
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 04 August 2022
Sec. Veterinary Epidemiology and Economics
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.957306
 Veerasak Punyapornwithaya1,2
Veerasak Punyapornwithaya1,2 Suvaluk Seesupa3
Suvaluk Seesupa3 Sitthinon Phuykhamsingha4
Sitthinon Phuykhamsingha4 Orapun Arjkumpa5
Orapun Arjkumpa5 Chalutwan Sansamur6
Chalutwan Sansamur6 Chaiwat Jarassaeng3*
Chaiwat Jarassaeng3*In 2021–2022, there were numerous outbreaks of lumpy skin disease (LSD) affecting cattle farms across Thailand. This circumstance was the country's first encounter with an LSD outbreak. Thus, a better understanding of LSD epidemiology is necessary. The aim of this study was to determine the spatio-temporal patterns of the LSD outbreaks in dairy farming areas. Data from LSD outbreak investigations collected from dairy farms in Khon Kean province, northeastern Thailand, were analyzed using spatio-temporal models including space-time permutation, Poisson, and Bernoulli models. LSD outbreaks were found in 133 out of 152 dairy farms from May to July, 2021. The majority of dairy farms (n = 102) were affected by the LSD outbreaks in June. The overall herd attack, morbidity and mortality rates were 87, 31, and 0.9%, respectively. According to the results of all models, the most likely clusters were found in the northern part of the study area. The space-time permutation and Poisson model identified 15 and 6 spatio-temporal outbreak clusters, respectively, while the Bernoulli model detected only one cluster. The most likely clusters from those models cover radii of 1.59, 4.51, and 4.44 km, respectively. All farms included in the cluster identified by the space-time permutation model were also included in the cluster identified by the Poisson model, implying that both models detected the same outbreak area. Furthermore, the study results suggested that farmers who own farms within a one km radius of the LSD outbreak farm should be advised to implement more stringent insect vector control measures to prevent disease spread. This study provides better insights into the spatio-temporal pattern of clusters of LSD in the outbreak area. The findings of this study can support authorities in formulating strategies to prevent and control future outbreaks as well as prioritizing resource allocation to high-risk areas.
According to the World Organization for Animal Health (WOAH), lumpy skin disease (LSD) is an emerging transboundary notifiable viral disease (1). This disease is caused by LSD virus (LSDV), a capripoxvirus within the Poxviridae family (2). The primary route of LSD transmission is driven by insect vectors such as stable files (3, 4) and mosquitoes (5, 6). Originating from the African continent, LSD has been reported in several countries in Europe, the Middle East and Asia (7). Currently, several Asian countries, including Bangladesh (8), China (9), India (10), Bhutan, Nepal (11), Vietnam (12), Myanmar (13), Hong Kong (14), Taiwan (15), Thailand (16) and Malaysia (17), are affected by this disease. The economic consequences of LSD are substantial, as the disease has a remarkable negative financial impact on cattle herd owners, customers, and the livestock industry (18, 19).
In Thailand, the first outbreak of LSD was found in beef farms located in the northeastern part of the country in March 2021 (16). Later, numerous LSD outbreaks were reported across the country (20, 21). Data collected by livestock authorities as part of the LSD outbreak investigation and control program in March 2022 revealed that the total number of beef and dairy cattle affected by LSD was 609,073, and 12,317, respectively (22). During the outbreaks, both beef and dairy cattle were affected by LSD, which resulted in poor health and decreased production (23).
Scan statistical methods (24) are increasingly being used in epidemiological research with the aim of detecting clusters in the spatial, temporal, or spatio-temporal aspects of infectious disease outbreaks (25–27). In purely spatial or temporal analysis, a scanning window moves across space or time to detect disease clusters within the target area whereas a cylinder moves both spatially and temporally to detect potential spatio-temporal clusters (28, 29). Space-time permutation, Poisson, and Bernoulli models are the most commonly used scan statical methods. These methods require different data inputs and have their own computation procedures. All models necessitate the use of geographic coordinate data. The number of cases is used in the space-time permutation model (STP), whereas the Poisson model requires both the number of cases and the total number of populations at risk (30). The Bernoulli model uses data from both cases and controls as its input data (24). Normally, these models are employed to determine clusters with a high rate of infection. The identified clusters can be thought of as a “hot zone” for disease outbreaks. Thus, intervention and resource allocations in these areas can be prioritized (24, 30).
Given that LSD was only recently discovered in the country, a better understanding of the epidemiology of LSD is necessary. However, there are very few reports on the epidemiology of LSD in Thailand. Even though a study of the spatio-temporal pattern of the outbreak in beef cattle farms was carried out (23), studies on the epidemiology of LSD outbreaks in dairy farms remain limited, allowing several knowledge gaps, particularly on how the LSD outbreaks distributed among dairy farms in terms of space and time. The findings of the study in beef farming areas (23) may not be extrapolated to dairy farming areas because beef and dairy cattle farms in Thailand are managed differently (31, 32). Furthermore, the spatial distributions of beef and dairy farms differ, with the majority of dairy farming areas being densely populated with dairy farms and beef farms being sparsely populated. Thus, it is essential to investigate spatio-temporal patterns of LSD outbreaks in dairy farming areas in order to gain a better understanding of the spatial epidemiology of LSD outbreaks affecting dairy farms.
The objective of this study was to determine spatio-temporal patterns of LSD outbreaks in dairy farms in northeastern Thailand using the STP, Poisson and Bernoulli models.
Outbreaks of LSD were reported in dairy farms located in dairy farming areas in Muang and Ban Haet districts, Khon Kean Province between May to July 2021. There was no prior history of LSD outbreaks in any dairy farms in these areas. The dairy farms are operated by dairy farmers who are members of the Khon Kean dairy cooperative. All dairy farms use a free-stall system; therefore, there is no chance of dairy cattle from one farm interacting with those from other farms.
During the outbreak, licensed veterinarians investigated the outbreak at every dairy farm in the outbreak areas with the support of the dairy cooperative committee. Additionally, the investigations were supervised by livestock authorities from the Khon Kean provincial livestock office, Department of Livestock Development (DLD).
All dairy cattle on each farm that participated in the study were examined by veterinarians for clinical signs of LSD. Similar to a previous report (23), an LSD animal case was defined as a dairy cow displaying the LSD clinical signs, which include raised firm nodules with a diameter of 1–7 cm on the head and/or body surface areas. Notably, veterinarians conducting outbreak investigations did not collect animal samples to confirm LSD infections. However, in order to verify this LSD outbreak, livestock authorities from the DLD collected blood and tissue samples from some dairy cattle from various farms in the outbreak areas. Thereafter, samples were processed in a laboratory by the National Institute of Animal Health (NIAH), which is part of the DLD. After that, the laboratory-confirmed results were made available to the public on the DLD website (22).
During the investigation, basic epidemiological data were collected, including the number of dairy cattle showing LSD clinical signs, the number of dairy cattle showing LSD clinical signs and then dying, and the number of all dairy cattle on the farm. The authorities also recorded the geographical coordinates of each farm in the study area.
In the present study, we analyzed data from such outbreak investigations. For each LSD outbreak farm, morbidity and mortality rates at the animal level were calculated. The herd attack rate was also determined to investigate how LSD affected all dairy farms in the study area. Several maps were created to present geographical information relevant to the LSD outbreaks. All maps were created by Quantum Geographic Information System (QGIS), which is an open-source software (33).
In addition to geographical coordinate data, the availability of (i) number of cases, (ii) number of cases and population at risk and (iii) number of cases for LSD outbreak farms and non-LSD outbreak farms (control farm) allows data to be analyzed in the dimensions of space and time using SPT, Poisson and Bernoulli models (34), respectively.
The SPT model evaluates LSD case data within each cylindrical window and calculates the ratio of the observed number of LSD cases to the expected number of LSD cases. The likelihood ratio test statistic was then determined from each scanning circular window. A cluster is detected when an elevated number of LSD cases are found within an area and within a certain time frame compared to the rest of the study area for the same time frame (28).
The Poisson model evaluates the number of dairy cattle populations at risk and the number of LSD cases within and outside the cylindrical windows in the dimensions of space and time. For each cylinder, the relative risk and log likelihood ratio (LLR) were calculated. The most likely cluster was determined to be the window with the maximum LLR value and a p-value <0.05.
For a given scanning window, the LLR is determined based on the following equation (35):
where n is the number of LSD cases within the scan window, N is the total number of dairy cattle on each farm, E is the expected number of LSD cases under the null hypothesis, and I denotes the indicator function (36).
The Bernoulli model used information from both LSD outbreak farms and control farms to detect spatio-temporal clusters. Similar to the Poisson, the LLR was calculated for each cylindrical window, and the cluster with the maximum LLR with a p-value <0.05 was justified as the most likely cluster. The likelihood function of this model is written as:
where C is the total number of LSD cases, c is the observed number of LSD cases within the window, n denotes the total number of LSD cases, N represents the combined total number of cases and controls within the dataset, and I() denotes the indicator of function I, which is equal to 1 in the case of c>C/N or () otherwise.
In the present study, SaTScan software was used to identify LSD spatio-temporal clusters (30). For parameter setting across all models, the maximum spatial cluster size was set as 50% of the population at risk, which is the default setting value. With this setting, a clusters size can contain at most 50% of the total population at risk (30). The maximum temporal cluster size was set at 7 days based on the LSD incubation period (7) and those used in the previous study (23). To determine the significance of candidate clusters, Monte Carlo simulation was employed (number of replications = 999). The clusters with p-value < 0.05 were considered as statistically significant clusters. Moreover, maps corresponding to the SaTScan results were created using QGIS software.
LSD outbreaks were found in 133 out of 152 dairy farms from May 1st to July 8th in the year 2021. The locations of dairy farms with and without LSD outbreaks are depicted in Figure 1. The herd attack rate was 87%. Overall, the morbidity and mortality rates were 31% (n =2,656/8,553) and 0.9% (n = 77/8,553), respectively. According to the epidemic curve, the highest number of LSD outbreaks were found in June followed by July. In June, more than 100 dairy herds were affected by LSD. Additionally, on several days in June, as many as ten farms reported LSD outbreaks (Figure 2).
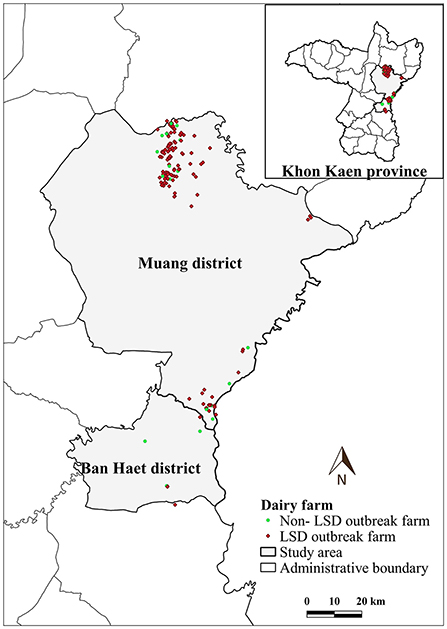
Figure 1. Study area, farm location and farm status based on lumpy skin disease (LSD) affecting status.
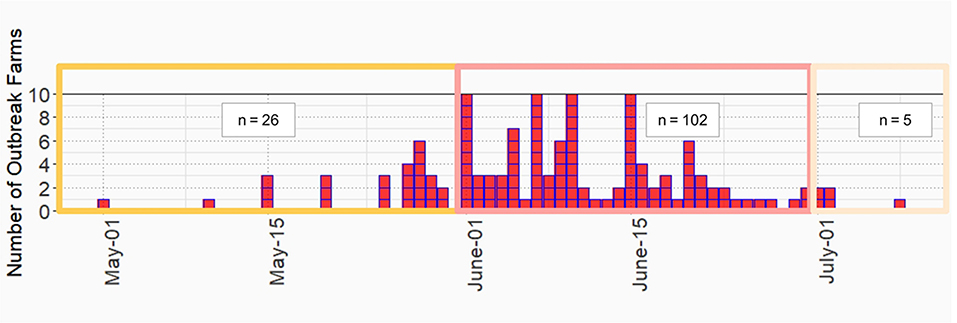
Figure 2. Epidemic curve of lumpy skin disease outbreak in dairy farms, Khon Kean province, Thailand.
The average total number of dairy cattle in dairy farms enrolled in this study was 57 heads. The average distance between a dairy farm and its nearest neighbor farm was 0.83 km (range: 0.12–7.31 km). Notably, in some areas, numerous farms are aggregated (Figure 1).
The most likely clusters detected by all models were located in the northern part of Muang district during the period of June, 2021. The STP model identified 16 clusters including the most likely cluster and 15 secondary clusters (Table 1) while the Poisson model yielded the most likely cluster and 6 secondary clusters (Table 2). The Bernoulli model detected only the most likely clusters (Table 3). Furthermore, the most likely cluster defined by the Poisson model had a size of 4.51 km, which is larger than the most likely clusters detected by the STP and Bernoulli models, which have sizes of 1.59 and 4.44 km, respectively.
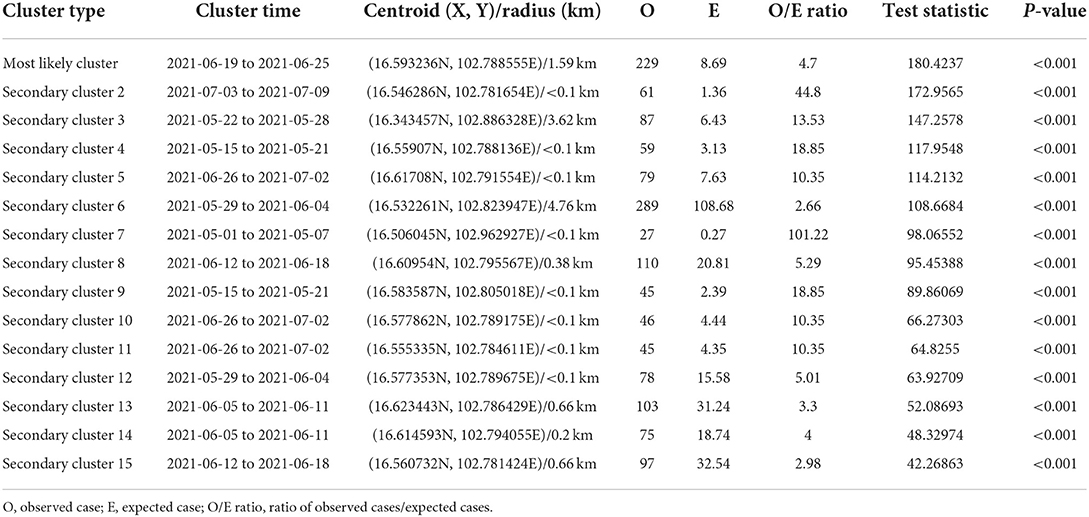
Table 1. Spatio-temporal analysis of lumpy skin disease outbreaks in dairy farms, Khon Kean province, Thailand by space-time permutation model.
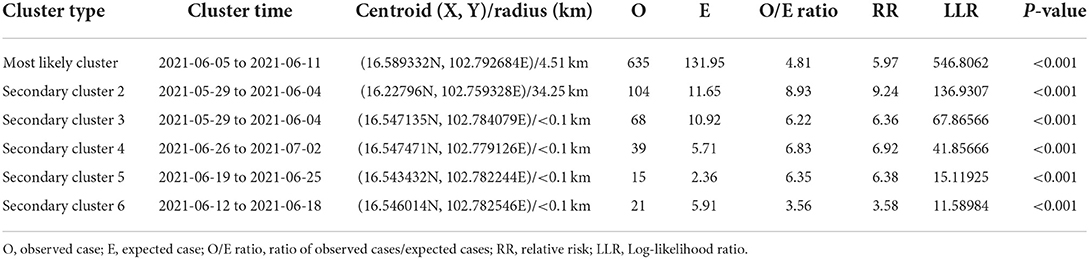
Table 2. Spatio-temporal analysis of lumpy skin disease outbreaks in dairy farms, Khon Kean province, Thailand by Poisson model.

Table 3. Spatio-temporal analysis of lumpy skin disease outbreaks in dairy farms, Khon Kean province, Thailand by Bernoulli model.
The most likely cluster identified by the STP model consisted of 229 LSD cases from 29 farms over a period of 7 days (Figure 3), whereas the Poisson model identified a larger cluster area consisting of 635 LSD cases from 103 farms (Figure 4) over the course of a week. The most likely clusters provided by the Bernoulli included 525 LSD cases from 79 farms and 231 dairy cattle without LSD clinical signs from 5 farms (Figure 5).
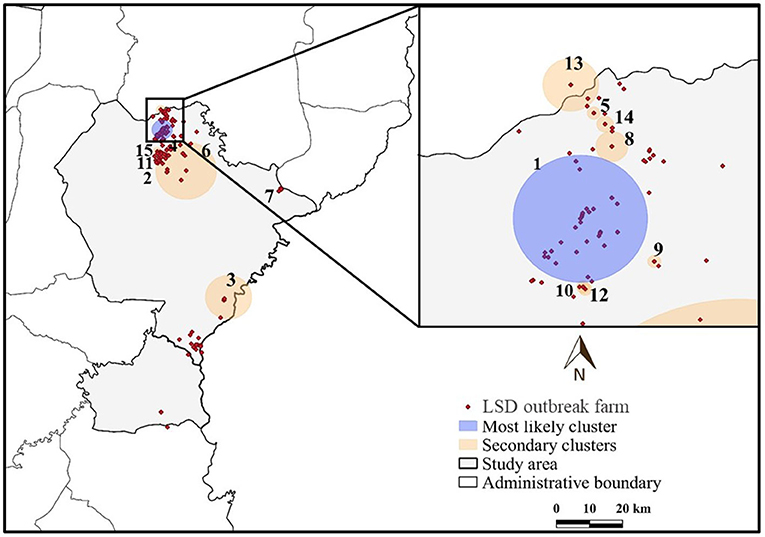
Figure 3. Spatio-temporal clusters of lumpy skin disease (LSD) outbreaks in dairy farms, Khon Kean province, Thailand detected by the space-time permutation model.
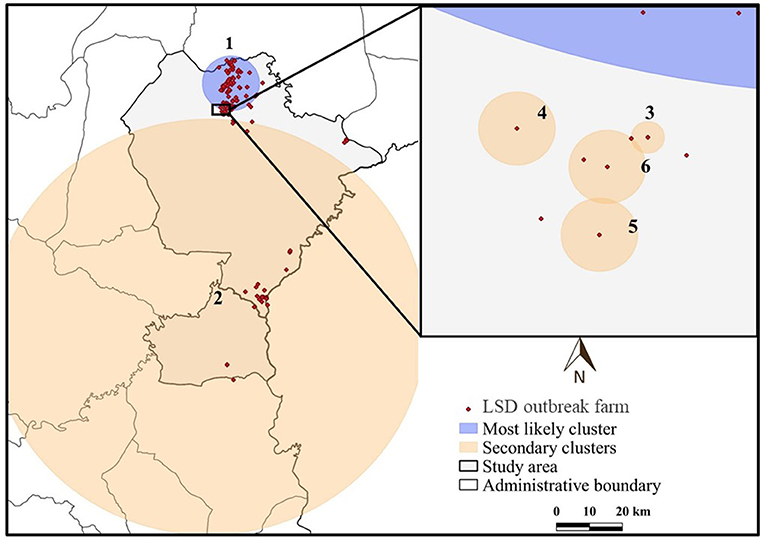
Figure 4. Spatio-temporal clusters of lumpy skin disease (LSD) outbreaks in dairy farms, Khon Kean province, Thailand detected by the Poisson model.
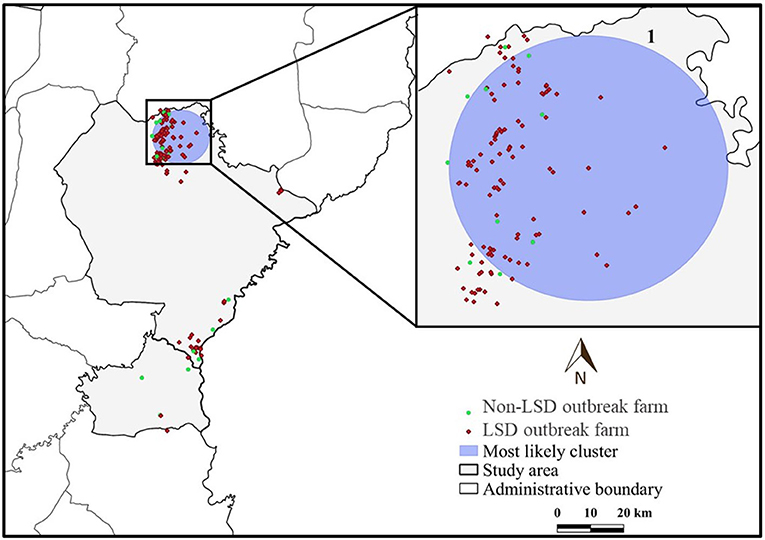
Figure 5. Spatio-temporal clusters of lumpy skin disease (LSD) outbreaks in dairy farms, Khon Kean province, Thailand detected by the Bernoulli model.
The most likely cluster from the STP model was located within the most likely cluster determined by the STP model. In other words, the same location was detected in both models, but the Poisson model included more LSD cases and covered a larger cluster area. In addition, the Poisson model detected the most likely spatio-temporal clusters earlier than the STP model. Nonetheless, the two most likely clusters were observed in June, when the largest number of LSD affected dairy farms was found. Furthermore, the Bernoulli model identified the most likely cluster observed during the same time period as the Poisson model.
The STP model identified 14 secondary clusters, 12 of which had a radius of <1 km (Table 1), whereas the Poisson model offered the largest secondary cluster (radius = 34.25 km) and 3 small clusters with a radius of <1 km (Table 2). The largest secondary cluster (Secondary cluster 6 in Table 1) derived from the STP model included a greater number of cases than those derived from the Poisson model.
To the best of our knowledge, this is the first study that utilized scan statistics including STP, Poisson and Bernoulli models to identify spatio-temporal clusters of LSD outbreaks in dairy farms in northeastern Thailand.
The most likely spatio-temporal clusters from all models were found in the northern part of the study area, where numerous dairy farms are aggregated. The most likely LSD clusters identified by STP were within the most likely cluster detected by the Poisson model, implying that both models suggested the same area for high LSD cases even though the temporal periods defined by both models were slightly different. Accordingly, the finding of the high number of cases and LSD-affected farms within a week suggests that LSD spreads rapidly among cattle both within and between farms.
The LSD virus can spread between cattle through close contact, insect-vectors, and other routes of transmission (3–6). In the study areas, because cattle movements among dairy farms are very low, the spread of LSD among dairy farms was less likely due to close contact between cattle from different farms. On the other hand, the spread of LSD is likely caused by insect vectors, which are abundant in most dairy farms in Thailand (37). Indeed, the finding that LSD outbreaks were found in a large number of farms within a short period of time and that several farms are aggregated in the area raises the possibility that the LSD viruses are most likely transmitted by insect vectors. This scenario was comparable to the findings of an Albanian study, which showed that if the distances between herds are short (<5 km), vector-borne contribute to the majority of LSD transmission (38). Our results are also consistent with those of a recent study conducted in Kazakhstan, which indicates that, in the absence of active animal movement, insect vectors may play a significant role in the spread of the LSD virus among cattle herds (39).
Regarding DLD control measures, DLD authorities recommended that if a farm encounters an LSD outbreak, neighboring farms within a 50 km radius should impose cattle movement restrictions, and the owners of cattle farms neighboring the outbreak farm should also take action to control such vectors (22). However, there was no guidance on the recommended size of the circle in which neighboring farms should be included. Regarding the approaches demonstrated herein, we suggested decision-makers or authorities utilize spatio-temporal models to determine the appropriate radius for the circle for each outbreak and use the results from the analyses to guide farmers. For example, for the study area, farmers who own farms within a 1-km radius of the outbreak farm should be guided to stricter insect vector control measures to prevent disease spread.
Although STP and Poisson models have been used in previous studies (23, 40), the use of the Bernoulli model for analyzing LSD outbreak data is very limited. In fact, the application of Bernoulli model to LSD outbreak data provides some advantages (41, 42). This model uses the number of cases and total population at risk from both control and LSD outbreak farms to determine clusters that include both control and outbreak farms, rather than just outbreak farms, as STP and Poisson do (41). Thus, the results from this model offer an opportunity to investigate the similarities and differences between control and LSD outbreak farms located in the same cluster (42). Due to the high herd attack rate, there are few farms without an LSD outbreak in this study. As a consequence of this, the Bernoulli model identified the most likely cluster that consisted of a limited number of non-LSD outbreak (control) farms, limiting the ability to compare potential factors associated with LSD outbreaks between non-LSD outbreak and LSD outbreak farms. However, in follow-up studies for other outbreaks where sample size is sufficient, we recommend further investigation of LSD outbreak farms (case) and non-LSD outbreak farms (control) located in the same spatio-temporal cluster to identify environmental factors, insect transmission-related factors (e.g., insect abundance and seasonality), and management practices that may be associated with outbreaks among cattle within or between farms.
It was demonstrated that the mortality and morbidity rates observed in the present study were in a range of previous reports from other countries (7, 13). These epidemiological parameters, on the other hand, were lower than those from a study conducted in beef farms in Thailand (23). This may be due to the fact that dairy farms are more intensive than beef farms, and therefore their disease prevention and control management practices are likely to be more rigorous (22). However, the differences in spatial characteristics between beef and dairy farms may also contribute to this comparison as well. For a better understanding of whether LSD affects beef and dairy cattle differently, follow up studies on this issue are necessary.
There were some limitations to the study that should be taken into consideration when interpreting the results. Even though many cattle showed LSD-like clinical signs, DLD livestock authorities collected blood and tissue samples from some cattle in some herds for further confirmation by laboratory results. Thus, the vast majority of LSD cattle cases were diagnosed based on clinical signs. However, this limitation has been encountered in many previous studies (11, 43–45). Moreover, despite the possibility that inaccurate diagnoses (such as misdiagnosis of other illnesses as LSD) in the current study may be a source of bias, this concern may not be a major issue given that the diagnoses were made by veterinarians under the supervision of the DLD livestock authorities. Furthermore, because dairy farming areas in Thailand differ geographically, the findings of this study should not be generalized to all dairy farms in the country. Indeed, epidemiological research on LSD outbreaks in other dairy farming areas with varying geographic and environmental conditions should be conducted in follow-up studies.
In this study, we demonstrated how commonly collected outbreak data can be utilized to gain additional insight into disease outbreak patterns using spatio-temporal analytical tools. We recommend incorporating the spatio-temporal analysis demonstrated herein into the current LSD surveillance system (22) to gain a better understanding of the epidemiology of LSD and generate results about hot zones or areas where resource allocations could be prioritized.
This study is the first to determine the burden of LSD outbreaks in dairy farms and provide insight into the spatio-temporal analysis of disease outbreaks in the northeastern region of Thailand. The findings from this study will benefit livestock authorities in better understanding LSD epidemiology, which is important information to enhance efforts and formulate an effective control strategy for preventing future LSD outbreaks.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
VP, CJ, SP, and SS designed the study. VP and CJ provided oversight for the project. VP, CJ, SP, SS, and OA gathered and organized the data from different sources. VP, CJ, SS, CS, and OA analyzed the data and interpreted the results. VP, CJ, SS, and CS prepared the initial draft of the manuscript. All authors participated in the editing of the submitted article and have given their final approval.
This research was funded by National Research Council of Thailand (NRCT), Thailand.
The authors are grateful to the staff from Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand and Khon Kaen Dairy Cooperative, Khon Kaen, Thailand for their assistance and necessary facilities. This manuscript was edited for proper English language by American Journal Experts (AJE) before submission. The certificate can be verified on the AJE website (https://www.aje.com/) using the verification code 115E-675A-08FD-B318-F1C6.
Author SP was employed by company Khon Kaen Dairy Cooperative.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sameea Yousefi P, Mardani K, Dalir-Naghadeh B, Jalilzadeh-Amin G. Epidemiological study of lumpy skin disease outbreaks in North-western Iran. Transbound Emerg Dis. (2017) 64:1782–9. doi: 10.1111/tbed.12565
2. Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, et al. Review: capripoxvirus diseases: current status and opportunities for control. Transbound Emerg Dis. (2017) 64:729–45. doi: 10.1111/tbed.12444
3. Sprygin A, Fedorova O, Nesterov A, Shumilova I, Byadovskaya O. The stable fly Stomoxys calcitrans L. as a potential vector in the spread of lumpy skin disease virus in Russia: short review. In: International Scientific and Practical Conference at Development of the Agro-lndustrial Complex in the Context of Robotization and Digitalization of Production in Russia and Abroa. E3S Web of Conference. Vol. 222. Moscow: EDP Sciences (2020). p. 06026. doi: 10.1051/e3sconf/202022206026
4. Kayesh MEH, Hussan MT, Hashem MA, Eliyas M, Anower AM. Lumpy skin disease virus infection: an emerging threat to cattle health in Bangladesh. Hosts Viruses. (2020) 7:97–108. doi: 10.17582/journal.hv/2020/7.4.97.108
5. Tuppurainen E, Dietze K, Wolff J, Bergmann H, Beltran-Alcrudo D, Fahrion A, et al. Vaccines and vaccination against lumpy skin disease. Vaccines. (2021) 9:1136. doi: 10.3390/vaccines9101136
6. Chihota C, Rennie L, Kitching R, Mellor P. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Med Vet Entomol. (2003) 17:294–300. doi: 10.1046/j.1365-2915.2003.00445.x
7. Tuppurainen E, Oura C. Lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transbound Emerg Dis. (2012) 59:40–8. doi: 10.1111/j.1865-1682.2011.01242.x
8. Hasib FMY, Islam MS, Das T, Rana EA, Uddin MH, Bayzid M, et al. Lumpy skin disease outbreak in cattle population of Chattogram, Bangladesh. Vet Med Sci. (2021) 7:1616–24. doi: 10.1002/vms3.524
9. Lu G, Xie J, Luo J, Shao R, Jia K, Li S. Lumpy skin disease outbreaks in China, since 3 August 2019. Transbound Emerg Dis. (2021) 68:216–9. doi: 10.1111/tbed.13898
10. Kumar N, Chander Y, Kumar R, Khandelwal N, Riyesh T, Chaudhary K, et al. Isolation and characterization of lumpy skin disease virus from cattle in India. PLoS ONE. (2021) 16:e0241022. doi: 10.1371/journal.pone.0241022
11. Koirala P, Meki IK, Maharjan M, Settypalli BK, Manandhar S, Yadav SK, et al. Molecular characterization of the 2020 outbreak of lumpy skin disease in Nepal. Microorganisms. (2022) 10:539. doi: 10.3390/microorganisms10030539
12. Tran HTT, Truong AD, Dang AK, Ly DV, Nguyen CT, Chu NT, et al. Lumpy skin disease outbreaks in Vietnam, 2020. Transbound Emerg Dis. (2021) 68:977–80. doi: 10.1111/tbed.14022
13. Das M, Chowdhury MSR, Akter S, Mondal AK, Jamal M. An updated review on lumpy skin disease: perspective of Southeast Asian countries. J Adv Biotechnol Exp Ther. (2021) 4:322–33. doi: 10.5455/jabet.2021.d133
14. Flannery J, Shih B, Haga IR, Ashby M, Corla A, King S, et al. A novel strain of lumpy skin disease virus causes clinical disease in cattle in Hong Kong. Transbound Emerg Dis. (2021) 1–8. doi: 10.1101/2021.04.20.440323
15. Huang CW, Ting LJ, Liu YP, Lin YJ, Lee F, Chiou CJ. Complete coding sequence of lumpy skin disease virus isolated from Kinmen Island, Taiwan, in 2020. Microbiol Resour Announc. (2022) 11:e0120421. doi: 10.1128/mra.01204-21
16. Arjkumpa O, Suwannaboon M, Boonrawd M, Punyawan I, Laobannu P, Yantaphan S, et al. First emergence of lumpy skin disease in cattle in Thailand, 2021. Transbound. Emerg. Dis. (2021) 68:3002–4. doi: 10.1111/tbed.14246
17. Khoo CK, Dahlan R, Desa ZM, Syarina PNA, Mohd Salim SSH, Barker Z, et al. Molecular detection of lumpy skin disease virus in Malaysia 2021. Int J Infect Dis. (2022) 116:S64. doi: 10.1016/j.ijid.2021.12.150
18. Gumbe A. Review on lumpy skin disease and its economic impacts in Ethiopia. J Dairy Vet Anim Res. (2018) 7:39–46. doi: 10.15406/jdvar.2018.07.00187
19. Gari G, Bonnet P, Roger F, Waret-Szkuta A. Epidemiological aspects and financial impact of lumpy skin disease in Ethiopia. Prev Vet Med. (2011) 102:274–83. doi: 10.1016/j.prevetmed.2011.07.003
20. Singhla T, Boonsri K, Kreausukon K, Modethed W, Pringproa K, Sthitmatee N, et al. Molecular characterization and phylogenetic analysis of lumpy skin disease virus collected from outbreaks in Northern Thailand in 2021. Vet Sci. (2022) 9:194. doi: 10.3390/vetsci9040194
21. Sariya L, Paungpin W, Chaiwattanarungruengpaisan S, Thongdee M, Nakthong C, Jitwongwai A, et al. Molecular detection and characterization of lumpy skin disease viruses from outbreaks in Thailand in 2021. Transbound Emerg Dis. (2022) 1–8. doi: 10.1111/tbed.14552
22. The Department of Livestock Development. Reports on lumpy skin disease in Thailand. (2022). Available online at: https://sites.google.com/view/dldlsd/home?authuser=0 (accessed September 01, 2021).
23. Arjkumpa O, Suwannaboon M, Boonrod M, Punyawan I, Liangchaisiri S, Laobannue P, et al. The first lumpy skin disease outbreak in Thailand (2021): epidemiological features and spatio-temporal analysis. Front Vet Sci. (2022) 8:799065. doi: 10.3389/fvets.2021.799065
24. Kulldorff M, Rand K, Gherman G, Williams G, DeFrancesco D. SaTScan-Spatial and Space-Time Scan Statistics: Software for Calculating the Spatial, Temporal and Space-Time Scan Statistics. Version 10.0.2. Bethesda, MD: National Cancer Institute (1998).
25. Minh PQ, Morris RS, Schauer B, Stevenson M, Benschop J, Nam HV, et al. Spatio-temporal epidemiology of highly pathogenic avian influenza outbreaks in the two deltas of Vietnam during 2003–2007. Prev Vet Med. (2009) 89:16–24. doi: 10.1016/j.prevetmed.2009.01.004
26. Kiani B, Raouf Rahmati A, Bergquist R, Hashtarkhani S, Firouraghi N, Bagheri N, et al. Spatio-temporal epidemiology of the tuberculosis incidence rate in Iran 2008 to 2018. BMC Public Health. (2021) 21:1093. doi: 10.1186/s12889-021-11157-1
27. Driciru M, Rwego IB, Asiimwe B, Travis DA, Alvarez J, VanderWaal K, et al. Spatio-temporal epidemiology of anthrax in Hippopotamus amphibious in Queen Elizabeth Protected Area, Uganda. PLoS ONE. (2018) 13:e0206922. doi: 10.1371/journal.pone.0206922
28. Yeom Y. Detecting spatial-temporal clusters of violent behavior in South Korea with space-time permutation scan statistics. Policing Int J. (2019) 42:490–502. doi: 10.1108/PIJPSM-07-2018-0085
29. Azage M, Kumie A, Worku A, Bagtzoglou AC. Childhood diarrhea exhibits spatiotemporal variation in northwest Ethiopia: a SaTScan spatial statistical analysis. PLoS ONE. (2015) 10:e0144690. doi: 10.1371/journal.pone.0144690
30. Kulldorff M. SaTDcanTM User Guide. (2021). Available online at: http://satscan.org (accessed April 1, 2022).
31. Punyapornwithaya V, Jampachaisri K, Klaharn K, Sansamur C. Forecasting of milk production in Northern Thailand using seasonal autoregressive integrated moving average, error trend seasonality, and hybrid models. Front Vet Sci. (2021) 8:775114. doi: 10.3389/fvets.2021.775114
32. Bunmee T, Chaiwang N, Kaewkot C, Jaturasitha S. Current situation and future prospects for beef production in Thailand: a review. Asian Aust J Anim Sci. (2018) 31:968–75. doi: 10.5713/ajas.18.0201
33. QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. (2022). Available online at: http://qgis.osgeo.org (accessed April 10, 2022).
34. Kulldorff M, Heffernan R, Hartman J, Assunçao R, Mostashari F. A space–time permutation scan statistic for disease outbreak detection. PLoS Med. (2005) 2:e59. doi: 10.1371/journal.pmed.0020059
35. Chen J, Roth RE, Naito AT, Lengerich EJ, Maceachren AM. Geovisual analytics to enhance spatial scan statistic interpretation: an analysis of US cervical cancer mortality. Int J Health Geogr. (2008) 7:57. doi: 10.1186/1476-072X-7-57
36. Amin R, Hendryx M, Shull M, Bohnert A. A cluster analysis of pediatric cancer incidence rates in Florida: 2000–2010. Stat Public Policy. (2014) 1:69–77. doi: 10.1080/2330443X.2014.928245
37. Changbunjong T, Weluwanarak T, Ratanakorn P, Maneeon P, Ganpanakngan M, Apiwathnasorn C, et al. Distribution and abundance of Stomoxyini flies (Diptera: Muscidae) in Thailand. Southeast Asian J Trop Med Public Health. (2012) 43:1400–10.
38. Gubbins S, Stegeman A, Klement E, Pite L, Broglia A, Abrahantes JC. Inferences about the transmission of lumpy skin disease virus between herds from outbreaks in Albania in 2016. Prev Vet Med. (2020) 181:104602. doi: 10.1016/j.prevetmed.2018.12.008
39. Issimov A, Kushaliyev K, Abekeshev N, Molla W, Rametov N, Bayantassova S, et al. Risk factors associated with lumpy skin disease in cattle in West Kazakhstan. Prev Vet Med. (2022) 29:105660. doi: 10.1016/j.prevetmed.2022.105660. [Epub ahead of print].
40. Ochwo S, VanderWaal K, Ndekezi C, Nkamwesiga J, Munsey A, Witto SG, et al. Molecular detection and phylogenetic analysis of lumpy skin disease virus from outbreaks in Uganda 2017–2018. BMC Vet Res. (2020) 16:66. doi: 10.1186/s12917-020-02288-5
41. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. (1997) 26:1481–96. doi: 10.1080/03610929708831995
42. Arjkumpa O, Sansamur C, Sutthipankul P, Inchaisri C, Na Lampang K, Charoenpanyanet A, et al. Spatiotemporal analyses of foot and mouth disease outbreaks in cattle farms in Chiang Mai and Lamphun, Thailand. BMC Vet Res. (2020) 16:170. doi: 10.1186/s12917-020-02392-6
43. Allam AM, Elbayoumy MK, Abdel-Rahman EH, Hegazi AG, Farag TK. Molecular characterization of the 2018 outbreak of lumpy skin disease in cattle in Upper Egypt. Vet World. (2020) 13:1262–8. doi: 10.14202/vetworld.2020.1262-1268
44. Mafirakureva P, Saidi B, Mbanga J. Incidence and molecular characterisation of lumpy skin disease virus in Zimbabwe using the P32 gene. Trop Anim Health Prod. (2017) 49:47–54. doi: 10.1007/s11250-016-1156-9
Keywords: lumpy skin disease, outbreak, cluster, spatio-temporal pattern, dairy cattle, Thailand
Citation: Punyapornwithaya V, Seesupa S, Phuykhamsingha S, Arjkumpa O, Sansamur C and Jarassaeng C (2022) Spatio-temporal patterns of lumpy skin disease outbreaks in dairy farms in northeastern Thailand. Front. Vet. Sci. 9:957306. doi: 10.3389/fvets.2022.957306
Received: 30 May 2022; Accepted: 11 July 2022;
Published: 04 August 2022.
Edited by:
Roswitha Merle, Freie Universität Berlin, GermanyReviewed by:
Maximilian Baumann, Freie Universität Berlin, GermanyCopyright © 2022 Punyapornwithaya, Seesupa, Phuykhamsingha, Arjkumpa, Sansamur and Jarassaeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaiwat Jarassaeng, chajar@kku.ac.th
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.