- 1Department of Rehabilitation, Beibei Traditional Chinese Medical Hospital, Chongqing, China
- 2Department of Radiology, Daping Hospital, Army Medical University, Chongqing, China
Background: Currently, many studies have been published on the relationship between the gut microbiome and knee osteoarthritis. However, the evidence for the association of gut microbiota with knee osteoarthritis has not been comprehensively evaluated.
Objective: This review aimed to assess existing results and provide scientific evidence for the association of low-grade inflammation caused by gut microbiota disturbances with knee osteoarthritis.
Methods: This study conducted an extensive review of the current literature using four databases, PubMed, EMBASE, Cochrane Library and Web of Science before 31 December 2021. Risk of bias was determined using ROBINS and SYRCLE, and quality of evidence was assessed using GRADE and CAMADARES criteria. Twelve articles were included.
Results: Studies have shown that a high-fat diet leads to a disturbance of the gut microbiota, mainly manifested by an increase in the abundance of Firmicutes and Proteobacteria, a decrease in Bacteroidetes, and an increase in the Firmicutes/ Bacteroidetes ratio. Exercise can reverse the pattern of gain or loss caused by high fat. These changes are associated with elevated levels of serum lipopolysaccharide (LPS) and its binding proteins, as well as various inflammatory factors, leading to osteoarthritis (OA).
Conclusion: This systematic review shows that a correlation between low-grade inflammation caused by gut microbiota disturbances and severity of knee osteoarthritis radiology and dysfunction. However, there was a very small number of studies that could be included in the review. Thus, further studies with large sample sizes are warranted to elucidate the association of low-grade inflammation caused by gut microbiota disturbances with osteoarthritis, and to explore the possible mechanisms for ameliorating osteoarthritis by modulating gut microbiota.
Introduction
Osteoarthritis (OA) is the most common musculoskeletal disease and one of the leading causes of disability (1). Epidemiological surveys show that more than 320 million people worldwide suffer from OA, and the prevalence is higher in women than men. Traditionally, mechanical and genetic factors have been considered important causes of OA (2, 3). However, emerging evidence suggests that low-grade inflammation plays an important role in the development of OA (4), and this inflammatory state is closely related to the gastrointestinal microbiota (5).
The gastrointestinal microbiota refers to the sum of all genetic material and its metabolites of all microbiota present in the gut (6, 7). The gut microbiota plays an important role in maintaining the body's homeostasis, which underlies human physiology, immune system development, digestion, fat storage, regulation of angiogenesis, behavior, development, and detoxification responses. The human gut microbiota is mainly composed of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Verrucobacterium. Among them, Bacteroidetes and Firmicutes account for more than 98% of the total number of intestinal symbiotic flora of more than 70 species (8, 9). Studies have shown that a variety of diseases are associated with specific bacterial sequences and alterations and disturbances in the composition of the microbiota (10, 11). At the same time, the gut microbiota plays a key role in the development and function of the immune system, as well as in allergic and inflammatory responses (12–15). Alterations in the microbiome activate the innate immune system, leading to increased pro-inflammatory cytokines, and these local and systemic low-grade inflammations contribute to the development and progression of OA (16, 17).
At present, there are more and more studies on the correlation between low-grade inflammation caused by intestinal flora disturbance and OA. It is difficult to draw conclusions about the consistency of the association due to different study designs and assessment methods, so it is unclear whether low-grade inflammation due to disturbances in the gut microbiota has a different effect on OA. Given the high prevalence of OA and its significant socioeconomic burden, it is important to explore the impact of low-grade inflammation caused by gut microbiota disturbances on OA.
Methods
Search strategy
We searched comprehensively for articles published before 31 December 2021 using four electronic medical databases (PubMed, EMBASE, Cochrane Library and Web of Science). Studies were identified using the search terms “('gut microbiota' or 'microbiome' or 'microbiota' or 'gut') and ('Osteoarthritis' or 'arthritis' or 'KOA' or 'OA') and ('Inflammation')”.
Selection criteria
Inclusion criteria: (1) clinical and basic research with any level of evidence; (2) English-language articles published in peer-reviewed journals; (3) studies on the association of low-grade inflammation caused by gut microbial imbalances with OA, and OA Pathogenesis or related-symptoms. Exclusion criteria: (1) studies with missing data; (2) studies with duplication and poor scientific method; (3) abstracts, case reports, conference reports, reviews, editorials, and expert opinions were excluded.
Literature screening and data extraction
Two investigators (WX and HX) independently searched, selected relevant articles according to the inclusion and exclusion criteria, read the full text, and extracted data from the final included literature. Any disagreements were resolved by an experienced systematic reviewer (BJJ). Differences in data extraction are resolved by consensus.
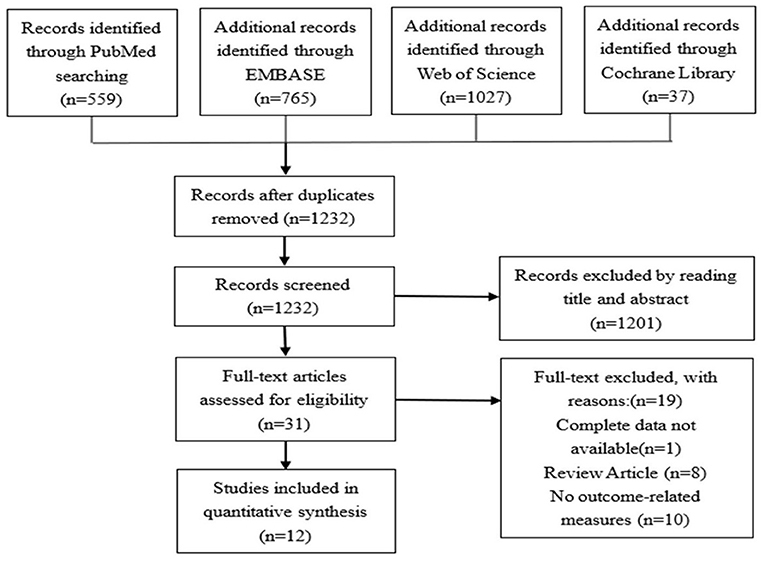
After extraction, the data was considered of heterogenous nature both by study design, measure, and method of assessment. Therefore, a descriptive analysis approach was preferred to a metanalysis. Figure 1 for details.
Risk of bias assessment
ROBINS was used to assess the risk of bias in non-randomized clinical studies (18), and RoB 2.0 (19) was used to assess the risk of bias in randomized clinical studies. Risk of bias in preclinical studies was assessed using SYRCLE (20). WX and HX conduct evaluations independently, and any disagreements are resolved by consensus.
Study quality assessment
The quality of clinical studies (n = 6) was assessed using the GRADE method (21) and each study was classified as 'low', 'moderate' or 'high'. All studies were ranked 'moderate' or 'high'. The quality of preclinical studies (n = 6) was assessed using the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMADARES) checklist (Supplementary material) (22, 23). Each study was scored on a scale from 0 to 10 points, and the overall quality of included studies was moderate (mean CAMADARES score 4.17, range 4–5). WX and HX conduct evaluations independently, and any disagreements are resolved by consensus.
Results
Study characteristics
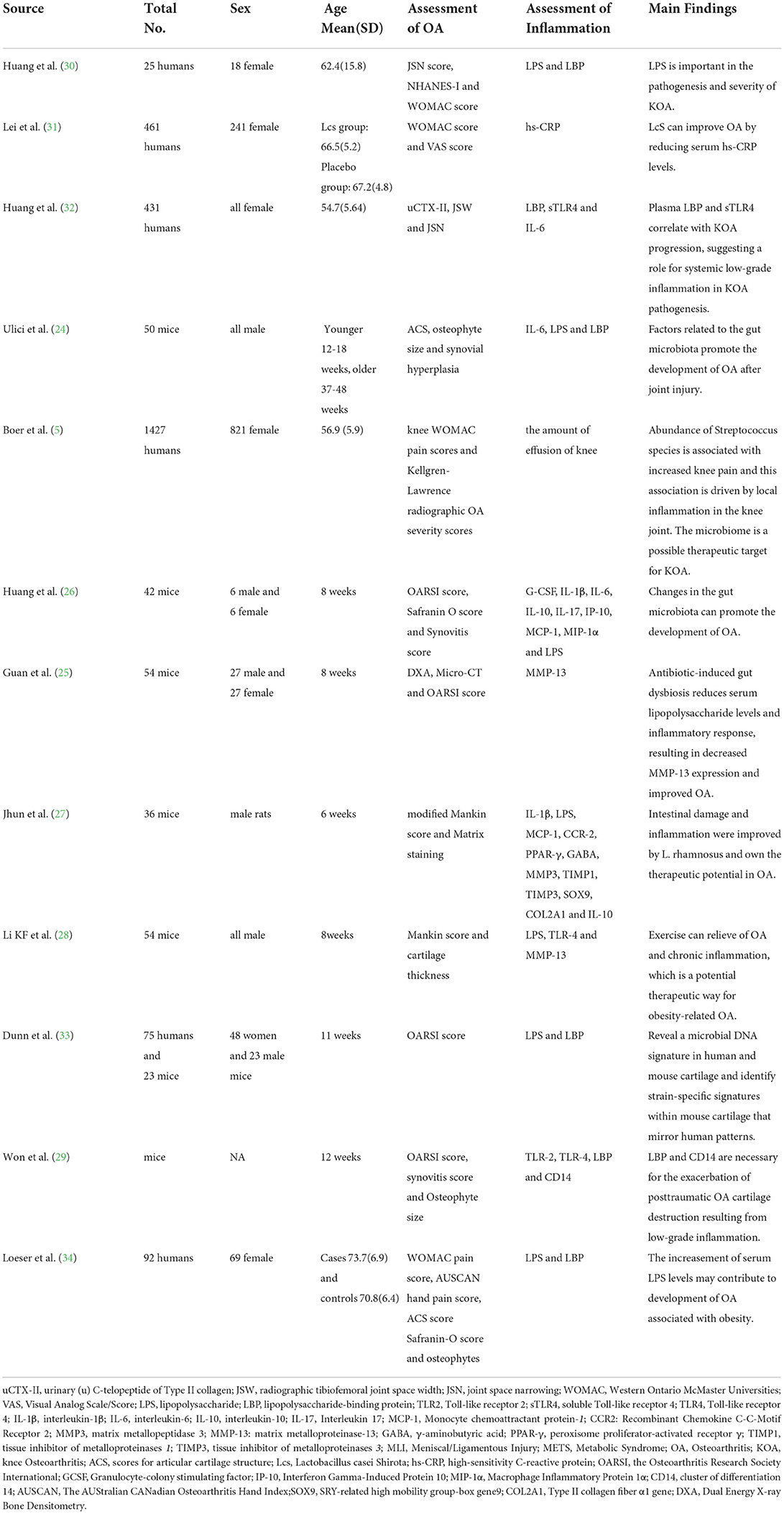
The final analysis included 12 studies, 6 of which were animal studies (24–29) and 6 were clinical studies (5, 30–34). Regarding clinical trials, 4 were non-randomized observational studies (5, 30, 33, 34) and 2 were randomized clinical trials (31, 32). The main characteristics of the included studies are reported in Table 1.
Most studies used 16S ribosomal RNA (rRNA) gene sequencing to examine gut microbiota and Enzyme-linked immunosorbent assay (ELISA) to measure inflammatory markers. Meanwhile, most studies assessed radiographic or symptom severity of OA using Western Ontario McMaster Universities (WOMAC) score, Visual Analog Scale (VAS) score, scores for articular cartilage structure (ACS) score, the Osteoarthritis Research Society International (OARSI) score, synovitis score and Osteophyte size. Overall, various studies have suggested that there is a certain relationship between inflammation caused by intestinal flora disturbance and OA.
Effects of diet, exercise or probiotics on gut microbiota
High-fat diet leads to gut microbiota disturbances and is a common model of low-grade inflammation (35). Firmicutes, Bacteroidetes and Proteobacteria are the three major phyla of the gut microbiota (28). High-fat diet cause disturbance of the gut microbiota, increase endotoxin-producing bacteria, and decrease bacteria protecting the intestinal barrier, thereby enhancing bone destruction on OA in mice. It is mainly manifested by an increase in the abundance of Firmicutes and Proteobacteria, but a decrease in Bacteroidetes, and an increase in the Firmicutes/Bacteroidetes ratio (28).
Exercise reverses high fat diet-induced gut microbiota disturbances, manifested by decreased abundances of Firmicutes and Proteobacteria, increased abundance of Bacteroidetes, and decreased Firmicutes/Bacteroidetes ratios. At the family level, exercise reversed the unclassified Bacteroidetes, Lachnospira, Desulfovibrio, Ruminococci, Lactobacillus, Prevotaceae, Peptostreptococcus, Bifidobacterium, and Staphylococcus (28).
Two studies suggest that probiotic supplementation reduces intestinal damage and inflammation, and has great potential in the treatment of osteoarthritis (27, 31).
The influence of intestinal flora disturbance on OA
Intestinal microbial disturbances increase intestinal permeability and cause low-grade inflammation throughout the body, thereby aggravating OA. By transplanting human microorganisms into mice, it was found that the abundance of Fusobacterium and Enterococcus faecalis in the transplanted mice increased, but the abundance of Ruminococcus decreased, the average systemic concentration of inflammatory markers increased, and the intestinal increased permeability is associated with more severe OA (26). At the same time, the serum estrogen level in OA rats was significantly decreased, which was correlated with the significant increase in LPS. In Lactobacillus rhamnosus-treated OA rats, the expression levels of Monocyte chemoattractant protein-1 (MCP-1) and its receptors Recombinant Chemokine C-C-Motif Receptor 2 (CCR2), interleukin-1β (IL-1β), matrix metallopeptidase 3 (MMP3) were decreased, while γ-aminobutyric acid (GABA) and peroxisome proliferator-activated receptor γ (PPAR-γ), tissue inhibitor of metalloproteinases 1 (TIMP1), tissue inhibitor of metalloproteinases 3 (TIMP3), SRY-related high mobility group-box gene9 (SOX9) and Type II collagen fiber α1 gene (COL2A1) and interleukin-10 (IL-10) increased expression levels (27).
The effect of inflammation on OA
Inflammation is a key link in the occurrence and development of OA. Whether it is inflammation in the plasma or in the local soft tissue of the joint, it can cause OA. Studies have shown that stimulation of toll-like receptor (TLR) signaling can exacerbate invasive OA in mice (29). At the same time, serum high-sensitivity C-reactive protein (hs-CRP) levels were correlated with bone and joint WOMAC score and VAS score (31). Research has shown that, LPS and lipopolysaccharide-binding protein (LBP) were significantly associated with activated macrophages and osteophyte severity in the joints of Knee Osteoarthritis (KOA) patients (30). Guan et al. also reported that the main indicators of OA, bone volume over total volume (BV/TV), trabecular thickness (Tb.Th), and medial femoral condyle (MFC) were positively correlated with LPS, IL-6, and Tumor necrosis factor-α (TNF-α), and negatively correlated with the ratio of Firmicutes and Bacteroidetes (25). However, not all studies have shown a correlation between inflammatory markers and osteoarthritis. Studies have shown no statistically significant association between soluble Toll-like receptor 4 (sTLR4) or IL-6 and radiographic progression of OA (32).
Discussions
Our systematic review suggests a link between low-grade inflammation caused by gut microbiota and osteoarthritis, but further research is needed in the future. Low-grade inflammation leads to OA through the production of inflammatory mediators, including innate immune activation, macrophage-dominated inflammatory response, Toll-like receptor (TLR) activation, and complement activation, among which TLR signaling plays an important role in the pathogenesis of OA (4, 36–38). Locally injured molecules activate TLRs, which trigger the secretion of pro-inflammatory substances and local inflammation in the joints (4, 38). It has been found that TLR expression is increased in areas of cartilage damage in OA patients (39). Upregulation of various TLR signaling components is seen in OA-associated chondrocytes, most notably LBP and cluster of differentiation 14 (CD14), which are accessory proteins of multiple TLRs and interact with multiple signaling molecules including LPS (37, 38).
Studies have shown that gut bacterial products such as LPS can enter the systemic circulation and affect many organs, including joints, by causing systemic low-grade inflammation (30, 40). LPS is an endotoxin associated with the outer membrane of various Gram-negative pathogens (41) and a classic innate immune system activator that activates host immune cells by binding to Toll-like proteins. Meanwhile, a correlation study between LPS and OA has shown that human serum LPS levels are associated with osteophyte severity in OA, and synovial fluid LPS is associated with osteophyte severity, joint space narrowing, and total pain/function severity scores (30).
Similar to LPS, LBP has also been shown to be associated with increased KOA severity in humans (30). LBP is mainly produced by hepatocytes and is a well-known acute phase reactant (42). LBP is activated by inflammatory mediators such as IL-6 and directly or indirectly by LPS itself (43–45). In humans, LBP triggers a dynamic endotoxin cascade by binding LPS and transferring it to CD14, which transfers LPS to the Toll-like receptor 4-myeloid differentiation protein-2 (TLR4-MD-2) receptor on immune cells; LBP thereby concentrates LPS on the cell membrane of immune cells, to induce an inflammatory response (46). LBP binds pro-inflammatory components of both Gram-positive and Gram-negative bacteria (47), making it a more prevalent marker of bacterial exposure than LPS derived only from Gram-negative bacteria (45). Meanwhile, other studies have shown that LBP is necessary for the inflammatory cascade triggered by saturated fatty acids and metabolic endotoxemia (48, 49).
A high-fat diet, an unhealthy dietary pattern that leads to obesity, altering microbial community structure and reduce microbial diversity, resulting in an increase in pro-inflammatory microbiota, thereby increasing intestinal permeability and circulating levels of LPS. In a high-fat diet model, TLR signaling plays a key role in low-grade inflammatory pathways (4, 50), such as toll-like receptor 4 (TLR4) (37, 51, 52), LPS, and LBP (31), and interleukin 6 (IL-6) (53–55), and have also been implicated in the inflammatory mechanisms of OA.
Exercise diversifies the gut microbiota and reduces the Firmicutes/Bacteroidetes ratio (56). This view was validated in our systematic review (28). At the same time, exercise produces high levels of endocannabinoids in arthritis patients, which mediate the gut microbiota to produce anti-inflammatory substances that reduce pain (57).
Gender variance is one of the factors affecting the prevalence of OA. A meta-analysis on global incidence and prevalence of OA in women is 1.69 and 1.39 times as much in males, respectively (58). Meanwhile, a study found that polymorphism in growth differentiation factor-5, estrogen-specific receptor-alpha, and calmodulin-1 has increased the disruption of cartilage and reduced mRNA and protein synthesis, which increased the risk of KOA in women (59). Moreover, A prevalence study on osteoporosis, hypovitaminosis D, and OA found higher rates of Vitamin D insufficiency and deficiency in women than in men (60), and there is a correlation between vitamin D deficiency and OA (61).
Limitations
First, In the analysis of microbial sequencing, the analytical methods were different across studies involving various regions (V3-V5) and cut-off points for clustering OTUs which may affect the results. Second, the gut microbial community analysis by 16S rRNA sequencing was not used in all studies, which may affect the consistency of the results. Third, most of them are animal studies, and there are fewer extensive studies in humans, and fewer studies on the complexity of the gut microbiota and its association with OA. Finally, most studies have only observed changes in gut microbiota and inflammatory factors, but the underlying mechanisms have not been further explored.
Conclusions
In conclusion, our systematic review provides evidence for the development of OA due to low-grade inflammation caused by intestinal flora disturbance. Further studies are needed to explore the mechanisms involved.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
WX and HX conceived and designed research, performed experiments, and edited and revised manuscript. YJ analyzed data. BJ and YJ interpreted results of experiments. WX prepared figures and drafted manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Chongqing Beibei District Science and Technology Bureau Project (2022-18).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.938629/full#supplementary-material
References
1. Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1603–58. doi: 10.1016/S0140-6736(16)31460-X
2. Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. (2011) 25:815–23. doi: 10.1016/j.berh.2011.11.013
3. Panoutsopoulou K, Zeggini E. Advances in osteoarthritis genetics. J Med Genet. (2013) 50:715–24. doi: 10.1136/jmedgenet-2013-101754
4. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:580–92. doi: 10.1038/nrrheum.2016.136
5. Boer CG, Radjabzadeh D, Gomez CM, Garmaeva S, Schiphof D, Arp P, et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. (2019) 10:4881. doi: 10.1038/s41467-019-12873-4
6. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. (2018) 555:210–15. doi: 10.1038/nature25973
7. Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, et al. A new genomic blueprint of the human gut microbiota. Nature. (2019) 568:499–504. doi: 10.1038/s41586-019-0965-1
8. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. (2006) 124:837-48. doi: 10.1016/j.cell.2006.02.017
9. Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
10. Frank DN, Amand AL, St Robert A, Feldman RA, Boedeker EC, Harpaz N, Pace N N R Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. (2007) 104:13780–85. doi: 10.1073/pnas.0706625104
11. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. (2015) 125:926–38. doi: 10.1172/JCI76304
12. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. (2011) 474:327–36. doi: 10.1038/nature10213
13. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. (2012) 336:1262–7. doi: 10.1126/science.1223813
14. Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. (2017) 18:851–60. doi: 10.1038/ni.3780
15. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. (2017) 152:327–39. doi: 10.1053/j.gastro.2016.10.012
16. Berenbaum F, Griffin TM, Liu-Bryan R. Review: metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol. (2017) 69:9–21. doi: 10.1002/art.39842
17. Krasnokutsky S, Oshinsky C, Attur M, Ma S, Zhou H, Zheng FF, et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol. (2017) 69:1213–20. doi: 10.1002/art.40069
18. Sterne JA, Hernán HA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
19. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:14898. doi: 10.1136/bmj.l4898
20. Hooijmans CR, Rovers MM, Vries RBD, Leenaars M, Hoitinga MR, Langendam MW, et al. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
21. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus AGRADE. guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64:380–2. doi: 10.1016/j.jclinepi.2010.09.011
22. Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. (2004) 35:1203–8. doi: 10.1161/01.STR.0000125719.25853.20
23. Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein P, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. (2012) 490:187–91. doi: 10.1038/nature11556
24. Ulici V, Kelley KL, Azcarate-Peril MA, Cleveland RJ, Sartor RB, Schwartz TA, et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage. (2018) 26:1098–109. doi: 10.1016/j.joca.2018.05.016
25. Guan Z, Jia JL, Zhang CG, Sun TT, Zhang W, Yuan WQ, et al. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin Sci (Lond). (2020) 134:3159–74. doi: 10.1042/CS20201224
26. Huang Z, Chen J, Li BL, Zeng BH, Chou CH, Zheng X, et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis. (2020) 79:646–56. doi: 10.1136/annrheumdis-2019-216471
27. Jhun J, Cho KH, Lee DH, Kwon JY, Woo JS, Kim J, et al. Oral administration of lactobacillus rhamnosus ameliorates the progression of osteoarthritis by inhibiting joint pain and inflammation. Cells. (2021) 10:1057. doi: 10.3390/cells10051057
28. Li K, Liu AL, Zong WH, Dai LL, Liu Y, Luo RP, et al. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci. (2021) 28:40–9. doi: 10.1016/j.sjbs.2020.08.027
29. Won Y, Yang JI, Park S, Chun JS. Lipopolysaccharide binding protein and CD14, cofactors of toll-like receptors, are essential for low-grade inflammation-induced exacerbation of cartilage damage in mouse models of posttraumatic osteoarthritis. Arthritis Rheumatol. (2021) 73:1451–60. doi: 10.1002/art.41679
30. Huang ZY, Stabler T, Pei FX, Kraus VB. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthritis Cartilage. (2016) 24:1769–75. doi: 10.1016/j.joca.2016.05.008
31. Lei M, Guo C, Wang D, Zhang C, Hua L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef Microbes. (2017) 8:697–703. doi: 10.3920/BM2016.0207
32. Huang ZY, Perry E, Huebner JL, Katz B, Li YJ, Kraus VB, et al. Biomarkers of inflammation—LBP and TLR- predict progression of knee osteoarthritis in the DOXY clinical trial. Osteoarthritis Cartilage. (2018) 26:1658–65. doi: 10.1016/j.joca.2018.08.005
33. Dunn CM, Velasco C, Rivas A, Andrews M, Garman C, Jacob PB, et al. Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis Rheumatol. (2020) 72:1111–22. doi: 10.1002/art.41210
34. Loeser RF, Arbeeva L, Kelley K, Fodor AA, Sun S, Ulici V, et al. Association of increased serum lipopolysaccharide, but not microbial dysbiosis, with obesity-related osteoarthritis. Arthritis Rheumatol. (2022) 74:227–36. doi: 10.1002/art.41955
35. Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, Herzog W, et al. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. (2019) 9:3893. doi: 10.1038/s41598-019-40601-x
36. Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. (2012) 12:168–79. doi: 10.1038/nri3151
37. Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis–finding targets for candidate DMOADs. Nat Rev Rheumatol. (2015) 11:159–70. doi: 10.1038/nrrheum.2014.209
38. van den Bosch MHJ, van Lent P, van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage. (2020) 28:532–43. doi: 10.1016/j.joca.2020.01.016
39. Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Kim HY, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. (2006) 54:2152–63. doi: 10.1002/art.21951
40. Creely SJ, McTernan PG, Kusminski CM, Fisherff M, Silva NF, Da Khanolkar M, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. (2007) 292:E740–7. doi: 10.1152/ajpendo.00302.2006
41. Miller SI, Ernst RK, Bader MWLPS. TLR4 and infectious disease diversity. Nat Rev Microbiol. (2005) 3:36–46. doi: 10.1038/nrmicro1068
42. Schumann R, Kirsching CJ, Lamping N, Amping N, Mping N, Unbehaun A, et al. The lipopolysaccharide-binding protein is a secretory class 1 acute-phase protein whose gene is transcriptionally activated by APRF/STAT-3 and other cytokine-inducible nuclear proteins. Mol Cell Biol. (1996) 16:3490–503. doi: 10.1128/MCB.16.7.3490
43. Brass DM, Savov JD, Whitehead GS, Maxwell AB, Schwartz DA. LPS binding protein is important in the airway response to inhaled endotoxin. J Allergy Clin Immunol. (2004) 114:586–92. doi: 10.1016/j.jaci.2004.04.043
44. Gamble L, Bagby GJ, Quinton LJ, Happel KI JP, Zhang P, et al. The systemic and pulmonary LPS binding protein response to intratracheal lipopolysaccharide. Shock. (2009) 31:212–7. doi: 10.1097/SHK.0b013e31817c0d7d
45. Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. (2011) 39:989–93. doi: 10.1042/BST0390989
46. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. (2013) 45:e66. doi: 10.1038/emm.2013.97
47. Tan Y, Kagan JCA. cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol Cell. (2014) 54:212–23. doi: 10.1016/j.molcel.2014.03.012
48. Chung S, LaPoint K, Martinez M, Kennedy A, Sandberg MB, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. (2006) 147:5340–51. doi: 10.1210/en.2006-0536
49. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
50. Sansone V, Applefield RC, Luca PD, Pecoraro V, Gianola S, Pascale W, et al. Does a high-fat diet affect the development and progression of osteoarthritis in mice? Bone Joint Res. (2020) 8:582–92. doi: 10.1302/2046-3758.812.BJR-2019-0038.R1
51. Barret G, Soininen A, Ylinen P, Sandelin J, Konttinen YT, Nordström DC, et al. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther. (2015) 17:379. doi: 10.1186/s13075-015-0902-0
52. Abella V, Scotece M, Conde J, López V, Pirozzi C, Pino J, et al. The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Sci Rep. (2016) 6:20356. doi: 10.1038/srep20356
53. Noss EH, Nguyen HN, Chang SK, Watts GF, Brenner MB. Genetic polymorphism directs IL-6 expression in fibroblasts but not selected other cell types. Proc Natl Acad Sci USA. (2015) 112:14948–53. doi: 10.1073/pnas.1520861112
54. Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. (2017) 76:748–55. doi: 10.1136/annrheumdis-2016-209757
55. Radojcic MR, Thudiuma CS, Henriksena K, Tanc K, Karlsten R, Dudley A, et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain. (2017) 158:1254–63. doi: 10.1097/j.pain.0000000000000908
56. Codella R, Luzi L, Terruzzi I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. (2018) 50:331–41. doi: 10.1016/j.dld.2017.11.016
57. Vijay A, Kouraki A, Gohir S, Turnbull J, Kelly A, Chapman V, et al. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. (2021) 13:e1997559. doi: 10.1080/19490976.2021.1997559
58. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies? EClinicalMedicine. (2020) 26:29–30. doi: 10.1016/j.eclinm.2020.100587
59. Mishra A, Srivastava RN, Awasthi S, Parmar D, Mishra P. Expression of genes and their polymorphism influences the risk of knee osteoarthritis? J Nucleic Acids. (2017) 2017:3138254. doi: 10.1155/2017/3138254
60. Ghosh B, Pal T, Ganguly S, Ghosh AA. study of the prevalence of osteoporosis and hypovitaminosis D in patients with primary knee osteoarthritis. J Clin Orthop Trauma. (2014) 5:199–202. doi: 10.1016/j.jcot.2014.09.002
Keywords: low-grade inflammation, gut microbiota, gut microbiota disturbances, osteoarthritis, systematic review
Citation: Xiang W, Ji B, Jiang Y and Xiang H (2022) Association of low-grade inflammation caused by gut microbiota disturbances with osteoarthritis: A systematic review. Front. Vet. Sci. 9:938629. doi: 10.3389/fvets.2022.938629
Received: 11 May 2022; Accepted: 24 August 2022;
Published: 12 September 2022.
Edited by:
Isaac Karimi, Razi University, IranReviewed by:
Vinod R. M. T. Balasubramaniam, Monash University Malaysia, MalaysiaMingxuan Zheng, Xuzhou Medical University, China
Copyright © 2022 Xiang, Ji, Jiang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han Xiang, OTAzMjc0MTg0QHFxLmNvbQ==
 Wu Xiang
Wu Xiang Bingjin Ji1
Bingjin Ji1