- 1Feed In Tech Join lab, 42 rue Georges Morel, Beaucouzé, France
- 2Nor Feed, 3 rue Amedeo Avogadro, Beaucouzé, France
- 3URSE, Ecole Supérieure d'Agricultures, University Bretagne Loire, Angers, France
For decades avian coccidiosis prevention was based on the use of synthetic coccidiostats. However, their intensive use led to the development of resistance phenomena. In addition, societal demand is increasing for antibiotic-free animal products. Thus, there is a need for a natural and efficient solution for coccidiosis management. Saponin-rich plants, like Yucca schidigera and Trigonella foenum-graecum, are promising tools for coccidiosis management. This study assessed the effects of supplementing broiler chickens with a commercial blend of these two plants (NorponinXO2) under an experimental Eimeria challenge and compared their effects to monensin supplementation. Three trials were performed. For each trial, chickens were divided into 4 groups, untreated uninfested control (UUC), infested untreated control (IUC), infested supplemented with 120 ppm of Monensin in feed (PM), and infested supplemented with 250 ppm of Norponin XO2 in the feed (PX). Chickens were raised in cages; experimental infestation was performed on d14. On d21, intestinal lesions (ILs) scores and growth performances were recorded. A statistical study was carried out on each trial, as well as data from the 3 trials. Experimental infestation reduced in a significant way final body weight in IUC broilers compared to UUC broilers. This loss was numerically compensated by PM and PX treatment. As expected, intestinal lesions were almost absent in the UUC group; however, broilers from the IUC group showed a higher intestinal lesion occurrence. Supplementations with Monensin and NPXO were able to reduce intestinal lesions occurrence. These results suggest that NPXO supplementation is as efficient as Monensin in managing coccidiosis.
Introduction
As broiler production intensified, several breaks in productivity appeared; Coccidiosis is among these breaks. This disease is caused by an apicomplexan parasite of the genus Eimeria (1). Animal infestation by the parasite seriously impairs broilers' growth performances (reduced body weight and feed efficiency) and negatively impacts their health status and welfare (2). In addition to negatively impacting the health and welfare of chickens, coccidiosis is a disease with a serious economic impact on poultry producers. This negative impact is linked to the cost of chemoprevention and loss due to decreased animal growth performances (3, 4). In a recent study, Blake et al. estimate the cost of coccidiosis at 0,21US$/bird (5).
The use of synthetic ionophores, like monensin in the chemoprevention of coccidiosis, helped the poultry industry to reach high levels of productivity while preventing coccidiosis (Chapman, 2009). However, this intensive use of these synthetic molecules for decades led to the development of resistant strains of Eimeria worldwide (6–10). Moreover, residues of these molecules in animal products and/or the environment are a serious issue (11–13). Facing these new challenges, poultry producers are looking for an efficient tool to add to their global strategy in managing coccidiosis (14). Plant and plant-extract feed additives are among the interesting approaches used to control coccidiosis in broiler flocks (15). The Saponins, thanks to their ability to disrupt cellular membranes, are a promising approach to managing coccidiosis in broiler chickens (16, 17). However, the perception of the effectiveness of these solutions by poultry producers is not always as positive as conventional solutions based on synthetic molecules according to a recent market survey (internal data). The reasons evoked by the professionals during this survey to explain this perception are mainly the rarity of data evidencing their efficacy using usual experimental methods and their mode of action. This highlights the need to generate data using experimental infestation methods to evaluate new solutions for coccidiosis management. The objective of this study was to evaluate and compare the effectiveness of feed supplementation with saponin-rich plant premixture (Norponin XO2®) to ionophore monensin under various experimental Eimeria challenges.
Materials and Methods
Experimental Design, Products, and Animal Management
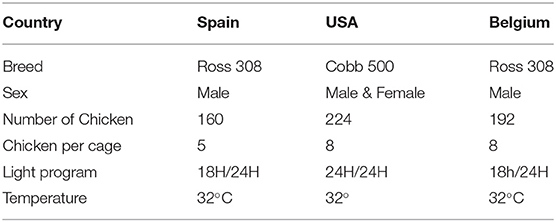
Three trials were carried out in three experimental facilities. The same experimental design was applied in each facility. The trials respectively took place in Belgium (Wolvenhof, Poulpharm animal site), the U.S.A (Willington, Colorado, COLORADO QUALITY RESEARCH, INC.), and Spain (Murcia, IMASDE Campus de Espinardo). These three centers were chosen to consolidate the results by maximizing the diversification of the breeding system (chick origin/strain, feed material, and operators). The trials were conducted according to the principles of GCP (2000) Guidelines on Good Clinical Practice for Clinical Trials for Registration of Veterinary Medicinal Products (VICH) and met appropriate current quality standards indicated by European Food Safety Authority. The experimental protocols used in this study were approved by the competent authorities of the country for each trial. Chickens were randomly distributed to either one of four treatments: Untreated Uninfested Control, Infested Untreated Control, Infested and supplemented with recommended inclusion rate in the feed of monensin (120 ppm), Infested and supplemented with the recommended inclusion rate in the feed of Norponin XO2® (250 ppm), and a premixture of saponin-rich plants. Broilers were raised to the age of 21 days in cages. For the supplemented groups, the supplementation started on the first day of the experiment. Feed and water were distributed ad libitum for all trials within the 3 experimental facilities. The experimental conditions (sex, strain, the number of animals, the number of replicates, and the size of the cage) are specified in Table 1.
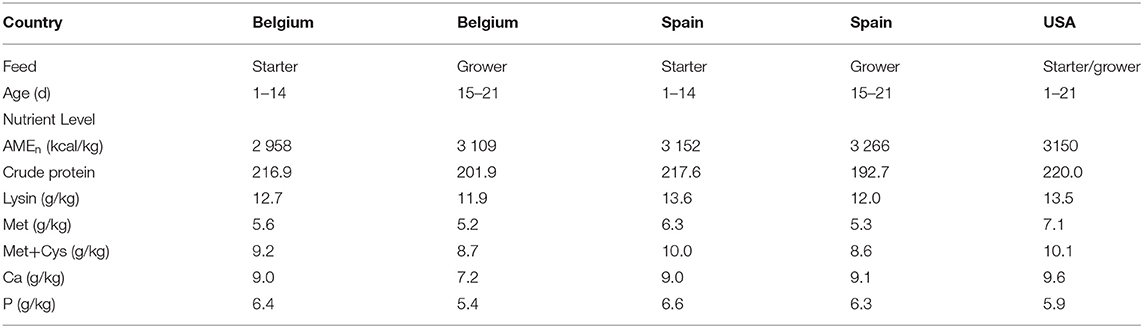
Monensin was purchased from Elanco (Greenfield, Indiana, USA) and Norponin XO2® from Nor-Feed SAS (Beaucouzé, France). The basal diets were formulated to meet or exceed the nutrient requirements recommended by the breed suppliers (Aviagen and Cobb) (Table 2).
Experimental Eimeria Challenge
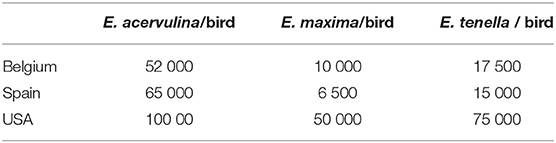
For the experimental challenge with wild-type Eimeria spp, oocytes from the field were used. As Eimeria. spp genetic background/virulence was proven to vary depending on geographical region (18), the number of sporulated oocysts per bird in the inoculum was defined by a preliminary dose titration study using susceptible broilers. This preliminary study aimed to determine the number of oocysts needed to obtain infestations leading to homogeneous lesions between the different trials and similar to those encountered in the field. Once the number of sporulated oocysts was determined, broilers were infested with sporulated oocysts in suspension by oral gavage at D14. The number of sporulated oocysts/birds is shown in Table 3. Chicks within the UUC group were gavaged with the same volume of distilled water.
Data Collection
Broilers were weighed per cage on days 1 and 21 to estimate BW. Feed intake was measured daily. These data were used to estimate average daily gain (ADG), average daily feed intake (ADFI), Feed Conversion Ratio (FCR), and European Production Efficiency Factor [EPEF, (19)]. EPEF is defined as follows:
Dead chicks were counted and removed daily. Broilers were euthanized at 21 days old to proceed to intestinal lesions scoring according to the scoring system published by Johnson and Reid (20).
Statistical Analysis
Data were analyzed using R (21) with emmeans and nnet packages. All quantitative data were analyzed using the following model:
where Yijk was a dependent variable of a repetition k, within treatment i and trial j. Treatment, trial, and their interaction were fixed factors, due to the low number of levels within each factor (22). All observations were weighted according to the number of broilers they represented. Body weight gain (BWG), ADG, ADFI, FCR, and EPEF were analyzed using a linear model. Results are reported as means and standard error of the means (mean ± SEM). Individual survival analysis and scores for the intestinal lesions were analyzed using logistic regression, results being reported as odds ratio (OR) to the base level and confidence interval (CI) [OR (lower confidence interval—upper confidence interval)]. Values for OR are given in scientific notation as they may be highly variable. The considered base level was IUC treatment for both cases, with a 0 score of lesions and being alive for survival analysis as standard. This allowed us to ensure that inoculation was successful in comparison to UUC treatment, and to assess the effects of both PX and PM treatments. Belgium was randomly chosen as a basal level for the trial effect. Survival analyses were analyzed using the same model as for quantitative data when the interaction was removed for the analysis of lesion scores to avoid error due to levels of scores not being presented for the given trial and treatment. A P-value of < 0.05 was used to indicate statistical significance, and between 0.05 and 0.1 was used to indicate a tendency.
Results
Growth Performances
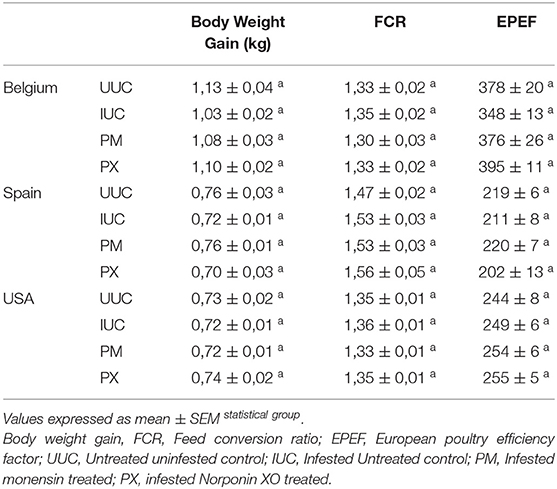
Initial BWs were not significantly different between treatments within a trial (P = 0.75 for trial x treatment interaction). The global analysis of from the 3 trials showed that BWs at the end of trials was higher (P < 0.05) in the UUC group than in the IUC group, with PX and PM being intermediate but not significantly different from other treatments (P > 0.10) (Table 1). The difference at the trial level was only numerical without statistical significance (Table 5). There was no significant difference due to treatments either in global analysis or trial level for ADG (P = 0.16), ADFI (P = 0.57), FCR (P = 0.24). or EPEF (P = 0.57) or showing similar growth performances results between treatments (Tables 4, 5). There was a tendency for ADFI (P = 0.10) for chicks within PX treatment to have a lower feed intake in comparison to chicks in PM treatment (48 ± 1.44 g vs. 52.8 ± 1.46 g), but this did not affect BWG or FCR. The mortality rate was very low, around 4%, whatever the considered effects. Survival analysis using logistic regression showed no differences in death OR due to treatment effect (P = 0.17), trial effect (P = 0.29), or their interaction (P = 0.53). This was due to the low number of deaths within each treatment. Survival rates were always higher than 90%, whatever the treatment or the trial.
Lesion Scores
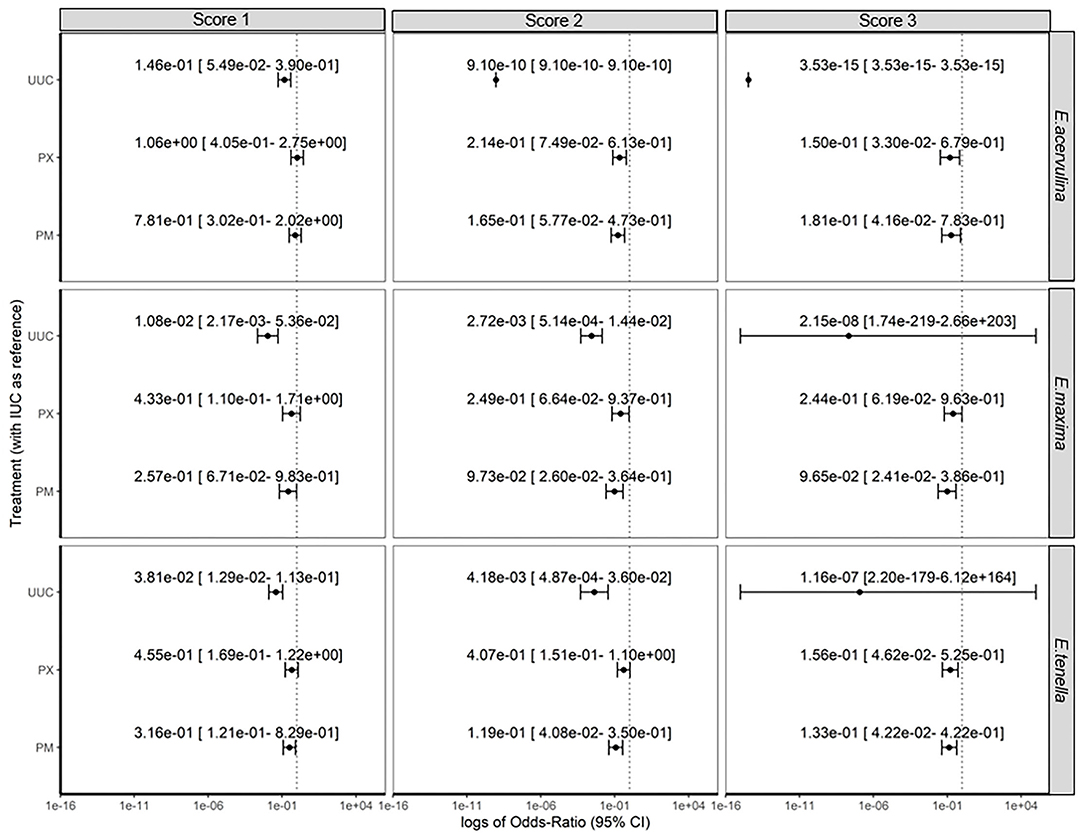
Odds of the apparition of lesions (Score 0 vs. Score 1 or higher) were affected by experimental treatments (Figure 1). Apparition of lesions due to E. acervulina was significantly lower for the UUC treatment (P < 0.001) in comparison to the IUC treatment. The OR was 3.21e-2 [1.25e-2–7.55e-2]. Odds of the apparition of the lesion were similar to IUC treatment for PM and PX groups, with respectively OR of 4.17e-1 [1.77e-1–9.38e-1] and 5.35e-1 [2.25e-1–1.23e+0], even OR for PM treatment was significantly lower. The trial effect was significant (P < 0.001), but there was no statistical difference when comparing OR.
Odds of apparition of lesions due to E. maxima were lower for UUC, PM, and PX groups in comparison to IUC group, with respective OR of 3.68e-3 [6.91e-4–1.46- 2], 1.30e-1 [3.34e-2–4.22-1] and 2.86e-1 [7.23e-2–9.44e-1]. Trial effects were also highly significant (P < 0.01), with higher odds of lesion apparition in the USA and Spain trials, with OR of 8.39e+0 [2.99e+0–2.83 e+1] and 3.89e+1 [1.28e+1–1.53e+2], respectively.
Treatment and trial effects significantly affected the odds of apparition of lesions due to E. tenella (P < 0.001). Like for the two other types of lesions, UUC treatment had the lowest OR, with a value of 1.52e-2[5.15e-3–3.97 e-2]. PM and PX groups also had lower odds of apparition than IUC group, with respective OR of 1.94e-1 [7.75e-2–4.49e-1] and 3.34e-1 [1.4e-1–8.41e-1]. The USA and Spain trials had higher OR of lesions apparition, with values of 3.01e0 [1.58e0–5.92e0] and 4.49e0 [1.99e0–1.08e1], respectively.
Analysis of the score of the lesions showed significant effects of trial and treatments (P < 0.001), whatever the considered type of lesion. Estimations and confidence intervals for each modality of treatment effect and each level of lesions are summarized in Figure 1. UUC treatment always had the lowest OR, whatever the level or type of lesions. For a score of 3+, estimations of UUC treatment were inefficient, infinite, or had a very large confidence interval, as this score was never observed for this treatment, whatever the trial. PM and PX had similar results on the score of lesions, reducing the odds of a higher score, 2 or 3+, in comparison to IUC treatment.
Discussion
Successful completion of an Eimeria. spp challenge is important for the interpretation of treatment results. In this study, we can state that the Eimeria. spp challenge was successful as evidenced by the significant decrease in final body weight between infested and non-infested chickens, even though the effects on other performance parameters, FCR, and EPEF, were not statistically significant. Most interestingly, the appearance of intestinal lesions was higher in infested chickens compared to non-infested chickens. The drop in final body weight caused by the experimental challenge in the IUC broilers tended to be compensated by the supplementation of monensin and saponin-rich plant premixture globally. At the trial level, there were some disparities (Table 5). However, this global compensation was only numerical and not statistically significant. It is well-known and documented that feed supplementation with monensin compensates for the loss of performance due to coccidiosis in broiler chicken (23). However, data remain scarce concerning the effect of the saponin-rich plant, Yucca schidigera, and Trigonella foenum greacum in Eimeria. Spp challenge conditions. The few available data report different results and conclusions. Our results are in line with recently published data concerning the use of saponin-rich plants (Quillaja.s) by the Bafundo team and those published by Saeed et al. (Yucca.s). (24, 25). On the other hand, a recent study shows that saponin-rich plant supplementation (Yucca.s) does not have a significant compensation effect on the loss of performance of chickens infested by Eimeria (26); thus, contradicting obtained data from the present study. An element that could explain this variability of results could be that the concentration of active compounds in the saponin-rich plants used is different from one study to another. Indeed, the concentration of these active compounds can be more or less high according to several parameters (type of plant, part of the plant used, harvesting period, etc.). In addition, unlike some cited studies, we used a formulation of 2 saponin-rich plants, namely, Yucca.s and Trigonella. f.g., thus, making the comparison of the obtained results quite difficult to the available scientific literature. This fact also highlights the importance of transparency and standardization in active compounds when natural plant-based products are used in animal nutrition. (27). The complexity of the parasites and the pathophysiology of coccidiosis infection can also play an important role in the observed variability of the results. In this study, we observed that both treatments (Monensin and Norponin XO2) reduced the appearance of intestinal lesions due to experimental Eimeria infestation. If these observations are well-documented for monensin supplementation in chicken (28, 29), data dealing with saponins deserve to be reinforced. The decrease in the occurrence of intestinal lesion scores for the saponin-rich plant treatment can be explained by the direct action of saponins. Indeed, saponins have the property to disrupt the cellular membrane of the parasite, thanks to their permeabilization effects (30). Interacting with the parasite and disrupting its membrane leading to the loss of its homeostasis could be a possible mechanism of action of saponin. Another mechanism of action of saponins can be the inhibition of the invasion step of the parasite. Felici et al. (31) evidenced the fact that saponins can inhibit the invasion process of the parasite. However, the cited studies are mainly in vitro studies that did not consider the possible degradation of the natural active compounds of saponin-rich plants. Moreover, in addition to saponin, these plants contain other actives compounds like flavonoids that certainly plays a role in the observed effect on animal and intestinal lesions. Thus, making the investigation of the mechanism of action of saponin-rich plants quite challenging. More studies are needed to better understand the mechanism of action behind the observed effects. Nevertheless, these experimental results showed that a 100% plant-based solution can be as efficient as a conventional coccidiostat in managing coccidiosis; thus, offering more agility for broiler chicken producers. Particulary, studies have shown that the use of alternative solutions to chemoprevention helps to restore the effectiveness of molecules, such as monensin and duclazuril (32, 33). Therefore, introducing a plant-based solution and coccidiosis vaccines could help to solve the resistance problem observed and described all over the world. However, we believe that in addition to the efficient and natural solution, there is a need to rethink coccidiosis management. Parameters like nutritional management (34), biosecurity, and chicken strain selection could help to raise chickens while limiting the use of synthetic and/or ionophore coccidiostats.
This study evidenced the fact that a natural solution formulated from Yucca schidigera and Trigonella foenum-graecum (Norponin XO2) is as efficient as a monensin supplementation in managing coccidiosis in an experimental-infestation model. These results should be confirmed in the field. Altogether, these elements will certainly help to achieve the ultimate goal of producing a sustainable broiler chicken at a reasonable cost for the continuously growing number of humans on earth.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by IMASDE and POULPHARME Commity.
Author Contributions
PC and CM reviwed the article. MB wrote the article. Statistical analysis made by PG and CM. Experiments were setup by MB. PC and MB made the experimental design. All authors contributed to the article and approved the submitted version.
Conflict of Interest
MB and PC work in the R&D departement of Nor Feed commercialize products based on sapoinns.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to give special thanks to the Poulpharm team in Belgium, IMASDE in Spain, and the Southern Poultry Research Center in the USA for their collaboration in putting together this large-scale study.
References
1. Shivaramaiah C, Barta JR, Hernandez-Velasco X, Téllez G, Hargis BM. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these apicomplexan parasites. Vet Med (Auckl). (2014) 5:23–34. doi: 10.2147./VMRR.S57839
2. Butterworth A, Weeks C. The impact of disease on welfare. In: Duncan I, HawkinP, editors. The Welfare of Domestic Fowl and Other Captive Birds. Animal Welfare, Vol. 9. Dordrecht: Springer (2010). p. 189–218. doi: 10.1007/978-90-481-3650-6_8
3. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. (2006) 5:143–63. doi: 10.1586/14760584.5.1.143
4. Chapman HDA. Landmark contribution to poultry science–prophylactic control of coccidiosis in poultry. Poult Sci. (2009) 88:813–5. doi: 10.3382/ps.2008-00316
5. Blake DP, Knox J, Dehaeck B, Huntington B, Rathinam T, Ravipati V, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. (2020) 51:115. doi: 10.1186./s13567-020-00837-2
6. Jeffers TK, Acervulina E, Maxima E. Incidence and anticoccidial drug resistance of isolants in major broiler-producing areas avian. Diseases. (1974) 18:331–42. doi: 10.2307/1589101
7. Jeffers TK. Eimeria tenella: incidence, distribution, and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis. (1974) 18:74–84. doi: 10.2307/1589244
8. Chapman HD. Drug resistance in avian coccidia (a review). Vet Parasitol. (1984) 15:11–27. doi: 10.1016/0304-4017(84)90106-7
9. Peek HW, Landman WJ. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. (2003) 32:391–401. doi: 10.1080/0307945031000121149
10. Peek HW, Landman WJ. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet Q. (2011) 31:143–61. doi: 10.1080/01652176.2011.605247
11. Li P, Wu Y, Wang Y, Qiu J, Li Y. Soil behaviour of the veterinary drugs lincomycin, monensin, and roxarsone and their toxicity on environmental organisms. Molecules. (2019) 24:24244465. doi: 10.3390./molecules24244465
12. Mainero Rocca L, Gentili A, Pérez-Fernández V, Tomai P. Veterinary drugs residues: a review of the latest analytical research on sample preparation and LC-MS based methods. Food Addit Contam Part A Chem Anal Control Expo Risk Asses. (2017) 34:766–84. doi: 10.1080/19440049.2017.1298846
13. Mortier L, Huet AC, Charlier C, Daeseleire E, Delahaut P, Van Peteghem C. Incidence of residues of nine anticoccidials in eggs. Food Addit Contam. (2005) 22:1120–5. doi: 10.1080/02652030500199355
14. Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. Biomed Res Int. (2015) 2015:430610. doi: 10.1155./2015/430610
15. El-Shall NA, El-Hack MEA, Albaqami NM, Khafaga AF, Taha AE, Swelum AA, et al. Phytochemical control of poultry coccidiosis: A review poultry Science. (2022) 101:101542. doi: 10.1016/j.psj.2021.101542
16. Bozkurt M, Giannenas I, Küçükyilmaz K, Christaki E, Florou-Paneri P. An update on approaches to controlling coccidia in poultry using botanical extracts. Br Poult Sci. (2013) 54:713–27. doi: 10.1080/00071668.2013.849795
17. Roussel P, Arnaiz V, Rodríguez V, García JM, Honorio Javes C, Chicoteau P. Replacing the coccidiostats: defining cost-effective surrogates. In: Planta Medica International Open. Verlag KG: Georg Thieme (2017).
18. Chengat Prakashbabu B, Venkatachalam T, Limon G, Kundu K, Kumar S, Garg R, et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. (2017) 233:62–72. doi: 10.1016/j.vetpar.2016.12.003
19. Marcu A, Vacaru-Opris L, Dumitrescu G, Ciochina LP, Marcu A, Nucula M, et al. The influence of the genotype on economic efficiency of broiler chickens growth scientific papers animal science and biotechnologies. Anim Sci Biotechnol. (2013) 46:339–46.
20. Johnson J, Reid WM. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. (1970) 28:30–6. doi: 10.1016/0014-4894(70)90063-9
21. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2008).
22. Bell A, Fairbrother M, Jones K. Fixed and random effects models: making an informed choice. Qual Quant. (2019) 53:1051–74. doi: 10.1007/s11135-018-0802-x
23. Chapman HD, Jeffers TK, Williams RB. Forty years of monensin for the control of coccidiosis in poult.ry Poult Sci. (2010) 89:1788–801. doi: 10.3382/ps.2010-00931
24. Saeed M, Arain MA, Naveed M, Alagawany M, Abd El-Hack ME, Bhutto ZA, et al. Yucca schidigera can mitigate ammonia emissions from manure and promote poultry health and production. Environ Sci Pollut Res. (2018) 25:35027–33. doi: 10.1007/s11356-018-3546-1
25. Bafundo KW, Gomez L, Lumpkins B, Mathis GF, McNaughton JL, Duerr I, et al. Concurrent use of saponins and live coccidiosis vaccines: the influence of a quillaja and yucca combination on anticoccidial effects and performance results of coccidia-vaccinated broilers. Poult Sci. (2020) 100:100905. doi: 10.1016/j.psj.2020.12.010
26. Oelschlager ML, Rasheed MSA, Smith BN, Rincker MJ, Dilger RN. Effects of Yucca schidigera-derived saponin supplementation during a mixed Eimeria challenge in broilers. Poult Sci. (2019) 98:3212–22. doi: 10.3382/ps/pez051
27. Scheurer W, Spring P, Maertens L. Effect of 3 dietary phytogenic products on production performance and coccidiosis in challenged broiler chickens. J Appl Poult Res. (2013) 22:591–9. doi: 10.3382/japr.2013-00726
28. Moraes PO, Cardinal KM, Gouvêa FL, Schroeder B, Ceron MS, Lunedo R, et al. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poult Sci. (2019) 98:5456–64. doi: 10.3382/ps/pez345
29. Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, et al. Safety and efficacy of ElancobanR G200 (monensin sodium) for chickens for fattening, chickens reared for laying and turkeys. EFSA J. (2019) 17:5891. doi: 10.2903./j.efsa.2019.5891
30. Lorent J, Le Duff CS, Quetin-Leclercq J, Mingeot-Leclercq MP. Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, α- and δ-Hederin. J Biol Chem. (2013) 288:14000–17. doi: 10.1074/jbc.M112.407635
31. Felici M, Tugnoli B, Ghiselli F, Massi P, Tosi G, Fiorentini L, et al. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult Sci. (2020) 99:5350–5. doi: 10.1016/j.psj.2020.07.035
32. Chapman HD, Jeffers TK. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int J Parasitol Drugs Drug Resist. (2014) 4:214–7. doi: 10.1016/j.ijpddr.2014.10.002
33. Peek HW, Landman WJ. Higher incidence of eimeria spp field isolates sensitive for diclazuril and monensin associated with the use of live coccidiosis vaccination with paracoxTM-5 in broiler farms. Avian Dis. (2006) 50:434–9. doi: 10.1637/7486-121205R.1
Keywords: cocccidia, Eimeria spp, gut, broiler–chicken, saponin, fenugeek, Yucca (Yucca schidigera)
Citation: Benarbia MeA, Gaignon P, Manoli C and Chicoteau P (2022) Saponin-Rich Plant Premixture Supplementation Is as Efficient as Ionophore Monensin Supplementation Under Experimental Eimeria spp Challenge in Broiler Chicken. Front. Vet. Sci. 9:946576. doi: 10.3389/fvets.2022.946576
Received: 17 May 2022; Accepted: 16 June 2022;
Published: 14 July 2022.
Edited by:
Guillermo Tellez-Isaias, University of Arkansas, United StatesReviewed by:
Roberto Senas Cuesta, University of Arkansas, United StatesSantiago Uribe-Diaz, University of Arkansas, United States
Copyright © 2022 Benarbia, Gaignon, Manoli and Chicoteau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed el Amine Benarbia, YW1pbmUuYmVuYXJiaWFAbm9yZmVlZC5uZXQ=
 Mohammed el Amine Benarbia
Mohammed el Amine Benarbia Pierre Gaignon3
Pierre Gaignon3 Claire Manoli
Claire Manoli Pierre Chicoteau
Pierre Chicoteau