- 1Department of Comparative, Diagnostic, and Population Medicine, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
- 2Seattle Aquarium, Seattle, WA, United States
- 3Department of Clinical Sciences and Center for Marine Sciences and Technology, College of Veterinary Medicine, North Carolina State University, Morehead City, NC, United States
- 4College of Veterinary Medicine, North Carolina State University, Raleigh, NC, United States
- 5ZooQuatic Laboratory, LLC, Baltimore, MD, United States
- 6OCEARCH, Park City, UT, United States
- 7Global Animal Welfare and Training, Charles River Laboratory, Wilmington, MA, United States
Welfare considerations and regulations for invertebrates have lagged behind those for vertebrates, despite invertebrates comprising more than 95% of earth's species. Humans interact with and use aquatic invertebrates for exhibition in zoos and aquaria, as pets, research subjects, and important food sources. Recent research has indicated that aquatic invertebrates, in particular cephalopod mollusks and decapod crustaceans, experience stress and may be able to feel pain. With this article, we present results of a survey on attitudes of aquatic animal health professionals toward aquatic invertebrate welfare and provide practical recommendations for advancing aquatic invertebrate welfare across four areas of opportunity: use of anesthesia, analgesia, and euthanasia; development of less invasive diagnostic and research sampling methods based on 3R principles; use of humane slaughter methods for aquatic invertebrates; and reducing impacts of invasive procedures in aquaculture and fisheries. We encourage consideration of these opportunities to achieve far-reaching improvements in aquatic invertebrate welfare.
Introduction
Despite comprising >95% of the animal species on Earth (1), attention to invertebrate animal welfare has lagged behind those for vertebrate animals. Although the interest in ethics and anesthesia related to vertebrate animal welfare has been increasing since the mid-twentieth century (2, 3), invertebrates have been less in the focus of welfare research and regulations. Aquatic invertebrates are displayed in zoos and aquaria, kept as pets, used as research animals, and serve as food sources for humans and other animals. Efforts to provide high quality care, to improve public perception and trust, and to extend ethical responsibilities to all veterinary patients and research subjects have driven the need to be mindful of aquatic invertebrate welfare.
Complicating our ability to discuss aquatic invertebrate welfare is the variable complexity of aquatic invertebrate nervous systems—from sponges which lack true nervous tissues (4) to cephalopods with roughly half a billion neurons (5). Cephalopods and decapod crustaceans are considered “advanced invertebrates” and have been the focus of the majority of research regarding invertebrate welfare. Cephalopods (e.g., cuttlefish, nautilus, octopus, and squid) have arguably the most complex nervous system found in invertebrates (6) and have a large body of literature devoted to exploring their nociceptive capabilities and pain perception (7–11). Decapod crustaceans (e.g., prawn, crab, lobster, and crayfish) have also been the subject of similar studies on nociceptive capabilities and pain perception (12–14) as well as indicators of stress (15–18). Less information is available for other taxa. Given the evidence of pain perception and stress in aquatic invertebrates, welfare considerations provide opportunities for advancements. In addition to the opportunities discussed in this paper, welfare can be improved through further minimization of stressors and the provision of species-appropriate housing, diet, water quality, social structure, and choices within an enriched environment, where appropriate (19–22).
Legal protections for aquatic invertebrates vary by country and whether the animals are used for research or human consumption. Cephalopods in research are protected in the European Union by Directive 2010/63/EU (23); decapod crustaceans were recommended for inclusion in this legislation (24) but were ultimately not included. This legal protection does not extend to animals intended for human consumption. Cephalopods and decapod crustaceans are protected in Switzerland (25), Norway (26), and New Zealand (27). Octopus are protected in the UK (28), although a recent publication by the London School of Economics reported strong evidence of sentience in cephalopod mollusks and decapod crustaceans (29). In Canada, cephalopods and “some other higher invertebrates” are protected (30). In the United States, invertebrates are not included in the Animal Welfare Act (31) but may be included for oversight by certain Institutional Animal Care and Use Committees if requested by the funding agency.
Here we present and discuss a survey on attitudes of aquatic animal health professionals toward aquatic invertebrate welfare and then provide practical recommendations for advancing aquatic invertebrate welfare across four areas of opportunity.
Current attitudes toward aquatic invertebrate welfare among aquatic animal health professionals
In November 2019, a 10-question informal, anonymous survey was distributed electronically to three veterinary medicine-focused professional email listservs to determine the attitudes of aquatic animal health professionals toward the welfare of aquatic invertebrates. The majority of the 112 respondents identified as veterinarians (87%) while others identified as animal care staff, pathologists, researchers, veterinary technicians or assistants, or veterinary students. Sixty-seven of 111 (60%) thought that invertebrates can feel pain and 52 of 61 (85%) thought that cephalopod mollusks could feel pain. Only 49% had attempted pain control in invertebrates. Seventy-five of 112 (67%) indicated that they strongly consider the welfare of the invertebrates when performing treatments, procedures, or euthanasia. Respondents indicated that they euthanized aquatic invertebrates most frequently due to illness (95%), followed by population control (20%), cosmetic reasons (15%), research (5%), diagnostics (2%), feed for other animals (2%), age-related reasons (2%), and health surveillance (1%). The most common methods for euthanasia, either individually or in combination, included immersion in tricaine methanesulfonate, otherwise known as MS-222 (58%), magnesium salts (52%), physical methods (30%), freezing (20%), immersion in alcohol (18%), and/or Aqui-S/clove oil/eugenol (13%). Less common methods included sodium pentobarbital (5%), removal from water (4%), isoflurane (2%), formalin (2%), 2-phenoxyethanol (1%), lidocaine (1%), propofol (1%), and “shock” (1%) which was not further defined. Fifty of 92 (54%) identified that they used a two-step process. The results of this survey highlighted the need for development and implementation of evidence-based guidelines to improve the welfare of aquatic invertebrates in various settings and as appropriate. Future research on the topic could benefit from a formal survey with more participants to enable further statistical analyses.
Opportunity 1: Promote the use of anesthesia, analgesia, and euthanasia
Anesthesia, analgesia, and euthanasia can provide great improvements to aquatic invertebrate welfare when appropriately implemented. Anesthesia can be used to immobilize aquatic invertebrates for physical examination, sample collection and procedures, and to reduce stress and the potential for injury for both animal and handler. Commonly used anesthetic concentrations have been previously reported for a limited number of species (32–36). The selection of anesthetic should be based on a knowledge of species biology, mechanisms of action of the agent, clinical judgment and if possible, recent literature, though even published methods should be critically evaluated. While hypothermia, carbon dioxide, and calcium-free seawater have been utilized as anesthetics, these procedures likely induce physiologic derangements, and their use may raise welfare concerns. Anesthesia for aquatic invertebrates typically involves immersing the animal in a solution (such as magnesium salts or 1–10% ethanol) or providing flow of anesthetic solution across the animal. Care should be taken that the solution is at the same temperature, pH, and osmolality as the animal's life support system water and is aerated to prevent hypoxia, and that water quality is monitored during prolonged procedures. Invertebrates should be frequently monitored and adjustments to the concentration of anesthetic made to maintain an optimal anesthetic plane. While MS-222 is commonly used in aquatic animal medicine, it may not be the best anesthetic choice for some invertebrate taxa as high concentrations are required which may lead to substantial changes in water chemistry that potentially impact animal health (37, 38).
When performing invasive or potentially painful procedures, the use of analgesic medications should be considered. However, there is a lack of information on appropriate analgesic medications for aquatic invertebrates. The few published research studies have focused on the use of local anesthetics such as lidocaine, given the conservation of sodium channels across species (39, 40). Lidocaine injections appear to have analgesic properties in cephalopods due to blocked afferent nerve signals and the prevention of behavioral responses to noxious stimuli (8, 11). Topical benzocaine decreased behavioral responses of glass prawn (Palaemon elegans) to noxious stimuli, also indicating potential analgesia (41). While morphine has been frequently used in decapod crustacean research, the observed results appear to be from sedation rather than analgesic properties (42). More evidence-based analgesic options are needed for all taxa of aquatic invertebrates.
Euthanasia is used to describe the act of ending the life of an animal in a manner that minimizes or eliminates pain and distress. Slaughter on the other hand is the act of killing animals for human or another animal's consumption (43) and is discussed in Opportunity 3. A follow-up anonymous survey was distributed electronically in May 2022 to four professional email listservs, predominantly aquatic animal veterinarians, to determine current euthanasia techniques for aquatic invertebrates. Of the 36 respondents who had euthanized an aquatic invertebrate in the previous 2 years, 92% identified as clinical veterinarians. The results of the survey are reported in Table 1.
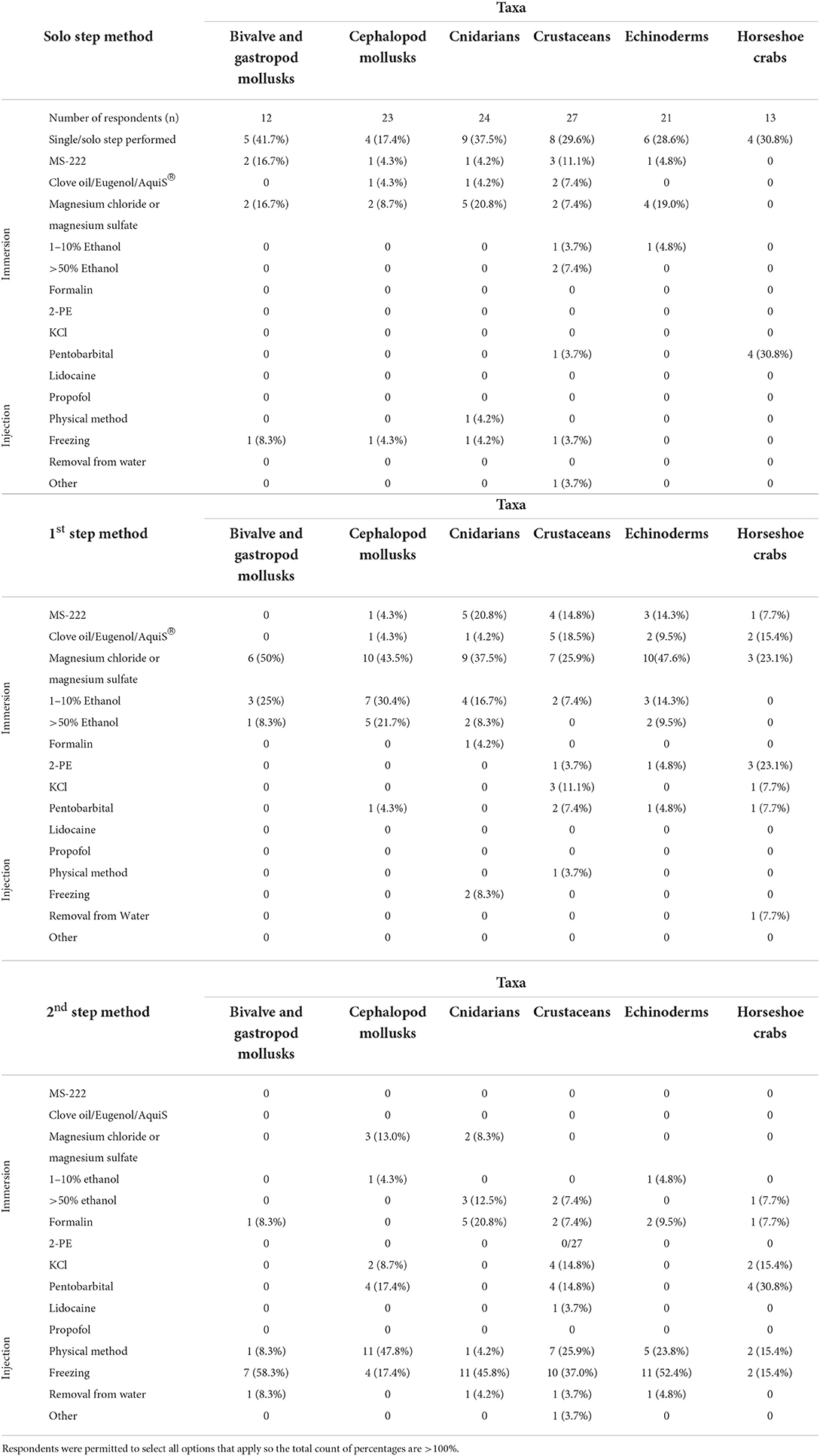
Table 1. Responses from an electronically delivered survey on currently used methods for euthanasia of aquatic invertebrates.
To be considered euthanasia, an animal should be quickly rendered non-responsive and the method should minimize stress, be reliable, reproducible, and irreversible (44). A two-step approach is recommended for the euthanasia of aquatic invertebrates by the American Veterinary Medical Association (43). The first step should render the animal non-responsive and can include immersion in anesthetics such as magnesium salts (MgCl2 or MgSO4), clove oil, eugenol, or ethanol (1-10%). Injections of potassium chloride in direct proximity to the ventral nerve chord or injectable anesthetics can be used in crustaceans (45, 46). The second step should be unsurvivable and include physical or chemical destruction of the brain or major ganglia. Acceptable options for the second step include immersion in 70% alcohol, 10% formalin, or physical methods such as pithing, freezing, boiling, or sharp dissection. Methods that are unacceptable as a first or solo step include removal from the water to die by desiccation and hypoxia, freezing, or immersion in caustic chemicals (such as tissue fixative or 70% ethanol) (43).
Opportunity 2: Development of less invasive sampling methods for research and diagnostic procedures
Research protocols and diagnostic procedures in aquatic invertebrates often involve terminally collected samples which may not be sustainable considering population declines in many invertebrate species. As of 2021, the International Union for Conservation of Nature (IUCN) lists 1,661 invertebrate species as critically endangered or endangered and an additional 1,326 species as threatened (47). Furthermore, lethal sampling may become unacceptable due to changing public attitudes and increasing animal welfare concerns by the scientific and animal health communities.
In animal research, scientists are obligated to use the 3Rs (replacement, reduction, and refinement) as a framework for the humane treatment of animals. The 3Rs were originally developed in 1959 by Russel and Burch (48) to improve laboratory animal welfare but are generally applicable to any situation where animals and humans interact. Replacement refers to replacing the use of animals; this can include the use of in vitro and in silico models. Reduction refers to the use of appropriate experimental design to appropriately power a study and optimize the number of animals used, as well as the data collected from each animal. Refinement refers to minimization of the pain, suffering, distress, and harm experienced by research animals (49).
We support application of the 3Rs principles across the animal kingdom. In various research settings and for diagnostic testing, lethal sampling techniques can be replaced with non-lethal procedures, including collection of hemolymph, coelomic fluid, or tissue biopsies. Current guidelines for blood collection in mammals limit removal to 10% of the total circulating blood volume (50), but very few analogous recommendations exist for invertebrates. Hemolymph and coelomic fluid removal should be limited to the minimum amount necessary, and perhaps no more than 10% of the circulating volume until safe guidelines can be established through research. Tissue biopsies should also be kept to the minimum practical size needed to fulfill sampling objectives. Only a few milligrams of tissue are necessary for conservation genetics and other molecular testing. Non-lethal sampling has been performed in sponges, corals, crustaceans, insects, echinoderms, and mollusks (51). Examples of non-lethal procedures include in vivo solid phase microextraction using a fiber inserted near the mouth of the animal to evaluate plastic contaminants in corals and bivalves (52) and the use of dragonfly fecal pellets and shed exoskeletons for DNA extraction (53). Further refinement can include the use of anesthetics and analgesics for invasive procedures. Handling techniques can be evaluated and improved to minimize stress and harm. If non-lethal sampling is performed but animals must be permanently removed from the wild, a plan to provide life-long care presents an opportunity for placement in educational or display settings. For example, if planned in advance, disposition to public aquaria may be an option for some non-releasable invertebrates, depending on capacity and institutional collection plans.
In cases where invasive sampling cannot be avoided, sharing samples with other researchers can reduce the need for additional specimen collection (54). If lethal sampling is required, aquatic invertebrates should be euthanized prior to sampling. Due to concerns over sample quality, invertebrates are often terminally sampled without methods rendering them non-responsive prior to sampling. However, several studies have demonstrated that high quality samples can still be obtained from euthanized animals. High quality RNA was successfully extracted from sea stars immersed in MgCl2 prior to sampling (55) and jellyfish immersed in MgCl2 provided useful samples for NMR-based metabolomics (56).
Opportunity 3: Use of humane slaughter methods for aquatic invertebrates
Aquatic invertebrates including non-cephalopod mollusks (e.g., bivalves and gastropods), crustaceans, cephalopod mollusks, jellyfish, sea cucumbers, and sea urchins are commonly consumed by humans with 41 million tons captured or cultured in 2018 (57). While euthanasia methods are published for many of these taxa, there is a lack of peer-reviewed literature evaluating humane stunning and slaughter methods. The only taxon with published information on humane slaughter are decapod crustaceans (58). Surprisingly, while cephalopods are the focus of much research on sentience and pain perception, no published article could be found on appropriate slaughter techniques for this taxon, as of April 2022. Methods for cephalopod slaughter include decapitation without prior stunning (59), “clubbing, slicing the brain, reversing the mantle, and asphyxiation in a suspended net bag”, none of which are considered to be humane (29).
The debate on humane slaughter methods for decapod crustaceans started in the 1950s with publications by Baker and Gunter (60, 61). There is contradictory evidence on whether slowly warming live animals or placing live animals in boiling water is humane (62, 63), but boiling lobsters alive has been banned in New Zealand (64), Norway (26), Switzerland (65), and certain parts of Italy (66). Ice slurries and electrical shock may paralyze crustaceans, but neural circuits still remain intact and functional so these methods are likely best used after rendering the animal insensible (67). Based on the available scientific evidence, all animals should have their nerve ganglia destroyed prior to cooking to prevent any potential suffering (60, 68). A commercially available stunning device, the CrustaStun (Mitchell & Cooper, Uckfield, England) that is recommended by the Royal Society for the Prevention of Cruelty to Animals (RSCPA), can be used to stun crustaceans prior to boiling and has been shown to arrest nervous activity after use (69).
Regardless of the method for slaughter chosen, stress should be minimized throughout the supply chain and animals should be killed quickly to avoid unnecessary suffering and pain. More evidence-based and species-appropriate methods are needed for practical humane slaughter of aquatic invertebrates, particularly for cephalopod mollusks.
Opportunity 4: Reduce the impact of invasive procedures in aquaculture and fisheries
Crustaceans have been shown to experience stress and likely have the capacity to feel pain, which should be considered during processes from collection to slaughter. Industry practices that might be adjusted to minimize stress include decreasing the trawling duration, live transport duration, and handling needs (70). Additional good practice recommendations include maintenance of the animals' thermal preference zone, provision of good water quality, and allowance of recovery periods (71). Anesthetics can also be used to decrease stress throughout the supply chain from collection to slaughter. Isobutanol, a food safe anesthetic, reduced ammonia concentrations and mortalities during live transport of tropical spiny lobsters (Panulirus spp.) (72).
Crabs in fisheries worldwide have their claws manually removed followed by release back into the water. Live declawing is performed with brown crabs (Cancer pagurus) (73), stone crabs (Menippe spp.) (74), and fiddler crabs (Uca tangeri) (75). While crustaceans do autotomize claws naturally, manual declawing is more stressful and causes significantly higher mortality than natural autotomy (73). This practice is often considered better than whole crab landing, based on the assumption that these animals survive and regenerate their claws, while remaining in the fishery. However, mortality was >60% in stone crabs with both claws removed (76) and regenerated claws only comprised 3% of legal stone crab landings (77), indicating that this practice is little or no more sustainable than whole crab harvest. Crabs that survive declawing show decreased feeding (74, 78) and decreased reproductive fitness (75, 79). Based on animal welfare concerns and negligible population benefits, declawing may not be preferable over humane harvest and slaughter.
Brown crabs are often transported alive, and mechanisms are needed to prevent them from damaging each other during transport. In the Irish fishery, they undergo a process known as nicking, which involves cutting the ligament under the dactylus of the claw since their claw shape makes traditional banding used in other crustaceans challenging (80). Nicking results in hemolymph loss, risk of infection, inability to molt, and increased mortality (81–83). Nicked crabs had higher hemolymph glucose, lactate, and refractive index, indicating they experienced increased stress (80). In the Norwegian fishery, the crabs are not nicked and are instead transported dry (84). However, emersion can also result in welfare issues, particularly at higher temperatures (85). Adapted banding techniques [e.g., Elastrator (castrator) rings combined with a wooden dowel through the claw] could be considered (86). Finding a solution that balances crab welfare with the needs of the fisheries offers an opportunity for research.
In shrimp aquaculture, eyestalk ablation is performed to induce female broodstock to spawn, since the eyestalks are a source of vitellogenesis-inhibiting hormone (VIH) which is a negative regulator of crustacean reproduction (87). Following eyestalk ablation, shrimp exhibit stress-related behaviors including erratic and spiral swimming, rubbing, and tail flicking, which are prevented by topical anesthetic application (88, 89). Beyond the stress and potential pain caused by handling and the procedure, eyestalk ablation can also impact the immune system of shrimp (90). Non-ablated broodstock females appear to perform at a similar level as ablated females, with larvae that are more resilient to typical pathogens and environmental stress (91, 92). As eyestalk ablation carries negative health and welfare consequences, evaluation of alternatives could be beneficial. Switching from a 1:1 ratio of females to males to a 1:2 ratio improves performance without ablation (91) and a single injection of anti-GIH monoclonal antibody was shown to have similar performance to eyestalk ablation (93).
Conclusions
Aquatic animal health professionals believe that aquatic invertebrates, especially cephalopods, can feel pain. However, <50% have used analgesia during invasive procedures with aquatic invertebrates, likely due to a dearth of well described effective options. This highlights the need for more research on appropriate anesthetic and analgesic options for aquatic invertebrates. While the discussion of pain perception in invertebrates is important, the ability to feel pain is not a prerequisite for promoting positive animal welfare in aquatic invertebrates. Many cost- and time-effective opportunities for the improvement of aquatic invertebrate welfare exist and can be appropriate in various settings. We advocate the use of these advancements and further investigations in this underrepresented but important field of animal welfare.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SW and NS conceptualized the presented idea. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the members of the Fish Club, World Aquatic Veterinary Medical Association, American Association of Zoo Veterinarians, and International Association for Aquatic Animal Medicine email listservs who took time out of their busy schedules to complete both surveys.
Conflict of interest
Author AN is employed by ZooQuatic Laboratory, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ruppert EE, Barnes RD. Invertebrate Zoology. 6th ed. New York, NY: Saunders College Publishing (1994). p. 1100.
2. Rollin BE. The regulation of animal research and the emergency of animal ethics: a conceptual history. Theor Med Bioeth. (2006) 27:285–304. doi: 10.1007/s11017-006-9007-8
3. Steffey EP. A history of veterinary anesthesia. In:Eger II EI, Saidman LJ, Westhorpe RN, , editors. The Wondrous Story of Anesthesia. New York, NY: Springer (2014). p. 293–302.
4. Leys SP. Elements of a “nervous system” in sponges. J Exp Biol. (2015) 218:581–91. doi: 10.1242/jeb.110817
5. Mather JA, Dickel L. Cephalopod complex cognition. Curr Opin Behav Sci. (2017) 16:131–7. doi: 10.1016/j.cobeha.2017.06.008
6. Hochner B, Shomrat T, Fiorito G. The octopus: a model for a comparative analysis of the evolution of learning and memory mechanisms. Biol Bull. (2006) 210:308–17. doi: 10.2307/4134567
7. Andrews PLR, Darmaillacq AS, Dennison N, Gleadall IG, Hawkins P, Messenger JB, et al. The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J Exp Mar Bio Ecol. (2013) 447:46–464. doi: 10.1016/j.jembe.2013.02.010
8. Butler-Struben HM, Brophy SM, Johnson NA, Crook RJ. In vivo recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front Physiol. (2018) 9:1–18. doi: 10.3389/fphys.2018.00109
9. Crook RJ, Walters ET. Nociceptive behavior and physiology of molluscs: animal welfare implications. ILAR J. (2011) 52:185–95. doi: 10.1093/ilar.52.2.185
10. Crook RJ, Hanlon RT, Walters ET. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J Neurosci. (2013) 33:10021–6. doi: 10.1523/JNEUROSCI.0646-13.2013
11. Crook RJ. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience. (2021) 24:102229. doi: 10.1016/j.isci.2021.102229
12. Elwood RW. Assessing the Potential for Pain in Crustaceans and Other Invertebrates. In:Carere C, Mather J, , editors. The Welfare of Invertebrate Animals. Animal Welfare, Vol. 18. Cham: Springer (2019). doi: 10.1007/978-3-030-13947-6_7
13. Elwood RW. Pain and suffering in invertebrates? ILAR J. (2011) 52:175–84. doi: 10.1093/ilar.52.2.175
14. Diggles BK. Review of some scientific issues related to crustacean welfare. ICES J Mar Sci. (2019) 76:66–81. doi: 10.1093/icesjms/fsy058
15. Bergmann M, Taylor AC, Geoffrey Moore P. Physiological stress in decapod crustaceans (Munida rugosa and Liocarcinus depurator) discarded in the Clyde Nephrops fishery. J Exp Mar Bio Ecol. (2001) 259:215–29. doi: 10.1016/S0022-0981(01)00231-3
16. Chang ES. Stressed-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr Comp Biol. (2005) 45:43–50. doi: 10.1093/icb/45.1.43
17. Chang ES, Keller R, Chang SA. Quantification of crustacean hyperglycemic hormone by ELISA in hemolymph of the lobster, Homarus americanus, following various stresses. Gen Comp Endocrinol. (1998) 111:359–66. doi: 10.1006/gcen.1998.7120
18. Lorenzon S, Edomi P, Giulianini PG, Mettulio R, Ferrero EA. Variation of crustacean hyperglycemic hormone (cHH) level in the eyestalk and haemolymph of the shrimp Palaemon elegans following stress. J Exp Biol. (2004) 207:4205–13. doi: 10.1242/jeb.01264
19. Monreal-Pawlowsky T, Vaicekauskaite R, Membrive GP, Delfour F, Manteca X. Goal-oriented behavioural and environmental enrichment in aquarium species. J Zoo Aquar Res. (2021) 9:273–80. doi: 10.19227/jzar.v9i4.588
20. Newberry RC. Environmental enrichment: Increasing the biological relevance of captive environments. Appl Anim Behav Sci. (1995) 44:229–43. doi: 10.1016/0168-1591(95)00616-Z
21. Kuba MJ, Gutnick T, Burghardt GM. Learning from Play in Octopus. In:Darmaillacq AS, Dickel L, Mather J, , editors. Cephalopod Cognition. Cambridge: Cambridge University Press (2014). p. 57–71.
22. Fernandez EJ. Training as enrichment: a critical review. Anim Welf. (2022) 31:1–12. doi: 10.7120/09627286.31.1.001
23. Directive 2010/63/EU of the European Parliament of the Council. (2010). Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed April 3, 2022).
24. Panel A. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to aspects of the biology and welfare of animals used for experimental and other scientific purposes. EFSA J. (2004) 2:1–46. doi: 10.2903/j.efsa.2004.44
25. Swiss Animal Protection Ordinance. (2008). Available online at: https://www.blv.admin.ch/blv/en/home/tiere/tierschutz.html (accessed April 3, 2022).
26. Norwegian Animal Welfare Act. (2009). Available online at: https://www.regjeringen.no/en/dokumenter/animal-welfare-act/id571188/.
27. New Zealand Animal Welfare Act. (1999). Available online at: http://www.legislation.govt.nz/act/public/1999/0142/latest/DLM49664.html?search=ts_act%40bill%40regulation%40deemedreg_animal+welfare_resel_25_a&p=1.
28. United Kingdom Animals (Scientific Procedures) Act (1986). Available online at: https://www.legislation.gov.uk/ukpga/1986/14/contents.
29. Birch J, Burn, C, Schnell, A, Browning, H, Crump, A,. Review of the Evidence of Sentience in Cephalopod Molluscs Decapod Crustaceans. LSE Consulting. LSE Enterprise Ltd. The London School of Economics Political Science. (2021). Available online at: https://www.lse.ac.uk/news/news-assets/pdfs/2021/sentience-in-cephalopod-molluscs-and-decapod-crustaceans-final-report-november-2021.pdf
30. Canadian Council on Animal Care. (1991). Available online at: https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf (accessed April 3, 2022).
31. United States Animal Welfare Act. (1970). Available online at: https://www.govinfo.gov/content/pkg/USCODE-2013-title7/pdf/USCODE-2013-title7-chap54.pdf.
32. Lewbart GA. Chapter 1 - invertebrates. In:Carpenter JW, Marion CJ, , editors. Exotic Animal Formulary. Philadelphia: Saunders (2018). p. 1–15.
33. Archibald A, Kate E, Gregory N, Kate M, Craig A. 2-Phenoxyethanol (2-PE) and tricaine methanesulfonate (MS-222) immersion anesthesia of American horseshoe crabs (Limulus polyphemus). J Zoo Wildl Med. (2019) 50:96. doi: 10.1638/2018-0085
34. Gorges MA, Martinez KM, Labriola NF, Phillips BE, Christian LS, Chen EP, et al. Effects of tricaine methanesulfonate in a managed collection of moon jellyfish (Aurelia aurita). J Zoo Wildl Med. (2022) 53:100–7. doi: 10.1638/2021-0028
35. Robertson JD, Delorme NJ, Hickey A, Jeffs AG. Assessment of the potential of the anesthetic AQUI-S for live transportation of the southern rock lobster, Jasus edwardsii. Bull Mar Sci. (2018) 94:1137–51. doi: 10.5343/bms.2017.1111
36. Abbo LA, Himebaugh NE, DeMelo LM, Hanlon RT, Crook RJ. Anesthetic efficacy of magnesium chloride and ethyl alcohol in temperate octopus and cuttlefish species. J Am Assoc Lab Anim Sci. (2021) 60:15–7. doi: 10.30802/AALAS-JAALAS-20-000076
37. Brown PB, White MR, Chaille J, Russell M, Oseto C. Evaluation of three anesthetic agents for crayfish (Orconectes virilis). J Shellfish Res. (1996) 15:433–5.
38. O'Neill PL. The effect of anaesthesia on spontaneous contraction of the body wall musculature in the asteroid Coscinasterias calamaria. Mar Behav Physiol. (1994) 24:137–50. doi: 10.1080/10236249409378887
39. Silva JJ, Scott JG. Conservation of the voltage-sensitive sodium channel protein within the Insecta. Insect Mol Biol. (2020) 29:9–18. doi: 10.1111/imb.12605
40. Stanley CE, Adams R, Nadolski J, Amrit E, Barrett M, Bohnett C, et al. The effects of tricaine mesylate on arthropods: crayfish, crab and Drosophila. Invertebr Neurosci. (2020) 20:1–16. doi: 10.1007/s10158-020-00243-5
41. Barr S, Laming PR, Dick JTA, Elwood RW. Nociception or pain in a decapod crustacean? Anim Behav. (2008) 75:745–51. doi: 10.1016/j.anbehav.2007.07.004
42. Barr S, Elwood RW. No evidence of morphine analgesia to noxious shock in the shore crab, 2Carcinus maenas. Behav Processes. (2011) 86:340–4. doi: 10.1016/j.beproc.2011.02.002
43. Leary S, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre C, et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Schaumburg, IL: AVMA (2020). p. 1–121.
44. Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, et al. Recommendations for euthanasia of experimental animals: Part 1. Lab Anim. (1997) 31:293–316. doi: 10.1258/002367796780739871
45. Quesada RJ, Smith CD, Heard DJ. Evaluation of parenteral drugs for anesthesia in the blue crab (Callinectes sapidus). J Zoo Wildl Med. (2011) 42:295–9. doi: 10.1638/2009-0071.1
46. Battison A, MacMillan R, MacKenzie A, Rose P, Cawthorn R, Horney B. Use of injectable potassium chloride for euthanasia of American lobsters (Homarus americanus). Comp Med. (2000) 50:545–50.
47. International Union for Conservation of Nature Natural Resources. (2022). Available online at: https://www.iucn.org/ (accessed April 6, 2022).
48. Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London: Methuen & CO LTD (1959). p. 197.
49. National Centre for the Replacement Refinemement and Reduction of Animals in Research. The 3Rs. Available online at: https://nc3rs.org.uk/ (accessed April 6, 2022).
50. Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. (2010) 1:87–93. doi: 10.4103/0976-500X.72350
51. Skillings DJ, Toonen RJ. It's just a flesh wound: non-lethal sampling for conservation genetics studies. Proc 29th Am Acad Underw Sci Symp. (2010) 9:1–9.
52. Saliu F, Montano S, Hoeksema BW, Lasagni M, Galli P, A. non-lethal SPME-LC/MS method for the analysis of plastic-associated contaminants in coral reef invertebrates. Anal Methods. (2020) 12:1935–42. doi: 10.1039/C9AY02621E
53. Monroe EM, Lynch C, Soluk DA, Britten HB. Nonlethal tissue sampling techniques and microsatellite markers used for first report of genetic diversity in two populations of the endangered Somatochlora hineana (Odonata: Corduliidae). Ann Entomol Soc Am. (2010) 103:1012–7. doi: 10.1603/AN10088
54. McLachlan RH, Dobson KL, Schmeltzer ER, Thurber RV, Grottoli AG, A. review of coral bleaching specimen collection, preservation, and laboratory processing methods. PeerJ. (2021) 9:1–21. doi: 10.7717/peerj.11763
55. Wahltinez SJ, Kroll KJ, Nunamaker EA, Denslow ND, Stacy NI. Practical euthanasia method for common sea stars (Asterias rubens) that allows for high-quality RNA sampling. Animals. (2021) 11:1–12. doi: 10.3390/ani11071847
56. Doerr AM, Stoskopf MK. Evaluation of euthanasia of moon jellyfish (Aurelia aurita) using simple salt solutions. J Zoo Wildl Med. (2019) 50:123. doi: 10.1638/2018-01510
57. FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome: FAO (2020). p. 244.
58. Conte F, Voslarova E, Vecerek V, Elwood RW, Coluccio P, Pugliese M, et al. Humane slaughter of edible decapod crustaceans. Animals. (2021) 11:1–13. doi: 10.3390/ani11041089
59. Kugino M, Kugino K, Ogawa T. Changes in microstructure and rheological properties of squid mantle during storage. Food Sci Technol Int Tokyo. (1997) 3:157–62. doi: 10.3136/fsti9596t9798.3.157
60. Baker JR. Experiments on the humane killing of crabs. J Mar Biol Assoc United Kingdom. (1955) 34:15–24. doi: 10.1017/S0025315400008572
61. Gunter G. Painless killing of crabs and other large crustaceans. Science. (1961) 133:327. doi: 10.1126/science.133.3449.327.a
62. Adams R, Stanley CE, Piana E, Cooper RL. Physiological and behavioral indicators to measure crustacean welfare. Animals. (2019) 9:914. doi: 10.3390/ani9110914
63. Fregin T, Bickmeyer U. Electrophysiological investigation of different methods of anesthesia in lobster and crayfish. PLoS ONE. (2016) 11:1–19. doi: 10.1371/journal.pone.0162894
64. New Zealand Animal Welfare (Care Procedures) Regulations. (2018). Available online at: https://www.legislation.govt.nz/regulation/public/2018/0050/23.0/LMS22832.html (accessed April 3, 2022).
65. Swiss Animal Protection Ordinance (2008). Available online at: https://www.blv.admin.ch/dam/blv/en/dokumente/tiere/rechts-und-vollzugsgrundlagen/tschv-en.pdf.download.pdf/AnimalProtectionOrdinance455.1.pdf.
66. Liuzzo G, Rossi R, Giacometti F, Mescolini G, Piva S, Serraino A. Analysis of provincial and municipal regulations governing crustacean welfare in Italy. Ital J Food Saf. (2017) 6:54–6. doi: 10.4081/ijfs.2017.6228
67. Weineck K, Ray AJ, Fleckenstein LJ, Medley M, Dzubuk N, Piana E, et al. Physiological changes as a measure of crustacean welfare under different standardized stunning techniques: Cooling and electroshock. Animals. (2018) 8:158. doi: 10.3390/ani8090158
68. Roth B, Øines S. Stunning and killing of edible crabs (Cancer pagurus). Anim Welf. (2010) 19:287–94.
69. Neil D. The effect of the Crustastun on nerve activity in crabs and lobsters. Proj Report Univ Glas Glas UK. (2010) 19.
70. Ridgway ID, Taylor AC, Atkinson RJA, Chang ES, Neil DM. Impact of capture method and trawl duration on the health status of the Norway lobster, Nephrops norvegicus. J Exp Mar Bio Ecol. (2006) 339:135–47. doi: 10.1016/j.jembe.2006.07.008
71. Paterson BD, Spanoghe PT. Stress indicators in marine decapod crustaceans, with particular reference to the grading of western rock lobsters (Panulirus cygnus) during commercial handling. Mar Freshw Res. (1997) 48:829–34. doi: 10.1071/MF97137
72. Pozhoth J, Jeffs A. Effectiveness of the Food-Safe Anaesthetic Isobutanol in the Live Transport of Tropical Spiny Lobster Species. Fishes. (2022) 7:1–9. doi: 10.3390/fishes7010040
73. Patterson L, Dick JTA, Elwood RW. Physiological stress responses in the edible crab, Cancer pagurus, to the fishery practice of de-clawing. Mar Biol. (2007) 152:265–72. doi: 10.1007/s00227-007-0681-5
74. Duermit E, Kingsley-Smith PR, Wilber DH. The consequences of claw removal on stone crabs Menippe spp. and the ecological and fishery implications. North Am J Fish Manag. (2015) 35:895–905. doi: 10.1080/02755947.2015.1064836
75. Oliveira RF, Machado JL, Jordão JM, Burford FL, Latruffe C, McGregor PK. Human exploitation of male fiddler crab claws: Behavioural consequences and implications for conservation. Anim Conserv. (2000) 3:1–5. doi: 10.1111/j.1469-1795.2000.tb00081.x
76. Gandy R, Crowley C, Chagaris D, Crawford C. The effect of temperature on release mortality of declawed Menippe mercenaria in the Florida stone crab fishery. Bull Mar Sci. (2016) 92:1–15. doi: 10.5343/bms.2015.1036
77. Duermit E, Shervette V, Whitaker JD, Kingsley-Smith PR, Wilber D, A. field assessment of claw removal impacts on the movement and survival of stone crabs Menippe spp. Fish Res. (2017) 193:43–50. doi: 10.1016/j.fishres.2017.03.019
78. Patterson L, Dick JTA, Elwood RW. Claw removal and feeding ability in the edible crab, Cancer pagurus: implications for fishery practice. Appl Anim Behav Sci. (2009) 116:302–5. doi: 10.1016/j.applanim.2008.08.007
79. McCambridge C, Dick JTA, Elwood RW. Effects of autotomy compared to manual declawing on contests between males for females in the edible crab Cancer pagurus: implications for fishery practice and animal welfare. J Shellfish Res. (2016) 35:1037–44. doi: 10.2983/035.035.0426
80. Welsh JE, King PA, MacCarthy E. Pathological and physiological effects of nicking on brown crab (Cancer pagurus) in the Irish crustacean fishery. J Invertebr Pathol. (2013) 112:49–56. doi: 10.1016/j.jip.2012.08.006
81. Jacklin M, Combes J. The Good Practice Guide to Handling and Storing Live Crustacea. Edinburgh: Sea Fish Ind Auth Publ UK (2007). p. 151.
82. Beaven GF, Truitt R V. Crab mortality on chesapeake bay shedding floats. Chesap Biol Lab. (1939) 33:3–14.
83. Johnson L, Coates CJ, Albalat A, Todd K, Neil D. Temperature-dependent morbidity of “nicked” edible crab, Cancer pagurus. Fish Res. (2016) 175:127–31. doi: 10.1016/j.fishres.2015.11.024
84. Kari Woll A, Marit Berge G. Feeding and management practices affect quality improvement in wild-caught edible crab (Cancer pagurus). Aquaculture. (2007) 269:328–38. doi: 10.1016/j.aquaculture.2007.04.022
85. Woll AK, Larssen WE, Fossen I. Physiological responses of brown crab (Cancer pagurus Linnaeus 1758) to dry storage under conditions simulating vitality stressors. J Shellfish Res. (2010) 29:479–87. doi: 10.2983/035.029.0226
86. Haefner PA. The use of elastrator rings for binding crab claws. Chesap Sci. (1971) 12:183–4. doi: 10.2307/1350780
87. Chen T, Zhang LP, Wong NK, Zhong M, Ren CH, Hu CQ. Pacific white shrimp (Litopenaeus vannamei) vitellogenesis-inhibiting hormone (VIH) is predominantly expressed in the brain and negatively regulates hepatopancreatic vitellogenin (VTG) gene expression. Biol Reprod. (2014) 90:1–10. doi: 10.1095/biolreprod.113.115030
88. Taylor J, Vinatea L, Ozorio R, Schuweitzer R, Andreatta ER. Minimizing the effects of stress during eyestalk ablation of Litopenaeus vannamei females with topical anesthetic and a coagulating agent. Aquaculture. (2004) 233:173–9. doi: 10.1016/j.aquaculture.2003.09.034
89. Diarte-Plata G, Sainz-Hernández JC, Aguiñaga-Cruz JA, Fierro-Coronado JA, Polanco-Torres A, Puente-Palazuelos C. Eyestalk ablation procedures to minimize pain in the freshwater prawn Macrobrachium americanum. Appl Anim Behav Sci. (2012) 140:172–8. doi: 10.1016/j.applanim.2012.06.002
90. Bae SH, Okutsu T, Kang BJ, Wilder MN. Alterations of pattern in immune response and vitellogenesis during induced ovarian development by unilateral and bilateral ablation in Litopenaeus vannamei. Fish Sci. (2013) 79:895–903. doi: 10.1007/s12562-013-0652-3
91. Zacarias S, Carboni S, Davie A, Little DC. Reproductive performance and offspring quality of non-ablated Pacific white shrimp (Litopenaeus vannamei) under intensive commercial scale conditions. Aquaculture. (2019) 503:460–6. doi: 10.1016/j.aquaculture.2019.01.018
92. Zacarias S, Fegan D, Wangsoontorn S, Yamuen N, Limakom T, Carboni S, et al. Increased robustness of postlarvae and juveniles from non-ablated Pacific whiteleg shrimp, Penaeus vannamei, broodstock post-challenged with pathogenic isolates of Vibrio parahaemolyticus (VpAHPND) and white spot disease (WSD). Aquaculture. (2021) 532:736033. doi: 10.1016/j.aquaculture.2020.736033
93. Treerattrakool S, Boonchoy C, Urtgam S, Panyim S, Udomkit A. Functional characterization of recombinant gonad-inhibiting hormone (GIH) and implication of antibody neutralization on induction of ovarian maturation in marine shrimp. Aquaculture. (2014) 428–9:166–73. doi: 10.1016/j.aquaculture.2014.03.009
Keywords: anesthesia, animal welfare, euthanasia, humane slaughter, refinement
Citation: Wahltinez SJ, Stacy NI, Hadfield CA, Harms CA, Lewbart GA, Newton AL and Nunamaker EA (2022) Perspective: Opportunities for advancing aquatic invertebrate welfare. Front. Vet. Sci. 9:973376. doi: 10.3389/fvets.2022.973376
Received: 20 June 2022; Accepted: 17 October 2022;
Published: 15 November 2022.
Edited by:
Nicole Kemper, University of Veterinary Medicine Hannover, GermanyReviewed by:
Eduardo J. Fernandez, University of Adelaide, AustraliaBerta Maria Heinzmann, Federal University of Santa Maria, Brazil
Copyright © 2022 Wahltinez, Stacy, Hadfield, Harms, Lewbart, Newton and Nunamaker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah J. Wahltinez, c2p3YWhsdGluZXpAZ21haWwuY29t
 Sarah J. Wahltinez
Sarah J. Wahltinez Nicole I. Stacy
Nicole I. Stacy Catherine A. Hadfield2
Catherine A. Hadfield2 Craig A. Harms
Craig A. Harms