
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 20 July 2022
Sec. Parasitology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.935428
This article is part of the Research Topic Trematode Infection in Ruminants, Volume II View all 5 articles
This review was conducted to provide an update on the status of the occurrence of Fasciola species in livestock, wildlife and humans, and the geographical distribution of snail intermediate host (IH) species in South Africa. The literature search was conducted on four electronic databases using the Boolean operators in combination with predetermined search terms for thematic analysis. Results showed that Fasciola species have been reported in six out of nine provinces of South Africa in the last six decades (1960–2021), with both F. hepatica and F. gigantica infecting vertebrate hosts and F. hepatica and Fasciola spp infecting humans. Results also showed that most studies relied on morphological identification of eggs and flukes without molecular confirmation, which might have led to the misidentification of specimens, especially when immature. Fasciola hepatica has been documented in Limpopo, Mpumalanga, and KwaZulu-Natal provinces. The occurrences of Galba truncatula as the probable snail IH for F. hepatica in the three provinces has been documented while Pseudosuccinea columella has only been documented in Mpumalanga and KwaZulu-Natal provinces. The occurrence of F. gigantica to date has been reported in Mpumalanga and KwaZulu-Natal provinces, with overlapping distribution with F. hepatica. Radix natalensis, the main IH of F. gigantica has been documented in all the three provinces, while the two alien Radix species (R. auricularia and R. rubiginosa) were documented in KwaZulu-Natal province and have been implicated elsewhere with the transmission of F. gigantica. The presence of Fasciola spp eggs and antibodies in humans were documented in the Eastern Cape and the Western Cape provinces, where both P. columella and G. truncatula are known to be present. The prevalence of Fasciola spp infection in livestock ranged from 9.1 to 37.67 %, with an estimated annual financial loss ranging from R44930.26-129901 in cattle production in the Eastern Cape province of South Africa. This review reaffirms the scarcity of information on the occurrence and burden of fasciolosis in South Africa, and further highlights the importance of future research covering all provinces of the country and assessing the public health significance of the disease in resource-poor livestock communities in the areas where the parasite is endemic.
Fasciolosis is an important food- and water-borne parasitic zoonosis caused by the liver flukes of the genus Fasciola (1–3). The two main species are Fasciola hepatica Linnaeus (1758) and F. gigantica Cobbold (1856) (1, 2, 4, 5), and the disease has been reported to affect a wide range of domestic and wildlife mammals, and humans as definitive hosts and infection rates in livestock in some endemic areas can reach up to 90% (6). According to Mas-Coma et al. (1), the global economic implications associated with the disease have been estimated to be US$3.2 billion annually. These production losses are due to reduced productivity, liver condemnation, and reduced carcass value, and mortality associated with fasciolosis (7, 8).
Fasciolosis has raised public health concerns due to its potential zoonotic nature (9). According to Mas-Coma et al. (6), human fasciolosis is emerging/re-emerging in many parts of the world, and has been reported to be endemic in many countries of the Middle East and North Africa (Ethiopia) (10) and South America (11). Nonetheless, previous research indicated that an estimated 17 million individuals are infected, with 180 million persons at risk of infection globally (8, 12, 13).
The epidemiology of fasciolosis is associated with the ecological characteristics of the snail intermediate hosts implicated in the transmission of Fasciola species (14, 15). Lymnaeidae snail species act as intermediate hosts of F. hepatica and F. gigantica, and play an important role in the geographical distribution of these two species (1, 16–19). However, the susceptibility of these snail vectors to Fasciola species infections may vary depending on the immunological responses inherent to these snail species (3, 18) such as those related to the role played by IL-1 of Biomphalaria glabrata in resistance to Schistosoma mansoni infections (20). Over 20 Lymnaeidae species have been linked with the transmission of Fasciola species globally (8, 19), however, their capacity to sustain the developmental stages of the trematodes varies and their dispersion is determined by the climatic and ecological factors such as temperature, rainfall, habitat and soil stratum type (8). Owing to the wide range and distribution of F. hepatica's intermediate hosts, the parasite is widely distributed and commonly found in temperate areas (18). In African countries, this species has been found predominantly in cold (temperate) regions with high altitudes in Ethiopia (1,800 m above the sea level) (21, 22), Tanzania (3,000 m above the sea level) (23), and Uganda (3,500 m above the sea level) (24). According to Malatji et al. (4), these high altitudes seem not to be conducive to the survival of Radix natalensis which is the main intermediate host of F. gigantica. Fasciola gigantica has been reported in areas of lower altitude of around 1,200 m above the sea level in Ethiopia, and between 1,000 and 1,500 m above sea level in Uganda (4, 25).
Previous studies have revealed that both F. hepatica and F. gigantica occur in South Africa (4), and infections in definitive hosts and intermediate hosts have been reported in six of nine provinces of South Africa (4, 26–28). Reports have also shown that these two species have a geographical overlap in their distribution in Mpumalanga and KwaZulu-Natal provinces (4, 27–29). Fasciola gigantica was reported to infect cattle (4, 27–29), while F. hepatica infections were documented in wildlife (4, 30), cattle (4, 27–29, 31), and horses (32). There have also been cases of human infections in the Western Cape (33) and Gauteng (34) provinces. According to de Kock and Wolmarans (35), the low number of reported human cases in South Africa as compared to the other countries such as Egypt and Ethiopia is likely due to underreporting.
Nonetheless, the availability of information on the prevalence of fascioliasis in livestock (31, 32, 36) and wildlife (30) in South Africa is still limited and fragmented and more so, there is a need to quantify economic losses and the public health risk due to fasciolosis in South Africa. Therefore, the aim of this study was to review the information available to date on the prevalence and burden of Fasciola spp infection in livestock, wildlife and human, and the geographical distribution of snail IH species in South Africa. Furthermore, the information generated from the study will create an awareness among policy makers and various stakeholders, of the importance of the disease in livestock and wildlife and its public health importance in South Africa.
Peer-reviewed research articles explicitly reporting on the occurrence, infection, and economic burden of Fasciola spp in livestock, wildlife, and humans, and the geographical distribution of their snail intermediate host species in South Africa, were reviewed following the scoping review framework as outlined by Arksey and O' Malley (37). The process included the following stages: (i) identifying the research questions; (ii) identifying relevant literature; (iii) literature selection; (iv) charting the data; and (v) collating, summarizing and reporting the results.
The following questions were identified: (i) What is the occurrence and geographical distribution of Fasciola spp in South Africa? (ii) What is the prevalence of Fasciola spp in livestock, wildlife, and humans in South Africa? (iii) What is the economic burden of fasciolosis in livestock and wildlife in South Africa? (iv) What is the occurrence and geographical distribution of intermediate hosts of Fasciola spp in South Africa?
Literature search was conducted on Google Scholar, PubMed, and SCOPUS using the Boolean operators (OR, AND) and the following terms: Fasciola OR fasciolosis AND livestock OR wildlife OR human, fasciolosis AND Prevalence, fasciolosis AND economic loss, Fasciola AND Lymnaea OR Radix OR Galba OR Pseudosuccinea species in South Africa. Literature search was limited to studies published from 1960 to 2021. Studies were initially identified by screening their titles and abstracts. Furthermore, the reference lists of the selected articles were screened as potential leads for additional relevant studies to include in the review. Full-text articles were retrieved and managed on Endnote reference manager version x9 (Clarivate Analytics, Philadelphia, PA, USA).
The review specifically included articles published in peer-reviewed journals reporting on any of the following: (i) the occurrence and prevalence of fasciolosis in humans, livestock, and wildlife; (ii) the economic burden associated with fasciolosis in livestock and wildlife; (iii) and the geographical distribution of Fasciola spp intermediate host snail species. (iv) All the studies should have been conducted in South Africa and published in English.
Studies were excluded if they, (i) reported infections in the intermediate hosts by other trematodes other than Fasciola spp; (ii) were books, review papers and dissertations/thesis; (iii) they did not contribute toward answering the review questions; and (iv) were conducted outside South Africa.
Data were extracted from the articles that met the inclusion criteria. Information of the author(s), year of publication, aims or objectives of the study, study location, the outcomes of the study, and any information that was relevant to the main objective of the review were recorded on a spreadsheet.
A total of 2,143 records were identified through database searching (PubMed, Science direct, and Google Scholar), and three additional articles were identified through screening the bibliography of included articles (Figure 1). Of these, 278 articles were excluded due to duplications, and the title and abstracts of the remaining 1,868 articles were screened and 1,744 were deemed ineligible and excluded. Full-text reprints of 124 articles assessed for eligibility, and 88 articles were excluded because they did not explicitly report on the distribution of Fasciola spp and their intermediate hosts (Radix spp, Galba sp, Pseudosuccinea sp or Lymnaea spp), prevalence of fasciolosis in humans, livestock and wildlife, the economic loss associated with fasciolosis in livestock and wildlife in South Africa, or did not contribute toward answering the review questions. The remaining 36 articles met the inclusion criteria in this review.
Of the 36 studies, 11 studies reported on Fasciola, while 25 reported on the occurrence of the intermediate snail hosts (Tables 1, 2, respectively). From the 11 studies reporting on Fasciola pp, three (n = 3) studies reported on F. hepatica alone, while five (n = 5) could not identify up to species level, and three (n = 3) documented on both F. hepatica and F. gigantica (Table 1). Fasciolosis was documented more in cattle (bovine) (n = 7), followed by human (n = 2), while infections in the horse (Equus caballus) and kudu (Tragelaphus strepsiceros) were documented in one study each. Only four (n = 4) studies reported on the infection burden (prevalence) of fasciolosis (Table 3), while two studies (n = 2) highlighted the financial loss associated with fasciolosis in cattle (Table 4).
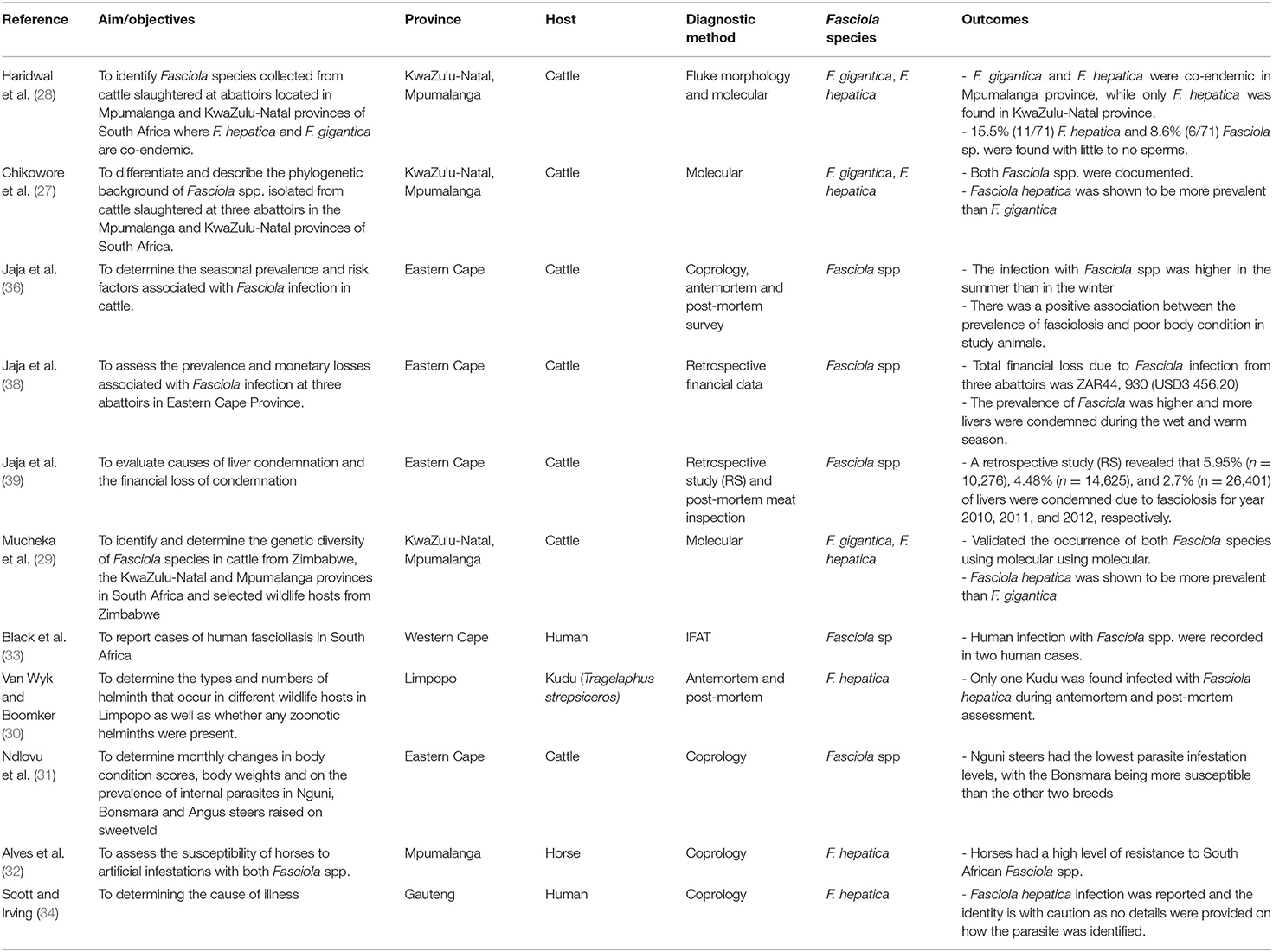
Table 1. Summary of studies which reported Fasciola spp. infection in livestock, wildlife and humans in different provinces of South Africa (1960–2021).
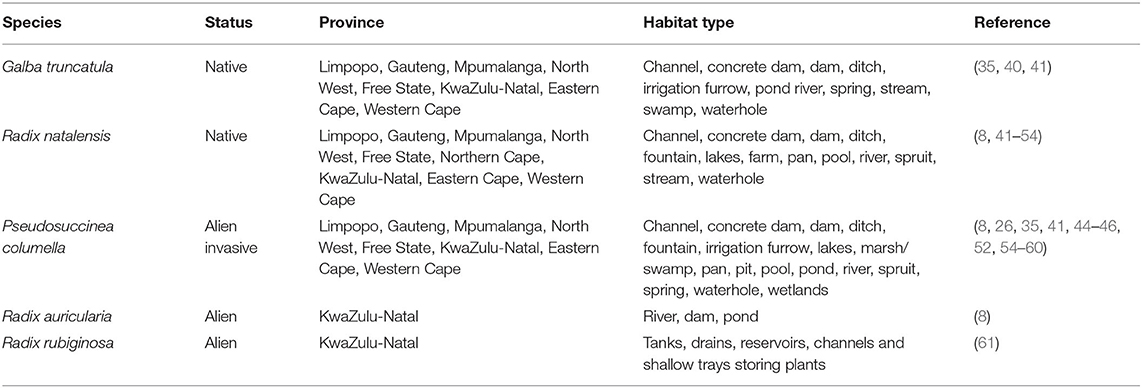
Table 2. Information from reviewed publications on geographical distribution of snail intermediate hosts of Fasciola spp. in South Africa.
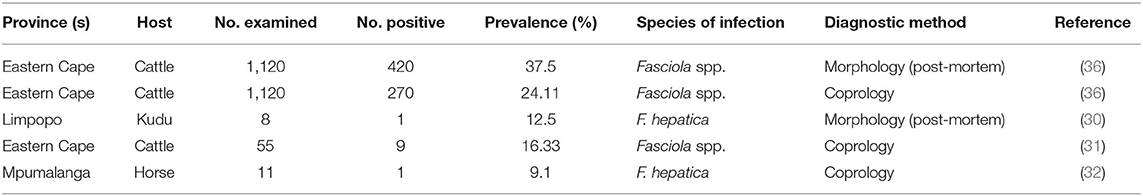
Table 3. Information from reviewed publications on the prevalence of Fasciola spp. in humans, wildlife and livestock from South Africa.
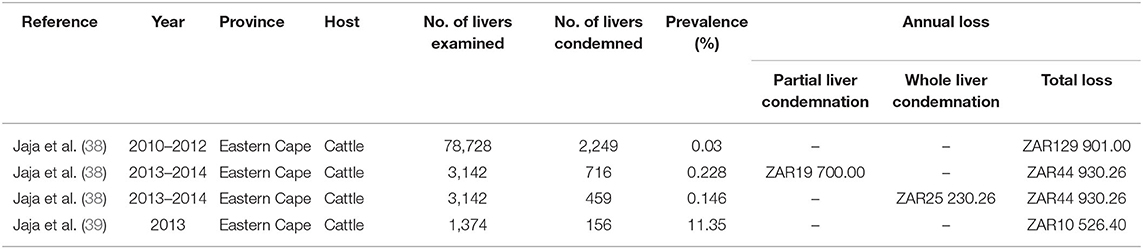
Table 4. Information from reviewed publications on the economic burden due to liver condemnation caused by fasciolosis in cattle reported in South Africa.
Results showed that fasciolosis has been reported in cattle, horses, kudu, and humans in Limpopo, Gauteng, Mpumalanga, KwaZulu-Natal, Eastern Cape, and Western Cape provinces (Table 1; Figure 2). Fasciola hepatica infection was documented in Kudu in Limpopo based on the morphological identification of the adult flukes post-mortem, in horses and humans from Mpumalanga and Gauteng provinces, respectively, based on the coprological technique, and cattle in Mpumalanga and KwaZulu-Natal provinces based on the morphological and molecular techniques. Results also showed the presence of the aspermic populations of F. hepatica in Mpumalanga and KwaZulu-Natal provinces, documented in the cattle and identified based on the morphological and molecular techniques (28). Infections due to F. gigantica were identified and recorded in cattle in Mpumalanga and KwaZulu-Natal, in conjunction with F. hepatica based on the morphological and molecular techniques (Table 1). Fasciola spp infections were reported in cattle in the Eastern Cape province based on coprological, antemortem, and post-mortem assessment, and in humans in the Western Cape province based on the antibody test (IFAT).
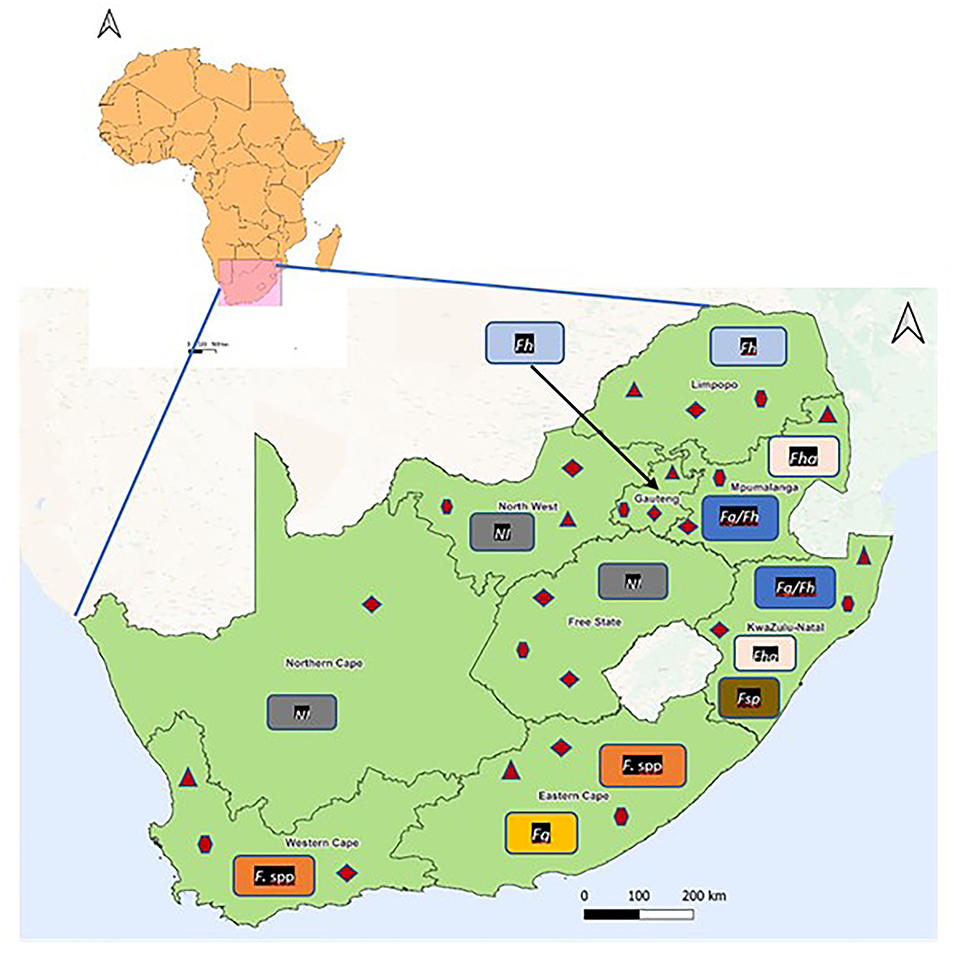
Figure 2. Reported geographical occurrence of Fasciola species and their lymnaeid intermediate hosts in the provinces of South Africa.  = Galba truncatula;
= Galba truncatula;  = Radix natalensis;
= Radix natalensis;  = Pseudosuccinea columella. Fg/Fh, Fasciola gigantica and F. hepatica; Fg, F. gigantica; Fh, F. hepatica; Fha, Fasciola hepatica aspermic; Fasciola spp, Fasciola species; Fasciola sp, Suspected Fasciola hybrid; NI, No information available on Fasciola species.
= Pseudosuccinea columella. Fg/Fh, Fasciola gigantica and F. hepatica; Fg, F. gigantica; Fh, F. hepatica; Fha, Fasciola hepatica aspermic; Fasciola spp, Fasciola species; Fasciola sp, Suspected Fasciola hybrid; NI, No information available on Fasciola species.
In total, five lymnaeid snail species, namely, Radix natalensis (Krauss, 1848), Galba truncatula (Muller, 1774), Pseudosuccinea columella (Say, 1817), Radix auricularia (Linnaeus, 1758), and Radix rubiginosa (Michelin, 1831) have been reported in South Africa (Table 2; Figure 2). Radix natalensis was documented in all the nine provinces of South Africa, viz Limpopo, Gauteng, Mpumalanga, North West, Free State, Northern Cape, KwaZulu-Natal, Eastern Cape, and Western Cape provinces. Galba truncatula was reported in Limpopo, Gauteng, Mpumalanga, North West, Free State, KwaZulu-Natal, Eastern Cape, and Western Cape provinces (Figure 2). Reviewed publications show that P. columella was distributed in the Limpopo, Mpumalanga, KwaZulu-Natal, Eastern Cape, and Western Cape provinces. In the Eastern Cape and KwaZulu-Natal provinces, this species was found naturally infected with F. gigantica and Fasciola sp (26). Radix rubiginosa and Radix auricularia were reported for the first time in South Africa in KwaZulu-Natal province.
From the studies reviewed, the prevalence of Fasciola hepatica infection ranged from 9.1% in horses in Mpumalanga based on coprology, to 12.5% in Kudu from Limpopo province, based on antemortem and post-mortem assessment (Table 3). The prevalence of Fasciola spp infection in cattle ranged from 16.33 to 37.67% based on coprology (31), and post-mortem survey (36) in the Eastern Cape province.
Host-based risk factors associated with the prevalence of fasciolosis in South Africa were only assessed in one study from the Eastern Cape province (36). The results showed that for both low-throughput abattoir (LTPA) and high-throughput abattoirs (HTPA), the prevalence of fasciolosis infection was generally higher in young animals (HTPA1 = 30.80%, HTPA2 = 23.20%, and LTPA = 18.20%) as compared with the older animals (HTPA1 = 8.80%, HTPA2 = 14.50%, LTPA = 14.10%). The HTPA1 showed no differences in the prevalence of infection between sex. However, HTPA2 showed high-infection rate in females (26.0%) than males (11.20%), and the LTPA showed a high prevalence in males (29.10%) as compared with females (3.20%). The HTPA abattoirs recorded high prevalence in animals with low body condition score (BCS) (26.60; 18.50%) followed by moderate BCS (10.40; 13.0%), whereas the prevalence of infections in the LTPA was high in animals with moderate BCS (15.90%) followed by those with good BCS (9.10%) (36).
Results showed that the financial impact due to fasciolosis from the cattle abattoir study was due to the partial or whole liver condemnation. Financial loss due to partial liver condemnation was estimated at ZAR19 700.00 from 716 condemned livers from 3,142 cattle slaughtered, and ZAR25 230.26 from full liver condemnation at cattle abattoirs in 2013 and 2014 in the Eastern Cape province (Table 4). The annual financial losses associated with the liver condemnation and carcass weight loss due to the chronic form of fasciolosis were estimated as ZAR129 901.00 from 2010 to 2012 and ZAR44 930.26 from 2013 to 2014 based on abattoir slaughter records (38). In addition, a financial survey from July to December showed that ZAR10 526.4 was lost due to liver condemnation caused by fasciolosis (39).
Results from reviewed studies showed that cases of Fasciola spp infections have been reported in Limpopo, Gauteng, Mpumalanga, KwaZulu-Natal, Eastern Cape and Western Cape provinces. Infections were reported in cattle, horse, kudu, and human, and corresponded with the distribution of the snail intermediate hosts implicated in the transmission of the species. Fasciola hepatica infections were documented in Limpopo, Gauteng, Mpumalanga, and KwaZulu-Natal provinces, where the native intermediate hosts of this species, G. truncatula, has been documented (40, 41). Furthermore, Haridwal et al. (28) also reported the presence of aspermic Fasciola sp specimens from F. hepatica population from cattle slaughtered at abattoirs in Mpumalanga and KwaZulu-Natal provinces. In addition, other snail species such as the native R. natalensis and the alien invasive P. columella were also documented in these provinces (32, 40, 41, 58), which explained the presence of F. gigantica in Mpumalanga and KwaZulu-Natal provinces and the overlapping distribution between the two species in these two provinces. Furthermore, P. columella has been previously found naturally infected with F. gigantica and Fasciola sp in KwaZulu-Natal (26). KwaZulu-Natal further documented two alien species, R. auricularia and R. rubiginosa which act as intermediate hosts of F. gigantica among other trematodes in their native origin and elsewhere (8, 61). Surprisingly, F. gigantica has not been documented in Limpopo and Gauteng, despite the presence of R. natalensis in these provinces.
Results also show that several studies did not identify the liver flukes up to species level. This was observed more especially in the Eastern Cape and Western Cape, where Fasciola spp infection was documented in cattle and humans, respectively. Lack of identification up to species level may have been attributed to the use of diagnostic tools such as coprology (31, 36, 38, 39) and antemortem and post-mortem assessment (36, 38, 39) in cattle and IFAT in humans (33) which can only confirm identity up to genus level. Considering that the intermediate hosts of both F. hepatica (G. truncatula) and F. gigantica (R. natalensis) including P. columella which have been confirmed to transmit F. gigantica in the Eastern Cape provinces, have been documented in these areas (26, 40, 59), it is possible that the Fasciola eggs or adult flukes reported could have been of any of the two Fasciola species.
Reviewed studies showed that although no peer-reviewed case reports or studies reported the occurrence of fasciolosis in some provinces, the intermediate hosts of these species were documented. This includes North West province, where the presence of G. truncatula and P. columella which are known intermediate host of F. hepatica and R. natalensis and P. columella, which are intermediate hosts of F. gigantica (41, 49, 56), and Free State province where G. truncatula (35, 40) and P. columella (55, 60) were previously documented. The presence of these snail species may indicate that fasciolosis in these provinces may be the presence, but unreported.
Only three cases of human fasciolosis have been documented in Gauteng (n = 1) and Western Cape (n = 2) provinces in South Africa from 1960 to 2013 (33, 34). Prior to 1960, only two cases in humans have been documented in the Eden district of the Western Cape province (62). According to Black et al. (33), the patient got infected by ingesting watercress purchased from the local markets and this corresponded with the observation made by Soliman (63) that infections in humans were linked to the consumption of watercress which is part of the regular diet of communities in several countries. Furthermore, more cases may be existing but a lack of awareness of the disease in the medical fraternity might be contributing to the low-human cases (33). This suggests that the Eden district of the Western Cape province may be a potential endemic area for human fasciolosis in South Africa.
According to Black et al. (33), the epidemiology of fasciolosis in livestock and humans in South Africa is still unknown. Results show that only four studies documented the prevalence of fasciolosis infection in horse, kudu, and cattle. Only one of eight Kudu (12.5%, 1/8) from Limpopo provinces was infected with F. hepatica (30). Although F. hepatica infections in Greater Kudu have only been reported in South Africa, F. gigantica infections have been documented in other southern African countries such as Zimbabwe (64) and Zambia (65). The low-infection rate may have been because fasciolosis is not common in kudus, due to browsing behavior which makes it less likely to get exposed to aquatic vegetations (66). Infection in these kudus may be attributed to the water source (dam), fed from a river, which was accessed by cattle and provided a favorable environment for freshwater snails that serve as IHs for the liver fluke (30).
Like kudu, only one (1/11, 9.1%) horse from Mpumalanga was found infected with F. hepatica (32). According to Alves et al. (32), the low-infection rate in horses may be due to the high-resistance level of horses to Fasciola spp strains present in South Africa after experimental infections failed to establish. Similar observations were made elsewhere, where horses were frequently resistant to infection with Fasciola (32, 67, 68), although high-infection rates have been documented especially in areas where the fasciolosis is endemic and horses share grazing with highly infected animals (69–71).
Prevalence of Fasciola spp infection in cattle ranged from 16.33 to 37.67%, although limited to the Eastern Cape province. The reviewed studies further indicated that the prevalence of fasciolosis is influenced by factors such as season, age, gender, and body condition scores of the animal. The prevalence was higher in the wet and warm season (31, 36), corresponding with the infection trend observed in Zimbabwe where the highest infection was in the month of February and December, and the lowest in September (72). Corresponding with the numerous studies from various countries, reviewed studies further showed that the infection rate was significantly higher in young animals than in old animals (73–75). However, only one study compared prevalence in males and female in cattle that showed high-prevalence of fasciolosis in males than females in the low-throughput abattoirs (LTPAs) which corresponded with reports from other studies (21, 74, 76–78), but the HTPA reported high infections in the females (36). Animals with poor body conditions scores showed to be more susceptible to infection (21, 74, 79, 80).
Fasciola infection causes enormous loss to the ruminant livestock production sector through liver condemnation, reduction in milk, meat and wool production, metabolic diseases, veterinary care, and mortality (81–84). Although several African countries have reported that millions of dollars were lost due to fasciolosis (72, 85, 86), the financial implication of fasciolosis in South Africa is still scanty (36). Results show that to date, only two studies assessed the economic loss associated with fasciolosis in South Africa with an annual financial loss associated with either partial or whole liver condemnation and carcass weight loss being reported (38, 39). However, these represent data from only three abattoirs in the Eastern Cape province of South Africa, and as such, cannot be extrapolated beyond this province.
The review showed Fasciola spp have been documented in six provinces of South Africa in the last six decades, and their geographical distribution correspond with the presence of their intermediate hosts. The review also showed the presence of intermediate hosts of Fasciola spp in provinces where infections in vertebrate hosts have not been documented. Furthermore, fasciolosis in South Africa was documented in cattle, horse, kudu, and humans, and the number of studies is scanty considering the geographical size of South Africa, and the economic and veterinary public health importance of the disease. The review showed the scarcity of information or studies, especially, on the epidemiology and cost-benefit analysis in the control and prevention of the disease in the animal production sectors. Hence, the authors recommend more research on the epidemiology of the disease covering all provinces and designing effective prevention and control strategies for the disease targeting resource-poor livestock farmers whose livestock are mostly negatively affected by fasciolosis. Future studies could also include surveillance of human fasciolosis, more especially in endemic areas, including areas where cases have been documented previously.
SM and MM conceptualized the study. IN and MM developed the concept note. IN conducted the search, selected studies under MM's supervision, and wrote the first draft of the manuscript. All author's contributed to the article, agreed on the final draft and approved the submitted version.
This project has received funding from the European Union's Horizon 2020 Research and Innovation Program under grant agreement No 101000365. The study also received financial assistance from SM's Research Productivity funds (UKZN).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. (2005) 35:1255–78. doi: 10.1016/j.ijpara.2005.07.010
3. Beesley NJ, Caminade C, Charlier J, Flynn RJ, Hodgkinson JE, Martinez-Moreno A, et al. Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound Emerg Dis. (2018) 65:199–216. doi: 10.1111/tbed.12682
4. Malatji MP, Pfukenyi DM, Mukaratirwa S. Fasciola species and their vertebrate and snail intermediate hosts in East and Southern Africa: a review. J Helminthol. (2020) 94. doi: 10.1017/S0022149X19000531
5. Mas-Coma S, Valero MA, Bargues MD. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet Parasitol. (2009) 163:264–80. doi: 10.1016/j.vetpar.2009.03.024
6. Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Organ. (1999) 77:340.
7. Mohammed BR. The impact of fasciolosis on food security in Nigeria: a review. Int J Multidiscip Curr Res. (2015) 758–61.
8. Malatji MP, Lamb J, Mukaratirwa S. Molecular characterization of liver fluke intermediate host lymnaeids (Gastropoda: Pulmonata) snails from selected regions of Okavango Delta of Botswana, KwaZulu-Natal and Mpumalanga provinces of South Africa. Vet Parasitol: Reg Stud Rep. (2019) 17:100318. doi: 10.1016/j.vprsr.2019.100318
9. Thanh NT. Zoonotic fasciolosis in Vietnam: Molecular Identification and Geographical Distribution (Doctoral dissertation), Ghent University, Bélgica (2012).
10. Nyindo M, Lukambagire AH. Fascioliasis: an ongoing zoonotic trematode infection. BioMed Res Int. (2015) 5:45–8. doi: 10.1155/2015/786195
11. Hillyer GV, de Galanes MS, Rodriguez-Perez J, Bjorland J, de Lagrava MS, Guzman SR, et al. Use of the Falcon™ assay screening test-enzyme-linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian altiplano. Am J Trop Med Hyg. (1992) 46:603–9. doi: 10.4269/ajtmh.1992.46.603
12. Ashrafi K. The status of human and animal fascioliasis in Iran: a narrative review article. Iran J Parasitol. (2015) 10:306.
13. Mas-Coma S, Valero MA, Bargues MD. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. (2009) 69:41–146. doi: 10.1016/S0065-308X(09)69002-3
14. Correa AC, Escobar JS, Durand P, Renaud F, David P, Jarne P, et al. Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis. BMC Evol Biol. (2010) 10:1–2. doi: 10.1186/1471-2148-10-381
15. Pino Santos A, Vázquez AA, Doménech I, Martínez R, Sánchez J, Martínez E. Natural infection with Fasciola hepatica in host-snails and cattle in ten dairy farms from a Western Municipality in Cuba. Rev Med Vet Zoot. (2018) 37:73–81. doi: 10.19052/mv.vol1.iss37.9
16. Mochankana ME, Robertson ID. A retrospective study of the prevalence of bovine fasciolosis at major abattoirs in Botswana. Onderstepoort J Vet Res. (2016) 83:1–5. doi: 10.4102/ojvr.v83i1.1015
17. Brown D. Freshwater Snails of Africa and their Medical Importance. London: Taylor & Francis Ltd. (1994).
18. Ngcamphalala PI, Malatji MP, Mukaratirwa S. Geography and ecology of invasive Pseudosuccinea columella (Gastropoda: Lymnaeidae) and implications in the transmission of Fasciola species (Digenea: Fasciolidae)–a review. J Helminthol. (2022) 96:1–18. doi: 10.1017/S0022149X21000717
19. Torgerson P, Claxton J, Dalton JP. Epidemiology and control. In: Fasciolosis. Oxon: CAB International (1999). p. 113–49.
20. Connors WO. Talerton RL. Interleukin 1 activity in haemolymph from strains of the snail Biomphalaria glabrata varying in susceptibility to the human blood fluke, Schistosoma mansoni: presence, differential expression, and biological function. Cytokine. (1994) 6:21–7. doi: 10.1016/1043-4666(94)90003-5
21. Assefa A, Assefa Z, Beyene D, Desiss F. Prevalence of bovine fasciolosis in and around Inchini town, West Showa Zone, Adaa Bega Woreda, Central Ethiopia. J Vet Med Anim Health. (2015) 7:241–8. doi: 10.5897/JVMAH2014.0352
22. Kedir S, Deressa B, Tigre W. Small ruminant fasciolosis in Jimma area of South Western Ethiopia: its epidemiology and minimum monetary loss. Glob Vet. (2012) 9:635–41.
23. Walker S, Makundi AE, Namuba FV, Kassuku AA, Keyyu J, Hoey EM, et al. Trudgett. A The distribution of Fasciola hepatica and Fasciola gigantica within southern Tanzania–constraints associated with the intermediate host. Parasitol. (2008) 135:495–503. doi: 10.1017/S0031182007004076
24. Stensgaard AS, Jørgensen A, Kabatereine NB, Rahbek C, Kristenses TK. Modeling freshwater snail habitat suitability and areas of potential snail-borne disease transmission in Uganda. Geospat Health. (2006) 1:93–104. doi: 10.4081/gh.2006.284
25. Howell A, Mugisha L, Davies J, LaCourse EJ, Claridge J, Williams DJ, et al. Bovine fasciolosis at increasing altitudes: parasitological and malacological sampling on the slopes of Mount Elgon, Uganda. Parasit Vectors. (2012) 5:196. doi: 10.1186/1756-3305-5-196
26. Malatji MP, Mukaratirwa S. Molecular detection of natural infection of Lymnaea (Pseudosuccinea) columella (Gastropoda: Lymnaeidae) with Fasciola gigantica (Digenea: Fasciolidae) from two provinces of South Africa. J Helminthol. (2019) 94:1–6. doi: 10.1017/S0022149X19000129
27. Chikowore TJ, Zishiri OT, Mukaratirwa S. Phylogenetic analysis of Fasciola spp. isolated from slaughtered cattle in KwaZulu-Natal and Mpumalanga provinces of South Africa based on the cytochrome coxidase subunit I mitochondrial marker Onderstepoort. J Vet Res. (2019) 86:1–1. doi: 10.4102/ojvr.v86i1.1706
28. Haridwal S, Malatji MP, Mukaratirwa S. Morphological and molecular characterization of Fasciola hepatica and Fasciola gigantica phenotypes from co-endemic localities in Mpumalanga and KwaZulu-Natal provinces of South Africa. Food Waterborne Parasitol. (2021) 22:e00114. doi: 10.1016/j.fawpar.2021.e00114
29. Mucheka VT, Lamb JM, Pfukenyi DM, Mukaratirwa S DNA. sequence analyses reveal co-occurrence of novel haplotypes of Fasciola gigantica with F. hepatica in South Africa and Zimbabwe. Vet Parasitol. (2015) 214:144–51. doi: 10.1016/j.vetpar.2015.09.024
30. Van Wyk IC, Boomker J. Parasites of South African wildlife. XIX The prevalence of helminths in some common antelopes, warthogs and a bushpig in the Limpopo province, South Africa Onderstepoort. J Vet Res. (2011) 78:308. doi: 10.4102/ojvr.v78i1.308
31. Ndlovu T, Chimonyo M, Muchenje V. Monthly changes in body condition scores and internal parasite prevalence in Nguni, Bonsmara and Angus steers raised on sweetveld. Trop Anim Health Prod. (2009) 41:1169–77. doi: 10.1007/s11250-008-9297-0
32. Alves RM, Van Rensburg LJ, Van Wyk JA. Fasciola in horses in the Republic of South Africa: a single natural case of Fasciola hepatica and the failure to infest ten horses either with F. hepatica or Fasciola gigantica. Onderstepoort J Vet Res. (1988) 55:157–63.
33. Black J, Ntusi N, Stead P, Mayosi B, Mendelson M. Human fascioliasis in South Africa. S Afr Med J. (2013) 103:658–9. doi: 10.7196/samj.7184
35. de Kock KN, Wolmarans CT. Distribution and habitats of three liver fluke intermediates in South Africa and their health implications involved: research and review article. S Afri J Sci Tech. (2008) 27:1–16. doi: 10.4102/satnt.v27i1.78
36. Jaja IF, Mushonga B, Green E, Muchenje V. Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Vet Anim Sci. (2017) 4:1–7. doi: 10.1016/j.vas.2017.06.001
37. Arksey H, O'Malley L. Scoping studies: toward a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
38. Jaja IF, Mushonga B, Green E, Muchenje V. Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite Epidemiol Control. (2017) 4:27–34. doi: 10.1016/j.parepi.2017.10.001
39. Jaja IF, Mushonga B, Green E, Muchenje V. A quantitative assessment of causes of bovine liver condemnation and its implication for food security in the Eastern Cape Province South Africa. Sustainability. (2017) 5:736. doi: 10.3390/su9050736
40. De Kock KN, Wolmarans CT, Bornman M. Distribution and habitats of the snail Lymnaea truncatula, intermediate host of the liver fluke Fasciola hepatica, in South Africa. J S Afr Vet Assoc. (2003) 74:117–22. doi: 10.4102/jsava.v74i4.523
41. Kemp M, De Kock KN, Zaayman JL, Wolmarans CT. A comparison of mollusc diversity between the relatively pristine Marico River and the impacted Crocodile River, two major tributaries of the Limpopo River, South Africa Water SA. (2016) 42:253–60. doi: 10.4314/wsa.v42i2.09
42. Wilken GB, Appleton CC. Avoidance responses of some indigenous and exotic freshwater pulmonate snails to leech predation in South Africa. S Afr J Zool. (1991) 26:6–10. doi: 10.1080/02541858.1991.11448226
43. de Kock KN, Wolmarans CT, Strauss HD, Killian M. Distribution and habitats of Lymnaea natalensis, snail intermediate host of the liver fluke Fasciola gigantica, in South Africa. S Afri J Sci Tech. (2001) 20:56–71. doi: 10.4102/satnt.v20i2.253
44. de Kock KN, Wolmarans CT A. re-evaluation of the occurrence of freshwater molluscs in the Kruger National Park. Koedoe. (1998) 41:1–8. doi: 10.4102/koedoe.v41i1.240
45. de Kock KN, Wolmarans CT, Du Preez LH. Freshwater mollusc diversity in the Kruger National Park: a comparison between a period of prolonged drought and a period of exceptionally high rainfall. Koedoe. (2002) 45:1–1. doi: 10.4102/koedoe.v45i2.23
46. Wolmarans C, de Kock KN. The current status of freshwater molluscs in the Kruger National Park. Koedoe. (2006) 49:39–44. doi: 10.4102/koedoe.v49i2.122
47. Moema EB, King PH, Baker C. Cercariae developing in Lymnaea natalensis Krauss, 1848 collected in the vicinity of Pretoria, Gauteng Province, South Africa. Onderstepoort J Vet Res. (2008) 75:215–23. doi: 10.4102/ojvr.v75i3.97
48. Miranda NAF, Perissinotto R, Appleton CC. Population structure of an invasive parthenogenetic gastropod in coastal lakes and estuaries of Northern KwaZulu-Natal, South Africa. PLoS ONE. (2011) 6:e24337. doi: 10.1371/journal.pone.0024337
49. Moema EB, King PH, Baker C. Descriptions of strigea cercariae from the Gauteng and North West Provinces, South Africa. Onderstepoort J Vet Res. (2012) 79:E1–8. doi: 10.4102/ojvr.v79i1.410
50. de Kock KN, Wolmarans CT, Kemp M, Roets W. 2013. Short-term threats for the sustained survival of freshwater Mollusca in the Olifants River and selected tributaries. S Afri J Sci Tech. (2013) 32:6. doi: 10.4102/satnt.v32i1.395
51. Perissinotto R, Miranda NA, Raw JL, Peer N. Biodiversity census of lake St Lucia, iSimangaliso wetland park (South Africa): gastropod molluscs. Zookeys. (2014) 440:1. doi: 10.3897/zookeys.440.7803
52. Wolmarans CT, Wepener V, Pretorius U, Erasmus JH, de Kock KN. A comparison of the Mollusca diversity in the Mooi River (North-West Province) as found during surveys conducted in 1963 and again 50 years later. S Afr J Sci Tech. (2015) 34:7. doi: 10.4102/satnt.v34i1.1294
53. Moema EB, King PH, Rakgole JN. Phylogenetic studies of larval digenean trematodes from freshwater snails and fish species in the proximity of Tshwane metropolitan, South Africa. Onderstepoort J Vet Res. (2019) 86:1–7. doi: 10.4102/ojvr.v86i1.1729
54. Erasmus JH, Lorenz AW, Zimmermann S, Wepener V, Sures B, Smit NJ, et al. diversity and functional approach to evaluate the macroinvertebrate responses to multiple stressors in a small subtropical austral river. Ecol Indic. (2021) 131:108206. doi: 10.1016/j.ecolind.2021.108206
55. De Kock KN, Joubert PH, Pretorius SJ. Geographical distribution and habitat preferences of the invader freshwater snail species Lymnaea columella (Mollusca: Gastropoda) in South Africa. Onderstepoort J Vet Res. (1989) 56:271–5. Available online at: http://hdl.handle.net/2263/42239
56. de Kock KN, Wolmarans CT, Nieuwoudt S, Smid MJ, Yssel E. A re-evaluation of the bilharzia risk in and around the Hartbeespoort Dam. Water SA. (1993) 19:89–91.
57. Dana P, Appleton CC. Observations on the population dynamics of the invasive freshwater snail Aplexa marmorata (Pulmonata: Physidae) in Durban, South Africa. S Afri J Sci. (2007) 103:493–6. Available online at: https://hdl.handle.net/10520/EJC96621
58. de Kock KN, Wolmarans CT. Invasive alien freshwater snail species in the Kruger National Park, South Africa. Koedoe: African Protected Area Conservation and Science. (2008) 50:49–53. doi: 10.4102/koedoe.v50i1.126
59. Mlambo MC, Bird MS, Reed CC, Day JA. Diversity patterns of temporary wetland macroinvertebrate assemblages in the South-Western Cape, South Africa. African J Aquat Sci. (2011) 36:299–308. doi: 10.2989/16085914.2011.636903
60. Foster L, Malherbe W, Ferreira M, Van Vuren JHJ. Macroinvertebrate variation in endorheic depression wetlands in North West and Mpumalanga provinces, South Africa. Afr J Aquat Sci. (2015) 40:287–97. doi: 10.2989/16085914.2015.1074060
61. Appleton CC, Miranda NAF. Two Asian freshwater snails newly introduced into South Africa and an analysis of alien species reported to date. Afr Invertebr. (2015) 56:1–17. doi: 10.5733/afin.056.0102
63. Soliman MF. Epidemiological review of human and animal fascioliasis in Egypt. J Infect Dev Ctries. (2008) 2:182–9. doi: 10.3855/jidc.260
64. Jooste R. A checklist of helminth parasites from the larger domestic and wild mammals of Zimbabwe. Trans Zim Sci Assoc. (1989) 64:5–32.
65. Zieger U, Boomker J, Cauldwell AE, Horak IG. Helminths and botfly larvae of wild ungulates on a game ranch in Central Province, Zambia. Onderstepoort J Vet Res. (1998) 65:137–41. Available online at: http://hdl.handle.net/2263/20196
66. Boomker J. Helminth infections: Wildlife. University of Pretoria, Pretoria, South Africa. (2007).
67. Soykan E, Öge H. The prevalence of liver trematodes in equines in different cities of Turkey. Türkiye Parazitolojii Dergisi. (2012) 36:152. doi: 10.5152/tpd.2012.36
68. Nansen P, Andersen S, Hesselholt M. Experimental infection of the horse with Fasciola hepatica. Exp Parasitol. (1975) 37:15–9. doi: 10.1016/0014-4894(75)90049-1
69. Boray JC. Experimental fascioliasis in Australia. Adv Parasitol. (1969) 7:95–210. doi: 10.1016/S0065-308X(08)60435-2
70. Busetti ET, Paske A, Thomaz V. Fasiolose hepática em Equus caballus no stado do Paraná Brasil. Arq Bras Med Vet Zootec. (1983) 35:193–6.
71. Rubilar L. San Martin M. Treating Fasciola hepatica in horses with Niclofolan [Bilevon (R); Chile]. Veterinaer Medizinische Nachrichten. (1982) 1:76–82.
72. Pfukenyi DM, Mukaratirwa S. A retrospective study of the prevalence and seasonal variation of Fasciola gigantica in cattle slaughtered in the major abattoirs of Zimbabwe between 1990 and 1999. Onderstepoort J Vet Res. (2004) 71:181–7. doi: 10.4102/ojvr.v71i3.258
73. Aregay F, Beleke J, Ferede Y, Hailemelekot M. Study on the prevalence of bovine fasciolosis in and around Bahir Dar, Ethiopia. Ethiop Vet J. (2013) 17:1–11. doi: 10.4314/evj.v17i1
74. Betebo T. Prevalence of fasciolosis in cattle slathered at Hosanna Municipal Abattoir, Southern Ethiopia. Int J Adv Res Biol Sci. (2017) 4:70–6. doi: 10.22192/ijarbs.2017.04.03.007
75. Regassa A, Woldemariam T, Damissie S, Moje N, Ayana D, Abunna F. Bovine fasciolosis, Coprological, Abattoir survey and financial loss due to liver condemnation in Bishooftu Municipal Abattoir, Central Ethiopia. Eur J Biol. (2012) 4:83–90. doi: 10.5829/idosi.ejbs.2012.4.3.63180
76. Kithuka J, Maingi N, Njeru FM, Ombui JN. The prevalence and economic importance of bovine fasciolosis in Kenya-an analysis of abattoir data. Onderstepoort J Vet Res. (2002) 69:255–62.
77. Munyeme M. Mingang'andu HM, Muma JB, Nambota AM, Biffa D, Siamudaala VM. Investigating effects of parasite infection on body condition of the Kafue lechwe (Kobus leche kafuensis) in the Kafue basin. BMC Res Notes. (2010) 3:346. doi: 10.1186/1756-0500-3-346
78. Njeruh FM, Kithuka JM, Maingi N, Ombui JN. Prevalence and economic importance of fascioliasis in cattle, sheep and goats in Kenya. Kenya Veterinarian. (2004) 27:118–23. doi: 10.4314/kenvet.v27i1.39574
79. Mebrahtu G, Beka K. Prevalence and economic significance of fas- ciolosis in cattle slaughtered at Dire Dawa Municipal Abattoir, Ethiopia. J Vet Adv. (2013) 3:319–24.
80. Moje N, Mathewos S, Desissa F, Regassa A Cross-sectional study on bovine fasciolosis: prevalence, corpological, abattoir survey and financial loss due to liver condemnation at Areka Municipal Abattoir, Southern Ethiopia. J Vet Med Anim Health. (2015) 7:33–8. doi: 10.5897/JVMAH2014.0342
81. Khanjari A, Bahonar A, Fallah S, Bagheri M, Alizadeh A, Fallah M, et al. Prevalence of fasciolosis and dicrocoeliosis in slaughtered sheep and goats in Amol Abattoir, Mazandaran, northern Iran. Asian Pac J Trop Dis. (2014) 4:120–4. doi: 10.1016/S2222-1808(14)60327-3
82. Terefe D, Wondimu A, Gachen DF. Prevalence, gross pathological lesions and economic losses of bovine fasciolosis at Jimma Municipal Abattoir, Ethiopia. J Vet Med Anim Health. (2012) 4:6–11. doi: 10.5897/JVMAH11.045
83. Toet H, Piedrafita DM, Spithill TW. Liver fluke vaccines in ruminants: strategies, progress and future opportunities. Int J Parasitol. (2014) 44:915–27. doi: 10.1016/j.ijpara.2014.07.011
84. Zeleke G, Menkir S, Desta M. Prevalence of ovine fasciolosis and its economic significance in basona worana district, central Ethiopia. SJZ. (2013) 2:81–94. doi: 10.14196/sjas.v2i8.944
85. Cadmus SI, Adesokan HK. Causes and implications of bovine organs/offal condemnations in some abattoirs in Western Nigeria. Trop Anim Health Prod. (2009) 41:1455–63. doi: 10.1007/s11250-009-9334-7
Keywords: fasciolosis, wildlife, human, livestock, intermediate hosts, distribution, prevalence, South Africa
Citation: Nyagura I, Malatji MP and Mukaratirwa S (2022) Occurrence of Fasciola (Digenea: Fasciolidae) Species in Livestock, Wildlife and Humans, and the Geographical Distribution of Their Intermediate Hosts in South Africa—A Scoping Review. Front. Vet. Sci. 9:935428. doi: 10.3389/fvets.2022.935428
Received: 03 May 2022; Accepted: 14 June 2022;
Published: 20 July 2022.
Edited by:
Khalid Mehmood, Islamia University of Bahawalpur, PakistanReviewed by:
Saima Masood, University of Veterinary and Animal Sciences, PakistanCopyright © 2022 Nyagura, Malatji and Mukaratirwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samson Mukaratirwa, TXVrYXJhdGlyd2FAdWt6bi5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.