- 1Animal Behaviour and Wildlife Conservation Group, School of Sciences, University of Wolverhampton, Wolverhampton, United Kingdom
- 2Animal Behaviour and Wildlife Conservation Group, School of Medicine, University of Wolverhampton, Wolverhampton, United Kingdom
- 3Dipartimento di Scienze Mediche Veterinarie, Universitá di Bologna, Bologna, Italy
- 4Behavioural, Ecology and Evolution Research Centre, Durham University, Durham, United Kingdom
The Lake Alaotra gentle lemur (Hapalemur alaotrensis) is one of the 25 most endangered primates in the world and shows low success rate in captive breeding programmes. It is therefore vital to further understand its reproductive biology. We studied a captive troop consisting of five individuals hosted at Jersey Zoo during breeding and non-breeding periods over 1 year. We collected behavioural data (n = 318 h) using all occurrence of some behaviours and ad libitum sampling methods, as well as faecal (n = 54) and anogenital scent (n = 35) samples of the breeding female. We measured sex hormone levels using enzyme immunoassay technique and investigated the volatile component of odour signals using solid-phase microextraction and gas chromatography-mass spectrometry. We observed sexual and aggressive behaviours occasionally during the breeding period. Our regression analysis showed that only period significantly predicted rates of female anogenital scent-marking, whereby the female performed anogenital scent-marking more frequently during the breeding rather than the non-breeding period. In contrast, female hormone levels did not significantly explain variation in rates of neither male nor female olfactory, sexual and affiliative behaviours, suggesting that individuals' behaviour alone is not an effective indicator of the ovulation window. The volatile chemical profile of anogenital odour secretions changed over the study, with four compounds distinguishing the fertile window during the breeding period. In conclusion, our findings suggest that anogenital scent-marking may signal the reproductive status of captive female gentle lemurs.
Introduction
Of 504 primate species recognised today worldwide, almost half are classified as endangered or critically endangered in the wild—primarily due to human impact; thus, raising global scientific and public awareness of the plight of the world's primates is now vital (1, 2). The most important actions needed for ensuring the survival of these irreplaceable species are conservation, research, public education and outreach, wherein zoos play a major role (3, 4).
Among captive animals, zoo populations are unique as they are usually managed to educate the public regarding wildlife and their habitats, and to preserve endangered species through captive breeding and reintroduction programmes (5–7). In this context, the maintenance of genetic variation, and thus high survival rate in case of reintroduction, is imperative (8, 9). However, captive populations, potentially serving as buffers against extinction, are experiencing problems that impair them from being viable for reintroduction into the wild. Particularly, zoo populations face reproductive challenges which may prevent them from serving as viable “reserve populations” (10).
To maintain captive healthy populations, modern zoos take part in conservation breeding programmes (5, 6). Moreover, as reproductive success is linked to how closely captive environmental conditions mirror those that primates would be experiencing in the wild (10), zoos also use environmental enrichments to manage captive populations [including scent enrichment programmes (e.g., (11))]. Environmental enrichments and conservation breeding programmes are linked, as enrichment is a dynamic process that changes an animal's environment, increasing its behavioural choices and prompting a wider range of natural and species-specific behaviours and abilities (12).
Among all primate species, lemurs are the most endangered. Almost a third of the 107 species living in Madagascar are listed as critically endangered, while 98% of them are threatened by extinction (13). Moreover, several captive lemur populations are struggling, in terms of abundance and demographic trend, and currently would not support reintroduction into the wild (10).
The Lake Alaotra gentle lemur (Hapalemur alaotrensis) is one of the five lemur species included in the 2018–2020 list of The World's 25 Most Endangered Primates (14). It is the only lemur species living exclusively in a wetland and its limited geographical range, combined with increasing habitat degradation and hunting pressure, have brought this species to the brink of extinction (15, 16). With a population continuously declining, it is estimated that only around 2,500 individuals remain in the wild (16).
Given the danger that it faces, the gentle lemur is now targeted by several conservation initiatives (16), such as the European Association of Zoos and Aquaria (EAZA)'s Ex-situ Programme (EEP), which aims to maintain captive healthy populations and reduce the loss of genetic variation, which can be rapid in small captive populations (8, 17, 18), through breeding management recommendations. Nevertheless, gentle lemurs are currently showing a low success rate in captive breeding across EAZA institutions (19). In this context, it is becoming increasingly important to fully understand their reproductive biology in order to try and affect positively their conservation status (20).
It is now widely acknowledged that olfaction plays an important role in socio-sexual communication and reproductive biology in non-human primates [e.g. (21–28)]. Although little is known about gentle lemurs, it is broadly established that lemurs rely heavily on olfaction [reviewed by (29)], with semiochemicals being important for territorial marking, social communication, kin recognition and mate choice (30–34). Among primates, lemurs show the utmost variability and specialization in chemosignalling, with a great diversity in design, delivery and perception of chemical signals. They actively scent mark, have a functional vomeronasal organ, which is very sensitive to chemical messages, and investigate scents via olfactory and gustatory means [reviewed by (35)]. Scent signals can be released through specialized glands, urine and faeces, sweat and skin (36); variation in scent mixing, delivery and multimodality alters signal longevity and intended receivers [reviewed by (37)]. Scent release can also vary both quantitatively (frequency of scent marking and amount of scent deposited) and qualitatively (spatial and seasonal distribution and substrate marked) [reviewed by (29)]. Moreover, signs of marking can be persistent in the environment [e.g., faecal or urinary latrines (38, 39)]. The information released with chemical signals changes the behaviour or perception of the receiver in a way that generally benefits both sender and receiver (40), typically in the service of mutually profitable, socio-reproductive functions (29). In several lemur species, female olfactory signals can advertise their reproductive status, varying between the breeding and non-breeding seasons (30, 41, 42). Thus, chemicals have also great potential as tools to trigger olfactory and sexual behaviours in lemurs (24, 36, 43), which is important in critically endangered species.
In this study, we combined behavioural observations of olfactory and sexual behaviours with the chemical investigation of anogenital odour secretions and the timing of fertility, obtained via measurement of sex hormone levels, in a zoo-housed gentle lemur. In particular, we aimed to:
• Detect the female fertile window using faecal progesterone and oestradiol levels.
• Examine the relationship between female and male behavioural patterns (focusing on olfactory and sexual behaviours) and female sexual cycle stages.
• Identify the key compounds that convey information about female fertility.
Materials and methods
Study subjects and periods
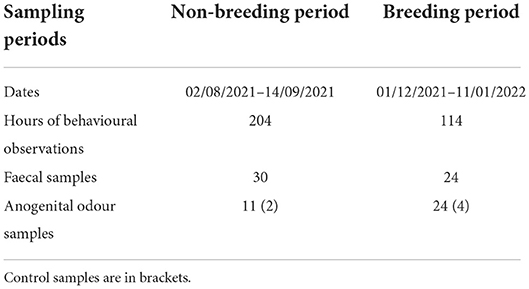
We studied a captive family group of gentle lemurs (n = 5), consisting of a breeding pair and their offspring (Table 1), hosted at Jersey Zoo—formerly Durrell Wildlife Park (Channel Islands).
Female gentle lemurs are able to reproduce at 2 years, whereas males are sexually mature at 3 years [wild: (44), captivity: (45)]. The average lifespan in captivity is 17.1 years for females and 12.8 years for males (46). Gentle lemurs are seasonally polyoestrous and mating occurs over 1 day per sexual cycle (47). They usually deliver offspring once per year (47) after an average gestation period of 145 days (45), with a high rate of twinning (44). In Madagascar the mating season occurs during the dry season [i.e., between April and September (44)], while there is no defined breeding season in captivity (45). We estimated the breeding and non-breeding periods on the basis of the last parturition of the female and the length of the weaning period (48, 49).
Sampling (breeding and non-breeding) periods, including behavioural observations as well as collected faecal and odour samples, are detailed in Table 2.
Data collection
Faecal hormone sampling and measurement
We collected faecal samples (n = 54) from the breeding female every morning during the behavioural observations, when defecation was observed, and the identity of the animal was certain. We immediately stored the samples in a −20°C freezer at the zoo and then transferred them, using a freezer box with ice packs to avoid any risk of defrosting, to the Rosalind Franklin Science Centre, University of Wolverhampton, for laboratory analyses.
Hormone analyses
We lyophilized the faecal samples for 72 h using a freeze-drying machine (Christ®, Beta 1–8 LSC plus, Osterode am Harz, Germany), then we pulverized and sieved them to separate the faecal residue from the fibrous material. The extraction methods were based on those detailed in Maréchal et al. (50). Briefly, we extracted 0.05–0.1 g of faecal powder in 3 ml of 80% methanol in a 15 ml plastic tube. Then, after vortexing for 15 min using a multi-tube vortexer (Grant Instruments®, Multi-Vortexer V-32, Cambridge, UK) and centrifugation for 20 min at 3,266 xg, we immediately stored the supernatant at −20°C. We excluded a faecal sample from the analysis as it was degraded.
Enzyme immunoassays
We measured progesterone metabolites and 17β-Estradiol levels using ELISA kits (DetectX Progesterone metabolites K068-H5 and DetectX 17β-Estradiol K030-H5, Arbor Assays®, USA) following the protocol detailed in (51). We diluted the samples 1:10 with the assay buffer and run all assays according to kit instructions. We assayed all faecal samples and standards in duplicate. We analysed assay data utilizing a 4-parameter logistic (4PL) fitting programme (MyAssays®, Brighton, UK). Concentrations were expressed as pg/mg.
Mean intra-assay coefficients of variation of four samples, tested with eight replicates within a single assay plate, was 10.2% for progesterone and 7.6% for oestradiol. Mean inter-assay coefficients of variation of four quality control samples, measured in duplicate across three assay plates, was 12.3% for progesterone and 8% for oestradiol.
Analytical validation
We conducted a parallelism test between serial dilutions of two samples (A and B) and the standard curves of each kit to validate the enzyme immunoassays (52). We performed a Pearson correlation test to assess the strength of the association between the slopes of the standard curves and that of the diluted samples [Progesterone-Sample A: r3 = 0.99, p = 0.001; Progesterone-Sample B: r3 = 0.99, p = 0.001; Oestradiol-Sample A: r3 = 0.96, p = 0.007; Oestradiol-Sample B: r3 = 0.97, p = 0.006].
Interpretation of faecal hormone profiles and definition of the oestrus cycle
We used the patterns of faecal progesterone metabolites and 17β-oestradiol levels to determine the occurrence of ovulatory windows and the timing of fertility in the breeding female [we considered the time lag between steroid secretion and excretion in faeces (53)]. We estimated an ovulatory window when oestradiol increased along with a progesterone decrease followed by a constant progesterone rise for at least 5 days. This approach was used to define the ovulatory phase of the ovarian cycle and has previously been shown to be valid for assessing the timing of ovulation in other lemur species (54–56).
Behavioural observations
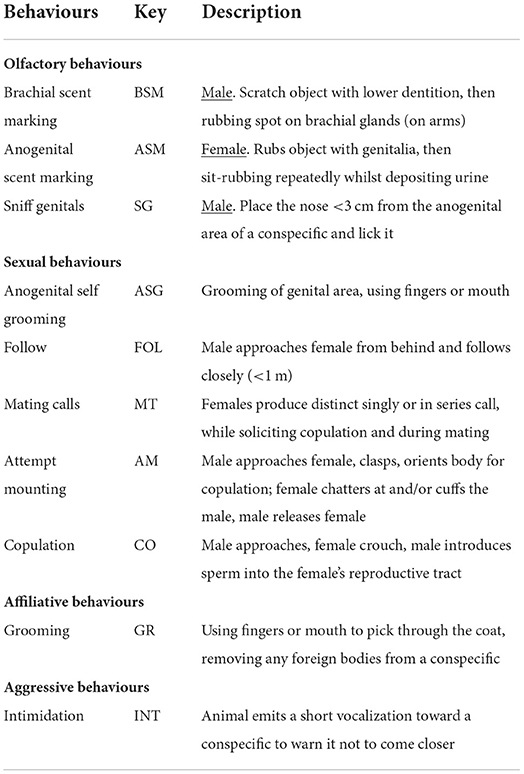
We collected behavioural data from early mornings to early afternoons (non-breeding period: 6 h/day; breeding period: 4 h/day), 5 days per week (non-breeding period: Mondays to Fridays; breeding period: Sundays to Tuesdays and Thursdays to Fridays), during two study periods (non-breeding period: August/September 2021; breeding period: December 2021/January 2022), for a total of 318 h. We conducted all occurrence and ad libitum sampling sessions (56, 57) to collect data on olfactory, sexual, affiliative and aggressive behaviours using an ethogram developed by Errington (58) and then modified using prior studies by other authors (45, 47, 59) (Table 3).
Odour sampling and investigation
Before data collection, we used positive reinforcement training (60) for 5 days to train the breeding female to allow us to collect anogenital odour secretions.
We collected anogenital odour samples (n = 35) every morning before behavioural observations by rubbing 10 times a sterile cotton swab around the wall of the vulva, using steady pressure, as previously described by Vaglio et al. (28). Moreover, we exposed control swabs to the air once a week to identify any compounds that did not derive from the lemurs. We placed all samples and controls into sterile vials (61) and immediately stored them in a −20°C freezer at the zoo. We then transferred the vials to the Rosalind Franklin Science Centre, University of Wolverhampton, using a freezer box with ice packs to avoid any risk of defrosting, for laboratory analyses.
Odour analyses
We investigated the volatile component of odour signals using solid-phase microextraction (SPME) and gas chromatography-mass spectrometry (GC-MS) techniques, as previously described by (61).
Briefly, we introduced a 65 μm polydimethylsiloxane/ divinylbenzene SPME syringe needle through the vial septum and exposed the fibre to the headspace above the sample in the vial for 15 min at 40°C. We analysed the adsorbed volatile analytes of all samples using a 5975C mass spectrometer (Agilent Technologies) EI, 70 eV, coupled directly to a 7890B gas chromatograph (Agilent Technologies) equipped with a fused silica HP5-MS UI capillary column (Agilent Technologies) 30 m × 0.25 mm crossbonded 5%-phenyl-95% dimethylpolysiloxane, film thickness 0.25 μm. We maintained the injector and transfer line temperatures at 270°C and 280°C, respectively.
We made injections in splitless mode (purge valve opened after 1 min) with a constant flow of helium carrier gas of 1 ml min −1. We started the oven temperature program at 45°C for 2 min, then raised it by 4°C min −1 to 170°C, and finally by 20°C min −1 to 300°C 40.
We assessed possible environmental contamination via blank analyses using an empty 10 ml vial (Supelco) and control swabs following the same procedure as for the samples and conditioned the fibre at 260°C pre-injection for 5 min and 260°C post-injection for 20 min to avoid any possible carry-over effects.
We standardized peak retention times using retention time locking to alpha pinene. We tentatively identified eluted compounds by comparing the experimental spectra with those of the mass-spectral library in ChemStation (Agilent Technologies) and NIST Database (National Institute of Standards and Technology), version MSD F.01.01.2317 (Agilent Technologies). We accepted a putative identification if the minimum matching factor was higher than 90%. To minimize the chance of misidentification and when more than one compound was a good match for the same GC peak, we considered the chromatographic retention time and compared it with those reported in the literature for the same chromatographic column type. We created a data matrix using the peak area relative to each identified compound using the integrated signal of the deconvoluted total ion current (TIC).
We analysed all samples in a short period of time to minimize inter-assay variability. We overlaid chemical profiles from control swabs on animal chemical profiles to identify compounds that did not derive from the animals and removed these from the swab results.
Data analysis
In order to assess whether male and female behaviours were significantly affected by female oestradiol levels and/or period (breeding vs. non-breeding), we conducted a series of linear regressions. All models included both female oestradiol concentrations and period as predictors, while, in separate models, we included as dependent variables female anogenital behaviour as well as male follow, sniffing, sexual inspection and grooming behaviours. We applied model validation on all regression models to verify the underlying assumptions, by checking whether residuals were normally distributed and by plotting residuals versus fitted values to assess homogeneity of variance. None of the assumptions were violated. We performed statistical analyses using the “lm” function implemented in R Studio software (version 4.1.1) (62).
Ethical statement
We conducted this study using non-invasive techniques, in compliance with the Directive 2010/63/EU and Decision 2014/11/EU. The study protocol was approved by the Life Sciences Ethics Committee of the University of Wolverhampton (UK) and the Ethics Committee of the Jersey Zoo—Durrell Wildlife Conservation Trust (Channel Islands).
Results
Faecal hormones
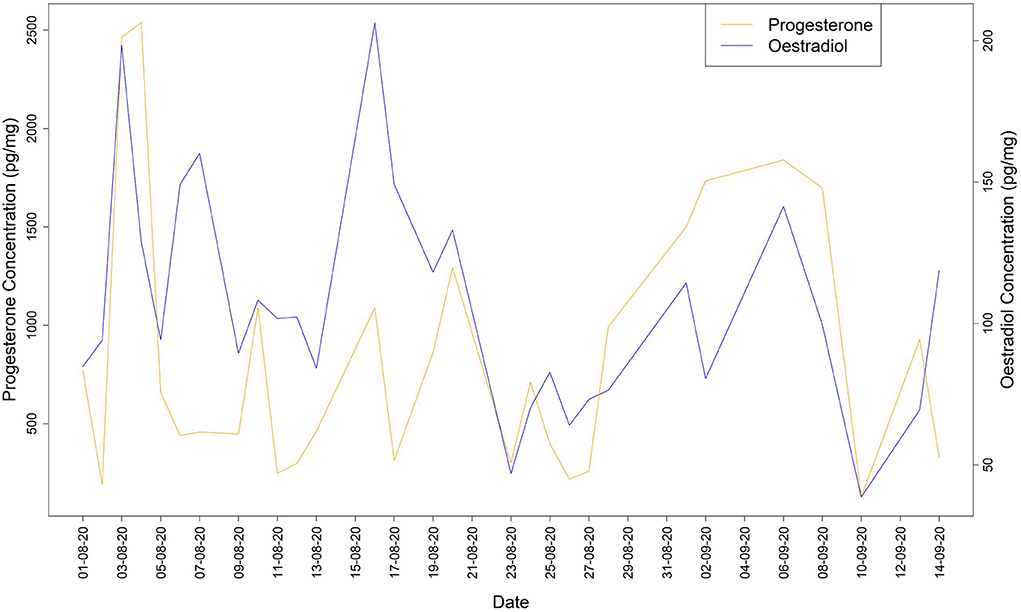
During the non-breeding period, the profiles of both sex hormones (progesterone and oestradiol) showed a synchronized pattern with daily variations, which did not indicate any ovulation (Figure 1).
During the breeding period an increase in oestradiol concentration, accompanied by a decrease of progesterone, occurred both on December 11th and 29th. In both cases oestradiol peaks were followed by a constant increase of progesterone levels that reached a peak after 8 days (Figure 2).

Figure 2. Oestradiol and progesterone concentrations during the breeding period. Vertical bars denote the ovulation window.
Olfactory, sexual and affiliative behaviours
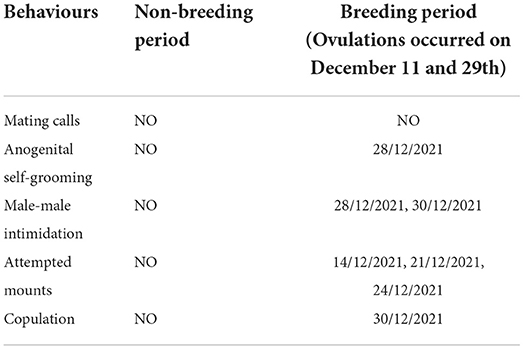
The regression analyses testing the effect of female oestradiol concentrations and period on male and female behaviours showed that the only model that was significant was the one that included rates of female anogenital scent-marking as dependent variable [F2, 34 = 8.98, p = 0.020, R2 = 0.16]. This model showed a significant effect of period (but not oestradiol levels) on rates of female anogenital scent-marking (Table 4), with higher frequencies of anogenital scent-marking occurring during breeding (0.86 N/h.) than non-breeding period (0.05 N/h.). Similarly, we found a trend in the model that included rates of male genital sniffing followed by scent-marking [F2, 34 = 3.11, p = 0.06, R2 = 0.10], with a significant effect, again, of period on male behaviour (Table 4): males performed more frequent genital sniffing followed by scent-marking during the breeding (1.98 N/h) than non-breeding period (0.97 N/h). None of the other regression models was significant (Table 4). We observed male and female anogenital self-grooming only during the breeding period, twice 2 days before copulation occurred. We observed intimidation behaviours from the adult male towards the subadult male three times 2 days before copulation and once the day of copulation. We observed attempted mounts on December 14th, 21st, and 24th, while copulation occurred five times with different durations, over an hour, on December 30th. We did not document any mating call. We did not observe any sexual behaviour after copulation occurred on December 30th. See Table 5 for a summary of such ad libitum observations.

Table 4. Summary of the linear regression results testing the effect of female oestradiol concentrations and periods (breeding vs. non-breeding) on female and male behaviours.
Odour secretions
We identified a total of 78 distinct peaks in 35 swab samples of gentle lemur anogenital secretions that were not present in the control swabs. These compounds included a series of hydrocarbons and fatty alcohols, organic aliphatic acid esters and carboxylic acid, aldehydes, and aliphatic ketones. Typical chromatograms from the fertile and non-fertile periods are shown in Figures 3, 4, respectively.
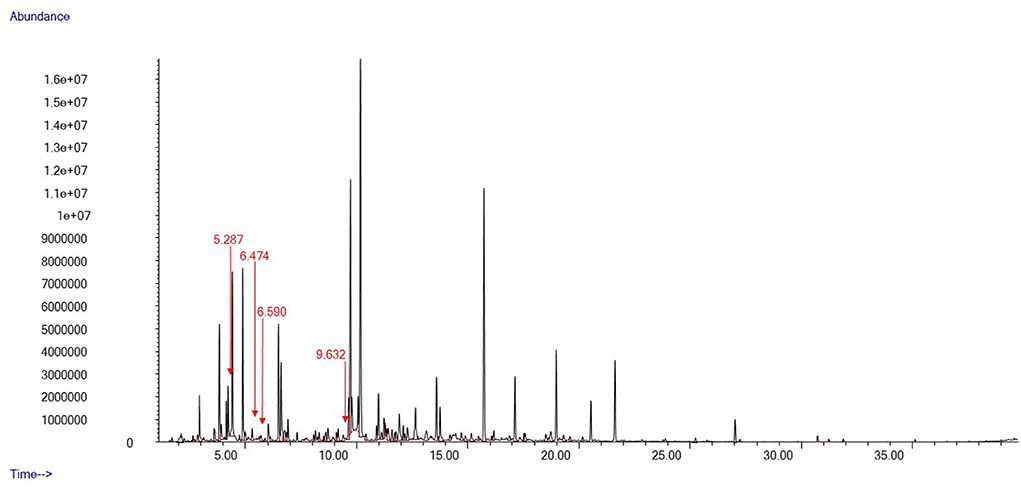
Figure 3. Oestradiol and progesterone concentrations during the breeding period. Vertical bars denote the estimated ovulation window.
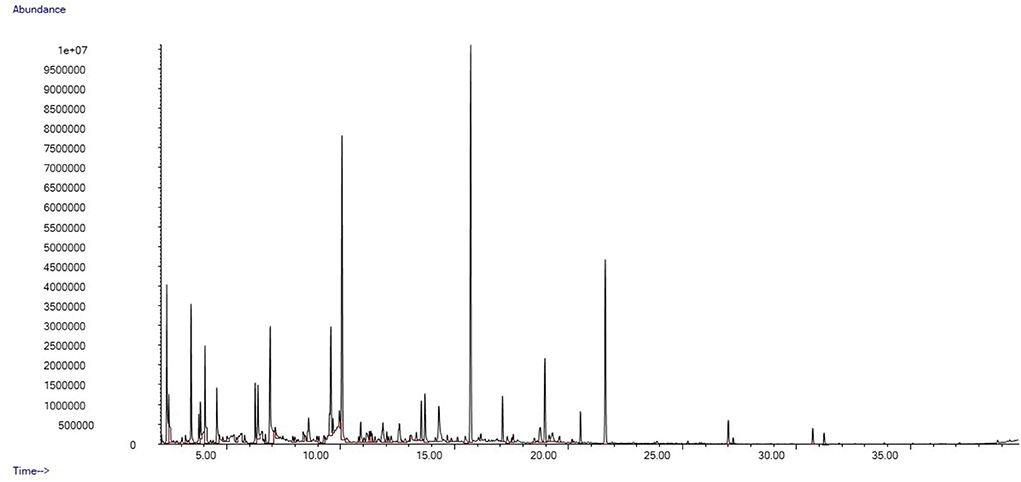
Figure 4. Example chromatogram from female gentle lemur, anogenital odour sample during the non-fertile period.
Particularly, a small pool of compounds was only present in the chemical profiles of anogenital odour samples collected during the second ovulation window (28th–30th December 2021). Three compounds were tentatively identified as 3-Heptanone, 2-Heptanone, and 3-Octanone, whilst one could not be given any tentative identification (Figure 3). These compounds were unique to the second ovulation window (28th–30th December 2021) during the breeding period.
Discussion and conclusions
Little is known about the chemical changes underlying reproductive quality in non-human primates due to methodological challenges in sampling and quantifying odour profiles (21). In this study we determined the timing of female fertility in a gentle lemur using observations of sexual behaviours and faecal endocrinology (63), and examined the relationship between the reproductive status of the female, anogenital scent, and both female and male behavioural patterns.
Faecal hormones are commonly used to acquire endocrine information on ovarian function in individual female primates (64). We found that faecal hormone trends were different between the study periods (non-breeding vs. breeding). Particularly, during the non-breeding period the profiles of progesterone and oestradiol showed a synchronized pattern, while during the breeding period two potential ovulatory events were estimated. In the context of the breeding period, as observed in other lemur species [e.g., Lemur catta (54, 55), Lemur variegatus (59), Eulemur mongoz (56)], the female oestradiol level increased, reaching a peak the day before mating occurred, alongside a decrease of the progesterone level followed by a slow steady rise until reaching a peak 8 days after ovulation. This is consistent with what has been reported in other lemurs; i.e., oestradiol peak usually occurs on the first day of vaginal oestrus and receptivity usually follows about 1 day after the oestradiol peak, oestradiol levels fall on the day after the oestradiol peak and females become sexually receptive during this period (55, 59). The tentative interval between the two ovulation windows was around 20 days. Although this interval is slightly different in comparison to what has been reported in other lemurs (i.e., Lemur macaco: 24–28 days, Lemur variegatus: 30–36 days, Eulemur mongoz: 26–35 days), a degree of variability both intra- and inter-species has also been suggested by other authors (54, 56, 59).
We also found that the average level of both hormones was higher during the non-breeding than the breeding period. This may be caused by several factors. First of all, the diet, as during the summer (non-breeding period) the daily diet of the study group included large amounts of bamboo; i.e., a plant rich in flavonoids (phytoestrogens) which may impact on female sex hormones. Particularly, it has been found that phytosteroids affect the reproductive hormone levels in both humans and non-human animals (65, 66), while many studies on dietary phytoestrogens highlight the potential effects of total flavonoids in terms of increasing progesterone and oestradiol levels (66). In addition, higher hormone levels over the summer can be due to seasonal variation, as progesterone and oestradiol tend to increase when the photoperiod lengthens (67), and the reproductive history of the female, who had gone into oestrus during previous summer seasons but at the time of this study was lactating over the summer and the presence of a suckling infant may have delayed the onset of ovulation (68).
Since we did not observe any attempted mounts or aggressive behaviours before the first ovulation window (9th–11th December 2021), we suggest that this was a silent ovulation event (i.e., an ovulation not accompanied by perceptible morphological changes in the anogenital area and during which no sexual behaviour occurs). Silent ovulations have been reported in several mammal species, including non-human primates (69, 70). In addition, based on our endocrinological and behavioural data, we estimated an ovulation window occurring on 28th–30th December 2021; as further verification that the female was ovulating, she became pregnant following copulation and gave birth to twins after 142 days of gestation. As in other lemur species [e.g., Lemur catta (71), Eulemur fulvus (72), Lemur variegatus (59)], within each breeding period the gentle lemur's female fertile window would then be restricted to 2 days per oestrus cycle (47).
With regards to the relationship between the reproductive status of the female and both female and male behavioural patterns, we found a significant effect of period (breeding vs. non-breeding) on female anogenital scent-marking and male sexual behaviour (i.e., sniff genital followed by scent-marking) but we did not find any significant effect of female hormones on either female marking behaviour or male olfactory, sexual and affiliative behaviours. These results suggest that male or female behaviours alone cannot be considered as an effective indicator of female fertility in gentle lemurs.
Although it is known that in other lemur species [e.g., Eulemur fulvus (73), Lemur variegatus (59)] male behavioural responses towards female fertile signals consist of increased frequency of behaviours such as follow, sniff genital and olfactory inspections of scent-marks, and more aggressive interactions between males [e.g., Microcebus murinus (74), Lemur catta (75), Propithecus verreauxi (76)] our findings are not unexpected. In gentle lemurs it is not unusual that activity levels remain mostly unchanged throughout the year (47), while oestrus cycles cannot be detected (45). Moreover, sexual behaviours may be missed or underestimated by the observers due to lemur cathemeral activity rhythm (77, 78). In addition, in some primate species, such as Hanuman langurs [Semnopithecus entellus (79)] and rhesus macaques [Macaca mulatta (80)], male behaviour seems not correlated with female ovulation.
Female primates use a variety of sexual signals to communicate their fertility to males, such as visual (81) [e.g., sexual swelling (82, 83) and proceptive behaviour (84)], acoustic [e.g., copulation calls (85–87)] and olfactory signals (24, 28, 88, 89). We investigated anogenital odour secretions released by a female gentle lemur and detected a small pool of compounds distinguishing the volatile chemical profile during the fertile period. Particularly, we found four volatile compounds (2-Heptanone, 3-Heptanone, 3-Octanone, and another compound that could not be identified) which were only present during the two-day ovulation window.
2-Heptanone, an aliphatic ketone insoluble in water, has been identified acting as a pheromone in both female and male mice urine (90). In females, it delays puberty in conspecifics (91) and promotes oestrous (92), while in males it has a function of stress indicator for conspecifics (91) and its content increases when the signaller is subjected to stressful events (93). It was also found having a putative semiochemical function in Macleay's marsupial mice (Antechinus stuartii), with a difference between intact and castrated males (94); in male brown rats (Rattus norvegicus), with the function of attracting females (95); and in male white-tailed deer (Odocoileus virginianus), with substantial differences between breeding and non-breeding seasons (96). It was also found in female giant panda (Ailuropoda melanoleuca) scent-marks (97), African elephants (Loxodonta Africana) musth (98) and wolf (Canis lupus) urine with higher levels in males than in females (99). Moreover, this compound was detected in several non-human primate species; namely in the urine of three strepsirrhine primates—Mohol bushbaby (Galago moholi), pygmy slow loris (Nycticebus pygmaeus) and fat-tailed dwarf lemur (Cheirogaleus medius) (100), in the scent gland secretions of owl monkeys (Aotus nancymaae) (101), and in both the urine and vaginal odour secretions of Japanese macaques (Macaca fuscata) during their fertile period (102). Finally, urinary 2-heptanone increases before menstruation and it is considered to play an important role in the functional changes preceding menstruation in women (103).
3-Heptanone, another aliphatic ketone, was detected in wolf urine (99) and African elephant musth with higher concentrations in older males (98), as well as in urine marks of the aye-aye (Daubentonia madagascariensis) (29, 104).
3-Octanone, a dialkyl ketone commonly used as a flavour and fragrance ingredient, has been found across a wide range of mammal species. For instance, it has been detected in the scent odour secretions of the giant panda (97) and in wolf urine (99), while it is involved in signalling musth among male African elephants (98). It was also found in the urine of the aye-aye (100, 105) and in the female anogenital odour secretions of crowned lemurs (Eulemur coronatus) (34).
Finally, some major limitations have to be acknowledged for this research work. First of all, the study focused on a small sample of subjects (i.e., one family troop), due to the limited number of breeding pairs hosted by EAZA institutions. Also, due to the seasonality and the constraints related to zoo daily routine, the daily diet and sampling hours varied between the two study periods (breeding vs. non-breeding). Additionally, less vaginal odour samples were collected during the non-breeding period because of the needed change of sampling protocol (i.e., from collection of spontaneously released anogenital scent-marks to training for vaginal odour sampling).
In conclusion, our findings suggest that female anogenital scent-marking and male genital sniffing followed by scent marking may signal the breeding period in gentle lemurs, but neither female nor male behaviours alone can be used as an effective indicator of female ovulation in this lemur species. On the other hand, our chemical investigation supports the hypothesis that anogenital odour may encode information about fertility in female gentle lemurs, with four volatile compounds distinguishing the ovulation window during their breeding period.
Future research work will focus on reproducing the chemical mixture, which may convey information about female fertility, to design, test and implement a new scent enrichment to trigger male mating behaviour and potentially enhance reproductive success in zoo-housed pairs of gentle lemurs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Life Sciences Ethics Committee, University of Wolverhampton (UK) and the Ethics Committee, and Jersey Zoo – Durrell Wildlife Conservation Trust (Channel Islands).
Author contributions
SF trained the study subjects, conducted the data collection, handling and analyses, and wrote the paper. SV designed the study, conducted the chemical analyses, and assisted with writing the paper. SK conducted the statistical analyses. PA and GM assisted with the endocrinological analyses and the interpretation of the results. All authors contributed to the article and approved the submitted version.
Funding
This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement no. 890341 to SF and SV and from the Primate Society of Great Britain's 2021 Captive Care grant scheme to SF. Lab work and publication fees were funded by the University of Wolverhampton's Research Investment Fund scheme – Phase 4 to SV.
Acknowledgments
We are grateful to the Durrell Wildlife Conservation Trust (Jersey), especially Dr. Eluned Price, Gale Glendewar, Rachel Cowen and the mammal keepers, for making this study possible. We thank the Wild Place Project (Bristol - UK), especially Will Walker, Daniella Pierce-Butler and the mammal keepers, for their help with the pilot study. We also thank Keith Holding and Dr. Matthew Palframan for their assistance with chemical analyses at the Rosalind Franklin Science Centre—University of Wolverhampton. Furthermore, we thank Wolverhampton University's Animal Behaviour and Wildlife Conservation group members for their constructive comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Estrada A, Garber PA. Principal drivers and conservation solutions to the impending primate extinction crisis: introduction to the special issue. Int J Primatol. (2022) 43:1–14. doi: 10.1007/s10764-022-00283-1
2. Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Fiore A, et al. Impending extinction crisis of the world's primates: why primates matter. Sci Adv. (2017) 3:e1600946. doi: 10.1126/sciadv.1600946
3. Mellor DJ, Hunt S, Gusset M. Caring for Wildlife: The World Zoo and Aquarium Animal Welfare Strategy; WAZA Executive Office. Gland: Wildlife Conservation. (2015). p. 87.
4. Farquharson KA, Hogg CJ, Grueber CE. Offspring survival changes over generations of captive breeding. Nat Commun. (2021) 12:3045. doi: 10.1038/s41467-021-22631-0
5. Tidière M, Lemaître JF, Douay G, Whipple M, Gaillard JM. High reproductive effort is associated with decreasing mortality late in life in captive ruffed lemurs. Am J Primatol. (2017) 79:e22677. doi: 10.1002/ajp.22677
6. Arumugam KA, Annavi G. Captive breeding of threatened mammals native to southeast asia – a review on their ex-situ management, implication and reintroduction guidelines. Annu Res Rev Biol. (2019) 30:1–16. doi: 10.9734/ARRB/2018/45921
7. Schulte-Hostedde AI, Mastromonaco GF. Integrating evolution in the management of captive zoo populations. Evol Appl. (2015) 8:413–22. doi: 10.1111/eva.12258
8. King T, Chamberlan C, Courage A. Assessing reintroduction success in long-lived primates through population viability analysis: Western lowland gorillas Gorilla gorilla gorilla in Central Africa. ORYX. (2014) 48:294–303. doi: 10.1017/S0030605312001391
9. Lacy RC. Stopping Evolution: Genetic Management of Captive Populations. In: Conservation Genetics in the Age of Genomics. New York Chichester, West Sussex: Columbia University Press (2009).
10. Meier K. Blue Eyes on Red Lists: Conservation and the Future of the Blue-Eyed Black Lemur. Saint Paul, MN: Macalester College (2016).
11. Vaglio S, Kaburu SSK, Pearce R, Bryant L, McAuley A, Lott A, et al. Effects of scent enrichment on behavioral and physiological indicators of stress in zoo primates. Am J Primatol. (2021) 83:e23247. doi: 10.1002/ajp.23247
12. Ben-Ari E. What's new at the zoo? Zoo biologists are taking a scientific approach to improving the quality of life for captive animals. BioScience. (2001) 51:172–7. doi: 10.1641/0006-3568
13. “The “The IUCN Red List of Threatened Species” che io sappia non e' pubblicato si trova a questo link: https://www.iucnredlist.org/species/9676/182236363 (2022).
14. Schwitzer C, Mittermeier RA, Rylands AB, Chiozza F, Williamson EA, Byler D, et al. Primates in Peril: The World's 25 Most Endangered Primates 2018–2020. Washington, DC: IUCN SSC Primate Specialist Group, International Primatological Society, Global Wildlife Conservation, and Bristol Zoological Society. (2019). p. 130.
15. Waeber PO, Reibelt LM, Randriamalala IH, Moser G, Raveloarimalala LM, Ralainasolo FB, et al. Local awareness and perceptions: consequences for conservation of marsh habitat at Lake Alaotra for one of the world's rarest lemurs. ORYX. (2018) 52:677–86. doi: 10.1017/S0030605316001198
16. Reibelt LM, Andrianandrasana HT, Ralainasolo F, Raveloarimalala LM, Lewis R, Ratsimbazafy J, et al. Lake Alaotra gentle lemur Hapalemur alaotrensis (Rumpler, 1975). In: Schwitzer C, Mittermeier RA, Rylands AB, Chiozza F, Williamson EA, Byler D, . editors. Primates in Peril: The World's 25 Most Endangered Primates 2018–2020. Washington, DC: IUCN SSC Primate Specialist Group, International Primatological Society, Global Wildlife Conservation, and Bristol Zoological Society (2019). p. 9–11.
17. Ballou JD, Lees C, Faust LJ, Long S, Lynch C, B LL, et al. Demographic and genetic management of captive populations. In: Kleiman DG, Thompson K, Baer CK, editors. Wild Mammals in Captivity: Principles and Techniques for Zoo Management. 2nd ed. Chicago, IL: University of Chicago Press (2010). p. 219–52.
18. Ralls K, Ballou JD. Captive Breeding and Reintroduction. In: Encyclopedia of Biodiversity: 2nd Ed. Waltham, MA: Academic Press (2013).
19. “Zoological Information Management System (ZIMS)”. E' un software, si trova a questo link: https://www.species360.org/products-services/zoo-aquarium-animal-management-software-2/ (2022).
20. Comizzoli P, Holt W. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol Reprod. (2019) 101:514–25. doi: 10.1093/biolre/ioz031
21. Heymann EW. The neglected sense-olfaction in primate behavior, ecology, and evolution. Am J Primatol. (2006) 68:519–24. doi: 10.1002/ajp.20249
22. Setchell JM, Vaglio S, Moggi-Cecchi J, Boscaro F, Calamai L, Knapp LA. Chemical composition of scent-gland secretions in an old world monkey (mandrillus sphinx): influence of sex, male status, and individual identity. Chem Senses. (2010) 35:205–20. doi: 10.1093/chemse/bjp105
23. Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, et al. Odour signals major histocompatibility complex genotype in an old world monkey. Proc R Soc B: Biol Sci. (2011) 278:274–80. doi: 10.1098/rspb.2010.0571
24. Drea CM. D'scent of man: A comparative survey of primate chemosignaling in relation to sex. Horm Behav. (2015) 68:117–33. doi: 10.1016/j.yhbeh.2014.08.001
25. Laska M, Hernandez Salazar LT. Olfaction in nonhuman primates. In: Doty RL, editor. Handbook of Olfaction and Gustation. 3rd Ed. Hoboken, NJ: John Wiley & Sons (2015). p. 605–21.
26. Setchell JM. Sexual selection and the differences between the sexes in Mandrills (Mandrillus sphinx). Am J Phys Anthropol. (2016) 159:S105–29. doi: 10.1002/ajpa.22904
27. Vaglio S, Minicozzi P, Romoli R, Boscaro F, Pieraccini G, Moneti G, et al. Sternal gland scent-marking signals sex, age, rank, and group identity in captive mandrills. Chem Senses. (2016) 41:177–86. doi: 10.1093/chemse/bjv077
28. Vaglio S, Minicozzi P, Kessler SE, Walker D, Setchell JM. Olfactory signals and fertility in olive baboons. Sci Rep. (2021) 11:8506. doi: 10.1038/s41598-021-87893-6
29. Drea CM. Design, delivery and perception of condition-dependent chemical signals in strepsirrhine primates: implications for human olfactory communication. Philos Trans R Soc Lond B Biol Sci. (2020) 375:20190264. doi: 10.1098/rstb.2019.0264
30. Scordato ES, Drea CM. Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim Behav. (2007) 73:301–14. doi: 10.1016/j.anbehav.2006.08.006
31. Charpentier MJE, Crawford JC, Boulet M, Drea CM. Message “scent”: lemurs detect the genetic relatedness and quality of conspecifics via olfactory cues. Anim Behav. (2010) 80:101–8. doi: 10.1016/j.anbehav.2010.04.005
32. delBarco-Trillo J, Drea CM. Socioecological and phylogenetic patterns in the chemical signals of strepsirrhine primates. Anim Behavi. (2014) 97:249–53. doi: 10.1016/j.anbehav.2014.07.009
33. Janda ED, Perry KL, Hankinson E, Walker D, Vaglio S. Sex differences in scent-marking in captive red-ruffed lemurs. Am J Primatol. (2019) 81:e22951. doi: 10.1002/ajp.22951
34. Elwell EJ, Walker D, Vaglio S. Sexual dimorphism in crowned lemur scent-marking. Animals. (2021) 11:2091. doi: 10.3390/ani11072091
35. Wyatt T. Pheromones and animal behaviour: communication by smell and taste. 1st ed. Cambridge Univ Press. (2003) 87–90. doi: 10.1017/CBO9780511615061
36. delbarco-Trillo J, Sacha CR, Dubay GR, Drea CM. Eulemur, me lemur: the evolution of scent-signal complexity in a primate clade. Philos Trans R Soc Lond B Biol Sci. (2012) 367:1909–22. doi: 10.1098/rstb.2011.0225
37. Wyatt TD. Pheromones and animal behavior: chemical signals and signatures. 2nd ed. Cambridge Univ Press. (2014) 10–23. doi: 10.1017/CBO9781139030748
38. Eppley TM, Ganzhorn JU, Donati G. Latrine behaviour as a multimodal communicatory signal station in wild lemurs: the case of Hapalemur meridionalis. Anim Behav. (2016) 111:57–67. doi: 10.1016/j.anbehav.2015.10.012
39. Irwin MT, Samonds KE, Raharison JL, Wright PC. Lemur latrines: observations of latrine behavior in wild primates and possible ecological significance. J Mammal. (2004) 85:420–7. doi: 10.1644/1545-1542085<0420:LLOOLB>2.0.CO;2
40. Hasson O. Towards a general theory of biological signaling. J Theor Biol. (1997) 185:139–56. doi: 10.1006/jtbi.1996.0258
41. Kappeler PM. A preliminary study of olfactory behavior of captive Lemur coronatus during the breeding season. Int J Primatol. (1988) 9:135–46. doi: 10.1007/BF02735733
42. Hayes RA, Morelli TL, Wright PC. Volatile components of lemur scent secretions vary throughout the year. Am J Primatol. (2006) 68:1202–7. doi: 10.1002/ajp.20319
43. Snowdon CT, Ziegler TE, Schultz-Darken NJ, Ferris CF. Social odours, sexual arousal and pairbonding in primates. Philos Trans R Soc Lond B Biol Sci. (2006) 361:2079–89. doi: 10.1098/rstb.2006.1932
44. Nievergelt CM, Mutschler T, Feistner ATC, Woodruff DS. Social system of the alaotran gentle lemur (Hapalemur griseus alaotrensis): genetic characterization of group composition and mating system. Am J Primatol. (2002) 57:157–76. doi: 10.1002/ajp.10046
45. Beattie JC, Feistner ATC. Husbandry and breeding of the alaotran gentle lemur: hapalemur griseus alaotrensis at jersey wildlife preservation trust. Int Zoo Yearb. (1998) 36:11–9. doi: 10.1111/j.1748-1090.1998.tb02880.x
46. Hakeem A, Sandoval R, Jones M, Allman J. Brain and life span in primates. In: Abeles RP, Catz M, Salthouse TT, editors. Handbook of the Psychology of Aging. San Diego: Academic Press (1996). p. 78–104.
47. Haring D, Davis K. Management of the grey gentle or eastern lesser bamboo lemur hapaletnur griseus griseus at duke university primate center, durham. Int Zoo Yearb. (1998) 36:20–34. doi: 10.1111/j.1748-1090.1998.tb02881.x
48. Wright PC. The evolution of female dominance and biparental care among non-human primates. In: Miller B, editor. Sex and Gender Hierarchies. Cambridge: University of Cambridge Press (1993). p. 127–45.
49. Rowe N, Myers M. All the world's primates. Pogonias Press. (2016) 777:45–46. doi: 10.1002/9781119179313.wbprim0086
50. Maréchal L, Semple S, Majolo B, Qarro M, Heistermann M, MacLarnon A. Impacts of tourism on anxiety and physiological stress levels in wild male barbary macaques. Biol Conserv. (2011) 144:2188–93. doi: 10.1016/j.biocon.2011.05.010
51. Fontani S, Vaglio S, Beghelli V, Mattioli M, Bacci S, Accorsi PA. Fecal concentrations of cortisol, testosterone, and progesterone in cotton-top tamarins housed in different zoological parks: relationships among physiological data, environmental conditions, and behavioral patterns. JAAWS. (2014) 17:228–52. doi: 10.1080/10888705.2014.916173
52. Gholib G, Wahyuni S, Kadar OH, Adam M, Lubis TM, Azhar A, et al. Measurement of serum testosterone in Kacang goat by using enzyme-linked immunosorbent assay (ELISA) technique: the importance of kit validation. IJVS. (2016) 10:32–6. doi: 10.21157/j.ked.hewan.v10i1.3367
53. Chen H, Yao H, Yang W, Fan P, Xiang Z. Assessing the utility of urinary and fecal cortisol as an indicator of stress in golden snub-nosed monkeys (Rhinopithecus roxellana). PeerJ. (2017) 5:e3648. doi: 10.7717/peerj.3648
54. Bogart MH, Kumamoto AT, Lasley BL. A comparison of the reproductive cycle of three species of lemur. Folia Primatol. (1977) 28:134–43. doi: 10.1159/000155803
55. van Horn RN, Resko JA. The reproductive cycle of the ring-tailed lemur (Lemur catta): sex steroid levels and sexual receptivity under controlled photoperiods. Endocrinology. (1977) 101:1579–86. doi: 10.1210/endo-101-5-1579
56. Curtis DJ, Zaramody A, Green DI, Pickard AR. Non-invasive monitoring of reproductive status in wild mongoose lemurs (Eulemur mongoz). Reprod Fertil Dev. (2000) 12:21–9. doi: 10.1071/RD99091
57. Altmann J. Observational study of behavior: sampling methods. Behaviour. (1974) 49:227–67. doi: 10.1163/156853974X00534
58. Errington M. An investigation into the effect of olfactory enrichment on the behaviour of Lac Alaotra gentle lemurs (Hapalemur alaotrensis), at Chessington Zoo, CWOAR. [Ph.D. Thesis]. Poole: Bournemouth University (2017).
59. Shideler SE, Lindburg DG, Lasley BL. Estrogen-behavior correlates in the reproductive physiology and behavior of the ruffed lemur (Lemur variegatus). Horm Behav. (1983) 17:249–63. doi: 10.1016/0018-506X(83)90024-7
60. Pomerantz O, Terkel J. Effects of positive reinforcement training techniques on the psychological welfare of zoo-housed chimpanzees (Pan troglodytes). A J Primatol. (2009) 71:687–95. doi: 10.1002/ajp.20703
61. Walker D, Vaglio S. Sampling and analysis of animal scent signals. J Vis Exp. (2021) 168:e60902. doi: 10.3791/60902
62. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
63. Aujard F, Heistermann M, Thierry B, Hodges JK. Functional significance of behavioral, morphological, and endocrine correlates across the ovarian cycle in semifree ranging female Tonkean macaques. Am J Primatol. (1998) 46:285–309. doi: 10.1002/(SICI)1098-2345(1998)46:4<285::AID-AJP2>3.0.CO;2-8
64. Ziegler T, Hodges K, Winkler P, Heistermann M. Hormonal correlates of reproductive seasonality in wild female hanuman langurs (Presbytis entellus). Am J Primatol. (2000) 51:119–34. doi: 10.1002/(SICI)1098-2345(200006)51:2<119::AID-AJP2>3.0.CO;2-O
65. Hodges J, Heisterman M. Field endocrinology: monitoring hormonal changes in free-ranging primates. In: Setchell J, Curtis DJ, editors. Field and Laboratory Methods in Primatology A practical guide. 2nd Edition. Cambridge: Cambridge University Press (2011). p. 353–70.
66. Liu H, Zhang C, Liu Y, Duan H. Total flavonoid contents in bamboo diets and reproductive hormones in captive pandas: exploring the potential effects on the female giant panda (Ailuropoda melanoleuca). Conserv Physiol. (2019) 7:coy068. doi: 10.1093/conphys/coy068
67. Perret M. Change in photoperiodic cycle affects life span in a prosimian primate (Microcebus murinus). J Biol Rhythms. (1997) 12:136–45. doi: 10.1177/074873049701200205
68. Walker ML, Wilson ME, Gordon TP. Endocrine control of the seasonal occurrence of ovulation in rhesus monkeys housed outdoors. Endocrinology. (1984) 114:1074–81. doi: 10.1210/endo-114-4-1074
69. Herndon JG, Turner JJ, Ruiz de Elvira MC, Collins DC. Silent ovulation in rhesus monkeys (M. mulatta): dissociation of hormonal and behavioral states. Physiol Behav. (1987) 40:665–72. doi: 10.1016/0031-9384(87)90115-6
70. Ungerfeld R. Socio-sexual signalling and gonadal function: opportunities for reproductive management in domestic ruminants. Soc Reprod Fertil Suppl. (2007) 64:207–21. doi: 10.5661/RDR-VI-207
71. Pereira ME. Asynchrony within estrous synchrony among ringtailed lemurs (Primates: Lemuridae). Physiol Behav. (1991) 49:47–52. doi: 10.1016/0031-9384(91)90228-G
72. Izard K, Coffman B, Katz A, Simons E. Reproduction in the collared lemur (Eulemur fulvus collaris). Am J Primatol. (1993) 30:320.
73. Overdorff DJ. Are eulemur species pair-bonded? Social organization and mating strategies in Eulemur fulvus rufus from 1988-1995 in southeast Madagascar. Am J Phys Anthropol. (1998) 105:153–66. doi: 10.1002/(SICI)1096-8644(199802)105:2<153::AID-AJPA4>3.0.CO;2-W
74. Perret M. Chemocommunication in the reproductive function of mouse lemurs. In: Alterman L, Doyle G, Izard M, editors. Creatures of the Dark. Boston, MA: Springer (1995).
75. Cavigelli SA, Pereira ME. Mating season aggression and fecal testosterone levels in male ring- tailed lemurs (Lemur catta). Horm Behav. (2000) 37:246–55. doi: 10.1006/hbeh.2000.1585
76. Kappeler PM, Mass V, Port M. Even adult sex ratios in lemurs: potential costs and benefits of subordinate males in Verreaux's sifaka (Propithecus verreauxi) in the Kirindy forest CFPF, Madagascar. Am J Phys Anthropol. (2009) 140:487–97. doi: 10.1002/ajpa.21091
77. Overdorff DJ, Rasmussen MA. Determinants of Nighttime activity in “diurnal” lemurid primates. In: Alterman L, Doyle G, Izard M, editors. Creatures of the Dark. Boston, MA: Springer (1995).
78. Donati G, Lunardini A, Kappeler PM. Cathemeral Activity of Red-Fronted Brown Lemurs (Eulemur Fulvus Rufus) in the Kirindy Forest/CFPF. In: New Directions in Lemur Studies. Boston, MA: Springer (1999).
79. Heistermann M, Ziegler T, van Schaik CP, Launhardt K, Winkler P, Hodges JK. Loss of oestrus, concealed ovulation and paternity confusion in free-ranging hanuman langurs. Proc R Soc B Biol Sci. (2001) 268:2445–51. doi: 10.1098/rspb.2001.1833
80. Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Horm Behav. (2012) 61:696–705. doi: 10.1016/j.yhbeh.2012.03.003
81. Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. (2001) 410:363–6. doi: 10.1038/35066567
82. Pagel M. The evolution of conspicuous oestrous advertisement in old world monkeys. Anim Behav. (1994) 47:1333–41. doi: 10.1006/anbe.1994.1181
83. Nunn CL. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav. (1999) 58:246–99. doi: 10.1006/anbe.1999.1159
84. Tiddi B, Wheeler BC, Heistermann M. Female behavioral proceptivity functions as a probabilistic signal of fertility, not female quality, in a new world primate. Horm Behav. (2015) 73:148–55. doi: 10.1016/j.yhbeh.2015.07.011
85. Semple S, McComb K. Perception of female reproductive state from vocal cues in a mammal species. Proc R Soc B Biol Sci. (2000) 267:707–12. doi: 10.1098/rspb.2000.1060
86. Maestripieri D, Roney JR. Primate copulation calls and postcopulatory female choice. Behav Ecol. (2005) 16:106–13. doi: 10.1093/beheco/arh120
87. Vaglio S, Ducroix L, Villanueva MR, Consiglio R, Kim AJ, Neilands P, et al. Female copulation calls vary with male ejaculation in captive olive baboons. Behaviour. (2020) 157:807–22. doi: 10.1163/1568539X-bja10024
88. Crawford JC, Boulet M, Drea CM. Smelling wrong: hormonal contraception in lemurs alters critical female odour cues. Proc R Soc B. (2011) 278:122–30. doi: 10.1098/rspb.2010.1203
89. Kücklich M, Weiß BM, Birkemeyer C, Einspanier A, Widdig A. Chemical cues of female fertility states in a non-human primate. Sci Rep. (2019) 9:131716. doi: 10.1038/s41598-019-50063-w
90. Spehr M, Spehr J, Ukhanov K, Kelliher KR, Leinders-Zufall T, Zufall F. Parallel processing of social signals by the mammalian main and accessory olfactory systems. Cell Mol Life Sci. (2006) 63:1476–84. doi: 10.1007/s00018-006-6109-4
91. Laska M. Olfactory sensitivity and odor structure-activity relationships for aliphatic ketones in CD-1 mice. Chem Senses. (2014) 39:415–24. doi: 10.1093/chemse/bju011
92. Liberles SD. Mammalian pheromones. Annu Rev Physiol. (2014) 76:151–75. doi: 10.1146/annurev-physiol-021113-170334
93. Gutiérrez-García AG, Contreras CM, Mendoza-López MR, García-Barradas O, Cruz-Sánchez JS. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav. (2007) 91:166–72. doi: 10.1016/j.physbeh.2007.02.006
94. Toftegaards CL, Moore C, Bradley AJ. Chemical characterization of urinary pheromones in brown antechinus, Antechinus stuartii. J Chem Ecol. (1999) 25:527–35. doi: 10.1023/A:1020901820044
95. Osada K, Kashiwayanagi M, Izumi H. Profiles of volatiles in male rat urine: the effect of puberty on the female attraction. Chem Senses. (2009) 34:713–21. doi: 10.1093/chemse/bjp058
96. Miller KV, Jemiolo B, Gassett JW, Jelinek I, Wiesler D, Novotny M. Putative chemical signals from white-tailed deer (Odocoileus virginianus): social and seasonal effects on urinary volatile excretion in males. J Chem Ecol. (1998) 24:673–83. doi: 10.1023/A:1022342219469
97. Hagey L, MacDonald E. Chemical cues identify gender and individuality in giant pandas (Ailuropoda melanoleuca). J Chem Ecol. (2003) 29:1479–88. doi: 10.1023/A:1024225806263
98. Rasmussen LEL, Wittemyer G. Chemosignalling of musth by individual wild African elephants (Loxodonta africana): implications for conservation and management. Proc R Soc B Biol Sci. (2002) 269:853–60. doi: 10.1098/rspb.2001.1933
99. Raymer J, Wiesler D, Novotny M, Asa C, Seal US, Mech LD. Chemical scent constituents in urine of wolf (Canis lupus) and their dependence on reproductive hormones. J Chem Ecol. (1986) 12:297–314. doi: 10.1007/BF01045612
100. Delbarco-Trillo J, Burkert BA, Goodwin TE, Drea CM. Night and day: the comparative study of strepsirrhine primates reveals socioecological and phylogenetic patterns in olfactory signals. J Evol Biol. (2011) 24:82–98. doi: 10.1111/j.1420-9101.2010.02145.x
101. Spence-Aizenberg A, Kimball BA, Williams LE, Fernandez-Duque E. Chemical composition of glandular secretions from a pair-living monogamous primate: sex, age, and gland differences in captive and wild owl monkeys (Aotus spp.). Am J Primatol. (2018) 80:e22730. doi: 10.1002/ajp.22730
102. Rigaill L, Vaglio S, Setchell JM, Suda-Hashimoto N, Furuichi T, Garcia C. Chemical cues of identity and reproductive status in Japanese macaques. Am J Primatol. (2022) e23411. doi: 10.1002/ajp.23411
103. Gutierrez-Garcia AG, Contreras CM. Mendoza-lopez R. 2-heptanone produces sensorial-emotional changes, depending on length of exposure. Rev Costarri Psicol. (2015) 34:1–14. doi: 10.22544/rcps.v34i01.01
104. Delbarco-Trillo J, Harelimana IH, Goodwin TE, Drea CM. Chemical differences between voided and bladder urine in the aye-aye (Daubentonia madagascariensis): implications for olfactory communication studies. Am J Primatol. (2013) 75:695–702. doi: 10.1002/ajp.22083
Keywords: captive breeding, chemical signalling, faecal endocrinology, Hapalemur alaotrensis, reproductive biology
Citation: Fontani S, Kaburu SSK, Marliani G, Accorsi PA and Vaglio S (2022) Anogenital scent-marking signals fertility in a captive female Alaotran gentle lemur. Front. Vet. Sci. 9:940707. doi: 10.3389/fvets.2022.940707
Received: 10 May 2022; Accepted: 30 June 2022;
Published: 28 July 2022.
Edited by:
Michał Dziẹcioł, Wroclaw University of Environmental and Life Sciences, PolandReviewed by:
Kathryn Wilsterman, Colorado State University, United StatesToni Lyn Morelli, United States Geological Survey (USGS), United States
Copyright © 2022 Fontani, Kaburu, Marliani, Accorsi and Vaglio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Vaglio, Uy5WYWdsaW9Ad2x2LmFjLnVr
 Sara Fontani
Sara Fontani Stefano S. K. Kaburu
Stefano S. K. Kaburu Giovanna Marliani
Giovanna Marliani Pier Attilio Accorsi3
Pier Attilio Accorsi3 Stefano Vaglio
Stefano Vaglio