and Syria, the incidence of brucellosis is high. In recent years, the world's map of brucellosis has changed remarkably.
Even though several countries have made significant progress in controlling the disease, in some parts of the world, there is evidence of the emergence of new hotspots (18). Brucella has a special place in history, epidemiology, phylogenetics, and pathogenicity, which put it in the spotlight of scientists (19). This study aimed to evaluate the prevalence, diagnosis, and manifestations of brucellosis.
This study was performed according to the Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) guideline (Supplementary material 1). Using the keywords such as “Brucella,” “Brucellosis,” “Malta Fever,” “Bang disease,”' “Brucella Infection,” “'Mediterranean remittent fever,” “Undulant Fever,” and “Gibraltar Fever,” all relevant articles were collected (IR.MEDILAM.REC.1400.130).
A complete and comprehensive search was conducted in international databases, including PubMed, Web of Science, Scopus, Embase, and Google scholar, from 1 January 2001 to 5 August 2021. In the search, the following Medical Subject Headings (MeSH) keywords, including “Brucellosis,” “Malta Fever,” “Gibraltar Fever,” “Mediterranean fever,” “Cyprus Fever,” “Rock Fever,” “Brucella Infection,” and “Undulant Fever” were used. In the present study, the PICOS included population: people in all age groups, intervention: none, comparison: none, outcome: prevalence of brucellosis, time: 1 January 2001 until 5 August 2021, and study design: observational study. The search method explained below was used based on PICOS for MEDLINE (MeSH), followed by other databases:
1. Brucella [text word] OR Brucella [MeSH term]
2. Brucellosis [text word] OR Brucellosis [MeSH term]
3. Malta fever [text word] OR Malta Fever [MeSH term]
4. Undulant Fever [text word] OR Undulant Fever [MeSH term]
5. 1 OR 2 OR 3 OR 4
6. Prevalence [text word] OR Prevalence [MeSH term]
7. Frequency [text word] OR Frequency [MeSH term]
8. Incidence [text word] OR Incidence [MeSH term]
9. 6 OR 7 OR 8
10. 5 AND 9
By using Google Scholar, we accessed gray literature. In addition, we consulted a bacteriology expert to find related articles. In this regard, by hand searching, we found multiple articles from the references list of related articles. All extracted data were then imported into Endnote X6. Thereafter, the duplicated articles were removed and the remaining studies (original Articles/abstracts published in English) were screened. Afterward, the titles of the articles were examined, and if the article was relevant, its abstract and full text were reviewed. These steps were carried out by two independent raters, “R-P” and “S-KH,” and inter-rater differences were resolved by the opinion of “M-H.” The inter-rater reliability was 89%. Masking and task separation were strategies employed in the selection of the study procedure.
All cross-sectional, prospective, and retrospective case-series studies which reported the prevalence of brucellosis were included. However, case reports and case series with less than five patients (as the study population) and also clinical trial studies were excluded. Also, studies without reported prevalence data, definite sample sizes, and clear correct estimates of the prevalence, as well as case–control studies and abstracts presented in scientific meetings without full texts, were excluded from further study.
In the current study, an excel data sheet was designed. From all eligible studies, general (author name, year, country, study design, sample size, or number of brucellosis patients, age, and sex) and specific (diagnosis method, therapy, symptoms, isolated bacteria, and comorbidities) data were extracted. The quality of these studies was appraised independently by two of the authors (R-P and S-KH) using the Joanna Briggs Institute checklist (20). The scale consisted of three parts, namely selection (four items), comparability (one item), and outcome (two items for cross-sectional and three items for cohort studies).
All data used in this study were analyzed using Stata software 14.0 (College Station, Texas, USA). The number of brucellosis cases and sample size were considered the same as in previous studies (21, 22). Heterogeneity and heterogeneity quantification were determined with the aid of Cochran's Q-test and I2 index, respectively. Based on the Higgins classification approach, I2 values of more than 0.7 were regarded as high heterogeneity. The “Metaprop” command and random-effect model were applied to calculate the pooled prevalence with a 95% confidence interval (CI) and to estimate the pooled prevalence, respectively. The “Freeman–Tukey double-arcsine transformation” method was also utilized to estimate 95% CI to keep the values between 0 and 100%. Factors (age, sample size, gender, and year) affecting heterogeneity among studies were examined by the meta-regression analysis. The publication bias was also checked by the “metabias” command. In case of any publication bias, “meta-trim” command using the trim-and-fill method was used to adjust the prevalence rate. In all analyses, a level of 0.05 was considered statistically significant.
Overall, 2,036 studies were found through databases. Following the exclusion of irrelevant articles, 1,802 studies were included. The screening was conducted in three steps. In the first step, 1,526 studies were excluded after reviewing the titles and abstracts. In the next step, the full texts of the remaining studies (n = 276) were evaluated and 233 studies were eliminated. In the last step, 43 studies with a total sample size of 52,502 were taken into consideration in the analysis. The flowchart of this selection process and the characteristic of studies are represented in Figure 1 and Table 1, respectively. Iran had the highest number of studies (n =18), followed by Pakistan with seven studies. The minimum age range of the subjects was 7.02 years in Pourakbari et al.'s (61) study, while the maximum age range was 68.7 years in the study of Sümer et al. (54). The results of the quality assessment of eligible studies are depicted in Supplementary Table 2. The quality of the studies was acceptable.
The brucellosis prevalence in all eligible studies and the forest plot of brucellosis prevalence are illustrated in Table 1 and Figure 2, respectively. The minimum prevalence (0.0%; 95% CI: 0–1.97) of brucellosis was reported by Alshehabat et al. (41) in Jordan, while the maximum prevalence (80.77%; 95% CI: 71.87–87.84) was reported by Kazemi et al. (60) in Iran. Based on the results of the random-effect model approach, as shown in Figure 2, the pooled estimate for brucellosis prevalence was 15.53% (95% CI: 10.97–20.70).
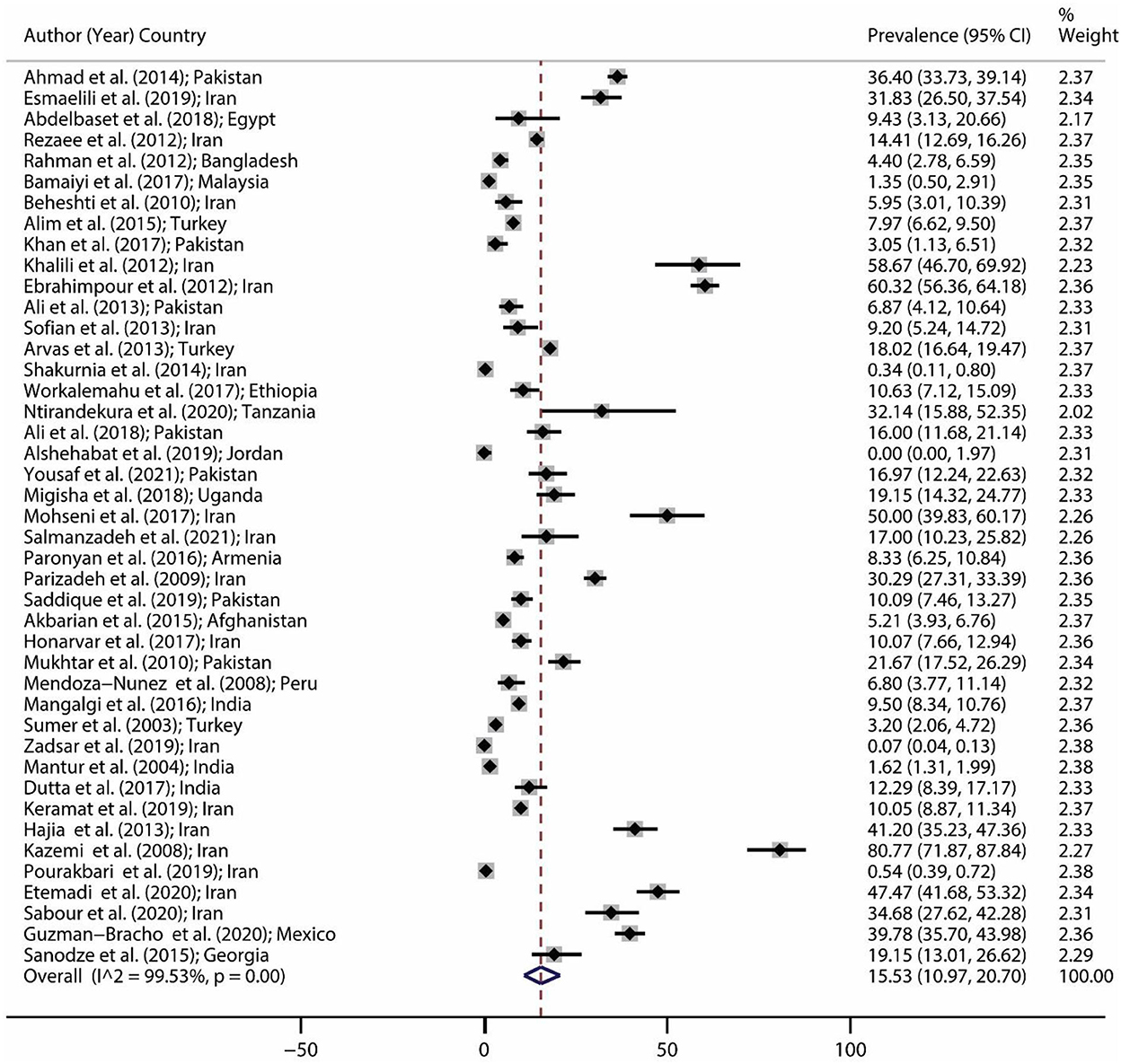
Figure 2. Forest plot for brucellosis prevalence in the world based on a random-effect model. Each study identifies the first author (year) and country. Each line segment's midpoint shows the prevalence estimate, the length of the line segment indicates a 95% confidence interval (CI) in each study, and the diamond mark illustrates the pooled estimate.
The pooled prevalence of brucellosis based on gender is demonstrated in Figure 3. The number of investigations on men and women was 16 and 12 studies, respectively. The pooled prevalence of brucellosis was 15.27% (95% CI: 9.68–21.86; heterogeneity I2 index: 97.43; p < 0.001) for men and 15.33% (95% CI: 7.19–25.75; heterogeneity I2 index: 98.19; p < 0.001) for women. Figure 4 shows the pooled prevalence of brucellosis based on country. As mentioned earlier, Iran has the highest number of studies (n = 18). The highest and lowest prevalence of brucellosis was observed in Mexico (39.78%; 95% CI: 35.7–43.98) and Jordan (0%; 95% CI: 0–1.97), respectively.
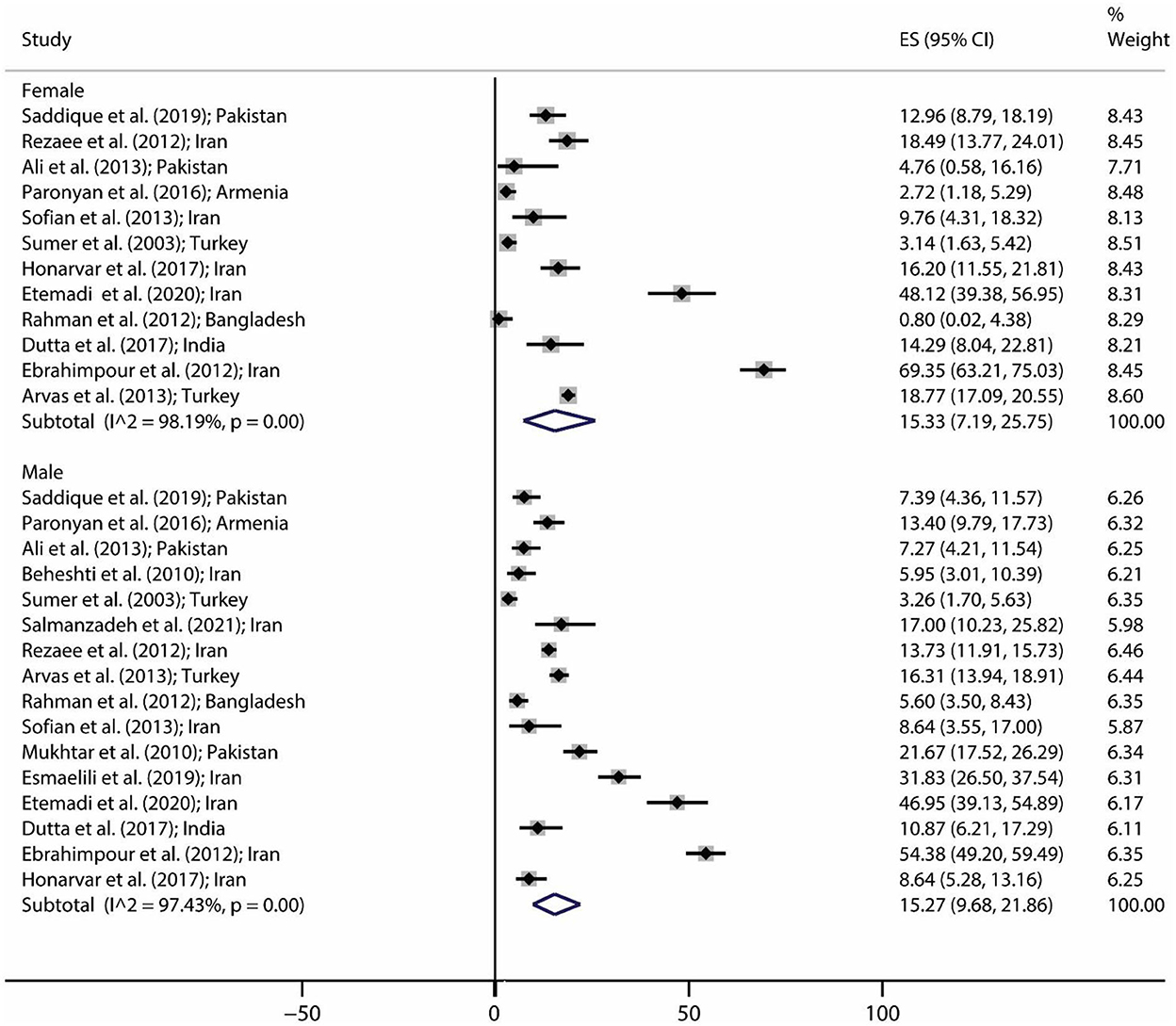
Figure 3. Pooled prevalence with 95% confidence interval (CI) of brucellosis prevalence based on gender. The diamond mark shows the pooled prevalence, and the length of the diamond indicates the 95% CI.
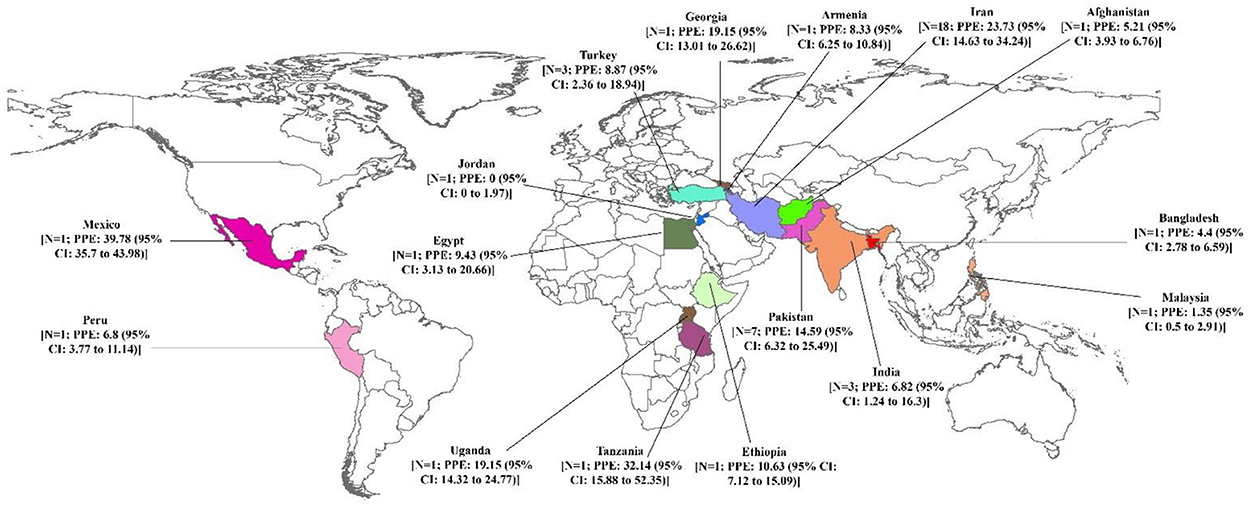
Figure 4. Pooled prevalence with 95% confidence interval (CI) of brucellosis prevalence based on country.
The results of heterogeneity are displayed in Table 2. Cochran's Q-test indicated significant heterogeneity among studies (p < 0.001). It means that the reported prevalence varies in primary studies and the main source of variation is caused by the difference in the true effects. The I2 index for the total prevalence of brucellosis was 99%. In other words, more than 99% of the variance in this study was due to heterogeneity. The results of the meta-regression exhibited that the sample size (coefficient: −0.002; p = 0.044) significantly affected heterogeneity among studies. The age (coefficient: 0.240; p = 0.480), gender (coefficient: −0.017; p = 0.800), publication year (coefficient: 0.114; p = 0.861), and quality of studies (coefficient: 0.034; p = 0.117) showed no significant effect on heterogeneity (Figures 5A, B).
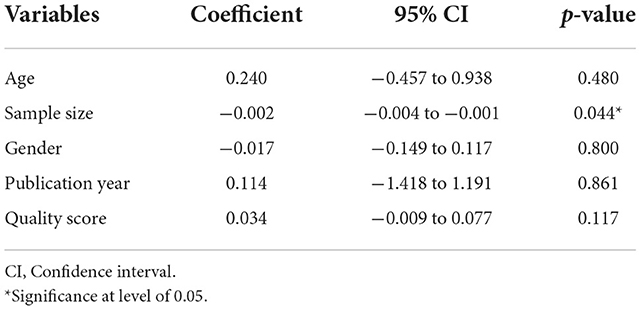
Table 2. The univariate meta-regression analysis on the heterogeneity of the determinants in included studies for the prevalence of brucellosis.
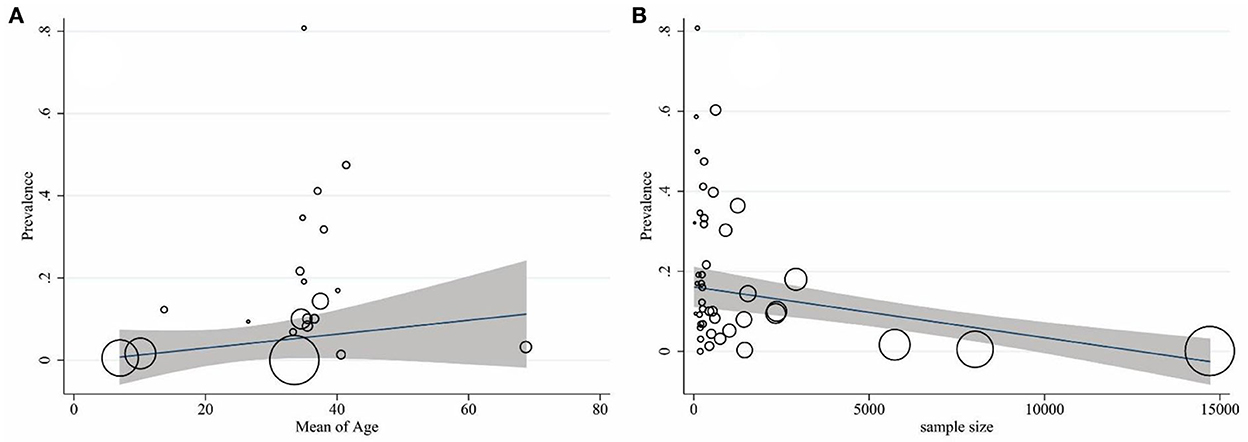
Figure 5. Association of the prevalence of age (A) and sample size (B) with that of brucellosis by using meta-regression. The size of the circles denotes the precision of each study.
Based on the results of Egger's test, a significant publication bias was observed for the prevalence of brucellosis (coefficient: 3.894; p < 0.001). The funnel plot (Figure 6) showed that some evidence might be missed due to not getting published. Therefore, the trim-and-fill-adjusted prevalence of brucellosis (18.30%; 95% CI: 14.10–22.52) was generated, which did not significantly differ from the original prevalence of brucellosis (15.53%; 95% CI: 10.97–20.70).
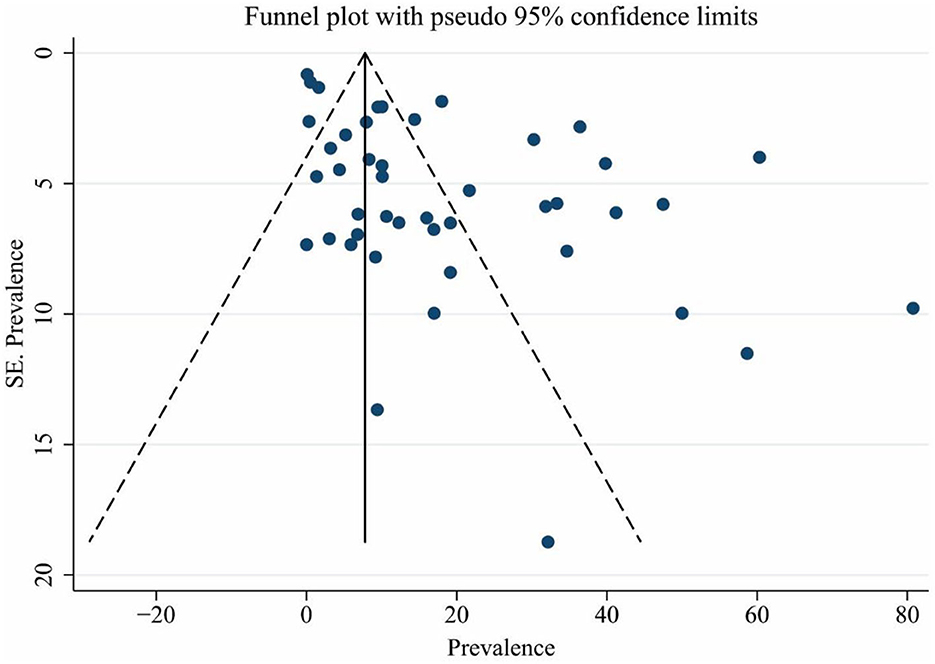
Figure 6. Funnel plot for assessing publication bias. The vertical and horizontal axes show the prevalence of brucellosis and the standard error of prevalence.
While brucellosis and its transmission routes have been discovered for more than 100 years, it is still a global concern, especially in low-income countries (1). Human brucellosis happens through direct or indirect contact with infected animals or their products. Various symptoms, such as fever, osteoarthritis, fatigue, and sweating, may occur in patients (66). Fever, back pain, cough, gastrointestinal symptoms, and blood disorders are multiple manifestations of brucellosis similar to those of other diseases, which often lead to misdiagnosis (3). For the treatment of brucellosis, a combination of tetracycline with rifampicin for 6 weeks or an alternative treatment with fluoroquinolone or co-trimoxazole in combination with rifampicin is commonly used. Medication reduces the length of the disease and prevents recurrence and complications such as arthritis and endocarditis (67). Given the importance and prominent position of brucellosis in the economies of countries, in this study, we evaluated the prevalence of brucellosis and its epidemiology. Transmission and prevalence of brucellosis depend on various factors comprising eating habits, processing methods of milk and its products, social customs, animal husbandry methods, climatic conditions, social and economic status, and environmental health (68).
The highest prevalence of brucellosis has been reported in countries, such as West Asia, India, the Middle East, Southern Europe, and Latin America. The main cause of the disease is B. melitensis (1), which has been eradicated in countries, such as Australia, Canada, Israel, Japan, and New Zealand (2). In industrialized and developed countries, the prevalence of brucellosis is low due to the careful screening program of infected animals and the vaccination of livestock (1). A number of countries have also attempted to eradicate brucellosis by implementing the following measures: setting up a strong system for continuous monitoring of animals and recording positive cases, close monitoring of slaughterhouses, markets, herds for quick and timely identification of positive cases, strict monitoring of herd movement to prevent the spread of infection, continuous and formal training of veterinarians, farmers, and supervisors, compensation of farmers in case of destruction of animals, and establishment of laws to support eradication programs to inhibit non-compliance with official actions (69). For four decades, developed countries have eradicated the infection using control and eradication programs, such as compulsory livestock vaccination; however, following this attempt, they limited and finally banned vaccination. In the continuation of the eradication program in developed countries, the test-and-slaughter policy was implemented, which usually requires more than 10 years to be completed, but the important point in the success of this plan is to compensate the financial losses of farmers (16). The Eastern Mediterranean, including countries, such as Iran, Jordan, Egypt, Palestine, Syria, and Lebanon, is an endemic area of brucellosis. More than half a million people in these countries are infected with brucellosis annually (70).
In endemic areas, there are several reasons for the persistence of infection, including low vaccination, differences between the number of positive animals expected and sampled due to insufficient monitoring, negative herds becoming positive due to the lack of continuous control, refusal of farmers to remove positive livestock owing to non-payment of compensation by regulatory agencies, cessation of eradication projects because of various reasons, for example, the absence of sufficient funds, recurrence of infection due to the lack of permanent monitoring, and problems in detecting positive cases arising from the low prevalence of infection (71). In developing countries, innovative approaches are needed; otherwise, it will be impossible to implement an effective eradication policy. Mongolia, with mass vaccination of livestock, and Tajikistan, with biennial vaccination of small ruminants with Rev1, reduced the prevalence of infection from 25.1 to 7.5% (16). In addition to the reasons mentioned earlier for the non-eradication of brucellosis, there are other reasons, such as the lack of public awareness of Malt fever, the absence of a proper eradication program, limited availability of animal vaccines in the market, the absence of a human vaccine, the maintenance of vaccinated animals more than the vaccine protection period, the use of traditional animal husbandry system, proximity of animal shelters to living areas, lack of border quarantine for the entry of livestock, and living in developing countries, which cause infection among animals and subsequently humans (72). The Middle East is a region with a high incidence of brucellosis, and its native countries, such as Syria, Iraq, Saudi Arabia, Turkey, and Iran, have the most reports of the disease (73).
In the present study, the highest (80.76%) and lowest (0%) prevalence of brucellosis was related to Iran and Jordan, respectively. The reason for the high incidence of the infection is that the majority of eligible articles have been published in Iran. According to the meta-analysis conducted in this study, the highest and lowest prevalence by country and the number of studies belonged to Mexico (39.78%) and Jordan (0%), respectively. The majority of countries surveyed in this study had only one article. Of note, if further studies are performed in a country, and if investigations are conducted in additional countries and different regions of the world, more accurate statistics on the prevalence of brucellosis can be provided. Unfortunately, in many countries that are economically poor and, of course, financial poverty affects the incidence of brucellosis, less information on brucellosis prevalence is available. The low incidence of the disease in some endemic areas is due to the lack or low level of monitoring and reporting (1); there is no exact estimate of the annual incidence of human brucellosis. The international community should allocate resources to understand and fill gaps in information because the existence of this information helps determine effective control strategies. The World Health Organization (WHO) will greatly contribute to the progress of this process by restoring human brucellosis as a neglected zoonotic disease priority (74).
According to the WHO, the prevalence of infection in the world varies between 0.01 and 200 people per 100,000 people. In the United Kingdom, the prevalence is 0.3 per million and in Germany, the prevalence is 0.03 per 100,000 (70). In this study, the global prevalence of brucellosis was estimated at 15.53%. Previous studies have shown that the prevalence of brucellosis is higher in men than in women. Occupational exposure and gender differences in access to healthcare have been cited as factors influencing this variation (1, 14). In the present study, gender had no effect on heterogeneity, and no significant difference was observed between the two gender groups. However, the prevalence was slightly higher in women (15.33%) than in men (15.27%).
In this systematic review, we found that the average age range varies from 7.02 to 68.7. In a review conducted in Kyrgyzstan, the results of a 10-year analysis revealed that all age groups, most of which were active workers, were affected by brucellosis (75). Brucellosis is detected in all age groups but is usually rare in children (1). Pediatric brucellosis is uncommon in areas where B. abortus is endemic, but in endemic areas of B. melitensis, cases are seen in children. One of the most important causes of infection in children is the consumption of raw milk (56). Socio-economic factors, improvement of monitoring systems, and animal control programs have led to the evolution of the epidemiology of human brucellosis (14). Certain population groups of countries, including immigrants and people with poor socio-economic status, are frequently more exposed to brucellosis than others (70). Another factor influencing the epidemiology of brucellosis is political change. For instance, before the collapse of the Soviet Union in 1991, the country was able to control the disease to a large extent, but after the dissolution of the Soviet Union, the prevalence increased in independent countries (14).
The incidence and prevalence of brucellosis can vary not only in different countries but also within a country (14). In a study conducted in Iran, the incidence of brucellosis was reported to be between 7.0 and 276.41 cases per 100,000 people (70). Various studies have also reported that the incidence of brucellosis depends on the seasons. Studies in Iran, Turkey, and Germany showed that the highest prevalence of the disease was in spring and summer, and the least occurred in winter and autumn (76–79). Spring and summer are the calving and lactation seasons for sheep (75, 80). In addition, during these seasons, the production and consumption of milk and dairy products increase, which, in turn, elevates the risk of brucellosis (80). To prevent the spread of the disease, a series of preventive measures should be taken into consideration. Since brucellosis is a zoonotic disease, it is critically important to control the disease in the animal host. Brucellosis is transmitted through the consumption of food, especially contaminated dairy products, which indicates the importance of heat treatment of dairy products. Another important point is occupational dependency in disease transmission, highlighting the need for appropriate and adequate health measures in people related to animals (1). For many years, two successful vaccines (Rev1 and B. abortus S19) have been used for the control of brucellosis in the world, but there are still disadvantages, such as abortion, bacterial removal from vaccinated animals, and induction of disease in humans (81). Appropriate control and preventive measures cause a significant decrease in the incidence of brucellosis. In a report published from Kyrgyzstan, the number of human brucellosis cases and its incidence increased between the years 1994 and 2010, but between 2010 and 2020, there was a continuous decline, which is due to effective preventive and control measures (75).
Limitations can be placed on this meta-analysis and systematic review. In this study, we observed the lack of high-quality scientific data and the absence of sufficient information from many countries, which did not allow a comprehensive analysis of all regions of the world. Another limitation was that we included only articles published in English. This is an important issue because, in many indigenous areas of brucellosis, articles have been published in the particular language of the region. Therefore, these articles have not been considered and evaluated in the present study. Despite many studies on brucellosis, the disease remains a health, economic, and social problem in many parts of the world, particularly in developing and low-income countries. Careful screening of brucellosis and its prevalence in the world and in different countries lead to accurate monitoring of the disease and its epidemic. Brucellosis is a long way from eradication. The important thing in controlling the disease is the strategies used for its prevention. Vaccination is the main strategy to control the disease (71). Prevalence rate, type of animal husbandry, availability of the vaccine, quality of consumed vaccine, amount of available financial and human resources and also legal authority, intersectoral cooperation, and surveillance identification are influential in the successful control of brucellosis (82).
While there is no human vaccine for brucellosis, regular and accurate vaccination of cattle can prevent this zoonotic disease. Educating people, especially villagers, about how to properly sterilize milk and its products can be a promising way to prevent the spread of the disease. With widespread alterations that occur annually in different parts of the world, the prevalence of brucellosis is changing and must be constantly monitored. An effective step to control brucellosis in future is to design and construct a novel vaccine that can create proper immunity in humans. Therefore, to encourage researchers to do this, an international call for the development of a new brucellosis vaccine with a significant price should be considered. In this call, researchers and scientists must compete to create the first licensed human vaccine (81).
Some effective factors in the prevalence of brucellosis were not covered in our study due to the lack of data, which is considered a significant limitation. Heterogeneity between the studies is another limitation. Therefore, we applied a random-effect model to combine the primary results in this meta-analysis. Studies written in English and the lack of studies from some regions of the world are other limitations of the present study.
The epidemiology of brucellosis is important because it is a common disease between humans and animals and can be transmitted to humans in different ways. In this study, the prevalence of brucellosis and factors affecting its incidence were investigated. The prevalence of brucellosis is closely related to the economic, cultural, and even educational status of communities. In developed countries, unlike developing ones, brucellosis is lower. Given this issue, the need for more extensive studies in low-income countries and the pursuit of strict control and prevention programs are recommended. A very prominent point that can be taken into account from the results of this study is that there are no data from some countries due to the lack of sufficient publication, which causes a big gap in the estimation of the annual prevalence of brucellosis in the world. To solve this problem, researchers need to cooperate in conducting more studies on brucellosis and subsequently publishing their data. The most significant matter about the eradication and prevention of brucellosis is the cooperation of the government and international sectors because this disease can affect the economy of countries so much that if these organizations do not take effective actions, many problems will befall the deprived and poor people.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
SK, RP, MK, MS, AA, GI, MM, IP, NS, and MH contributed to the revisions of the article. All authors approved the final version of the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.976215/full#supplementary-material
1. Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. (2007) 25:188–202. doi: 10.1016/S0255-0857(21)02105-8
2. Al Jindan R. Scenario of pathogenesis and socioeconomic burden of human brucellosis in Saudi Arabia. Saudi J Biol Sci. (2021) 28:272–9. doi: 10.1016/j.sjbs.2020.09.059
3. Zheng R, Xie S, Lu X, Sun L, Zhou Y, Zhang Y, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. BioMed Res Int. (2018) 2018:5712920. doi: 10.1155/2018/5712920
4. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. (2007) 7:775–86. doi: 10.1016/S1473-3099(07)70286-4
5. Godfroid J, Nielsen K, Saegerman C. Diagnosis of brucellosis in livestock and wildlife. Croatian Med J. (2010) 51:296–305. doi: 10.3325/cmj.2010.51.296
6. Smirnova EA, Vasin AV, Sandybaev NT, Klotchenko SA, Plotnikova MA, Chervyakova OV, et al. Current methods of human and animal brucellosis diagnostics. Adv Infect Dis. (2013) 3:177–84. doi: 10.4236/aid.2013.33026
7. Avijgan M, Rostamnezhad M, Jahanbani-Ardakani H. Clinical and serological approach to patients with brucellosis: a common diagnostic dilemma and a worldwide perspective. Microbial Pathog. (2019) 129:125–30. doi: 10.1016/j.micpath.2019.02.011
8. Khan MZ, Zahoor M. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop Med Infect Dis. (2018) 3:65. doi: 10.3390/tropicalmed3020065
9. Omer M, Skjerve E, MacMillan A, Woldehiwet Z. Comparison of three serological tests in the diagnosis of Brucella infection in unvaccinated cattle in Eritrea. Prev Vet Med. (2001) 48:215–22. doi: 10.1016/S0167-5877(00)00185-9
10. Hassan L, Ali S, Syed MA, Shah AA, Abbasi SA, Tabassum S, et al. Risk factors for acute brucellosis in patients on the day of admission at selected hospitals of Abbottabad, Pakistan. Front Public Health. (2021) 9:669278. doi: 10.3389/fpubh.2021.669278
11. Nielsen K. Diagnosis of brucellosis by serology. Vet Microbiol. (2002) 90:447–59. doi: 10.1016/S0378-1135(02)00229-8
12. Abdel-Hamid NH, Ghobashy HM, Beleta EI, Elbauomy EM, Ismail RI, Nagati SF, et al. Risk factors and Molecular genotyping of Brucella melitensis strains recovered from humans and their owned cattle in Upper Egypt. One Health. (2021) 13:100281. doi: 10.1016/j.onehlt.2021.100281
13. Pappas G, Papadimitriou P, Christou L, Akritidis N. Future trends in human brucellosis treatment. Expert Opin Invest Drugs. (2006) 15:1141–9. doi: 10.1517/13543784.15.10.1141
14. Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2012) 6:e1929. doi: 10.1371/journal.pntd.0001929
15. Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. (2010) 34:379–94. doi: 10.1111/j.1574-6976.2010.00211.x
16. Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. (2013) 2013:743509. doi: 10.1155/2013/743509
17. Lalsiamthara J, Lee JH. Development and trial of vaccines against Brucella. J Vet Sci. (2017) 18:281–90. doi: 10.4142/jvs.2017.18.S1.281
18. Wang X, Jiang H. Global prevalence of human brucellosis. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:1717–22. doi: 10.3760/cma.j.cn112338-20191022-00751
19. Pappas G, Papadimitriou P. Challenges in Brucella bacteraemia. Int J Antimicrob Agents. (2007) 30:29–31. doi: 10.1016/j.ijantimicag.2007.06.011
20. Institute JB. Joanna Briggs Institute Reviewers' Manual: 2014 Edition. Australia: The Joanna Briggs Institute (2014). p. 88–91.
21. Soltani S, Zandi M, Faramarzi S, Shahbahrami R, Vali M, Rezayat SA, et al. Worldwide prevalence of fungal coinfections among COVID-19 patients: a comprehensive systematic review and meta-analysis. Osong Public Health Res Perspect. (2022) 13:15–23. doi: 10.24171/j.phrp.2021.0293
22. Pakzad R, Malekifar P, Shateri Z, Zandi M, Akhavan Rezayat S, Soleymani M, et al. Worldwide prevalence of microbial agents' coinfection among COVID-19 patients: a comprehensive updated systematic review and meta-analysis. J Clin Lab Anal. (2022) 36:e24151. doi: 10.1002/jcla.24151
23. Ahmad B, Jamil S, Bashir S, Bilal M, Hassan S, Khan J. Incidence of Brucella abortus and Brucella melitensis in Peshawar and identification of active and passive infection. Life Sci J. (2014) 11:1–5. Available online at: http://www.dx.doi.org/10.7537/marslsj1110s14.01
24. Esmaeili S, Bagheri Amiri F, Mokhayeri H, Kayedi MH, Maurin M, Rohani M, et al. SeroepidemiologicalstudyofQfever, brucellosisandtularemiainbutchers andslaughterhousesworkersinLorestan, westernofIran. Comp Immunol Microbiol Infect Dis. (2019) 66:101322. doi: 10.1016/j.cimid.2019.06.003
25. Abdelbaset AE, Abushahba MF, Hamed MI, Rawy MS. Sero-diagnosis of brucellosis in sheep and humans in Assiut and El-Minya governorates, Egypt. Int J Vet Sci Med. (2018) 6:S63–7. doi: 10.1016/j.ijvsm.2018.01.007
26. Rezaee MA, Rashidi A, Motaharinia Y, Hossaini W, Rahmani MR. Seroprevalence study of brucellosis among high-risk groups in comparison with other people of the population in Sanandaj (West of Iran). Afr J Microbiol Res. (2012) 6:1985–9. doi: 10.5897/AJMR11.1095
27. Bamaiyi P, Hassan L, Bejo SK, Zainalabidin M, Adzhar A, Mokhtar N, et al. Seroprevalence of Brucellosis among farmers and veterinary technical staff in Peninsular Malaysia. Sains Malaysiana. (2017) 46:933–43. doi: 10.17576/jsm-2017-4606-13
28. Beheshti S, Rezaian G, Azad F, Faghiri Z, Taheri F. Seroprevalence of brucellosis and risk factors related to high risk occupational groups in Kazeroon, South of Iran. Int J Occup Environ Med. (2010) 1:62–8.
29. Alim A, Oguzkaya-Artan M, Artan C. The seroprevalence of brucellosis among undiagnosed family members of brucellosis positive patients. Nigerian J Clin Pract. (2015) 18:620–5. doi: 10.4103/1119-3077.154206
30. Khan AQ, Haleem SK, Shafiq M, Khan NA, ur Rahman S. Seropositivity of brucellosis in human and livestock in Tribal-Kurram Agency of Pakistan indicates cross circulation. Thai J Vet Med. (2017) 47:349.
31. Khalili M, Sami M, Aflatoonian MR, Shahabi-Nejad N. Seroprevalence of brucellosis in slaughterhouse workers in Kerman city, Iran. Asian Pac J Trop Dis. (2012) 2:448–50. doi: 10.1016/S2222-1808(12)60098-X
32. Ebrahimpour S, Youssefi MR, Karimi N, Kaighobadi M, Tabaripour R. The prevalence of human Brucellosis in Mazandaran province, Iran. Afr J Microbiol Res. (2012) 6:4090–4. doi: 10.5897/AJMR11.1076
33. Rahman AA, Dirk B, Fretin D, Saegerman C, Ahmed MU, Muhammad N, et al. Seroprevalence and risk factors for brucellosis in a high-risk group of individuals in Bangladesh. Foodborne Pathogens Dis. (2012) 9:190–7. doi: 10.1089/fpd.2011.1029
34. Ali S, Ali Q, Neubauer H, Melzer F, Elschner M, Khan I, et al. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Pathogens Dis. (2013) 10:500–5. doi: 10.1089/fpd.2012.1360
35. Sofian M, Safaeipour L, Aghakhani A, Sharif MR, Banifazl M, Sharif A, et al. Screening of family members of patients with acute brucellosis in an endemic area of Iran. Iran J Microbiol. (2013) 5:215.
36. Arvas G, Akkoyunlu Y, Berktas M, Kaya B, Aslan T. The prevalence of brucellosis in adults in northeastern region of Turkey. Jundishapur J Microbiol. (2013) 6:262. doi: 10.5812/jjm.5147
37. Shakurnia A, Qasemzadeh A, Afra M, Kohshour MO. Sero-prevalence of brucellosis among blood donors in Ahvaz, Southwest Iran. Asian Pac J Trop Dis. (2014) 4:S307–10. doi: 10.1016/S2222-1808(14)60461-8
38. Workalemahu B, Sewunet T, Astatkie A. Seroepidemiology of human brucellosis among blood donors in Southern Ethiopia: Calling attention to a neglected zoonotic disease. Am J Trop Med Hygiene. (2017) 96:88. doi: 10.4269/ajtmh.15-0702
39. Ntirandekura JB, Makene VA, Kasanga CJ, Matemba LE, Kimera SI, Muma JB, et al. Molecular characterization of Brucella species detected in humans and domestic ruminants of pastoral areas in Kagera ecosystem, Tanzania. Vet Med Sci. (2020) 6:711–9. doi: 10.1002/vms3.298
40. Ali S, Nawaz Z, Akhtar A, Aslam R, Zahoor MA, Ashraf M. Epidemiological investigation of human brucellosis in Pakistan. Jundishapur J Microbiol. (2018) 11:e61764. doi: 10.5812/jjm.61764
41. Alshehabat M, Obaidat M, Hayajneh W. Seroprevalence of Brucella canis in dogs and at-risk humans in Jordan. Vet Med. (2019) 64:260–5. doi: 10.17221/67/2018-VETMED
42. Yousaf R, Khan I, Shehzad W, Hussain R, Ali S, Neubauer H, et al. Seroprevalence and molecular detection of brucellosis in hospitalized patients in Lahore hospitals, Pakistan. Infect Dis Rep. (2021) 13:166–72. doi: 10.3390/idr13010018
43. Migisha R, Nyehangane D, Boum Y, Page A-L, Zúñiga-Ripa A, Conde-Álvarez R, et al. Prevalence and risk factors of brucellosis among febrile patients attending a community hospital in south western Uganda. Sci Rep. (2018) 8:1–8. doi: 10.1038/s41598-018-33915-9
44. Mohseni K, Mirnejad R, Piranfar V, Mirkalantari S. A comparative evaluation of ELISA, PCR, and serum agglutination tests for diagnosis of Brucella using human serum. Iran J Pathol. (2017) 12:371. doi: 10.30699/ijp.2017.28293
45. Salmanzadeh S, Aliakbarian Z, Mostafavi E, Salehi-Vaziri M, Moogahi S. A cross-sectional study of sero-prevalence and risk factors of brucellosis and haemorrhagic fever in slaughterhouse staff in Ahvaz City, Iran. Med Stud Studia Medyczne. (2020) 37:7–15. doi: 10.5114/ms.2021.104995
46. Paronyan L, Zardaryan E, Bakunts V, Gevorgyan Z, Asoyan V, Apresyan H, et al. A retrospective chart review study to describe selected zoonotic and arboviral etiologies in hospitalized febrile patients in the Republic of Armenia. BMC Infect Dis. (2016) 16:1–8. doi: 10.1186/s12879-016-1764-z
47. Parizadeh SMJ, Seyednozadi M, Erfanian MR, Nezhad MA. A survey on antibody levels among individuals at risk of brucellosis in Khorasan Razavi Province, Iran. Pak J Nutr. (2009) 8:139–44. doi: 10.3923/pjn.2009.139.144
48. Saddique A, Ali S, Akhter S, Khan I, Neubauer H, Melzer F, et al. Acute febrile illness caused by Brucella abortus infection in humans in Pakistan. Int J Environ Res Public Health. (2019) 16:4071. doi: 10.3390/ijerph16214071
49. Akbarian Z, Ziay G, Schauwers W, Noormal B, Saeed I, Qanee AH, et al. Brucellosis and Coxiella burnetii infection in householders and their animals in secure villages in Herat province, Afghanistan: a cross-sectional study. PLoS Negl Trop Disases. (2015) 9:e0004112. doi: 10.1371/journal.pntd.0004112
50. Honarvar B, Moghadami M, Lankarani K, Davarpanah M, Ataolahi M, Farbod A, et al. Brucellosis as a neglected disease in a neglected population: a seroepidemiological study of migratory nomads in the Fars province of Iran. Epidemiol Infect. (2017) 145:491–7. doi: 10.1017/S0950268816002600
51. Mukhtar F. Brucellosis in a high risk occupational group: seroprevalence and analysis of risk factors. J Pak Med Assoc. (2010) 60:1031.
52. Mendoza-Núñez M, Mulder M, Franco MP, Maas KS, Castañeda ML, Bonifacio N, et al. Brucellosis in household members of Brucella patients residing in a large urban setting in Peru. Am J Trop Med Hyg. (2008) 78:595–8. doi: 10.4269/ajtmh.2008.78.595
53. Mangalgi SS, Sajjan AG, Mohite ST, Gajul S. Brucellosis in occupationally exposed groups. J Clin Diagn Res. (2016) 10:DC24. doi: 10.7860/JCDR/2016/15276.7673
54. Sümer H, Sümer Z, Alim A, Nur N, Özdemir L. Seroprevalence of Brucella in an elderly population in mid-Anatolia, Turkey. J Health Popul Nutr. (2003) 21:158–61.
55. Zadsar M, Shirzadi MR, Zeynali M, Rasouli M, Karimi G. Human Brucellosis: risks and prevalence among Iranian blood donors residing in endemic areas. Transfusion Med Hemother. (2020) 47:103–9. doi: 10.1159/000500248
56. Mantur B, Akki A, Mangalgi SS, Patil S, Gobbur R, Peerapur B. Childhood brucellosis—a microbiological, epidemiological and clinical study. J Trop Pediatr. (2004) 50:153–7. doi: 10.1093/tropej/50.3.153
57. Dutta D, Sen A, Gupta D, Kuila P, Chatterjee D, Sanyal S, et al. Childhood brucellosis in eastern India. Indian J Pediatr. (2018) 85:266–71. doi: 10.1007/s12098-017-2513-z
58. Keramat F, Karami M, Alikhani MY, Bashirian S, Moghimbeigi A, Adabi M. Cohort profile: famenin brucellosis cohort study. J Res Health Sci. (2019) 19:e00453.
59. Hajia M, Fallah F, Angoti G, Karimi A, Rahbar M, Gachkar L, et al. Comparison of methods for diagnosing brucellosis. Lab Med. (2013) 44:29–33. doi: 10.1309/LM4J9MWOBIPA6RBN
60. Kazemi B, Yousefi Namin S, Bandepour M, Kafilzadeh F, Gachkar L, Mahmoudinejad F, et al. Detection of Brucella by peripheral blood PCR and comparison with culture and serological methods in suspected cases. Iran J Public Health. (2003) 37:96–102.
61. Pourakbari B, Abdolsalehi M, Mahmoudi S, Banar M, Masoumpour F, Mamishi S. Epidemiologic, clinical, and laboratory characteristics of childhood brucellosis. Wiener Medizinische Wochenschrift. (2019) 169:232–9. doi: 10.1007/s10354-019-0685-z
62. Etemadi A, Moniri R, Saffari M, Akbari H, Alamian S, Behrozikhah AM. Epidemiological, molecular characterization and risk factors of human brucellosis in Iran. Asian Pac J Trop Med. (2020) 13:169. doi: 10.4103/1995-7645.280224
63. Sabour S, Arzanlou M, Jeddi F, Azimi T, Hosseini-Asl S, Naghizadeh-Baghi A, et al. Evaluating the efficiency of TaqMan real-time PCR and serological methods in the detection of Brucella spp. in clinical specimens collected from suspected patients in Ardabil, Iran. J Microbiol Methods. (2020) 175:105982. doi: 10.1016/j.mimet.2020.105982
64. Guzmán-Bracho C, Salgado-Jiménez B, Beltrán-Parra LG, Hernández-Monroy I, Vargas-Pino F, Rodríguez D, et al. Evaluation of serological diagnostic tests of human brucellosis for prevention and control in Mexico. Eur J Clin Microbiol Infect Dis. (2020) 39:575–81. doi: 10.1007/s10096-019-03760-3
65. Sanodze L, Bautista CT, Garuchava N, Chubinidze S, Tsertsvadze E, Broladze M, et al. Expansion of brucellosis detection in the country of Georgia by screening household members of cases and neighboring community members. BMC Public Health. (2015) 15:459. doi: 10.1186/s12889-015-1761-y
66. Zheng R, Xie S, Niyazi S, Lu X, Sun L, Zhou Y, et al. Meta-analysis of the changes of peripheral blood T cell subsets in patients with brucellosis. J Immunol Res. (2018) 2018:8439813. doi: 10.1155/2018/8439813
67. Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. BMJ. (2008) 336:701–4. doi: 10.1136/bmj.39497.500903.25
68. Joint FAO/WHO expert committee on brucellosis. World Health Organ Tech Rep Ser. (1986) 740:1–132.
69. Zamri-Saad M, Kamarudin M. Control of animal brucellosis: the Malaysian experience. Asian Pac J Trop Med. (2016) 9:1136–40. doi: 10.1016/j.apjtm.2016.11.007
70. Mirnejad R, Jazi FM, Mostafaei S, Sedighi M. Epidemiology of brucellosis in Iran: a comprehensive systematic review and meta-analysis study. Microbial Pathog. (2017) 109:239–47. doi: 10.1016/j.micpath.2017.06.005
71. Zhang N, Huang D, Wu W, Liu J, Liang F, Zhou B, et al. Animal brucellosis control or eradication programs worldwide: a systematic review of experiences and lessons learned. Prev Vet Med. (2018) 160:105–15. doi: 10.1016/j.prevetmed.2018.10.002
72. Golshani M, Buozari S. A review of Brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran Biomed J. (2017) 21:349–59. doi: 10.18869/acadpub.ibj.21.6.349
73. Bagheri Nejad R, Krecek RC, Khalaf OH, Hailat N, Arenas-Gamboa AM. Brucellosis in the middle east: current situation and a pathway forward. PLoS Negl Trop Dis. (2020) 14:e0008071. doi: 10.1371/journal.pntd.0008071
74. Laine CG, Scott HM, Arenas-Gamboa AM. Human brucellosis: widespread information deficiency hinders an understanding of global disease frequency. PLoS Negl Trop Dis. (2022) 16:e0010404. doi: 10.1371/journal.pntd.0010404
75. Kydyshov K, Usenbaev N, Sharshenbekov A, Aitkuluev N, Abdyraev M, Chegirov S, et al. Brucellosis in humans and animals in Kyrgyzstan. Microorganisms. (2022) 10:1293. doi: 10.3390/microorganisms10071293
76. Moosazadeh M, Abedi G, Kheradmand M, Safiri S, Nikaeen R. Seasonal pattern of brucellosis in Iran: a systematic review and meta-analysis. Iran J Health Sci. (2016) 4:62–72. doi: 10.18869/acadpub.jhs.4.1.62
77. Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, Evirgen O, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. (2010) 14:e469–78. doi: 10.1016/j.ijid.2009.06.031
78. Al Dahouk S, Neubauer H, Hensel A, Schöneberg I, Nöckler K, Alpers K, et al. Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerg Infect Dis. (2007) 13:1895. doi: 10.3201/eid1312.070527
79. Minas M, Minas A, Gourgulianis K, Stournara A. Epidemiological and clinical aspects of human brucellosis in Central Greece. Jpn J Infect Dis. (2007) 60:362.
80. Amro A, Mansoor B, Hamarsheh O, Hjaija D. Recent trends in human brucellosis in the West Bank, Palestine. Int J Infect Dis. (2021) 106:308–13. doi: 10.1016/j.ijid.2021.04.037
81. O'callaghan D. Human brucellosis: recent advances and future challenges. Infect Dis Poverty. (2020) 9:1–2. doi: 10.1186/s40249-020-00715-1
Keywords: brucellosis, diagnosis, prevalence, Brucella, review
Citation: Khoshnood S, Pakzad R, Koupaei M, Shirani M, Araghi A, Irani GM, Moradi M, Pakzad I, Sadeghifard N and Heidary M (2022) Prevalence, diagnosis, and manifestations of brucellosis: A systematic review and meta-analysis. Front. Vet. Sci. 9:976215. doi: 10.3389/fvets.2022.976215
Received: 24 June 2022; Accepted: 17 November 2022;
Published: 22 December 2022.
Edited by:
Domenico Vecchio, Experimental Zooprophylactic Institute of Southern Italy (IZSM), ItalyReviewed by:
Tariq Jamil, Friedrich Loeffler Institut, GermanyCopyright © 2022 Khoshnood, Pakzad, Koupaei, Shirani, Araghi, Irani, Moradi, Pakzad, Sadeghifard and Heidary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsen Heidary, bW9oc2VuaGVpZGFyeTQwQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.