
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 18 January 2023
Sec. Veterinary clinical, anatomical, and comparative pathology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.971813
This article is part of the Research Topic Reviews in Pathology of Infectious Diseases View all 10 articles
 Muhammad Noman Naseem1
Muhammad Noman Naseem1 Rachel Allavena2
Rachel Allavena2 Ali Raza1
Ali Raza1 Constantin Constantinoiu3
Constantin Constantinoiu3 Michael McGowan2
Michael McGowan2 Conny Turni1
Conny Turni1 Muhammad Kamran1
Muhammad Kamran1 Ala E. Tabor1,4
Ala E. Tabor1,4 Peter James1*
Peter James1*Haematobia irritans exigua, commonly known as buffalo fly, is the major hematophagous ectoparasite of north Australian cattle herds. Lesions associated with buffalo fly infestation are generally alopecic, hyperkeratotic, or scab encrusted wounds with variable hemorrhagic ulceration. Buffalo flies can transmit a filarial nematode, Stephanofilaria sp., which has been implicated in the pathogenesis of buffalo fly lesions, but Stephanofilaria infection has not been detected in all lesions suggesting that other causal factors may be involved. This study characterized the pathology of buffalo fly lesions to identify the role of Stephanofilaria in lesion development, as well as to identify other potential agents. Lesion biopsies were collected from north and south Queensland and tested for the presence of Stephanofilaria by qPCR. Each lesion was scored grossly (0–4) for hemorrhage, ulceration, exudation, and alopecia. Lesions were also scored microscopically (0–4) for epidermal and dermal damage and inflammatory characters. Stephanofilaria infection was detected in 31% of lesion biopsies. Grossly, Stephanofilaria-infected lesions had significantly larger lesion area and higher scores for alopecia and hyperkeratosis than lesions where no nematodes were found (P < 0.05). Histologically, epidermal, dermal, and adnexal damage was significantly higher in Stephanofilaria infected lesions than lesions without nematodes. Eosinophils, macrophages, and lymphocytes were significantly more abundant in Stephanofilaria positive lesions as compared to negative lesions. This study also noted bacterial infection with colonies of coccoid bacteria, observed in skin sections from 19 lesions. Grossly, lesions with bacterial infection had significantly higher ulceration scores compared to Stephanofilaria positive lesions, and histologically epidermal disruption was significantly greater in bacteria-infected lesions. We found no evidence of bacteria or Stephanofilaria infection in 49% of the lesions assessed and tissue damage patterns and eosinophilic inflammation suggested hypersensitivity to buffalo fly feeding as a possible cause of these lesions. These findings suggest that although the presence of Stephanofilaria infection may increase the severity of lesion pathology, it is not essential for lesion development. These outcomes also suggest a potential role of bacteria and hypersensitivity in pathogenesis of some lesion. A better understanding of buffalo fly lesion etiology will contribute to the optimal treatment and control programmes.
Flies from the genus Haematobia are obligate hematophagous ectoparasites that feed mainly on cattle and buffalo (1). Two major and closely related species of this genus are the buffalo fly (Hematobia irritans exigua) (BF) prevalent in the tropical and subtropical parts of Asia, Australia and other parts of Oceania, and the horn fly (Haematobia irritans irritans) (HF) widespread throughout Europe, Africa and the Americas (1). Buffalo flies (BFs) are considered a major pest affecting animal production and welfare, particularly in northern Australian herds (2). Infestation with BFs is frequently accompanied by the development of skin lesions associated with BF feeding that occur mainly near the medial canthus of the eye, along the lateral and ventral neck and on the abdomen. These lesions can range from raised dry, alopecic, hyperkeratotic or scab encrusted to severe haemorrhagic ulcerated areas (3, 4) and occur in up to 95% of northern Australian herds (5). When surveyed, northern dairy farmers noted BF as a problem on 91% of farms and animal welfare aspects due to BF infestation were noted as the most serious BF-related issue (6). Often the key concern of cattle producers are the lesions associated with BF feeding because of their visual appearance (most particularly the open and suppurating wounds) and the associated irritation to the animal (3). Buffalo fly lesions can also penetrate the dermis, affecting hide quality, cattle saleability, and can increase animal susceptibility to secondary infections (7).
In Australia, BFs transmit an unnamed species of filarial nematode, Stephanofilaria sp., which has been associated with the pathogenesis of BF lesions (5, 7, 8). This nematode is closely related to, or potentially the same species as Stephanofilaria stilesi vectored by horn flies (HFs)in the northern hemisphere and South America (3, 9). However, Stephanofilaria nematodes and their microfilariae were detected in only 40% of the lesions examined histologically in a study by Johnson et al. (5) and Naseem et al. (10) found that only 11% of 120 BF lesions assessed were positive for Stephanofilaria infection by qPCR. In addition, nematode distribution was limited to northern and central Queensland, and qPCR testing found no Stephanofilaria in either lesions or BF from southern Queensland although BF-associated lesions are prevalent in these areas (10).
Although lesion development is associated with BF feeding, there is a poor correlation between BF numbers and lesion development (7, 11) suggesting that there are additional contributing factors. In addition, although HFs have been reported to vector Stephanofilaria stilesi causing granular abdominal dermatitis, udder and teat lesions in cattle in North and South America (9, 12–14), the nematode was not found in all cases (14, 15). Thus these lesions were suggested to be potentially due to hypersensitivity induced by HF feeding (16) and infection with Staphylococcus spp. bacteria vectored by HFs (17). These observations taken together suggest that Stephanofilaria infection may not be essential for the pathogenesis of BF lesions, and that other causal factors may be involved.
In this study, we utilized a recently developed Stephanofilaria specific qPCR (18) to identify the presence of nematodes in lesions, and characterized and compared the gross and microscopic pathology of BF-associated lesions with various pathogens present to clarify the key factors involved in the pathogenesis of these lesions.
Lesion samples (n = 86) were collected from skins of recently slaughtered cattle at a commercial abattoir in north Queensland (n = 62) as well as from biopsies from live cattle (n = 24). All abattoir samples were from lesions near the medial canthus of the eyes whereas biopsies from live cattle were from two herds kept in southern Queensland (Pinjarra Hills −27.50OS, 152.91OE and Forest Hill −27.60OS, 152.39 OE) and were collected from the neck (n = 17), shoulder (n = 2), belly (n = 3) and from near the eye (n = 2). All biopsies were taken from the center of the lesion using 8 mm sterile skin punches (Paramount Surgimed Ltd., New Delhi, India) together with a control skin biopsy from unaffected skin 3–4 cm away from each lesion. A sub sample was taken from each of the 86 samples collected and confirmed as positive or negative for Stephanofilaria by TaqManTM qPCR assay (18). All samples were preserved in 10% neutral buffered formalin until histological processing. These studies were conducted under The University of Queensland Animal Ethics approval no. 2021/AE000054.
The initial gross appearance of each lesion was scored at the time of sample collection according to a de novo scoring scheme shown in Table 1. Each lesion was scored (0–4) grossly for the presence of ulceration, exudation, hemorrhage, alopecia, scab formation and hyperkeratosis. All lesions were also photographed alongside a measurement scale before collection of the biopsies and the area of each sampled lesion was measured from photographs using an online tool SketchAndCalc (https://www.sketchandcalc.com/).
Skin biopsies were dehydrated and cleared using an automated Tissue Processor (Shandon Excelsior ES, Thermofisher Scientific, Waltham, MA, USA). The biopsies were then embedded in paraffin using a tissue embedding machine (Leica EG1160, Leica Biosystem, Wetzlar, Germany). A 4 μm thick section was taken from each biopsy using a manual microtome (Leica RM2235, Leica Biosystem, Wetzlar, Germany) and stained with hematoxylin and eosin. Staining was performed on an auto-stainer (Leica ST5020, Leica Biosystems, Wetzlar, Germany) according to the manufacturer's instructions. Each section was scanned (Leica Aperio CS2, Leica Biosystems, Wetzlar, Germany) and examined on a computer screen.
All 86 samples were reviewed for the presence of Stephanofilaria adult nematodes or microfilariae, and bacteria. For quantitation of the tissue damage, all epidermal and dermal changes were scored by the scoring systems given in Table 2. Epidermal changes including epidermal disruption, crust over epidermis, hyperkeratosis, acanthosis and spongiosis were scored individually for each sample and a total epidermal damage score for each sample was calculated by summing the individual score of all parameters. Similarly dermal damage was scored for adnexal destruction, endothelial activity, vascular changes and collagenolysis, and the total dermal damage was determined for each section by summing the individual scores of all assessed dermal parameters. Differential inflammatory cell scores and total inflammatory response in each lesion were scored according to the scoring scheme shown in Table 3. The scoring schemes for histological changes in this study were created with reference to observations of the biopsies from unaffected skin areas processed in this study. All parameters for histological changes were scored in ten different fields selected randomly from three fields each from the left and right side, and four from the center of each biopsy. A final score was calculated as an average of the ten individual field scores.
All the gross and histological scores were compared between different groups using the Mann–Whitney U-test (19) and the lesion areas were compared by two tailed t-tests in GraphPad Prism version 9.1.0 (GraphPad Software, La Jolla, CA; by www.graphpad.com). The level of statistical significance was P < 0.05.
Of 62 lesion samples collected from north Queensland, 27 were positive for Stephanofilaria infection by TaqManTM qPCR while the adult nematodes or its microfilariae or both were detected in the histological sections of only 14 samples. Of these 14 histologically positive samples, adult nematodes and microfilariae were observed in seven and four respectively, while both were observed in three samples (Figure 1). The number of adult Stephanofilaria nematodes ranged from 1 to 11 in individual sections, and microfilariae ranged from 5 to 15. No false PCR positive samples were recorded and none of the 24 lesions tested from south Queensland was positive for Stephanofilaria infection by either qPCR or histological examination.
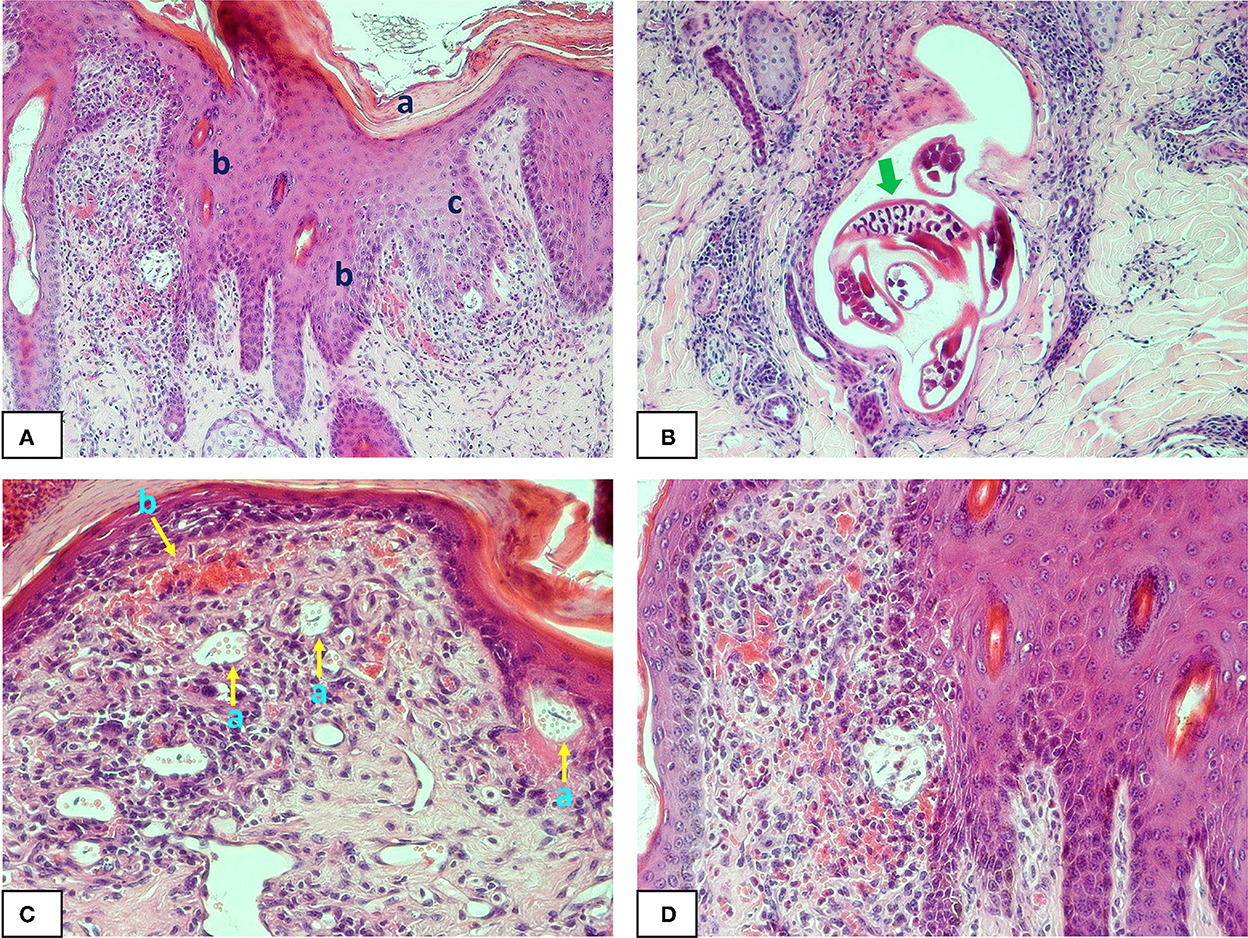
Figure 1. Photomicrographs of buffalo fly lesion histology: (A) shows hyperkeratosis (a) acanthosis (b) and spongiosis (c) in the epidermal layer of a Stephanofilaria infected lesion (100X); (B) shows adult Stephanofilaria in a cyst-like structure surrounded by inflammatory cells. The arrow shows the microfilariae within the gravid uterus of an adult female nematode (200X); (C) Shows microfilariae close to the epidermis and enclosed in a round to oval-shaped vitelline membrane that also contains numerous small spherical eosinophilic bodies (a) and hyperaemia (b) with extensive inflammation in the superficial dermis (200X); (D) shows acanthosis, severe hyperaemia and eosinophilic inflammation in Stephanofilaria-infected lesion (200X).
Multiple clusters of ~1.5 μm purple-stained cocci were observed within the superficial serocellular crust of 19 lesion samples (Figure 2). This included 11 samples from south Queensland and eight from north Queensland. Of these bacteria-positive samples, only two were also positive for Stephanofilaria by qPCR.

Figure 2. Photomicrograph (400X) of buffalo fly lesions shows multiple clusters of coccoid shaped bacteria (arrow) within the superficial crust.
The BF lesions examined ranged from dry, hyperkeratotic hairless areas to severe open suppurative wounds with hemorrhagic or scab encrusted surface (Figure 3). Body lesions including lesions sampled from the neck, dewlap and belly, had areas ranging from 5.14 to 40 cm2, while the lesions sampled near the eyes of cattle had lesion areas ranging from 3.04 to 54.44 cm2. There were no obvious differences in the gross pathology of lesions sampled from different anatomical locations.
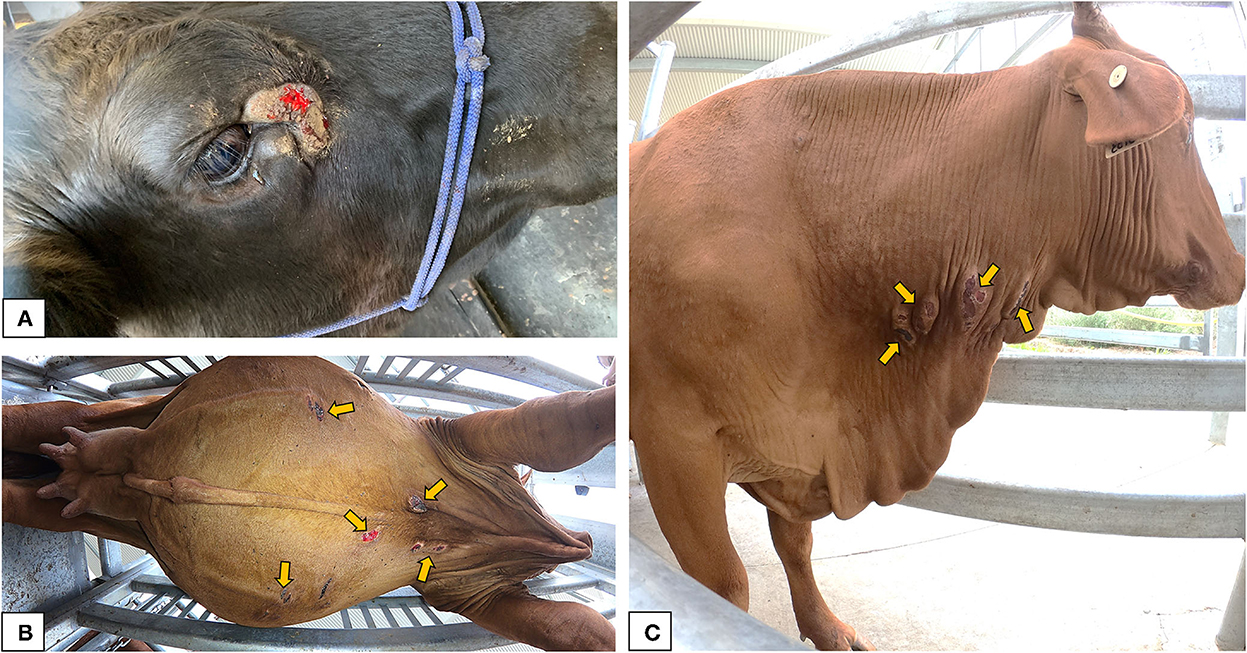
Figure 3. Gross appearance of the buffalo fly lesions: (A) lesion adjacent to the medial canthus of the eye in a Brangus steer indicating raised, circumscribed, hairless, ulcerative area partially covered with scab forming from the periphery; (B) multiple ulcerative to scab encrusted lesions near the ventral midline of a Droughtmaster cow; (C) multiple raised scabbed lesions on the neck of a Droughtmaster cow.
When the lesion areas and scores for total gross damage, alopecia, ulceration and hyperkeratosis from north Queensland were compared between BF lesions which tested positive (n = 25) and negative (n = 29) for Stephanofilaria infection with PCR, Stephanofilaria positive lesions had significantly larger lesion areas (ranging from 7.02 to 54.44 cm2) as compared to negative lesions (3.04 to 15.07 cm2) (Figure 4A). This comparison excluded two lesions that were positive for both Stephanofilaria and bacterial infection to avoid any confounding effects due to bacterial infection. Total gross damage, alopecia and hyperkeratosis were significantly higher in Stephanofilaria positive lesions (Figures 4B–D). Notably, all of the animals in the Stephanofilaria positive group had a score of 4 for alopecia (>80% of the lesion area affected), whereas the occurrence of alopecia was more variable (score 2–4) in the Stephanofilaria negative group. There was only one animal with ulceration score >0 in the Stephanofilaria positive group and this animal had severe (score 4) ulceration whereas in the Stephanofilaria negative group, more animals had a score of 1–3 for ulceration, but none had a score 4 (Figure 4E).
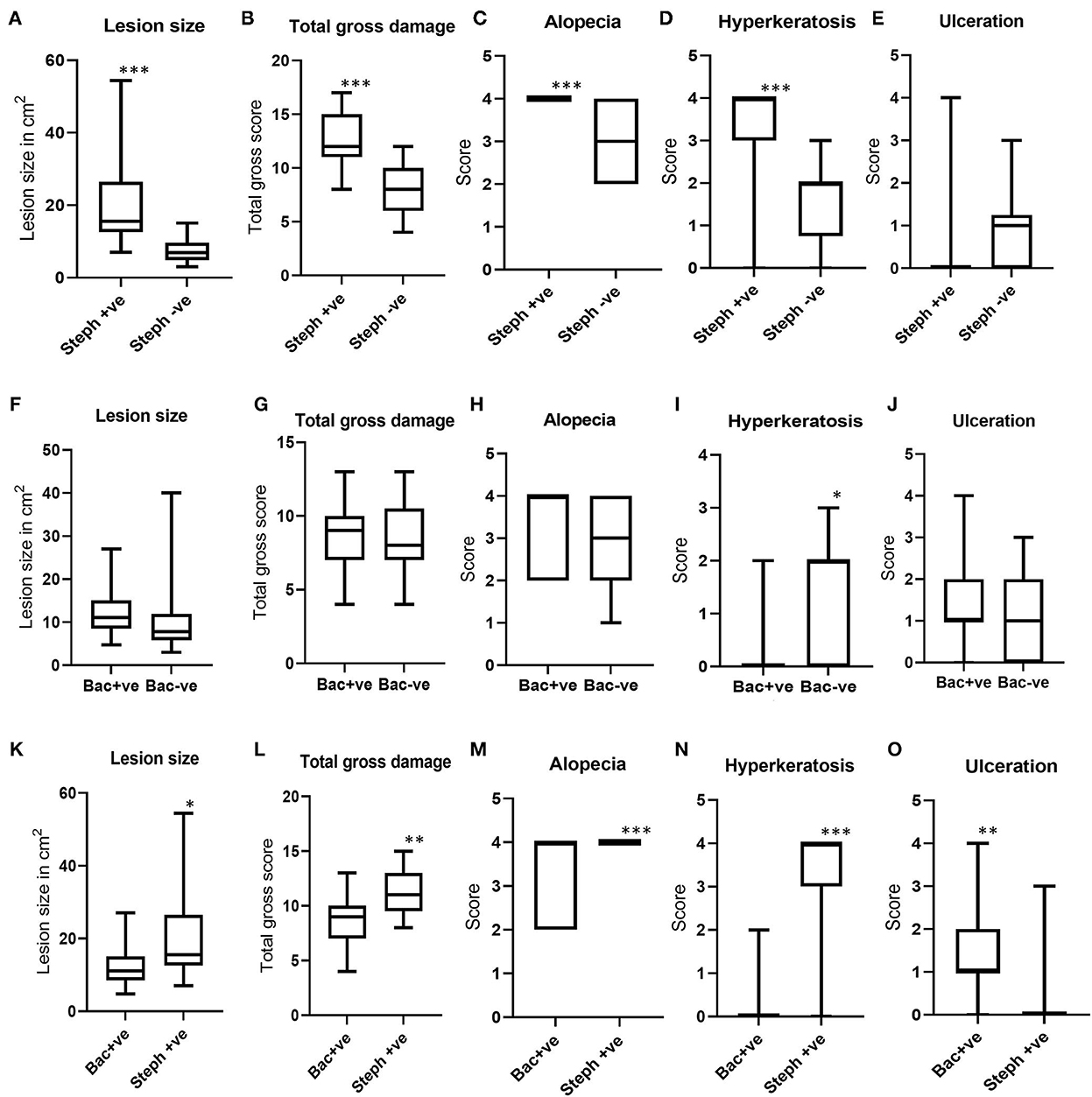
Figure 4. Boxplots showing the distribution of lesion areas and pathology scores for total gross damage, alopecia, ulceration, and hyperkeratosis. (A–E) Show gross pathology comparisons between Stephanofilaria positive (Steph +ve) and negative (Steph -ve) lesions; (F–J) Show gross pathology comparisons between bacteria positive (Bac +ve) and negative (Bac-ve) lesions; (K–O) Show gross pathology comparisons between Steph +ve and Bac +ve lesions (*P < 0.05, **P < 0.01, ***P < 0.001).
In the comparison between BF lesions with bacterial infection (n = 17) and without bacterial infection (n = 42), a non-significant difference was noted in lesion areas between lesions positive (ranged 4.77–27 cm2) and negative (3.04 to 40 cm2) for bacterial infection (Figure 4F). The scores for total gross damage, alopecia and ulceration were not significantly different between these two groups (Figures 4G, H, J). Lesions with no bacterial growth had significantly higher hyperkeratosis as compared to bacteria-infected lesions (Figure 4I).
When the gross pathology scores for BF lesions positive for Stephanofilaria but not bacteria (n = 25) were compared with lesions observed with bacterial infection but not Stephanofilaria (n = 17), Stephanofilaria-infected lesions had significantly larger lesion areas (7.02 to 54.44 cm2) as compared to bacterial infected lesions (4.77 to 27 cm2) (Figure 4K). The scores for total gross damage, alopecia and hyperkeratosis were also significantly higher in Stephanofilaria-infected lesions (Figures 4L–N), whereas bacteria-infected lesions had significantly higher ulceration scores (Figure 4O). For this comparison, there was only one animal in the Stephanofilaria group with ulceration (score 3) whereas in the bacteria affected lesions ulceration was more common with most animals affected (only one animal with score 0) and scores ranging from 0 to 4.
The most consistent differences in the epidermis between lesion-affected and unaffected skin included the degree of hyperkeratosis, acanthosis, spongiosis, epidermal disruption and formation of a serocellular crust of varying thickness. There were also varying degrees of cellular infiltration in lesion-affected areas with cell composition including necrotic epidermal cells, neutrophils, and eosinophils (Figure 1A). Epidermal disruption was most commonly observed in sections biopsied from body lesions from south Queensland. Changes observed in the dermis included varying degrees of adnexal destruction, endothelial reactivity, vascular changes and infiltration of inflammatory cells. Acute lesions had moderate to severe superficial dermal collagenolysis whereas varying degrees of fibrosis were observed in chronic wounds indicating the commencement of scarring. Cellular infiltrate was predominately eosinophils along with macrophages, neutrophils and lymphocytes.
Adult Stephanofilaria nematodes were observed up to 0.5–2.5 mm deep in the dermis, mostly within cysts formed at the base of the damaged hair follicles (Figure 1B). In two lesion sections, adult nematodes were observed close to the epidermis with necrotic tracts in the dermal layer suggesting nematode migration through the dermis. Microfilariae were mostly observed close to the epidermis in the dermal papillae and rete pegs and enclosed in a round to oval-shaped vitelline membrane that also contained numerous small spherical eosinophilic bodies (Figures 1C, D). Similar membrane-enclosed microfilariae were also observed in the uteri of gravid female nematodes. Eosinophils were dispersed throughout the superficial dermal layer with markedly abundant numbers near Stephanofilaria adults and microfilariae.
To characterize the microscopic pathology of BF lesions with different causal factors, histopathological scores were compared among lesions grouped according to potential causal factors.When histopathological scores for north Queensland lesions qPCR positive for Stephanofilaria were compared with lesions without Stephanofilaria infection, total epidermal damage in Stephanofilaria positive lesions was significantly higher than in the negative lesions (Figure 5A). Epidermal disruption was not significantly different between these two groups (Figure 5B). Total dermal damage and particularly adnexal damage was significantly higher in Stephanofilaria positive lesions (Figures 5C, D). Similarly, Stephanofilaria-infected lesions had significantly higher inflammation compared to non-infected lesions (Figure 5E). Although infiltration of eosinophils, neutrophils, macrophages, lymphocytes and plasma cells was evident in both lesion groups, the scores for eosinophils, macrophages and lymphocytes were significantly higher in lesions with Stephanofilaria (Figures 5F–J).

Figure 5. Boxplots (A–D) show the distribution of scores for total epidermal damage, epidermal disruption, total dermal damage and adnexal destruction scores for Stephanofilaria positive (Steph +ve) and negative (Steph -ve) lesions. The boxplots (E–J) show the total inflammation and individual inflammatory cell scores of these two groups (**P < 0.01, ***P < 0.001).
When the histological scores for BF lesions with and without bacterial infection were compared, total epidermal damage, epidermal disruption, total dermal damage, and adnexal destruction in BF lesions with bacteria were significantly higher compared to non-infected lesions (Figures 6A–D). However, total inflammation scores and the differential leukocytic counts were not significantly different, except for macrophages, which were significantly higher in lesions where bacteria were present (Figures 6E–J). Notably, there was no difference in eosinophil count between lesions with and without bacteria, in contrast to the difference observed between Stephanofilaria positive and negative lesions.
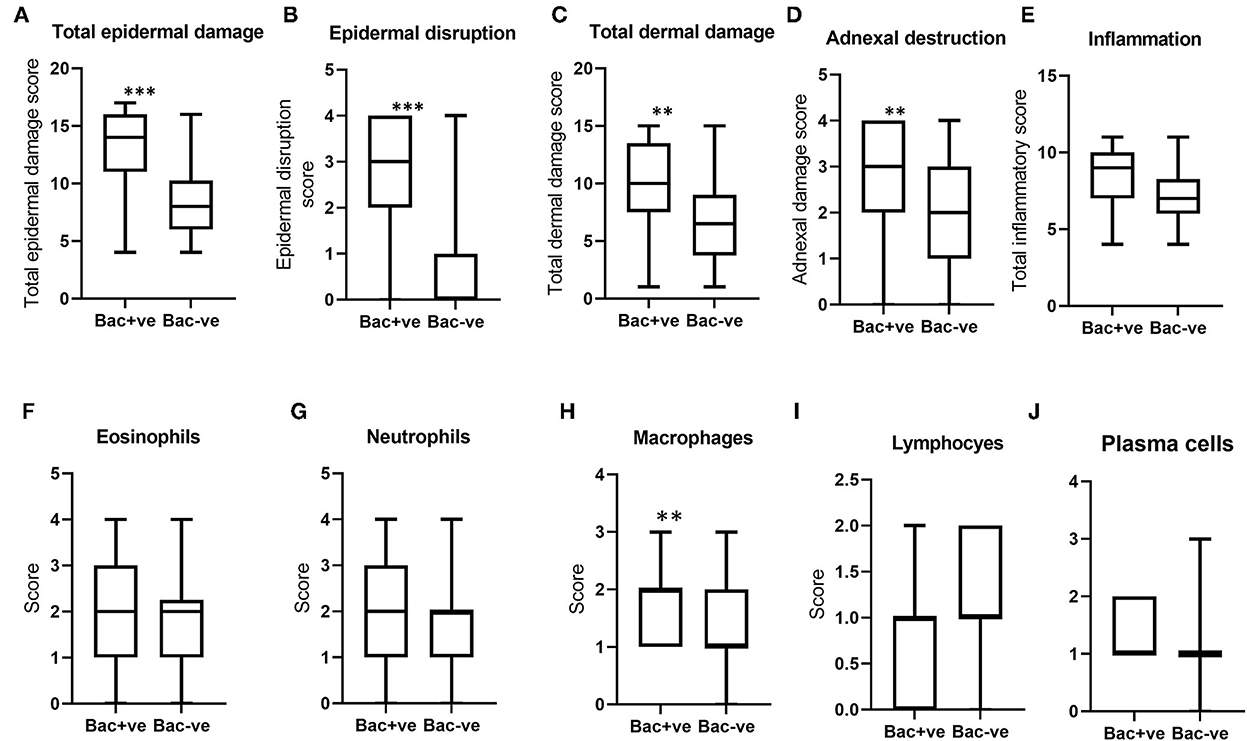
Figure 6. Boxplots (A–D) indicate the distribution of scores for total epidermal damage, epidermal disruption, total dermal damage and adnexal destruction for bacterial positive (Bac+ve) and negative (Bac-ve) lesions. Boxplots (E–J) show total inflammation and individual inflammatory cell scores of these two groups (**P < 0.01, ***P < 0.001).
When the histopathological scores of Stephanofilaria positive BF lesions were compared with bacteria-infected lesions, although epidermal disruption was significantly higher in lesions with bacteria (Figure 7B), total epidermal damage was not significantly different (Figure 7A). Out of 17 bacteria-positive lesions, only two had zero scores for epidermal disruption. The score for total dermal damage was not significantly different between bacteria and Stephanofilaria positive lesions (Figure 7C). Adnexal damage was significantly higher in Stephanofilaria-infected lesions than in bacteria-infected lesions (Figure 7D) and Stephanofilaria-infected lesions had a significantly higher inflammation score (Figure 7E). Infiltration of eosinophils, neutrophils, macrophages, lymphocytes, and plasma cells was evident in both lesion groups but the scores for eosinophils, lymphocytes and plasma cells were significantly higher in Stephanofilaria-infected lesions (Figures 7F–J).
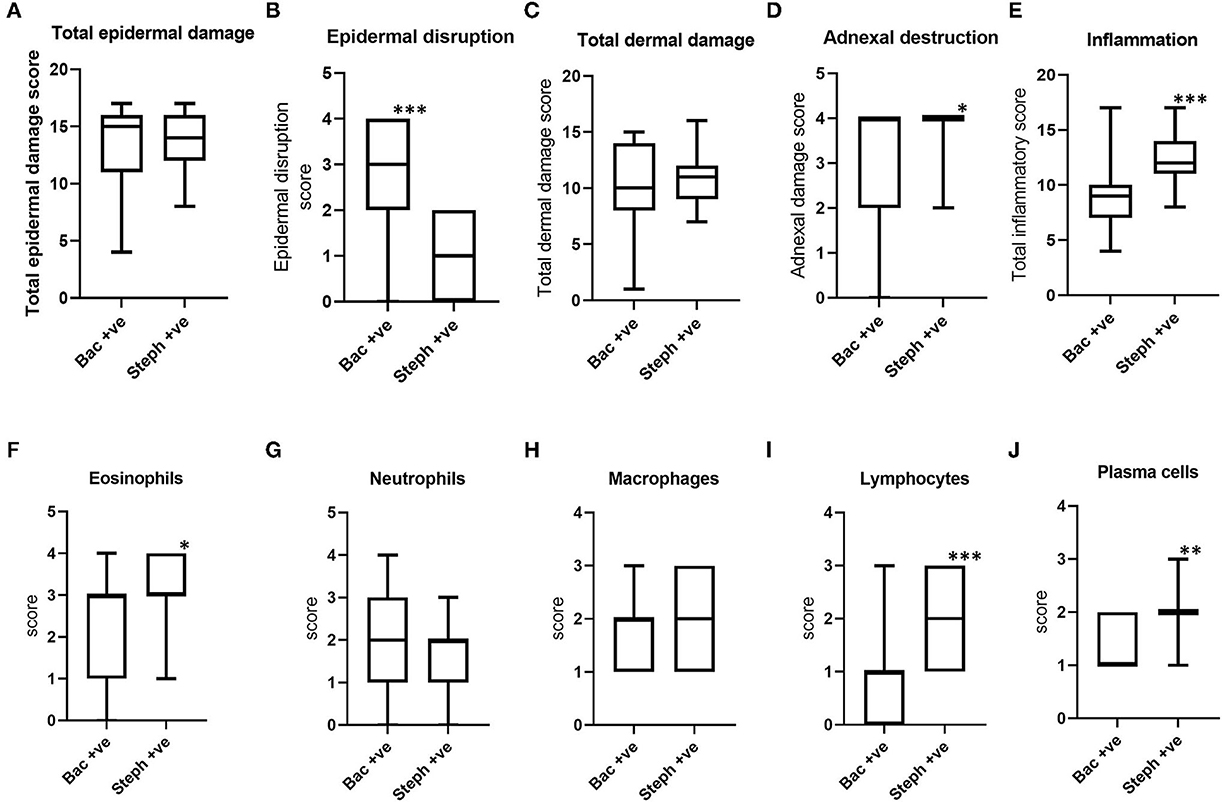
Figure 7. Boxplots (A–D) indicating the distribution of scores for total epidermal damage, epidermal disruption, total dermal damage and adnexal destruction scores for bacterial positive lesions (Bac+ve) and Stephanofilaria positive lesions (Steph +ve). Boxplots (E–J) show total inflammation and individual inflammatory cell scores of these two groups (*P < 0.05, **P < 0.01, ***P < 0.001).
Our study has highlighted the complex interplay amongst BFs, Stephanofiliaria and bacteria in BF lesion development. Johnson et al. (4) were the first to report the association between BF lesions and Stephanofilaria infection, which at that time was considered to be the main etiological agent of these lesions. However, Johnson et al. (5) only detected Stephanofilaria in 40% of the lesions they examined, an observation which the authors (5) attributed to the low sensitivity of histology and saline extraction techniques they used, the only detection methods available at that time. It is notable however that in controlled studies Johnson (3) was able to induce lesions similar in appearance to field BF lesions by exposing cattle held in fly proof cages to high numbers of BF not known to be infected with Stephanofilaria sp.
The recent development of a more sensitive qPCR for detecting the presence of Stephanofilaria in lesions and BFs (18) provided further evidence that Stephanofilaria is not present in all BF lesions and indeed appears to be completely absent from BF populations in some regions where BF lesions are prevalent (10). The availability of this test provided the opportunity to further examine the importance of potential causal factors in the development of BF lesions. Our study extended on previous work, comprehensively describing and differentiating the gross and microscopic pathological features of BF lesions involving potential comorbid etiological factors including Stephanofilaria sp., bacteria, and host immune response.
When the size of the BF lesions which had Stephanofilaria was compared to bacterial infected lesions or lesions negative for both Stephanofilaria and bacteria, the area of the lesions with Stephanofilaria was significantly greater than for either of the other two groups, extending up to 54 cm2 area in some instances. However, even in the absence of both Stephanofilaria and bacteria, BF lesions could attain a lesion size up to 40 cm2 and it is hypothesized that this could be a function of individual animal hypersensitivity to BF antigens as has been previously suggested for HF-associated lesions in some instances (16, 20). In addition, the presence of BF lesions causes mild to severe pruritus (7) manifesting as frequent scratching and rubbing of the lesions which could also act to increase the lesion area and the severity of the tissue damage.
Hyperkeratosis and alopecia were more obvious gross features in Stephanofilaria infected lesions than in lesions without nematodes and there was no difference between bacteria-infected and non-infected lesions for these gross changes. These observations are consistent with previous reports for Stephanofilaria-infected lesions (21, 22) and consistent with our casual observations that hyperkeratotic hairless lesions are commonly present in cattle in northern Queensland where Stephanofilaria is prevalent, but not commonly seen in southern Queensland where no Stephanofilaria was found (10). The occurrence of ulceration in bacteria-infected lesions is consistent with observations of Devriese and Derycke (23) and Hazarika et al. (24), who isolated Staphylococcus hyicus from ulcerative cattle skin lesions. Although the difference in ulceration scores between bacteria positive and negative lesions was not significant in our study, ulceration was significantly higher in lesions with bacteria than in lesions with Stephanofilaria.
Epidermal disruption was the most noticeable epidermal change associated with the bacterial infection in our study, but was not evident in Stephanofilaria-infected lesions. Johnson (3) also observed breached epidermis associated with bacterial growth in some lesions, but in common with the current study rarely observed this change in the Stephanofilaria-infected lesions. Naseem et al. (25) isolated Staphylococcus hyicus and S. agnetis from BF lesions and both these bacterial species were found to have exfoliative toxin type A and C genes. These toxins are epidermolytic serine proteases that can digest skin desmoglein, destroying keratinocyte adhesion and can cause epidermal damage (26). Similar toxins have been identified in S. hyicus isolates associated with exudative epidermitis in pigs (“greasy pig disease”) (27). However, clinical expression of exfoliative toxins has not yet been confirmed and it is possible that these toxins are not actively involved in lesion development as we observed some uncircumscribed ulcerated lesions. Based on the isolation of Staphylococcus spp. from ulcerative BF lesions by Naseem et al. (25), it appears that the cocci-shaped bacterial colonies observed in this study are likely to be S. hyicus or S. agnetis, although S. aureus was also reported from similar lesions in dairy cattle associated with HF (17).
The varying degrees of hyperkeratosis and serocellular crust formation observed in all Stephanofilaria positive lesions are consistent with the epidermal changes described with typical Stephanofilaria spp. associated lesions (3, 21, 22). However, there was also a significant difference between bacteria negative and positive lesions in the hyperkeratotic score. Mild to moderate epidermal changes including spongiosis, hyperkeratosis, acanthosis, epidermal disruption and formation of serocelluar crust, were also noted in sections examined from BF lesions negative for Stephanofilaria and bacteria. Similar epidermal changes have also been reported by Mosca et al. (20) who attributed these lesions to an allergic response to HF feeding. The failure to detect either Stephanofilaria or bacteria in these lesions and the resemblance of these epidermal changes to those seen in the HF-associated lesions suggests that these changes could be immunopathological effects resulting from an immune response to BF feeding.
Both Stephanofilaria-infected and bacteria-infected lesions had more severe dermal damage and adnexal destruction than lesions without infection. Johnson (3) suggests that in Stephanofilaria infected lesions, this could be due to a severe localized host immune reaction, comprising histiocytes, lymphocytes and eosinophils, elicited by the adult nematodes present at the base of the hair follicle. A similar response was seen in our study where eosinophils were seen clustered around Stephanofilaria adult nematodes and microfilariae. This could subsequently result in the destruction of the hair follicles. Our study also observed complete loss or early signs of adnexal destruction in the BF lesions without Stephanofilaria infection. Thus adnexal destruction could result from an allergic response triggered by BF feeding in addition to the response to Stephanofilaria. Further evidence for this is provided by Mosca et al. (20) and Guglielmone et al. (28) who reported severe dermal oedema, folliculitis and furunculosis in HF-associated skin lesions without the presence of Stephanofilaria. There was also a significant difference in dermal damage and adnexal destruction scores between bacteria-infected and noninfected lesions, which suggest that bacterial infection could also play a role.
Although eosinophil infiltration in the superficial dermal layer was observed in all the BF lesions examined in this study, Stephanofilaria infection produced significantly higher eosinophilic infiltration, especially around the adult nematode and microfilariae. Eosinophilic dermatitis has also been reported by Whittier et al. (22) and Watrelot-Virieux and Pin (21) in Stephanofilaria-infected lesions. In contrast to our finding of significantly higher numbers of eosinophils, macrophages, and lymphocytes in Stephanofilaria positive lesions, Johnson (3) indicated eosinophils and neutrophils as the major inflammatory cells in lesions without Stephanofilaria whereas he observed histiocytes and lymphocytes were the predominant inflammatory cells in Stephanofilaria-infected lesions. The difference between our observations and those of Johnson (3) could be explained by difference in lesion stage when biopsied, as Patnaik (29) also observed lymphocytes and histiocytes dominated inflammatory response around dead worms in chronic infections of Stephanofilaria assamensis. The eosinophil-dominant inflammatory reaction in the BF lesions, without nematode or bacterial infection, may also indicate that hypersensitivity responses to BF feeding play a major role in lesion pathology, as a similar inflammatory pattern was reported for Stephanofilaria sp.-negative HF associated lesions (20, 28).
Overall our findings suggest that both Stephanofilaria and bacteria can play a role in BF lesion development. However, it appears that neither of these factors is essential for lesion development as we did not find either in 49% of the samples studied. This suggests that either there is a further unidentified factor involved, or that BF feeding can initiate lesion development without the involvement of other factors. Stephanofilaria infection caused more severe damage to the dermal layer, and could be a key factor in the formation of dry crusted type lesions often described in association with Stephanofilaria infection, whereas bacteria-infected lesions had more severe epidermal damage, which may drive the development of more open ulcerative lesions. Hypersensitivity to BF feeding is hypothesized to be an important contributing factor, particularly in the case of highly allergic individuals, as tissue damage and eosinophilic inflammation were important histological features in the absence of Stephanofilaria or bacteria. In addition, pruritis and rubbing of lesion areas, likely mediated by IgE based responses, may exacerbate the severity of lesions and resultant hemorrhage. Another possible explanation for the causality of lesions with unidentified co-factors could be the physical damage to skin caused by abrading mouthparts of BFs during feeding. As BFs have been shown to vector both Stephanofilaria (3, 8) nematodes and Staphylococcus spp. bacteria (25) they could also contribute to the development and severity of lesions by this means. Furthermore, lesions are often seen to persist well after the BF season has ended, suggesting that infection with Stephanofilaria or bacteria or any unknown factor may increase the longevity of lesions.
Treatment of cutaneous lesions associated with BF or HF, most commonly targeting Stephanofilaria nematodes, have given variable results (3, 14). Notably, the best effect has generally been seen with macrocyclic lactones that affect both the nematodes and flies (3, 14, 30), reported from the areas where both flies and Stephanofilaria are prevalent. Our results suggest that approaches that directly target BF, in addition to Stephanofilaria, could give more consistent treatment results and including a bacteriaocide, may also help to limit the severity of lesions.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the University of Queensland Animal Ethics approval no. 2021/AE000054.
MN, RA, and PJ contributed to conceptualization and design of study. MN, RA, AR, CC, MM, and MK contributed to methodology. MN and RA contributed to investigations and data visualization. MN and PJ contributed to statistical analysis. PJ, RA, AR, CT, MM, CC, and AT supervised this study. PJ and AT contributed to project administration and acquisition of funding. MN contributed to writing original draft, review, and editing. RA, CC, PJ, AR, AT, CT, and MM contributed to review and editing. All authors contributed to the article have approved the submitted version.
This study was funded as part of the Meat and Livestock Australia (MLA) Donor company-funded project (Grant No. P.PSH.798).
The authors thank the Meat and Livestock Australia (MLA) Donor Company, Grant P.PSH.0798, for funding this research. We thank the JBS Townville staff for access to cattle hides for sample collection. We are also grateful to the University of Queensland Pinjarra Hills Research Precinct staff (Ms. Alison Moore and Mr. Tom Connelly) for their help in sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. James P, Madhav M, Brown G. Buffalo flies (Haematobia exigua) expanding their range in Australia. In:Hendrichs J, Pereira R, Vreysen MJB, , editor. Area-wide Integrated Pest Management: Development and Field Application. FL: CRC Press (2020). 463–82. doi: 10.1201/9781003169239-24
2. Jonsson NN, Mayer DG. Estimation of the effects of buffalo fly (Haematobia irritans exigua) on the milk production of dairy cattle based on a meta-analysis of literature data. Med Vet Entomol. (1999) 13:372–6. doi: 10.1046/j.1365-2915.1999.00179.x
3. Johnson SJ. Studies of stephanofilariasis in Queensland. [PhD thesis]. Douglas, QLD: James Cook University (1989).
4. Johnson SJ, Parker RJ, Norton JH, Jaques PA, Grimshaw AA. Stephanofilariasis in cattle. Aust Vet J. (1981) 57:411–3. doi: 10.1111/j.1751-0813.1981.tb00544.x
5. Johnson SJ, Arthur RJ, Shepherd RK. The distribution and prevalence of stephanofilariasis in cattle in Queensland. Aust Vet J. (1986) 63:121–4. doi: 10.1111/j.1751-0813.1986.tb07679.x
6. Jonsson NN, Matschoss AL. Attitudes and practices of Queensland dairy farmers to the control of the cattle tick, Boophilus microplus. Aust Vet J. (1998) 76:746–51. doi: 10.1111/j.1751-0813.1998.tb12306.x
7. Sutherst RW, Bourne AS, Maywald GF, Seifert GW. Prevalence, severity, and heritability of Stephanofilaria lesions on cattle in central and southern Queensland, Australia. Aust J Agric Res. (2006) 57:743–50. doi: 10.1071/AR05265
8. Shaw SA, Sutherland IA. The prevalence of Stephanofilaria in buffalo fly, Haematobia irritans exigua, in central Queensland. Aust J Entomol. (2006) 45:198–201. doi: 10.1111/j.1440-6055.2006.00545.x
9. Hibler CP. Development of Stephanofilaria stilesi in the horn fly. J Parasitol. (1966) 52:890–898. doi: 10.2307/3276527
10. Naseem MN, Raza A, Fordyce G, McGowan M, Constantinoiu C, Turni C, et al. Detection and distribution of Stephanofilaria sp. in buffalo flies and buffalo fly skin lesions in cattle in Queensland, Australia. Vet Parasitol. (2022) 305:109715. doi: 10.1016/j.vetpar.2022.109715
11. Holroyd RG, Hirst DJ, Merrifield AW, Toleman MA. The effect of spraying for buffalo fly (Haematobia irritans exigua) on infestations, growth rate and lesion development on Bos indicus x B. taurus cattle in the dry tropics of north Queensland. Aust J Agric Res. (1984) 35:595–608. doi: 10.1071/AR9840595
12. Foil LD. Tabanids as vectors of disease agents. Parasitol. (1989) 5:88–96. doi: 10.1016/0169-4758(89)90009-4
13. Newsholme SJ, Verster AJ, Jacobs JC. Bovine skin lesions of possible filarial origin associated with heavy horn fly infestations (Haematobia meridiana). Onderstepoort J Vet Res. (1983) 50:73–5.
14. Silva L, Elias RR. de-Moura I, Fioravanti MCS, Borges M, de-Oliveira L, et al. Epidemiological aspects and treatment of parasitic lesions similar to stephanofilariasis disease in nursing cows. Semin Cienc Agrar. (2010) 31:689–98. doi: 10.5433/1679-0359.2010v31n3p689
15. Miyakawa VI, Reis ACF, Lisbôa JAN. Epidemiological and clinical features of stephanofilariasis in dairy cows and diagnostic methods. Pesqui Vet Bras. (2009) 29:887–93. doi: 10.1590/S0100-736X2009001100004
16. Edwards JF, Wikse SE, Field RW, Hoelscher CC. Herd DB. Bovine teat atresia associated with horn fly (Haematobia irritans irritans (L))-induced dermatitis. Vet Pathol. (2000) 37:360–4. doi: 10.1354/vp.37-4-360
17. Owens WE, Oliver SP, Gillespie BE, Ray CH, Nickerson SC. Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers. Am J Vet Res. (1998) 59:1122–4.
18. Naseem MN, Raza A, Allavena R, McGowan M, Morgan JAT, Constantinoiu C, et al. Development and validation of novel PCR assays for the diagnosis of bovine stephanofilariasis and detection of Stephanofilaria sp. nematodes in vector flies. Pathogens. (2021) 10:1211. doi: 10.3390/pathogens10091211
19. Meyerholz DK, Tintle NL, Beck AP. Common pitfalls in analysis of tissue scores. Vet Pathol. (2019) 56:39–42. doi: 10.1177/0300985818794250
20. Mosca M, Vabret M, Randleff-Rasmussen P, Pin D. Skin lesions in Aubrac cows strongly associated with fly bites (Haematobia irritans). Vet Dermatol. (2018) 29:254–e94. doi: 10.1111/vde.12530
21. Watrelot-Virieux D, Pin D. Chronic eosinophilic dermatitis in the scrotal area associated with stephanofilariasis infestation of charolais bull in France. J Vet Med B Infect Dis Vet Public Health. (2006) 53:150–2. doi: 10.1111/j.1439-0450.2006.00923.x
22. Whittier CA, Murray S, Holder K, McGraw S, Fleischer R, Cortes-Rodriguez N, et al. Cutaneous filariasis in free-ranging Rothschild's Giraffes (Giraffa camelopardalis rothschildi) in Uganda. J Wildl Dis. (2020) 56:234–8. doi: 10.7589/2018-09-212
23. Devriese LA, Derycke J. Staphylococcus hyicus in cattle. Res Vet Sci. (1979) 26:356–8. doi: 10.1016/S0034-5288(18)32893-5
24. Hazarika RA, Mahanta PN. Dutta, GN, Devriese LA. Cutaneous infection associated with Staphylococcus hyicus in cattle. Res Vet Sci. (1991) 50:374–5. doi: 10.1016/0034-5288(91)90146-F
25. Naseem MN, Turni C, Gilbert R, Raza A, Allavena R, McGowan M, Constantinoiu C, Ong CT, Tabor AE, James P. The role of Staphylococcus agnetis and Staphylococcus hyicus in the pathogenesis of buffalo fly skin lesions in cattle. Microbiol Spectr. (2022) 10:e00873-22. doi: 10.1101/2022.03.11.483979
26. Fudaba Y, Nishifuji K, Andresen LO, Yamaguchi T, Komatsuzawa H, Amagai M, et al. Staphylococcus hyicus exfoliative toxins selectively digest porcine desmoglein 1. Microb Pathog. (2005) 39:171–6. doi: 10.1016/j.micpath.2005.08.003
27. Wegener HC, Andresen LO, Bille-Hansen V. Staphylococcus hyicus virulence in relation to exudative epidermitis in pigs. Can J Vet Res. (1993) 57:119–25.
28. Guglielmone AA, Gimeno E, Idiart J, Fisher WF, Volpogni MM, Quaino O, et al. Skin lesions and cattle hide damage from Haematobia irritans infestations. Med Vet Entomol. (1999) 13:324–9. doi: 10.1046/j.1365-2915.1999.00167.x
29. Patnaik MM. Histopathology of lesions in stephanofilariasis and onchocerciasis in buffalo and cattle. Ind J Anim Sci. (1982) 52:159–66.
Keywords: haematobia, Stephanofilaria, buffalo fly, skin lesions, histopathology
Citation: Naseem MN, Allavena R, Raza A, Constantinoiu C, McGowan M, Turni C, Kamran M, Tabor AE and James P (2023) Pathology and pathogenesis of cutaneous lesions in beef cattle associated with buffalo fly infestation. Front. Vet. Sci. 9:971813. doi: 10.3389/fvets.2022.971813
Received: 17 June 2022; Accepted: 29 December 2022;
Published: 18 January 2023.
Edited by:
Marie Christine Cadiergues, Ecole Nationale Vétérinaire de Toulouse (ENVT), FranceReviewed by:
Didier Pin, VetAgro Sup, FranceCopyright © 2023 Naseem, Allavena, Raza, Constantinoiu, McGowan, Turni, Kamran, Tabor and James. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter James,  cC5qYW1lczFAdXEuZWR1LmF1
cC5qYW1lczFAdXEuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.