
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 21 October 2022
Sec. Parasitology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.949462
 Zhao-Jun Heng1†
Zhao-Jun Heng1† Jian-Fa Yang1,2†
Jian-Fa Yang1,2† Xin-Yan Xie1
Xin-Yan Xie1 Cui-Rong Xu1
Cui-Rong Xu1 Jun-Rong Chen1
Jun-Rong Chen1 Jun Ma1
Jun Ma1 Jun-Jun He1*
Jun-Jun He1* Hua-Ming Mao2*
Hua-Ming Mao2*Giardia duodenalis is an important zoonotic protozoon, which can infect a variety of animals, causing diarrhea and even death of animals or humans. Dairy cattle have been implicated as important sources of human G. duodenalis. However, the information about the prevalence and genetic diversity of G. duodenalis in dairy cattle in China's Yunnan Province remains limited. This study investigated the occurrence and multilocus genotyping of G. duodenalis of Holstein cattle in Yunnan Province, China. A total of 524 fresh fecal samples of Holstein cattle were randomly collected from 8 farms in Yunnan. In this study, 27.5% (144/524) of tested samples were positive for G. duodenalis infection. The highest infection ratio was found in preweaned calves (33.7%), and the infection rates of postweaned calves, growing cattle, and adult cattle were 24.5%, 23.0%, and 17.3%, respectively. The sequence analysis of SSU rRNA gene showed that the predominant assemblage of G. duodenalis in this study was assemblage E (97.9%, 141/144), whereas assemblage A was identified only in three samples (2.1%, 3/144). All G. duodenalis-positive samples were further assayed with nested polymerase chain reaction (PCR) targeting β-giardin (bg), triosephosphate isomerase (tpi), and glutamate dehydrogenase (gdh) genes, and 87, 41, and 81 sequences were obtained, respectively. Mixed infection of assemblages A and E of G. duodenalis was detected in three samples. Multilocus genotyping yielded 23 multilocus genotypes (MLGs). This is the first study that reveals the prevalence data of G. duodenalis in Holstein cattle in Yunnan Province, and the results of this study provided baseline data for the prevention and control of G. duodenalis infection in Holstein cattle in Yunnan Province, China.
Giardia duodenalis is one of the most common parasitic protists that can infect humans, livestock, companionn animals, and wildlife (1). G. duodenalis has a simple life cycle that consists of two stages of development (trophozoite and cyst) (2–4). Trophozoite is the replicative stage that can cause clinical symptoms of giardiasis, while cyst is the main stage of infection (5, 6), and the cysts can excyst in the small intestine when exposed to bile salts and gastric acid. One G. duodenalis cyst releases two trophozoites that parasitize the intestinal epithelia of duodenum and jejunum. Cysts are environmentally resistant and they can survive at 0–8°C for 2 months. Successful infection of G. duodenalis can be established by ingestion of 10–25 cysts (2, 7). G. duodenalis could lead to acute or chronic diarrhea, abdominal cramps, nausea, vomiting, weight loss, and malabsorption in the infected hosts (8, 9), and the severity of clinical symptoms of Giardiasis is related to the virulence of the genotype of G. duodenalis (10).
G. duodenalis consists of eight assemblages (A–H), and some of those aggregates display host specificity (6, 11). Assemblages A and B of G. duodenalis infect various mammals (e.g., bovines) (7), assemblages C and D infect dogs and other canines, assemblage E infects hoofed animals, assemblage F infects cats, assemblage G infects rodents, and assemblage H infects marine vertebrates (1, 12). Recently, assemblages C, D, E, and F have also been found in humans (13–16). Dairy cows are dominantly infected with G. duodenalis of assemblages A, B, and E (11, 17). Calves are more frequently infected with zoonotic assemblages A and B than adult cattle (18). G. duodenalis is one of the most important parasitic pathogens that causes calf diarrhea (19). The infection rate of G. duodenalis in cattle ranges from 2 to 89% (20–27). There was a relatively high prevalence of G. duodenalis infection in cattle in China (28–36). Humans and other animals can be infected by ingesting food or water contaminated with Giardia cysts (8, 37). It is clear that cattle is an important zoonotic reservoir of G. duodenalis and plays important roles in the cross transmission between humans and cattle (38, 39).
A previous study has shown that the occurrence of mixed infection with different assemblages is common in animals (1). Since the use of multiple markers can obtain more reliable results for genotyping (1, 40, 41), multilocus sequence typing (MLST) or multilocus genotypes (MLGs) has been widely applied to study the population genetic structure of parasites, detecting and discriminating the mixed infections of different assemblages (or subassemblages) (42, 43). SSU rRNA, β-giardin (bg), triosephosphate isomerase (tpi), and glutamate dehydrogenase (gdh) genes are four commonly used genetic markers in the genotyping of G. duodenalis. Mixed infection of G. duodenalis will result in inconsistent genotyping results of different loci for that bg, tpi, and gdh genes show high genetic polymorphism (41).
Up to now, the infection data of G. duodenalis in dairy calves mainly focus on the difference between preweaned and postweaned stages in China. The prevalence data of G. duodenalis in dairy cattle remain to be limited in Yunnan, especially the molecular data. In this study, we investigated the infection of G. duodenalis in Holstein cattle in some areas of Yunnan Province by using nested polymerase chain reaction (PCR) targeting the small subunit ribosomal RNA (SSU rRNA) gene of G. duodenalis. All positive samples were further subjected to the gene analysis of bg, tpi, and gdh genes for the genotyping of G. duodenalis.
This study was approved by the Life Science Ethics Committee of Yunnan Agricultural University with the ethical code 202109003. Fecal samples were collected from the Holstein cattle with the permission of the farm owners or managers.
From July to November 2021, a total of 524 fecal samples of Holstein cattle were randomly collected from 8 farms, including one free-ranging farm and seven intensive feeding farms. The age of Holstein cattle ranged from newborn to 2 years old. The collection sites included Dali, Kunming, Qujing, and Chuxiong. Feces were collected from 422 females and 102 males, and only 18 of them had obvious clinical symptoms of diarrhea. Fresh fecal samples (10–20 g per cattle) were collected directly from the rectum using disposable gloves and then transferred separately into disposable plastic bags, marked with the date, age, and geographical information. Fecal samples were stored at 4°C until used for DNA extraction.
The age of Holstein cattle was classified according to the Technical Specification for Standardized Scale Breeding and Production of Dairy Cows (Trial) issued by the Ministry of Agriculture of the People's Republic of China. The cattle ≤ 60 days old are preweaned calves, 61–180 days old are postweaned calves, 181–450 days old are growing cattle, and ≥ 450 days old are adult cows.
Before DNA extraction, stored feces were washed with distilled water and centrifuged at 3,000 × g for 3 min; 250 mg of each washed sample was used for DNA extraction individually. The DNA of the collected sample was extracted by using E.Z.N.A.R® Stool DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) according to the manufacturer's instructions. The extracted DNA was stored at −20°C until use.
All DNA samples were tested with nested PCR that targets the SSU rRNA of G. duodenalis to determine the infection of G. duodenalis (44). The bg (45), tpi (46), and gdh (41) genes were used to determine the subtypes of G. duodenalis. The primers, annealing temperatures, and the expected product sizes of nested PCR are listed in Table 1. The nested PCR reaction of SSU rRNA, bg, gdh, and tpi loci was conducted in a 25-μl reaction system containing 10 × PCR buffer, 200 μM of each dNTP, 0.4 μM of each primer, 1 unit of TaKaRa r-Taq DNA polymerase (TaKaRa Shuzo Co., Ltd.), and 2 μl of DNA sample. Dimethyl sulfoxide (DMSO) was added to enhance the amplification efficiency of nested PCR. The products of the second nested PCR were subjected to electrophoresis on 1% agarose gel and photographed by using a gel imaging system.
The positive secondary nested PCR products were sent to Shenggong Bioengineering (Shanghai) Co., Ltd. for bidirectional sequencing. All sequences obtained in this study were searched against GenBank by using BLAST, and the Clustal X software was used for sequence alignment analysis. All representative nucleotide sequences generated at bg, tpi, and gdh loci in this study were deposited in the GenBank database under accession numbers ON773555–ON773581.
The chi-square (χ2) test was used to analyze the differences in G. duodenalis infection in Holstein cattle among different regions, age, sex, and farming methods. The confidence interval was set as 95%, and P < 0.05 was considered statistically significant. All statistical analyses were performed by using the SPSS20.0 statistical software.
In this study, 144 samples were G. duodenalis positive, and the global positive ratio of G. duodenalis was 27.5% (144/524) (Table 2). G. duodenalis infection was found in all 8 farms, while the prevalence of G. duodenalis varied from farm to farm, and the infection rate ranged from 5.6 to 43.7% (Table 2). The highest infection rate was found in Dali (44.0%, 40/91), followed by Shilin (40.4%, 23/57) and Wuding (40%, 4/14). The lowest infection rate was found in Qijiashan Ranch (5.6%, 5/89) in Qujing. This study showed that the prevalence of G. duodenalis among different regions was significantly different (χ2 = 57.74, df = 8, P < 0.01). Infection of G. duodenalis was found in Holstein cattle in all age groups, among which the highest infection rate (33.7%) was found in preweaned calves. Adult cattle showed the lowest infection rate (17.3%). The χ2 test showed that the prevalence of G. duodenalis among the four age groups was significantly different (χ2 = 11.56, df = 3, P < 0.01) (Table 3). No significant difference was found between intensive feeding and free-ranging farms (χ2 = 2.95, df = 1, P > 0.05) (Table 3). By comparing the infection in different sexes, we found that the infection rate in females and males was 25.6% and 35.3%, respectively. The difference was significant between female and male (χ2 = 3.88, df = 1, P < 0.05) (Table 3). In addition, in this study, no significant difference was found between diarrhea sample (22.2%) and normal feces (27.7%) (χ2 = 0.26, df = 1, P > 0.05) (Table 3).
Sequence analyses of the amplified 144 SSU rRNA genes showed that three of them were classified into G. duodenalis assemblage A (2.1%, 3/144) and the rest of them were a member of assemblage E (97.9%, 141/144). Of the 144 positive fecal samples, 60.4% (n = 87) were bg positive, 28.5% (n = 41) were tpi positive, and 56.3% (n = 81) were gdh positive. Notably, 20.1% (n = 29) of samples were positive for all four genes in this study.
The bg subtype analysis showed that 9 subtypes of assemblage E and 1 subtype of assemblage A were identified, among which 5 subtypes of assemblage E had previously been identified; for that, there was a 100% similarity to the sequences available in GenBank with accession numbers of E9 (KY769091, n = 8), E3 (MK252653, n = 7), E5 (KY769092, n = 6), E1 (MK252651, n = 2), and E2 (MK252652, n = 1); the remaining 4 subtypes represent novel subtypes (Table 4). Assemblage A shares the same sequence with MK610391 in GenBank. Of the tpi subtype, 16 subtypes of assemblage E and 2 subtypes of assemblage A were observed, including 3 subtypes of assemblage E and 1 subtype of assemblage A with sequences identical to those in GenBank, namely, E34 (MK252659, n = 9), E17 (MK252661, n = 3), E2 (EF654683, n = 1), and A1 (MK639171, n = 2). The remaining 13 subtypes of assemblage E and 1 subtype of assemblage A represented novel sequences. At the gdh locus, 13 subtypes of assemblage E and 1 subtype of assemblage A were identified, including 5 known subtypes of assemblage E with sequences identical to those in GenBank, namely, E3 (KY769099, n = 9), E1 (KY769096, n = 8), E14 (KY769097, n = 1), E15 (KY432839, n = 1), and E (MH794177, n = 1). The remaining 8 subtypes of assemblage E and 1 subtype of assemblage A represented novel subtypes in this study (Table 4). Of these subtypes, E9 (n = 8, bg subtype), E34 (n = 6, tpi subtype), and E3 (n = 8, gdh subtype) were the predominant subtypes.
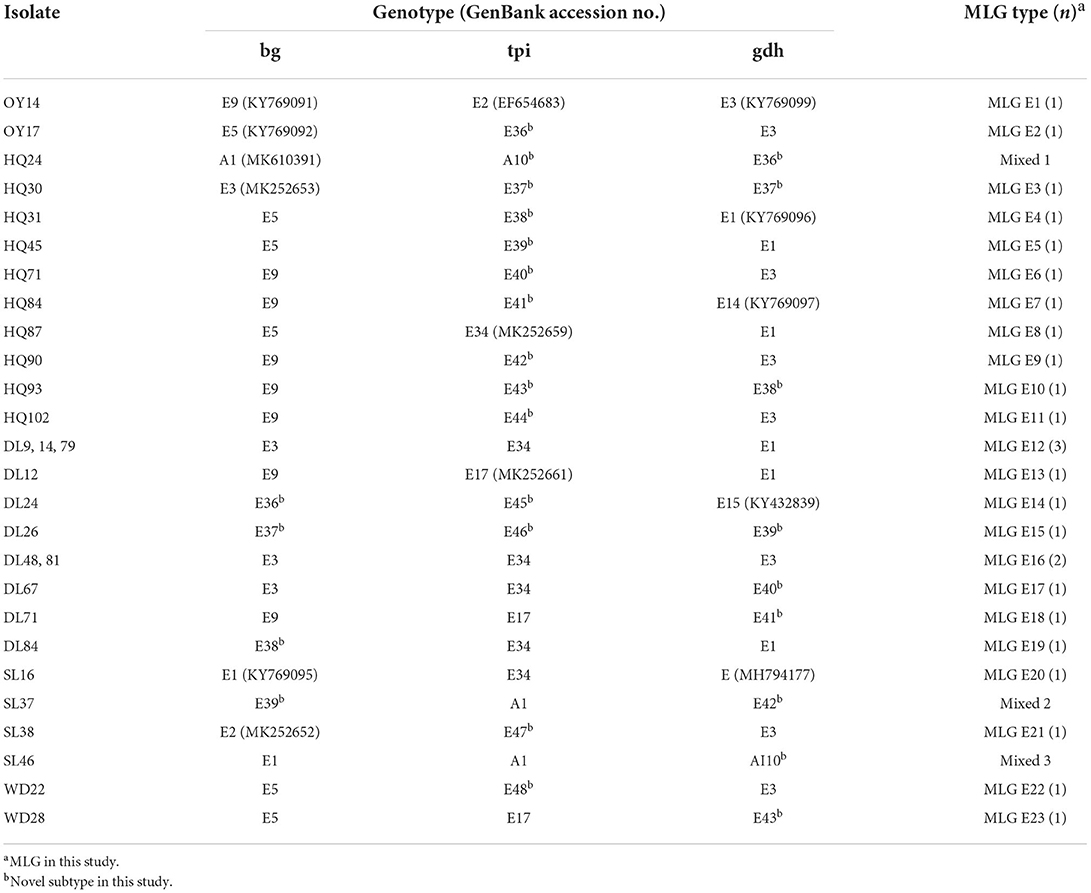
Table 4. Multilocus sequence genotypes of Giardia duodenalis assemblage E in Holstein cattle in Yunnan Province.
In this study, 29 samples were simultaneously amplified at all three intra-assemblage variation genetic loci. Notably, 26 samples' G. duodenalis belonged to assemblage E, including 23 novel assemblage E MLGs (named MLG-E1 to MLG-E23). Three samples showed mixed infection with assemblages E and A (Table 4). MLG-E1 and MLG-E2 were the predominant MLGs in this study. MLGs were detected only in preweaning calves and postweaning calves.
This study indicated that 27.5% of the tested Yunnan Holstein cattle were infected with G. duodenalis, which is similar to the result of dairy cows reported in Hubei (22.6%, 77/339) (33). The infection rate of G. duodenalis (27.5%) observed in this study was higher than that of most provinces in China, such as Jiangsu (20.6%, 281/1,366) (34), Henan (7.2%, 128/1,777) (35), Xinjiang (13.4%, 69/514) (36), and Hebei and Tianjin (4.7%, 49/1,040) (47). It was also higher than that of yak in Qinghai (2.04%, 21/1,027) (48), Tibetan cattle in Tibet (3.8%, 17/442 ) (49), and Yunling cattle in Yunnan (10.49%, 41/391) (30) but lower than that of calf in Sichuan (41.2%, 26/306) (50), Guangdong (74.2%, 288/388) (31), and Shanghai (60.1%, 492/818) (32). Compared with G. duodenalis infection in other countries, the overall infection rate in this study was higher than that of Thailand (5.0%, 45/900) (51), South Korea (5.6%−12.7%) (52, 53), Iran (4.2%, 8/192) (54), and Egypt (13.3%, 33/248) (55). Infection rates are affected by a series of factors, such as geographical and ecological conditions, animal age, health status, sampling season, and diagnostic and research methods.
Previous studies have revealed that the prevalence of G. duodenalis is associated with animal age (35, 56, 57), and the infection is inversely associated with animal age (8, 58, 59). As shown in Table 3, the prevalence of preweaned calves in this study was significantly higher than that of postweaned calves, and the infection rate of G. duodenalis gradually decreased with the increase of age. The finding of this study is consistent with previous reports (34, 60–62). It could be the result that calves are more susceptible to G. duodenalis than adult cows. In this study, no significant difference was observed between intensive feeding and free-ranging farms (χ2 = 2.95, df = 1, P > 0.05), which is consistent with the findings of a previous study in Sichuan (50). By comparing the infection ratio between different sexes, the results of this study showed that the infection rate in females was 25.59%, while the infection rate in males was 35.29%, which is statistically significant (χ2 = 3.88, df = 1, P < 0.05) and contrary to the prevalence data of G. duodenalis in Hubei (33). G. duodenalis infection showed no significant correlation between the stool sample types in this study. This result agreed with the findings of the previous study in Korea (63), although some studies showed that there was a statistical association between G. duodenalis and the type of fecal sample (53).
At present, a total of 8 assemblages (A–H) have been found in G. duodenalis. In this study, two assemblages (assemblages A and E) were detected in dairy cows, and assemblage E was the dominant assemblage in this study. In other studies, assemblage E was also the predominant genotype in dairy cows (1, 32, 35, 36). It has been generally believed that assemblage E is animal-specific and mostly infects ungulates. However, the occurrence of assemblage E in human in Australia (9), Brazil (64), and Egypt (16, 65) has been reported. In this study, zoonotic assemblage A was observed in Dali and Kunming areas, and assemblage A found in this study is close to assemblage A found in the human body. These results indicate that the infected Holstein cattle of Dali and Kunming could be a potential source of zoonotic G. duodenalis.
In this study, SSU gene loci-positive samples were further analyzed by multilocus genes to reveal genetic variation in G. duodenalis. A total of 9 assemblage E subtypes and 1 assemblage A subtype were identified by using bg locus, 16 assemblage E subtypes and 2 assemblage A subtypes were identified by using tpi locus, and 13 assemblage E subtypes and 1 assemblage A subtype were identified by using gdh locus. The combination of sequence polymorphisms at these three loci led to the identification of 23 E MLGs, and three samples had different assemblages at three loci (Table 4). In this study, G. duodenalis A+E mixed infection was detected in preweaned and postweaned calves, which is consistent with other studies in Xinjiang (36), Henan, (35) and Shaanxi (62) provinces of China and Europe (57). All three genes were successfully amplified and sequenced from 29 isolates. A total of 23 MLGs of assemblage E and 3 MLGs of assemblage E+A were identified by three loci, among which MLG-E12 and MLG-E16 were the dominating MLGs in this study.
In this study, there was less overlap for MLGs among samples. It might be the result that G. duodenalis of Holstein cattle in Yunnan is rich in genetic diversity (66). In Guangdong and Sichuan Provinces of China, there was also a very high genetic diversity of assemblage E, and the three genetic loci (bg, tpi, and gdh) show high sequence polymorphism (31, 50). Assemblage E intra-assemblage genetic recombination may be the cause of high subtype diversity (41, 67). Previous studies have also shown that successful amplification rates of the gdh, bg, and tpi loci varied from 8% to 58% (68, 69). The samples that were positive for SSU showed the negative result for the other 3 genetic loci (bg, tpi, and gdh), possibly due to the limited sensitivity of PCR in testing the single-copy gene. This is probably the main limitation of this study. Despite this drawback, MLGs provide a necessary tool to identify different genetic variants within G. duodenalis (4). Further molecular epidemiological studies are needed to be performed to reveal the molecular characteristics of G. duodenalis in Holstein cattle in Yunnan Province, southwestern China.
This is the first MLG characterization study of G. duodenalis in Holstein cattle in Yunnan Province, southwestern China. In addition, the factors associated with G. duodenalis infection were also analyzed. In this study, two assemblages (A and E) of G. duodenalis were found in Holstein cattle, and assemblage E was identified as the dominating genotype. The presence of zoonotic assemblage A in Heqing and Shilin cattle suggests their zoonotic potential. Multilocus genotyping at bg, tpi, and gdh loci revealed 23 novel assemblage E MLGs and 3 E+A mixed infection in Holstein cattle. These findings indicate that G. duodenalis of Holstein cattle in Yunnan is rich in genetic diversity, and the sequence of each gene locus is quite different. For the limited sensitivity of PCR, intensive study is required to reveal the molecular characteristics of G. duodenalis in Holstein cattle in Yunnan, and it is important to strengthen the surveillance of this parasitic disease to ensure the health of livestock and human beings.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary materials.
The animal study was reviewed and approved by the Life Science Ethics Committee of Yunnan Agricultural University.
H-MM, J-FY, and J-JH designed the study. J-FY performed fecal sample collection. Z-JH, X-YX, C-RX, and J-RC performed the molecular genetic studies. JM analyzed sequences. J-JH, J-FY, and H-MM provided laboratory supplies and revised the manuscript. Z-JH interpreted the results and wrote the manuscript. All authors have read and approved the final manuscript.
This study was funded by the Scientific and Technological Mission of Dairy Cow Industry in Heqing County, Yunnan Province (Grant No. 202204BI090005), the Veterinary Public Health Innovation Team of Yunnan Province (Grant No. 202105AE160014), and the Yunnan Expert Workstation (Grant No. 202005AF150041).
The authors would like to thank the managers of farms involved in this study for providing assistance during fecal sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. (2011) 24:110–40. doi: 10.1128/CMR.00033-10
2. Huang DB, White AC. An updated review on Cryptosporidium and Giardia. Gastroenterol Clin North Am. (2006) 35:291–314. doi: 10.1016/j.gtc.2006.03.006
3. Einarsson E. Ma'ayeh S, Svärd SG. An up-date on Giardia and giardiasis. Curr Opin Microbiol. (2016) 34:47–52. doi: 10.1016/j.mib.2016.07.019
4. Thompson RCA, Ash A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect Genet Evol. (2016) 40:315–23. doi: 10.1016/j.meegid.2015.09.028
5. Ryan U, Cacciò SM. Zoonotic potential of Giardia. Int J Parasitol. (2013) 43:943–56. doi: 10.1016/j.ijpara.2013.06.001
6. Cacciò SM, Lalle M, Svärd SG. Host specificity in the Giardia duodenalis species complex. Infect Genet Evol. (2018) 66:335–45. doi: 10.1016/j.meegid.2017.12.001
7. Abeywardena H, Jex AR, Gasser RB. A perspective on Cryptosporidium and Giardia, with an emphasis on bovines and recent epidemiological findings. Adv Parasitol. (2015) 88:243–301. doi: 10.1016/bs.apar.2015.02.001
8. Li J, Wang H, Wang R, Zhang L. Giardia duodenalis infections in humans and other animals in China. Front Microbiol. (2017) 8:2004. doi: 10.3389/fmicb.2017.02004
9. Vivancos V, González-Alvarez I, Bermejo M, Gonzalez-Alvarez M. Giardiasis: characteristics, pathogenesis and new insights about treatment. Curr Top Med Chem. (2018) 18:1287–303. doi: 10.2174/1568026618666181002095314
10. Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. (2008) 160:75–80. doi: 10.1016/j.molbiopara.2008.04.006
11. Heyworth MF. Giardia duodenalis genetic assemblages and hosts. Parasite. (2016) 23:13. doi: 10.1051/parasite/2016013
12. Ryan U, Zahedi A. Molecular epidemiology of giardiasis from a veterinary perspective. Adv Parasitol. (2019) 106:209–54. doi: 10.1016/bs.apar.2019.07.002
13. Zhang Y, Mi R, Yang L, Gong H, Xu C, Feng Y, et al. Wildlife is a potential source of human infections of Enterocytozoon bieneusi and Giardia duodenalis in southeastern China. Front Microbiol. (2021) 12:692837. doi: 10.3389/fmicb.2021.692837
14. Zahedi A, Field D, Ryan U. Molecular typing of Giardia duodenalis in humans in Queensland-first report of assemblage E. Parasitology. (2017) 144:1154–61. doi: 10.1017/S0031182017000439
15. Gelanew T, Lalle M, Hailu A, Pozio E, Cacciò SM. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. (2007) 102:92–9. doi: 10.1016/j.actatropica.2007.04.003
16. Foronda P, Bargues MD, Abreu-Acosta N, Periago MV, Valero MA, Valladares B, et al. Identification of genotypes of Giardia intestinalis of human isolates in Egypt. Parasitol Res. (2008) 103:1177–81. doi: 10.1007/s00436-008-1113-2
17. Bartelt LA, Sartor RB. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep. (2015) 7:62. doi: 10.12703/P7-62
18. Khan SM, Debnath C, Pramanik AK, Xiao L, Nozaki T, Ganguly S. Molecular evidence for zoonotic transmission of Giardia duodenalis among dairy farm workers in West Bengal, India. Vet Parasitol. (2011) 178:342–5. doi: 10.1016/j.vetpar.2011.01.029
19. Thompson RC. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. (2004) 126:15–35. doi: 10.1016/j.vetpar.2004.09.008
20. Bartley PM, Roehe BK, Thomson S, Shaw HJ, Katzer F. Detection of potentially human infectious assemblages of Giardia duodenalis in fecal samples from beef and dairy cattle in Scotland. Parasitology. (2019) 146:1123–30. doi: 10.1017/S0031182018001117
21. Mahato MK, Singh DK, Rana HB, Acharya KP. Prevalence and risk factors associated with Giardia duodenalis infection in dairy cattle of Chitwan, Nepal. J Parasit Dis. (2018) 42:122–6. doi: 10.1007/s12639-017-0975-6
22. Wegayehu T, Adamu H, Petros B. Prevalence of Giardia duodenalis and Cryptosporidium species infections among children and cattle in North Shewa Zone, Ethiopia. BMC Infect Dis. (2013) 13:419. doi: 10.1186/1471-2334-13-419
23. Hailu M, Asmare K, Gebremedhin EZ, Sheferaw D, Gizaw D, Di Marco V, et al. Cryptosporidium and Giardia infections in dairy calves in southern Ethiopia. Parasite Epidemiol Control. (2020) 10:e00155. doi: 10.1016/j.parepi.2020.e00155
24. Lichtmannsperger K, Hinney B, Joachim A, Wittek T. Molecular characterization of Giardia intestinalis and Cryptosporidium parvum from calves with diarrhoea in Austria and evaluation of point-of-care tests. Comp Immunol Microbiol Infect Dis. (2019) 66:101333. doi: 10.1016/j.cimid.2019.101333
25. Becher KA, Robertson ID, Fraser DM, Palmer DG, Thompson RC. Molecular epidemiology of Giardia and Cryptosporidium infections in dairy calves originating from three sources in Western Australia. Vet Parasitol. (2004) 123:1–9. doi: 10.1016/j.vetpar.2004.05.020
26. Squire SA, Yang R, Robertson I, Ayi I, Ryan U. Molecular characterization of Cryptosporidium and Giardia in farmers and their ruminant livestock from the Coastal Savannah zone of Ghana. Infect Genet Evol. (2017) 55:236–43. doi: 10.1016/j.meegid.2017.09.025
27. Onder Z, Simsek E, Duzlu O, Yetismis G, Yildirim A. Molecular prevalence and genotyping of Giardia duodenalis in cattle in central Anatolia region of Turkey. Parasitol Res. (2020) 119:1–8. doi: 10.1007/s00436-020-06771-8
28. Li F, Wang H, Zhang Z, Li J, Wang C, Zhao J, et al. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Beijing, China. Vet Parasitol. (2016) 219:61–5. doi: 10.1016/j.vetpar.2016.01.023
29. Wang Y, Cao J, Chang Y, Yu F, Zhang S, Wang R, et al. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Gansu, northwest China. Parasite. (2020) 27:62. doi: 10.1051/parasite/2020058
30. Liang XX, Zou Y, Li TS, Chen H, Wang SS, Cao FQ, et al. First report of the prevalence and genetic characterization of Giardia duodenalis and Cryptosporidium spp. in Yunling cattle in Yunnan province, southwestern China. Microb Pathog. (2021) 158:105025. doi: 10.1016/j.micpath.2021.105025
31. Feng Y, Gong X, Zhu K, Li N, Yu Z, Guo Y. et al. Prevalence and genotypic identification of Cryptosporidium spp. Giardia duodenalis and Enterocytozoon bieneusi in pre-weaned dairy calves in Guangdong, China. Parasit Vectors. (2019) 12:41. doi: 10.1186/s13071-019-3310-5
32. Wang X, Cai M, Jiang W, Wang Y, Jin Y, Li N, et al. High genetic diversity of Giardia duodenalis assemblage E in pre-weaned dairy calves in Shanghai, China, revealed by multilocus genotyping. Parasitol Res. (2017) 116:2101–10. doi: 10.1007/s00436-017-5509-8
33. Fan Y, Wang T, Koehler AV, Hu M, Gasser RB. Molecular investigation of Cryptosporidium and Giardia in pre-and post-weaned calves in Hubei province, China. Parasit Vectors. (2017) 10:519. doi: 10.1186/s13071-017-2463-3
34. Wang R, Li N, Jiang W, Guo Y, Wang X, Jin Y, et al. Infection patterns, clinical significance, and genetic characteristics of Enterocytozoon bieneusi and Giardia duodenalis in dairy cattle in Jiangsu, China. Parasitol Res. (2019) 118:3053–60. doi: 10.1007/s00436-019-06426-3
35. Wang H, Zhao G, Chen G, Jian F, Zhang S, Feng C, et al. Multilocus genotyping of Giardia duodenalis in dairy cattle in Henan, China. PLoS ONE. (2017) 9:e100453. doi: 10.1371/journal.pone.0100453
36. Qi M, Wang H, Jing B, Wang R, Jian F, Ning C, et al. Prevalence and multilocus genotyping of Giardia duodenalis in dairy calves in Xinjiang, northwestern China. Parasit Vectors. (2016) 9:1–6. doi: 10.1186/s13071-016-1828-3
37. Dixon BR. Giardia duodenalis in humans and animals—transmission and disease. Res Vet Sci. (2020) 135:283–9. doi: 10.1016/j.rvsc.2020.09.034
38. Smith HV, Cacciò SM, Tait A, McLauchlin J, Thompson RC. Tools for investigating the environmental transmission of Cryptosporidium and Giardia infections in humans. Trends Parasitol. (2006) 22:160–7. doi: 10.1016/j.pt.2006.02.009
39. Jian YN, Zhang XY, Li XP, Karanis G, Ma LQ, Karanis P. Prevalence and molecular characterization of Giardia duodenalis in cattle and sheep from the Qinghai-Tibetan Plateau Area (QTPA), northwestern China. Vet Parasitol. (2018) 250:40–4. doi: 10.1016/j.vetpar.2017.12.001
40. Sprong H, Cacciò SM, van der Giessen JW. ZOOPNET network and partners. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl Trop Dis. (2009) 3:e558. doi: 10.1371/journal.pntd.0000558
41. Cacciò SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int J Parasitol. (2008) 38:1523–31. doi: 10.1016/j.ijpara.2008.04.008
42. Liang WT, Liu H, Deng Y. Multilocus sequence typing and its application on population genetic structure analysis of parasites. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. (2014) 26:449–52. doi: 10.16250/j.32.1374.2014.04.008
43. Akinkuotu OA, Greenwood SJ, McClure JT, Takeet MI, Otesile EB, Olufemi F. Multilocus genotyping of Giardia duodenalis infecting rabbits in Ogun State, Nigeria. Vet Parasitol Reg Stud Rep. (2018) 13:171–6. doi: 10.1016/j.vprsr.2018.06.005
44. Appelbee AJ, Frederick LM, Heitman TL, Olson ME. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet Parasitol. (2003) 112:289–94. doi: 10.1016/S0304-4017(02)00422-3
45. Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Cacciò SM. Genetic heterogeneity at the β-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. (2005) 35:207–13. doi: 10.1016/j.ijpara.2004.10.022
46. Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, et al. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis. (2003) 9:1444–52. doi: 10.3201/eid0911.030084
47. Hu S, Liu Z, Yan F, Zhang Z, Zhang G, Zhang L. et al. Zoonotic and host-adapted genotypes of Cryptosporidium spp, Giardia duodenalis and Enterocytozoon bieneusi in dairy cattle in Hebei and Tianjin, China. Vet Parasitol. (2017) 248:68–73. doi: 10.1016/j.vetpar.2017.10.024
48. Song JK, Wang D, Ren M, Yang F, Wang PX, Zou M, et al. Seasonal prevalence and novel multilocus genotypes of Giardia duodenalis in Yaks (Bos grunniens) in Qinghai province, western China. Iran J Parasitol. (2021) 16:548–54. doi: 10.18502/ijpa.v16i4.7865
49. Wu Y, Chen Y, Chang Y, Zhang X, Li D, Wang L., et al. Genotyping and identification of Cryptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi from free–range Tibetan yellow cattle and cattle–yak in Tibet, China. Acta Tropica. (2020) 212:105671. doi: 10.1016/j.actatropica.2020.105671
50. Dan J, Zhang X, Ren Z, Wang L, Cao S, Shen L, et al. Occurrence and multilocus genotyping of Giardia duodenalis from post-weaned dairy calves in Sichuan province, China. PLoS One. (2019) 14:e0224627. doi: 10.1371/journal.pone.0224627
51. Inpankaew T, Jiyipong T, Thadtapong N, Kengradomkij C, Pinyopanuwat N, Chimnoi W, et al. Prevalence and genotype of Giardia duodenalis in dairy cattle from northern and northeastern part of Thailand. Acta Parasitol. (2015) 60:459–61. doi: 10.1515/ap-2015-0063
52. Lee YJ, Ryu JH, Shin SU, Choi KS. Prevalence and molecular characterization of Cryptosporidium and Giardia in pre-weaned native calves in the Republic of Korea. Parasitol Res. (2019) 118:3509–17. doi: 10.1007/s00436-019-06482-9
53. Oh SI, Jung SH, Lee HK, Choe C, Hur TY, So KM. Multilocus genotyping of Giardia duodenalis occurring in Korean native calves. Vet Sci. (2021) 8:118. doi: 10.3390/vetsci8070118
54. Kiani-Salmi N, Fattahi-Bafghi A, Astani A, Sazmand A, Zahedi A, Firoozi Z, et al. Molecular typing of Giardia duodenalis in cattle, sheep and goats in an arid area of central Iran. Infect Genet Evol. (2019) 75:104021. doi: 10.1016/j.meegid.2019.104021
55. Naguib D, El-Gohary AH, Mohamed AA, Roellig DM, Arafat N, Xiao L. Age patterns of Cryptosporidium species and Giardia duodenalis in dairy calves in Egypt. Parasitol Int. (2018) 67:736–41. doi: 10.1016/j.parint.2018.07.012
56. Cui Z, Wang L, Cao L, Sun M, Liang N, Wang H, et al. Genetic characteristics and geographic segregation of Giardia duodenalis in dairy cattle from Guangdong province, southern China. Infect Genet Evol. (2018) 66:95–100. doi: 10.1016/j.meegid.2018.09.019
57. Geurden T, Vanderstichel R, Pohle H, Ehsan A, von Samson-Himmelstjerna G, Morgan ER, et al. A multicentre prevalence study in Europe on Giardia duodenalis in calves, with molecular identification and risk factor analysis. Vet Parasitol. (2012) 190:383–90. doi: 10.1016/j.vetpar.2012.06.039
58. Muhid A, Robertson I, Ng J, Yang R, Ryan U. Prevalence of Giardia spp. infection in pre-weaned and weaned calves in relation to management factors. Vet J. (2012) 191:135–7. doi: 10.1016/j.tvjl.2011.01.007
59. Santín M, Trout JM, Fayer R. A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet Parasitol. (2009) 162:40–5. doi: 10.1016/j.vetpar.2009.02.008
60. Huang J, Yue D, Qi M, Wang R, Zhao J, Li J, et al. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Ningxia, northwestern China. BMC Vet Res. (2014) 10:292. doi: 10.1186/s12917-014-0292-6
61. Liu G, Su Y, Zhou M, Zhao J, Zhang T, Ahmad W, et al. Prevalence and molecular characterization of Giardia duodenalis isolates from dairy cattle in northeast China. Exp Parasitol. (2015) 154:20–4. doi: 10.1016/j.exppara.2015.03.020
62. Wang XT, Wang RJ, Ren GJ Yu ZQ, Zhang LX, Zhang SY, et al. Multilocus genotyping of Giardia duodenalis and Enterocytozoon bieneusi in dairy and native beef (Qinchuan) calves in Shaanxi province, northwestern China. Parasitol Res. (2016) 115:1355–61. doi: 10.1007/s00436-016-4908-6
63. Lee YJ, Han DG Ryu JH, Chae JB, Chae JS Yu DH, et al. Identification of zoonotic Giardia duodenalis in Korean native calves with normal feces. Parasitol Res. (2018) 117:1969–73. doi: 10.1007/s00436-018-5863-1
64. Fantinatti M, Bello AR, Fernandes O, Da-Cruz AM. Identification of Giardia lamblia assemblage E in humans points to a new anthropozoonotic cycle. J Infect Dis. (2016) 214:1256–9. doi: 10.1093/infdis/jiw361
65. Abdel-Moein KA, Saeed H. The zoonotic potential of Giardia intestinalis assemblage E in rural settings. Parasitol Res. (2016) 115:3197–202. doi: 10.1007/s00436-016-5081-7
66. Chen D, Zou Y, Li Z, Wang SS, Xie SC, Shi LQ, et al. Occurrence and multilocus genotyping of Giardia duodenalis in black-boned sheep and goats in southwestern China. Parasit Vectors. (2019) 12:102. doi: 10.1186/s13071-019-3367-1
67. Aguiar JM, Silva SO, Santos VA, Taniwaki SA, Oliveira TM, Ferreira HL, et al. Evidence of heterozygosity and recombinant alleles in single cysts of Giardia duodenalis. Rev Bras Parasitol Vet. (2016) 25:187–95. doi: 10.1590/S1984-29612016031
68. Rafiei A, Baghlaninezhad R, Köster PC, Bailo B, Hernández de. Mingo M, Carmena D, et al. Multilocus genotyping of Giardia duodenalis in southwestern Iran. A community survey. PLoS ONE. (2020) 15:e0228317. doi: 10.1371/journal.pone.0228317
Keywords: Giardia duodenalis, prevalence, multilocus genotypes (MLGs), Holstein cattle, genotyping
Citation: Heng Z-J, Yang J-F, Xie X-Y, Xu C-R, Chen J-R, Ma J, He J-J and Mao H-M (2022) Prevalence and multilocus genotyping of Giardia duodenalis in Holstein cattle in Yunnan, China. Front. Vet. Sci. 9:949462. doi: 10.3389/fvets.2022.949462
Received: 21 May 2022; Accepted: 28 September 2022;
Published: 21 October 2022.
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Catalina Avendano, Beckmaan Research Institute, City of Hope, United StatesCopyright © 2022 Heng, Yang, Xie, Xu, Chen, Ma, He and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Jun He, aGVqdW5qdW42MTdAMTYzLmNvbQ==; Hua-Ming Mao, bWFvaG1AdmlwLnNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.