Abstract
Foot-and-mouth disease is an acute, highly infectious, and economically significant transboundary animal disease. Vaccination is an efficient and cost-effective measure to prevent the transmission of this disease. The primary way that foot-and-mouth disease spreads is through direct contact with infected animals, although it can also spread through contact with contaminated environments. This paper uses a diffuse foot-and-mouth disease model to account for the efficacy of vaccination in managing the disease. First, we transform an age-space structured foot-and-mouth disease into a diffusive epidemic model with nonlocal infection coupling the latent period and the latent diffusive rate. The basic reproduction number, which determines the outbreak of the disease, is then explicitly formulated. Finally, numerical simulations demonstrate that increasing vaccine efficacy has a remarkable effect than increasing vaccine coverage.
1. Introduction
The foot-and-mouth disease virus (FMDV), a spherical, capsule-free, single-stranded RNA virus, is an infectious disease that causes foot-and-mouth disease (FMD). Both domestic and wild animals with cloven hooves are susceptible to FMDV infection (1). FMD frequently causes dairy cattle to produce less milk and beef cattle and pigs to lose weight. The efficacy of vaccination against infection is frequently weakened by temporal and spatial variations in FMDV antigenicity (2). Consequently, the presence of FMD poses a significant barrier to international trade, has a negative impact on the livestock industry, and results in significant economic losses for animal products (3). Therefore, the World Organization for Animal Health (WOAH) has ranked FMD as the top animal disease.
Animals with a clinical infection always have FMDV in their excretions and secretions, contaminating the environment (4). There are three different types of FMD transmission routes: (1) Direct transmission: the infection spreads through direct contact between infected animals and naive animals (5); (2) Indirect transmission: the infection spreads through indirect contact via fomites (6); (3) Airborne transmission: the transmission of virus-carrying particles through aerosols (7). It has been shown that FMDV can survive various conditions and maintain a longer survival cycle. Therefore, it is important to acknowledge that contaminated environments can transmit FMD infection to animals as a risk factor. Colenutt et al. found that the estimated reproduction number is 1.65, which is significantly lower than the amount for direct animal to animal transmission. However, it would be sufficient to sustain an outbreak even if control measures to prevent direct transmissions, including animal movement and culling restrictions, are implemented (6).
Vaccination is a very effective measure of preventing FMD outbreaks in field conditions and lab settings (8). Evidence has shown that FMDV was radically eliminated in cattle after vaccination (4, 9). It can effectively lower the cost of agricultural production and the cost of health from an economic standpoint. According to reports, FMD has seven serotypes: A, O, C, Asial, SAT1, SAT2, and SAT3, all of which are highly mutagenic (2). Generally, vaccination with one serotype of these seven strains does not protect against other serotypes and does not provide complete protection from a single shot. Hence, to limit FMD infection, emergency ring vaccination and culling of infected animals have been executed. Vaccinating and restricting the movement of infected animals and their products is crucial when dealing with an outbreak of FMD transmission (10, 11). Several studies have quantified the efficacy of FMD vaccinations and evaluated the comprehensive economic consequences from a statistical point of view. The major concern is whether vaccinating all susceptible animals is required to limit the spread of FMD or if vaccinating only against certain agents could be adequate. It is essential to employ mathematical models to qualitatively assess the comprehensive efficacy of FMD vaccination and provide guidance for policymakers. For example, Mushayabasa et al. proposed a basic compartment model to investigate the effects of vaccination and the impact of seasonal conditions on the spread of foot-and-mouth disease (12). De Rueda et al. estimated that in mixed cattle-sheep populations with at least 14% of cattle, vaccination of cattle is sufficient to lower to be less than 1 (8). The causes of FMD outbreaks have been explained in detail by Lyons et al. to demonstrate the effectiveness of vaccines for FMD control (13).
Many dynamic models have been explored for examining long-term FMD behaviors according to their transmission mechanisms. Mathematical models can be used to build preparedness plans in advance of an outbreak epidemic, anticipate outbreaks, and evaluate the efficacy of control measures. Researchers proposed several models to forecast FMD development trends in response to the UK's 2001 FMD epidemics (14–17). For instance, Ferguson et al. built an empirical model to forecast changes in the foot-and-mouth disease outbreak (18). Keeling et al. used the Cambridge–Edinburgh model to address the long tail property of foot-and-mouth disease cases in the UK in 2001 (19, 20). Morris et al. developed the inter-spread model to evaluate the transmission of temporal-spatial foot-and-mouth disease (21). Lewis and Ward adopted a logistic regression model to ascertain whether a collection of explanatory factors was associated with an outbreak of foot-and-mouth disease (22). Ringa and Bau created a pair approximation model to examine the role of vaccination in the optimal long-term prevention of the spread of foot-and-mouth disease (23). Most of these models ignore animal heterogeneities and assume all animals are mixed homogeneously. Jolles et al. found that FMD viruses cannot persist among infected hosts without environmental transmission through experimental and theoretical methods (24). Colenutt et al. found that environmental transmission has been linked to long-lasting FMD outbreaks (6). Bravo de Rueda et al. quantified the FMDV transmission process and showed that the environment is responsible for approximately half of FMDV transmission (25).
Animal movements significantly impact the FMD transmission pattern since it was revealed that FMD had displayed geographical diversity. Mathematical models must be used to reveal the mechanisms of spatial transmission for FMD infection. Three basic models are being used to analyze such temporal-spatial features. Spatial diffusive models investigate the temporal-spatial dynamics described by partial differential equations (26). The main focus of percolation theory is the impact on the farming landscape. Network models examine short- or long-distance transmissions starting from stochastic events (27). The information for the last two models was frequently obtained from a statistical physics point of view (28).
In this paper, we build a linked model of FMD transmission from animal to animal and from FMD virus to animal with an age-space structure. We offer a diffusive mathematical model with partial immunity from vaccination, which implies that the vaccinated animals may catch infection again once they come in contact with the infected ones. The FMD vaccine cannot provide total immunity against FMD transmission. According to numerical analysis, increasing vaccine efficacy has a greater impact than increasing vaccination coverage.
2. Method
The qualitative analysis of the evolution of FMDV transmission relies heavily on mathematical models since they offer a conceptual framework for understanding a particular system's language and making a large-scale prediction. FMDV prevalence is significantly influenced by spatial effects, animal movements, and vaccine efficacy. Identifying the FMDV transmission mechanisms in the UK can be done with the help of a spatial diffusion model (19, 20). In this paper, we used a spatial diffusion model to investigate the efficacy of the vaccination against FMDV infection. The model complies with the “compartmental concepts” proposed by Kermack and McKendrick (29), which couples with the Laplace operator Δ to describe an animal's random movements. This model provides the most accurate representation of spatial FMDV propagation due to diffusion.
2.1. Model formulation
The main concern of this paper is to reveal the temporal and spatial patterns of FMD transmission. According to the compartmental modeling rules, we categorize the total cattle population N(t, x) into three subgroups: susceptible animals, vaccinated animals, and infected animals. S(t, x)(V(t, x)) denotes the space density of susceptible (vaccinated) animals at time t in position where Ω⊂ℝn is a bounded subset of ℝn.
The early detection of the incursion as well as the ability to efficiently trace and identify animals that have been exposed to the source of infection is crucial for curbing FMD transmission. There exists a high-risk period from the first infection to the detected first case, which lasts about 0.5 days after susceptible animals contact infected animals. During such period, there are potentially subtle or unapparent clinical signs of infection and it causes an underestimation of the infection. For the description of such period, we employ an age of infection to investigate the preclinical transmission process. i(t, a, x) represents the age-space density of infected animals with since infection age a, at time t in position x. B(t, x) denotes the space density of foot-and-mouth virus (FMDV) in a contaminant environment at time t in position x∈Ω.
We hypothesize that susceptible cattle directly contact infected cattle and get an infection at rate β(a), where a is the age since infection, and moreover, susceptible cattle can get infection indirectly contacting by fomites in the contaminated environment at rate . Conversely, we assume that vaccinated cattle can be infected both by infected cattle and FMDV at a discount rate σ compared with the original infection. The infection force is defined by
Motivated by the above, the mechanisms of a foot-and-mouth disease model are characterized in the following equations (see Figure 1):
Figure 1
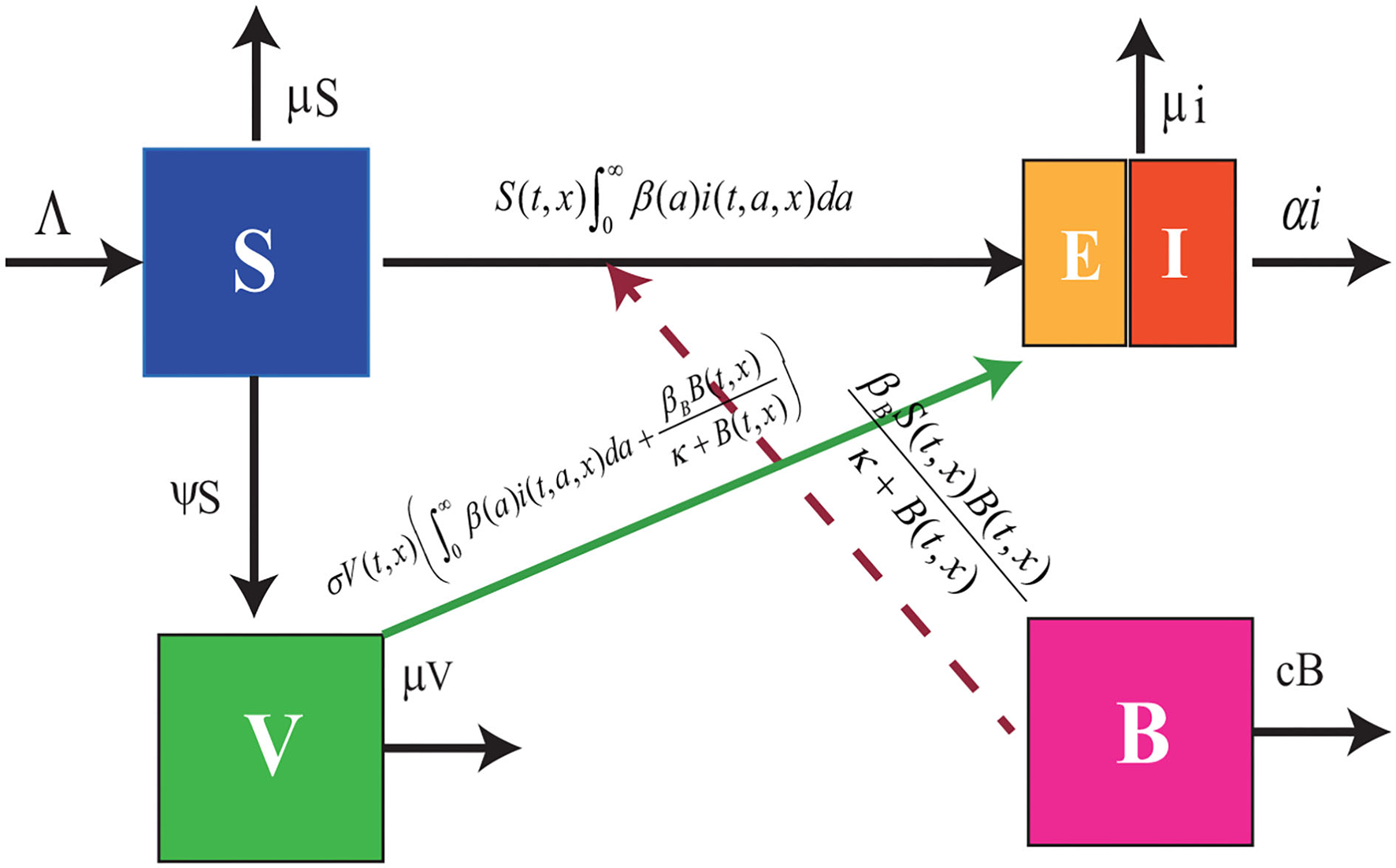
Flowchart of model (Equation 1). The blue box denotes the susceptible cattle, the green box represents the vaccinated cattle, the purple box stands for the density of FMDV in the contaminated environment, and the combined box denotes the infected cattle including the exposed cattle and the symptomatic cattle.
where dj(J = S, V, i, B) denotes the diffusion coefficients of susceptible, vaccinated, infected animals, and foot-mouth viruses, Λ denotes the produce rate, μ and c denote slaughter rate of cattle and the degradation rate of FMDV, respectively. α(·) stands for the death rate caused by FMD. The infected animals release the FMDV into the environment at rate p(·). ∂/∂n denotes the derivative along the outward unite normal vector n.
By Theorem 1.5 in Pazy (33), the operator djΔ(j = S, V, i, B) with the zero flux boundary condition generate the following compact and strongly positive semigroups
where Γj(j = S, V, i, B) are Green functions. Assume that the latent period is τ, then we can separate the infected cattle into two subgroups:
where E(t, x) represents the space density of latent cattle at time t in position x, I(t, x) denotes the space density of infected cattle at time t and position x. Integrating the third equation of model (1) with its initial and boundary conditions, we have that
where
represents the probability of an infected animal survives until infect age a. If we set
Based on the above assumptions on β(·), di(·), and α(·), then the evolution of the latent and infected cattle satisfies
The detailed derivations of E and I are enclosed in Appendix A. From Equation (3), it is easy to see that the compartment E is decoupled, but the latent information is inclosed in the term
where we have assumed that the latent cattle has no infected ability. Replacing i in Equation (1) and ignoring equation E, one arrives at
where we have assumed that pE = 0 suggesting that latent cattle do not release the FMDV into the environment. In what follows, we will focus on the efficacy of vaccination and the diffusion of the latent cattle on the temporal-spatial patterns of FMD transmission.
2.2. Basic reproduction number
The basic reproduction is the average number of secondary cases produced by an infected individual at a completely susceptible environment during his infectious period, which provides an overall measure of the potential for transmission of an infection in a population. Generally, if it is less than one, the disease dies out; otherwise, it invades the host population.
Lemma 0.3 in Appendix B implies that system (Equation (4)) has a disease-free steady state . Linearizing system (Equation (4)) around the disease-free equilibrium E0, we obtain
Let us introduce a newly infection operator
where
Moreover, let us introduce a transition operator
where
Besides, the transit operator B generates the following positive and compact semigroup
Then
Hence, the next-generation operator G can be defined by
where
Therefore, the basic reproduction number is defined by
From the property of Γj, we have concluded that the next operator G is positive and compact. Employing Krein–Rutman Theorem, is a positive eigenvalue with respect to a positive eigenvector ϕ, which suggests that
Letting ϕ = 1, then
From the epidemiological view of points, we introduce the other reproduction number by
where
Theorem 0.0.1. Let and be defined by Equations (6) and (7). The following statements are true:
(1)
(2)
(3)
From what has been discussed, we return to give a detailed explanation for In fact, βe−(μ+α)τ gives the average number of the secondary cases produced by one infected animal and it is still alive after the latent period τ. Hence, gives the average number of the secondary infected animals produced by an infected animal during its infectious period. Similarly, means that the average number of the secondary cases produced by a typical FMDV during its period.
3. Results
3.1. Theoretical results
In this section, we will show the basic reproduction number is a threshold index for disease extinction or persistence.
Lemma 0.0.2. For any the following items hold.
(1) For any t∈ℝ+, S(t, ·)>0 and V(t, ·)>0. Moreover, there exists a positive value such that
(2) If there exists some t0≥0 such that I(t0, ·)≢0 or B(t0, ·)≢0, then
Proof. In the proof of Lemma 0.1 in Appendix B, there exist two positive constants T and M such that for any
In view of the first equation of (4), we note that
By Lemma 4.1, the following system
has a unique equilibrium which is globally asymptotically stable in By the standard parabolic comparison theorem, we conclude that
From Lemma 0.3 in the Appendix B, it follows that
The part (2) is a direct result of Theorem 3 and Theorem 4 in Protter and Weinberger (34) replacing t = 0 by t = t0.
Theorem 0.0.3. Suppose is defined in Equation (6). Then the following results hold.
(1) If then the virus-free equilibrium E0 is globally asymptotically stable;
(2) If then there exists a positive value ϵ>0 such that for all ϕ3(x)≢0 and ϕ4(x)≢0
uniformly for all Moreover, system (Equation 4) has at least one endemic equilibrium E*.
The detailed proof of Theorem 0.0.3 is enclosed in Appendix C.
3.2. Numerical results
In this section, we have conducted numerical examples to show some significant results. First, we fix some parameters in Table 1. Henec, we pick up
Table 1
| Parameters | Biological meanings | Values | Unit | References |
|---|---|---|---|---|
| Λ | Produce rate | 38,340 | day−1 | (30) |
| βB | The transmission rate from FMDV to cattle | 1.3348 × 10−6 | day−1 | (30) |
| κ | The half-saturation concentration of the FMDV | 108 | copies/cattle | (30) |
| μ | The slaughter rate | 0.0018 | day−1 | (5) |
| α | The death rate due to FMD | 1/3.5 | day−1 | (30) |
| c | The natural decay rate of FMDV | 1/30 | day−1 | (31) |
| p | The pathogen production rate of an infected cattle | 104.3 | day−1 | (30) |
| ψ | the vaccinated rate | 0.8 | day−1 | (32) |
| 1−σ | the efficacy of vaccination | 0.5 | day−1 | (11) |
| dS | The diffusion coefficient of susceptible cattle | 0.0005 | - | Assumed |
| dV | The diffusion coefficient of vaccinated cattle | 0.0005 | - | Assumed |
| dI | The diffusion coefficient of infected cattle | 0.0003 | - | Assumed |
| dB | The diffusion coefficient of FMDV | 0.001 | - | Assumed |
List of parameter values.
The initial values are chosen as follows:
3.3. The dynamics of the system
Next, if we choose β = 3.0 × 10−8, then From Theorem 0.0.3 (1), it follows that the virus-free steady state E0 is globally attractive. Figures 2A,B show that the densities of infected animals and the FMD virus decay to zero as time goes to infinity. Enlarging β = 1.0 × 107, we calculate Theorem 0.0.3 (2) ensures that the disease persists when and ϕ∈W0. Figures 2C,D display that the densities of I(t, x) and B(t, x) gradually decay to a positive distribution when time evolves.
Figure 2
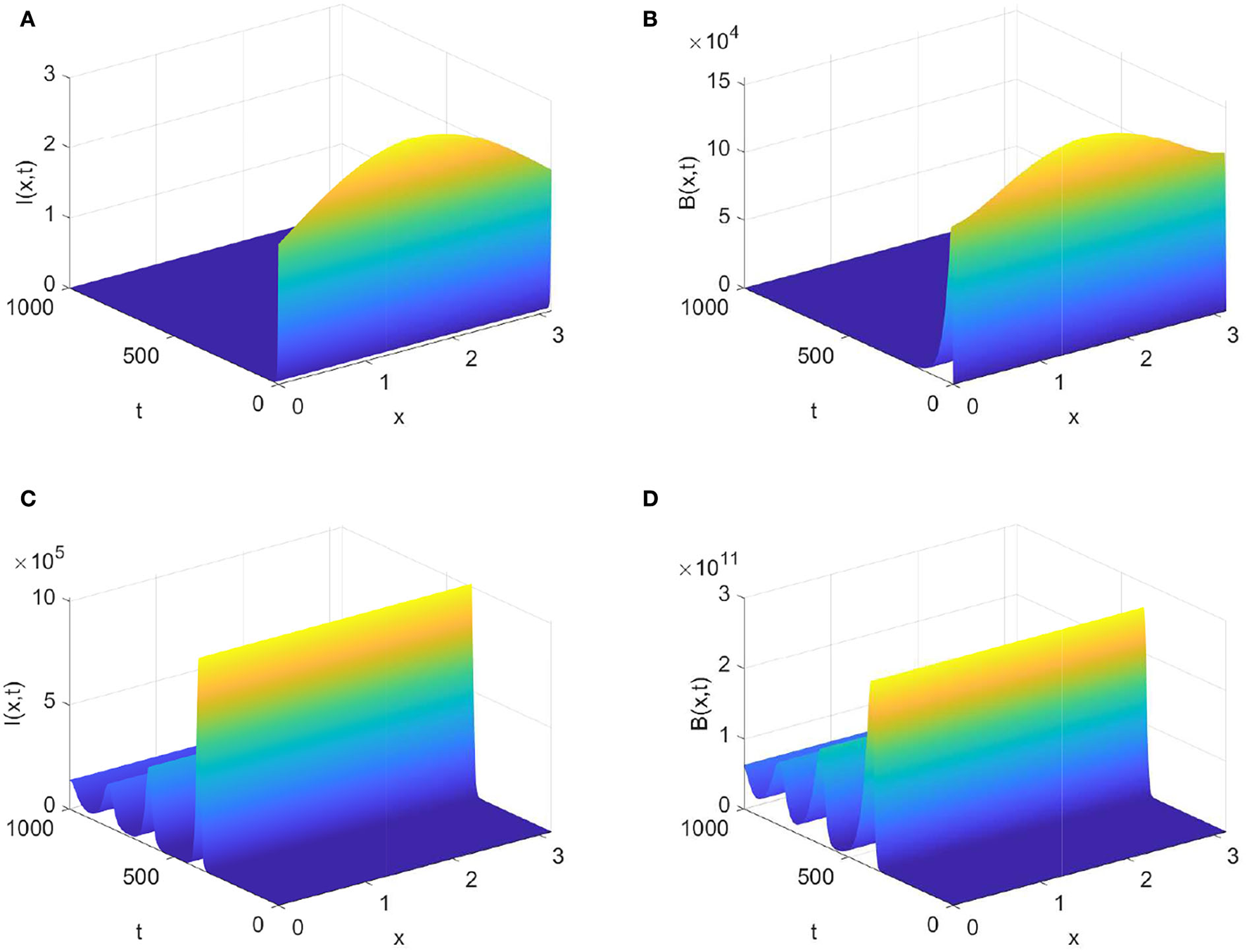
Evolution of infected animals and FMD virus with parameters in Table 1. (A,B) with (C,D) with .
3.4. Sensitivity analysis
Note that model (Equation 4) contains fifteen parameters. It is necessary to find which parameters are more sensitive than other parameters in affecting evolution of FMD infection. Theorem 0.0.3 shows that plays a significant role in determining the outbreak of FMD. Hence, we need to seek the sensitivity analysis of on each parameter. To achieve this aim, we select Latin hypercube sampling (LHS) to identify the rank of key factors that affect the basic reproduction number. In this process, we use partial rank correlation coefficient (PRCC) with 1,000 samples to give a tornado plot, which provides a visible figure to show the importance of every parameter's uncertainty. Figure 3A shows that reducing the transmission from animal to animal, improving the efficacy of vaccination, enlarging the curing rate, and lengthening the latent period are helpful for reducing the size of Moreover, reducing transmission rate from animal to animal has the most importance than other control measures. The samples of converge a normal distribution with an average value 10.1404 [95% CI (10.014–1.02668)] and a variance 2.0369 [95% CI(1.9514–2.1304)] (see Figure 3B).
Figure 3
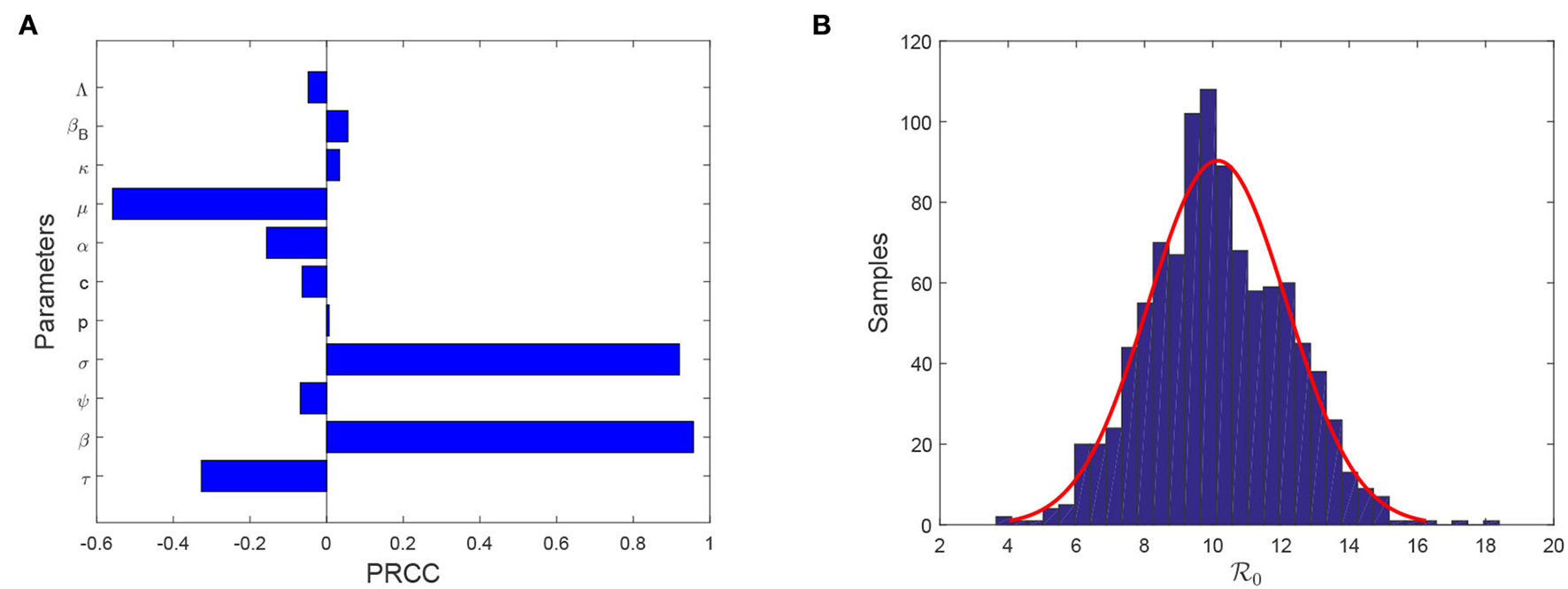
(A) Tornado plot of PRCC for model parameters associated with (B) Samples of .
To evaluate each effective control measure, we verify parameters β, μ, σ, and τ to detect the sensitivity analysis of the dynamics of system (Equation 4). From Figures 4A,B, we find that reducing the transmission risk from animal to animal and improving the efficacy of the vaccination can delay the fist peak arrival time and reduce the sizes of peaks, but such two prevention measures enhance the frequencies of temporal oscillations. Figures 4C,D expound that improving the slaughter rate and lengthening the latent period can reduce the size of the final prevalence, delay fist peak arrival time, and decrease the size of each peak. However, lengthening the latent period enhances the frequency of the temporal oscillations; increasing the slaughter rate has a side effect on the frequency of oscillation patterns.
Figure 4
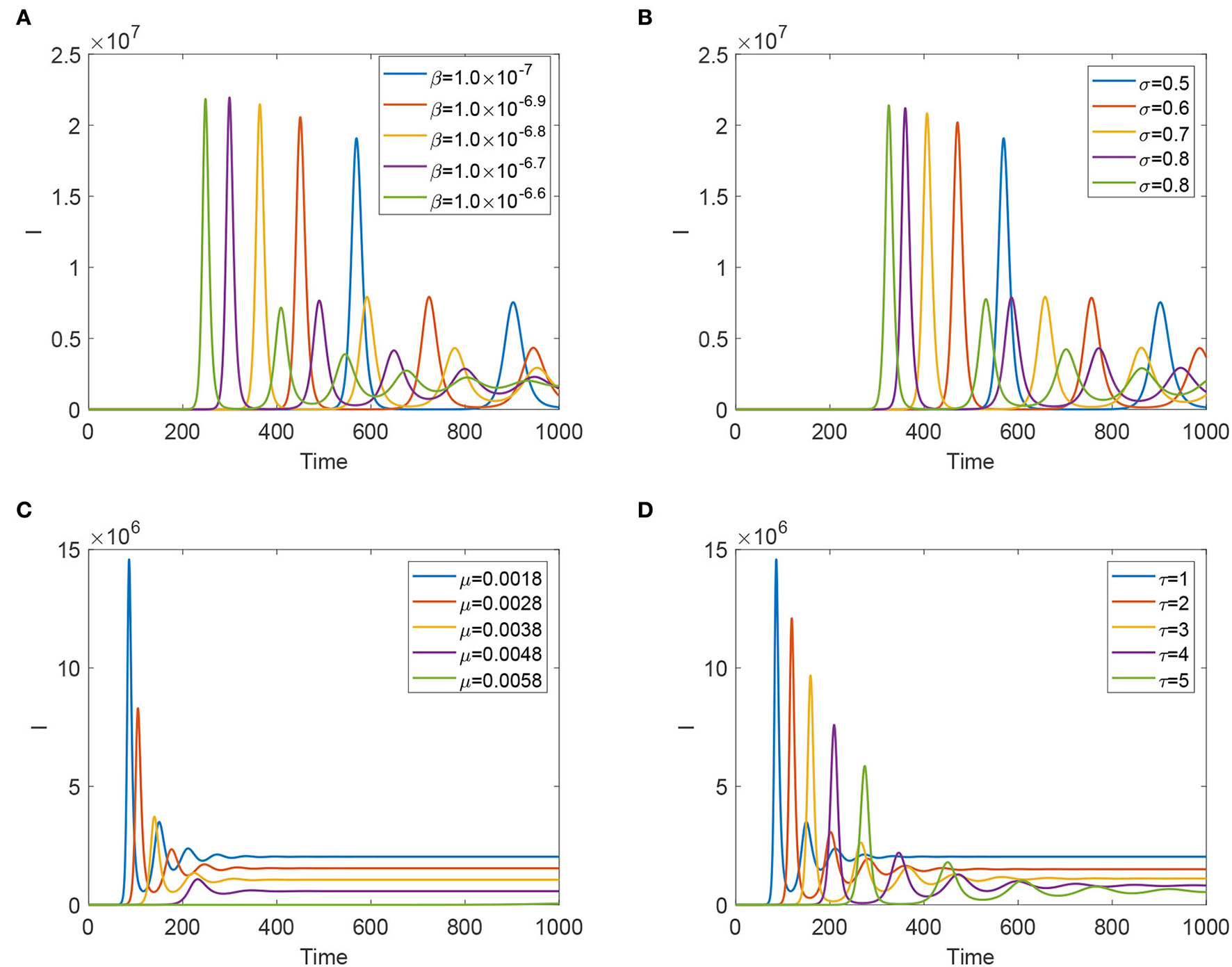
Evolution of infected animals and FMD virus with some significant parameters. (A) With different β. (B) With different σ. (C) With different μ. (D) With different τ.
4. Conclusion and discussion
This paper proposes a nonlocal, diffusive foot-and-mouth disease model that couples the animal to animal and FMDV-to-animal transmission modes. We derived the basic reproduction number using the next generation operator theory, whose characteristic is equivalent to a principal eigenvalue problem. The basic reproduction number is a threshold value determining the outbreak of FMD infection. If the disease ends; otherwise, it persists.
Vaccination is one of the most important preventive measures for curbing FMD prevalence. However, the evaluation of the FMD vaccination's effectiveness plays a significant role in preventing disease transmission since the vaccine does not provide full immunity against FMD. Theorem 0.0.3 states that is a necessary condition for eradicating FMD in a region. is a declining function concerning ψ, as seen in Figure 5. Hence, ψ effectively decreases the size of by increasing vaccination coverage. Moreover, increasing vaccination coverage ψ can delay the first peak arrival period and lower the final prevalence (see Figure 5B). Compared to Figures 4B, 5B, we found that increasing the efficacy of the FMD vaccine has a greater impact on preventing infection than increasing vaccine coverage. The development of more potent vaccines will offer the best defense against FMDV invasion. The first one has a notable accomplishment for reducing the value of , which suggests that slaughtering the animals and purifying the environment play an effect in the face of an outbreak of an emerging FMD. This contrasts the effects of improving the slaughter rate μ and the vaccination rate ψ. However, such measures will inevitably result in significant economic losses. Long-term, increasing vaccination coverage rates may have a greater economic impact on preventing FMD infection.
Figure 5
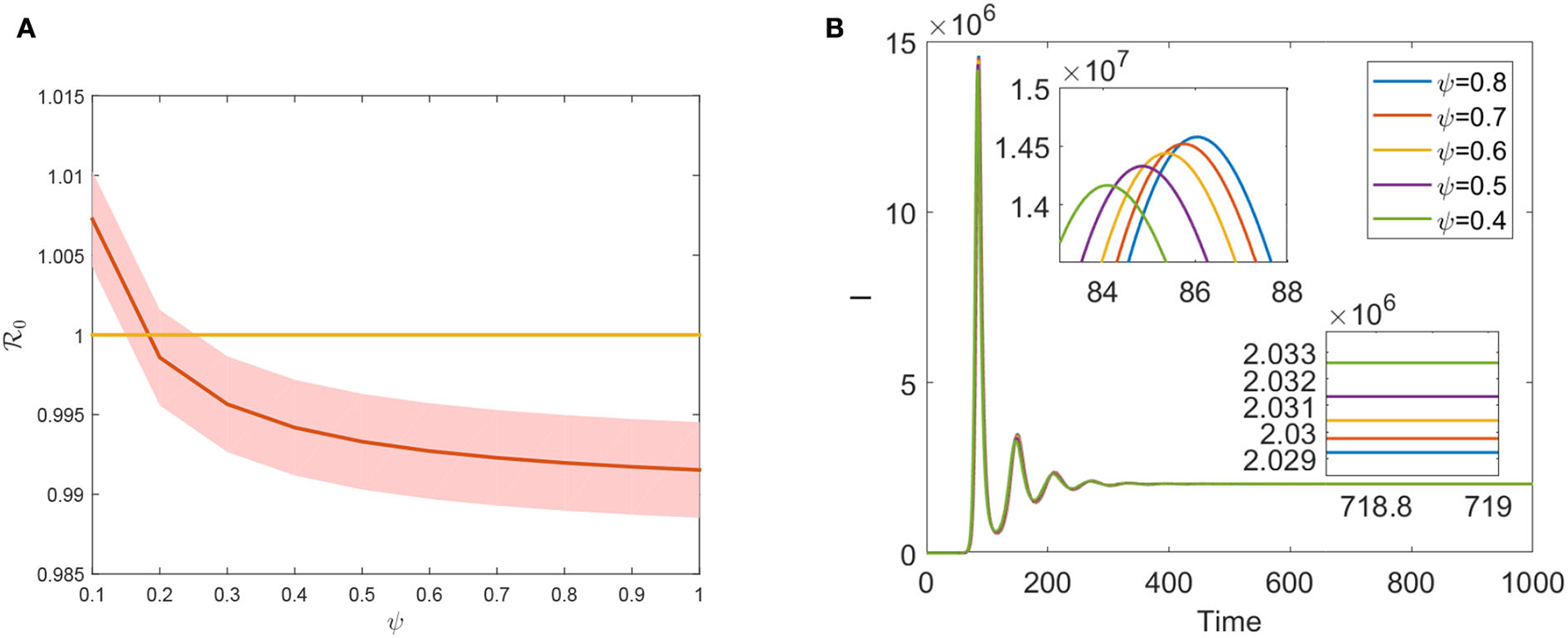
(A) The plot of via ψ. (B) Evolution of total infected cattle varies with different ψ.
The reviews of the expression of have no relation with any diffusive coefficient. As we know, stochastic movement's speed does have an impact on how FMD transmission scenarios develop. We conducted computational experiments to alter the values of dL and dI to understand how diffusive coefficients affect the dynamics of FMD. The scenarios of infected animals eventually flatten (see Figures 6A,B). Increasing the diffusive rate of infected animals is advantageous for reducing the prevalence of FMD.
Figure 6
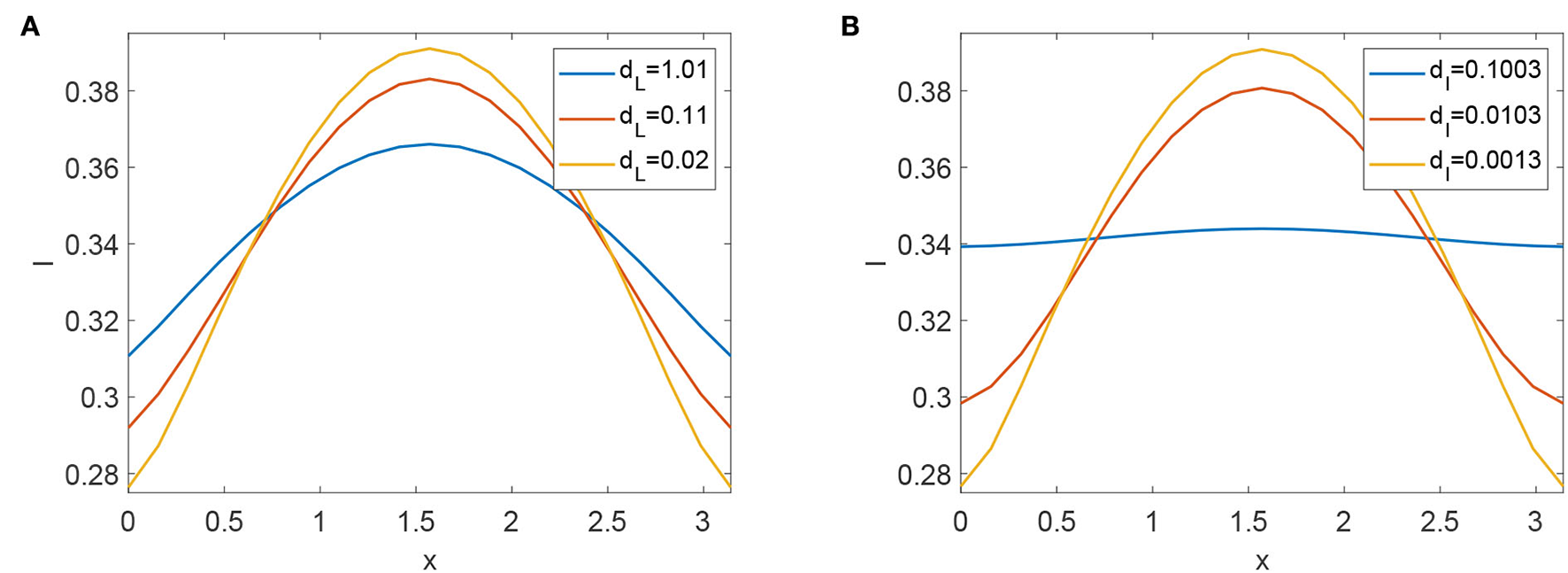
(A) Profiles of infected animals with different diffusive coefficients dL. (B) Profiles of infected animals with different diffusive coefficients dI.
The carriers of FMDV is defined by confirmed ones if the virus or viral genomes are isolated from the esophageal-pharyngeal fluid more than 28 days after infection. Several experimental evidence shows that carriers may be the main reason of the occasional cause of outbreaks (35, 36). Although the role of carriers in the occurrence of new outbreaks is still a matter of debate (37), it is useful to study the risk of carriers on the persistence of FMDV from a cost-benefit perspective (24) and quantify the risk of infection from carriers to susceptible cattle. We will leave these work in future.
Funding
This work was partially supported by Humanities and Social Foundation of Ministry of Education of China (22YJAZH129), the National Natural Science Foundation of China (Nos. 12001339 and 12271143), the Shanxi Province Science foundation (20210302123454), and the Shanxi Province Science Foundation for Youths (No. 201901D211413).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JY wrote the manuscript. XW designed the numerical algorithms. KL reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.952382/full#supplementary-material
References
1.
BelshamGJ. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog Biophys Mol Biol. (1993) 60:241–60. 10.1016/0079-6107(93)90016-D
2.
PatonDSumptionKChrlestonB. Options for control of foot-and-mouth disease: knowledge, capability and policy. Philos Trans R Soc B. (2009) 364:2657–67. 10.1098/rstb.2009.0100
3.
MardonesFPerezASanchezJet al. Parameterization of the duration of infection stages of serotype O foot-and-mouth disease virus: an analytical review and meta-analysis with application to simulation models. Veterinary Res. (2010) 41:45. 10.1051/vetres/2010017
4.
SutmollerPBartelingSOlascoagaRSumptionK. Control and eradication of foot-and-mouth disease. Virus Res. (2003) 91:101–44. 10.1016/S0168-1702(02)00262-9
5.
AlexandersenS DAZhangZ. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol. (2003) 129:1–36. 10.1016/S0021-9975(03)00041-0
6.
ColenuttCBrownENelsonNPatonDEblePDekkerAet al. Quantifying the transmission of foot-and-mouth disease virus in cattle via a contaminated environment. MBio. (2020) 129:11. 10.1128/mBio.00381-20
7.
SchijvenJRijsGBJde Roda HusmanAM. Quantitative risk assessment of FMD virus transmission via water. Risk Anal Int J. (2005) 25:13–21. 10.1111/j.0272-4332.2005.00563.x
8.
De RuedaCDekkerAEblePDe JongM. Vaccination of cattle only is sufficient to stop FMDV transmission in mixed populations of sheep and cattle. Epidemiol Infect. (2015) 143:143. 10.1017/S0950268814003033
9.
LeforbanY. Review of the status of foot and mouth disease and approach to ontrol/eradication in Europe and Central Asia. Revue Scientifique et Technique. (2002) 21:477–492. 10.20506/rst.21.3.1345
10.
HalasaLBoklundACoxSEnoeC. Meta-analysis on the efficacy of foot-and-mouth disease emergency vaccination. Preventive Vet Med. (2011) 98:1–9. 10.1016/j.prevetmed.2010.08.005
11.
ParidaS. Vaccination against foot-and-mouth disease virus: strategies and effectiveness. Expert Rev Vaccines. (2011) 8:347–65. 10.1586/14760584.8.3.347
12.
MushayabasaSPosnyDWangJ. Modeling the intrinsic dynamics of foot-and-mouth disease. Math Biosci Engin. (2016) 13:425. 10.3934/mbe.2015010
13.
LyonsNStärkKvan MaanenCThomasSChepkwonyESangulaAet al. Epidemiological analysis of an outbreak of foot-and-mouth disease (serotype SAT2) on a large dairy farm in Kenya using regular vaccination. Acta Trop. (2015) 143:103–11. 10.1016/j.actatropica.2014.09.010
14.
McCauleyEHAulaqiNASundquistWBNewJCMillerWM. A study of the potential economic impact of foot and mouth disease in the United States. Proc Annu Meet U S Anim Health Assoc. (1977) 81:284–96.
15.
KeelingMJ. Models of foot-and-mouth disease. Proc R Soc B Biol Sci. (2005) 272 :1195–202. 10.1098/rspb.2004.3046
16.
FergusonNMGhaniACDonnellyCAet al. BSE in Northern Ireland: epidemiological patterns past, present and future. Proc R Soc Lond B Biol Sci. (1998) 265:545–54. 10.1098/rspb.1998.0329
17.
DurandBMahulO. An extended state-transition model for foot-and-mouth disease epidemics in France. Prevent Vet Med. (2000) 47:121–39. 10.1016/S0167-5877(00)00158-6
18.
FergusonNMDonnellyCAAndersonRM. Transmission intensity and impact of control policies on the foot and mouth epidemic in Great Britain. Nature. (2001) 413:542–8. 10.1038/35097116
19.
KeelingMJet al. Dynamics of the 2001 UK foot and mouth epidemic: stochastic dispersal in a heterogeneous landscape. Science. (2001) 294:813–7. 10.1126/science.1065973
20.
KeelingMJWoolhouseMEJMayRMDaviesGGrenfellBT. Modelling vaccination strategies against foot-and-mouth disease. Nature. (2003) 421:136–42. 10.1038/nature01343
21.
MorrisRSWilesmithJWSternMWSansonRLStevensonMA. Predictive spatial modelling of alternative control strategies for the foot-and-mouth disease epidemic in Great Britain, 2001. Vet Rec. (2001) 149:137–44. 10.1136/vr.149.5.137
22.
LewisFIMichaelPW. Improving epidemiologic data analyses through multivariate regression modelling. Emerg Themes Epidemiol. (2013) 10:1–10. 10.1186/1742-7622-10-4
23.
RingaNBCT. Impacts of constrained culling and vaccination on control of foot and mouth disease in near-endemic settings: A pair approximation model. Epidemics. (2014) 9:18–30. 10.1016/j.epidem.2014.09.008
24.
JollesAGorsichEGubbinsSBeechlerBBussPJuleffNet al. Endemic persistence of a highly contagious pathogen: foot-and-mouth disease in its wildlife host. Science. (2021) 374:104–9. 10.1126/science.abd2475
25.
Bravo de RuedaCde JongMEbléPLDekkerA. Quantification of transmission of foot-and-mouth disease virus caused by an environment contaminated with secretions and excretions from infected calves. Vet Res. (2015) 46:1–12. 10.1186/s13567-015-0156-5
26.
WangXSunHYangJ. Temporal-spatial analysis of an age-space structured foot-and-mouth disease model with Dirichlet boundary condition. Chaos. (2021) 31:053120. 10.1063/5.0048282
27.
AhmedEHegaziA. On the dynamics of some numerical methods. Int J Mod Phys C. (2000) 11:1481–7. 10.1142/S0129183100001310
28.
KaoR. Landscape fragmentation and foot-and-mouth disease transmission. Vet Rec. (2001) 148:746–7. 10.1136/vr.148.24.746
29.
KermackWMcKendrickA. A contribution to mathematical theory of epidemics. Proc Roy Soc Lond A. (1927) 115:700–21. 10.1098/rspa.1927.0118
30.
ZhangJJinZYuanY. Assessing the spread of foot and mouth disease in mainland China by dynamical switching model. J Theor Biol. (2019) 460:209–19. 10.1016/j.jtbi.2018.09.027
31.
PirtleEBeranG. Virus survival in the environment. Revue Scientifique et Technique. (1991) 10:733–48. 10.20506/rst.10.3.570
32.
JoHYouSChoiJKoMKShinSHSongJet al. Evaluation of novel inactivated vaccines for the SAT, SAT2 and SAT3 serotypes of foot-and-mouth disease in pigs. Virol J. (2019) 16:156. 10.1186/s12985-019-1262-1
33.
PazyA. Semigroups of Linear Operators and Application to Partial Differential Equations. New York, NY: Springer-Verlag (1983).
34.
ProtterMWeinbergerH. Maximum Principles in Differential Equations. Berlin: Springer-Verlag (1984).
35.
SaltJ. The epidemiological significance of FMDV carriers-A review. Soc Vet Epidemiol Prevent Med Proc. (1994) 1:71–84.
36.
Van BekkumJGrenkelHFrederiksHFrenkelS. Observations on the carrier state of cattle exposed to foot-and-mouth disease virus. Tijdschr Diergeneeskd. (1959) 20:1159–64.
37.
TenzinDekkerAVernooijHBoumaAStegemanA. Rate of foot-and-mouth disease virus transmission by carriers quantified from experimental data. Risk Anal. (2008) 28:303–9. 10.1111/j.1539-6924.2008.01020.x
Summary
Keywords
foot-and-mouth disease, the basic reproduction number, vaccination coverage, diffusion, latent period
Citation
Yang J, Wang X and Li K (2022) Temporal-spatial analysis of a foot-and-mouth disease model with spatial diffusion and vaccination. Front. Vet. Sci. 9:952382. doi: 10.3389/fvets.2022.952382
Received
25 May 2022
Accepted
16 November 2022
Published
05 December 2022
Volume
9 - 2022
Edited by
Mariano Pérez-Filgueira, Instituto Nacional de Tecnología Agropecuaria, Argentina
Reviewed by
Joshua Macdonald, Tel Aviv University, Israel; Yiltawe Wungak, National Veterinary Research Institute (NVRI), Nigeria
Updates
Copyright
© 2022 Yang, Wang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyuan Yang yjyang66@sxu.edu.cn
This article was submitted to Veterinary Epidemiology and Economics, a section of the journal Frontiers in Veterinary Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.