
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 16 June 2022
Sec. Parasitology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.929443
 Michele Capasso1,2
Michele Capasso1,2 Lavinia Ciuca1*
Lavinia Ciuca1* Isabel Guadano Procesi3,4
Isabel Guadano Procesi3,4 Francesco Zinno2
Francesco Zinno2 Federica Berrilli4
Federica Berrilli4 Giuseppe Cringoli1
Giuseppe Cringoli1 Laura Rinaldi1
Laura Rinaldi1The aim of this study was to assess the single and synergistic effects of fenbendazole (Fenb) and metronidazole (Metro) for the treatment of Giardia duodenalis infection in different species of non-human primates (NHPs) housed in a zoological garden of southern Italy. Moreover, the study also aimed to better define the circulation of G. duodenalis zoonotic assemblages in NHP and the potential occurrence of zoonotic transmission between the staff from the zoo and NHP. Briefly, six species that belonged to four families (Lemuridae, Cercopithecidae, Atelidae, and Hylobatidae) of NHP and housed in six cages (CG) were identified as Giardia positive and divided into two groups. Group F (N = 16 animals) was treated with Fenb (50 mg/kg, every 24 h for 5 consecutive days) and Group M (N = 7 animals) was treated with Metro (25 mg/kg, two times a day for 5 consecutive days). After the first round of therapy, all the animals were retreated for 5 days by inverting the drugs in each group. On each sampling day [study days (SDs) 3–24], the samples were tested for the presence of Giardia cysts using the FLOTAC technique. Multiple fecal tests for the antigen detection of Giardia, such as rapid ELISA and direct immunofluorescence (IFA), were performed at each sampling point only on samples that resulted in positive for Giardia cysts with FLOTAC. The efficacy of Fenb ranged from 30 to 67% and for Metro ranged from 82 to 96%. The results showed the synergistic effects of Metro and Fenb (98–100%) over the combination of Fenb and Metro (52–90%) against the infection by Giardia in NHPs. The overall k agreement between FLOTAC and IFA was reached 0.858 (p = 0.0001). In contrast, all the samples had a negative antigen result when using ELISA. At molecular analysis, six samples were confirmed positive for Giardia by nested PCR. Only two positive samples were successfully sequenced that showed 100% of identity with assemblage B. All the samples from the humans included in the study resulted in negative for Giardia cysts. Overall, the study emphasizes the need for regular monitoring of Giardia infections in NHP housed in zoos by traditional diagnostic tools combined with molecular characterization of the parasite.
Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis) is a ubiquitous enteric flagellated protozoan of global importance that infects a wide range of hosts, i.e., >40 animal species, such as humans. It is a common leading cause of infection known as giardiosis (or giardiasis) and infects up to ≈ 28.2 million people worldwide, with 500,000 new cases every year (1). Giardia duodenalis has been frequently identified as a pathogen in non-human primates (NHPs) (2–6). The prevalence of G. duodenalis in NHP that were kept in zoos in different European countries was ranged between 6 and 70% in studies conducted in Belgium (7), Croatia (8), Poland (9), Slovakia (10), Spain, and Brazil (11, 12). A recent study conducted in six European zoological gardens (located in France, Germany, and Spain) reported an 18.1% prevalence of G. duodenalis with the presence of both assemblages A and B (6). In Italy, the presence of G. duodenalis has been reported in NHP that were kept in the Bioparco in Rome with a prevalence of 47.0% in Lemur catta (3), and recently also in NHP were kept in four zoos in central and southern Italy with an overall prevalence of 3.3% (13), whereas the parasite was not reported in captive cynomolgus macaques (Macaca fascicularis) imported from registered breeding facilities in China (14). The assemblages of G. duodenalis found in the NHP in Italy are mainly A and B, with the dominant assemblage B (3, 15, 16). Giardiosis in NHP causes diarrhea and slow growth, especially in juvenile animals (17). However, few studies have demonstrated an association between the presence of G. duodenalis infection and the occurrence of clinical manifestations (18), strongly suggesting that the pathogenic role of G. duodenalis in captive NHP is limited (6). Nevertheless, NHPs play an important role as reservoirs of zoonotic Giardia infections in the zoological gardens (6, 19–21). The most common therapy used against giardiosis in veterinary medicine is based on metronidazole (Metro) and fenbendazole (Fenb) (22). Metro, such as tinidazole, is used as a single dose in the treatment of giardiosis in humans with high rates of healing (about 90%) and low complications (23, 24). Additionally, tinidazole has been used in the treatment of giardiosis in Barbary macaque (25). Though Giardia is a common parasite of zoonotic relevance in NHP, a limited number of field studies have been conducted on the efficacy of antiparasitic treatments in NHPs. Both Fenb and Metro are recommended as therapy for protozoa infections in NHP (26–28). The aim of this study was to evaluate the single and synergistic effects of Fenb and Metro for the treatment of G. duodenalis subclinical infection in different species of NHPs that were housed in a zoological garden in southern Italy. Moreover, the study also aimed to better define the circulation of G. duodenalis zoonotic assemblages in NHPs and the potential occurrence of zoonotic transmission between the staff (zookeepers and veterinarians) from the zoo and NHPs.
The study was conducted in a zoological garden that is located in the Benevento province of southern Italy (Pesco Sannita, 41°13'57”N; 14°48'40”E). Husbandry in this zoo is based on the European Association of Zoos and Aquaria (EAZA) Best Practice Guidelines for each species or similar, providing the best possible care with good levels of welfare and with sanitary safety for animals, staff, and visitors. Employees and visiting staff working with NHPs wore personal protective equipment when in contact with the animals or their fecal material (13).
This study was performed under the annual preventive medicine plan of the facility recognized by the National/International Regulations for the EU Zoo Directive (DL73/2005 and 92/65 CEE). The Zoo includes a variety of 115 species, housing over 500 animals, and is in continuous expansion. Currently, there are 42 NHPs belonging to 11 species of the following families: Lemuridae, Cercopithecidae, Atelidae, Hylobatidae, and Cebidae. Animals' food consists of specific commercial food combined with daily fresh fruit, vegetables, seeds, eggs, and/or mealworms. Freshwater is provided daily ad libitum in polycarbonate water bottles. The cages are cleaned three times a week and disinfected every 2 weeks.
All the NHPs from the zoo were firstly screened for identification of G. duodenalis. Fecal samples (pools) were collected from 11 cages (N = 42 subjects of NHPs belonging to 11 species) and subjected to copromicroscopic analysis using the FLOTAC technique as detailed below (29, 30). Six cages (CG) out of 11 (N = 23 animals) resulted positive for Giardia cysts [mean cyst per gram (CPG) of Giardia in each cage: 300 CPG in CG1; 1,200 CPG in CG2; 240 CPG in CG3; 2,280 CPG in CG4; 950 CPG in CG5; and 273 CPG in CPG6]. One of the cages was positive also for Blastocystis sp. All the NHPs that resulted positive for Giardia cysts were included in the treatment groups. In addition, all the zookeepers (N = 4) and veterinarians (N = 2) from the zoo were tested for the detection of Giardia cysts using the same technique that was used for NHPs (30). All the human stool samples resulted in negative for Giardia cysts.
Fecal samples analyzed at each sampling point were represented by pools (5–10 g) and collected from the inner core of the cages. Analyses were performed within 24 h of sampling. On each sampling day, the fecal samples were tested for the presence of Giardia cysts using the FLOTAC technique based on zinc sulfate (specific gravity = 1.350) flotation solution with a detection limit of 1 CPG of feces (29, 30). Moreover, multiple fecal tests for the antigen detection of Giardia, such as rapid enzyme immunoassay (Remel Xpect Giardia/Cryptosporidium, Thermo Fisher) and direct immunofluorescence (IFA; MeriFluor Giardia/Cryptosporidium, Bioscience), were performed at each point of the study only on samples that resulted positive for Giardia cysts with FLOTAC (29).
Genomic DNA was extracted from six fecal samples of NHPs (pools from each cage) using the QIAamp DNA Stool Mini Kit (Qiagen, Italy) following the instructions of the manufacturer. To identify G. duodenalis, the triosephosphate isomerase (TPI) fragment was amplified by nested PCR using the protocol described by Sulaiman et al. (31). Briefly, for the primary PCR, a PCR product of 605 bp was amplified by using primers AL3543 [5′-AAATIATGCCTGCTCGTCG-3′] and AL3546 [5′-CAAACCTTITCCGCAAACC-3′]. For the secondary PCR, a fragment of 530 bp was amplified by using 2.5 μl of primary PCR reaction and primers AL3544 [5′-CCCTTCATCGGIGGTAACTT-3′] and AL3545 [5′-GTGGCCACCACICCCGTGCC-3′]. Molecular analyses were carried out by amplifying an 18S rRNA fragment for Blastocystis sp. The specific primer BhRDr (GAGCTTTT-TAACTGCAACAACG) and the broad-specificity eukaryote-specific primer RD5 (ATCTGGTT-GATCCTGCCAGT) were used in a standard PCR reaction with Taq DNA polymerase (BIOTAQ, Bioline, UK) using the protocol described by Stephanie et al. (32).
PCR products were analyzed by agarose gel electrophoresis and visualized after ethidium bromide staining. Subsequently, all the secondary PCR products were sent for sequencing to the Bio-Fab Research, Rome, Italy. Sequences for each amplified region were compared to those previously published in the GenBank database. Identities at the assemblage/subtype level were verified using the Basic Local Alignment Search Tool (BLAST). Sequences were submitted to GenBank under Accession number ON246260-ON246261 for G. duodenalis TPI locus and ON215732 for Blastocystis 18S rRNA fragment.
The study design is summarized in Table 1. Six species that belonged to four families (Lemuridae, Cercopithecidae, Atelidae, and Hylobatidae) of NHP (N = 23 animals) and housed in six cages (CG) were identified as Giardia positive and divided into two groups: the Group F treated with Fenb (Panacur®, 2.5%, Intervet Italia Srl; 50 mg/kg, orally, every 24 h for 5 consecutive days) and the Group M treated with Metro (ERADIA®, Virbac Italia Srl; 25 mg/kg, orally, two times a day for 5 consecutive days). After 5 days from the first round of therapy, all the animals were retreated for 5 days by inverting the drugs in each group, as follows: Metro (25 mg/kg, orally, every 24 h for 5 consecutive days) in Group F and Fenb (50 mg/kg, orally, two times day for 5 consecutive days) in Group M. The treatment groups were allocated based on the distance and position of the cages in the zoo where the NHPs were housed according to the species. Specifically, the Group F included three cages housing the following species of NHP each: 12 male dogs of Lemur catta (ring-tailed lemur) in CG1; one male and one female of Cercopithecus mona (mona monkey) in CG2 and two female dogs of Alouatta caraya (black howler) in CG3. Group M included three CG with the following species of NHP: one male and one female of Nomascus concolor (black crested gibbon) in CG4; one male and one female of Colobus Guereza (mantled guereza) in CG5; and two female dogs and one male of Semnopithecus entellus (gray langur) in CG6. For ethical reasons, no untreated control group of animals was available. All the animals were monitored for the presence of Giardia cysts and Giardia antigen for 24 study days (SDs) as follows: before treatment (SD3–SD1), during the first treatment (T1) (SD2–SD6); during post-treatment-T1 (SD7–SD13); during the second treatment (T2) (SD14–SD18), and post-treatments (SD19–SD24). All the animals included in the study were healthy animals as determined by a physical examination performed before (3–1 days) the beginning of the study. Moreover, all the animals received physical examinations by a veterinarian during the treatments and on the last day of the trial.
The medicated meal was obtained using specific “meatballs” consisting of a moistened mixture of primate-pellets, honey, yogurt, or chopped fruit, which easily allowed the administration of the drugs to the animals. Noteworthy, this type of drug administration allowed the veterinarians to apply the correct amount of the drug for each animal (individually) according to their weight.
Treatment efficacy was evaluated based on Giardia CPG of feces on SDs 7–12 and SDs 19–24 for both groups (33).
CPG = cysts per gram feces.
Statistical analysis was performed using Windows SPSS® (version 17.0). The non-parametric Mann-Whitney U test was used to determine the level of significant difference between groups of treatment (F and M). Moreover, Kappa (k) statistic was employed to determine the strength of agreement between FLOTAC/IFA and FLOTAC/rapid ELISA, using the following criteria: ≤ 0.2 = poor; 0.21–0.40 = fair; 0.41–0.60 = moderate, 0.61–0.80 = good, and ≥0.80 = very good (34). The level of significance was set at a p-value of 0.05.
The values of Giardia CPG in both groups (F and M) and for each SD are shown in Tables 2, 3. Tables 4, 5 show data of mean Giardia CPG and efficacies (%) of Fenb and Metro treatments calculated at different post-treatment days. Briefly, in Group F, the results of the parasitological analyses on the SD1 (pre-treatment) revealed a mean value of 2,160 CPG of Giardia and on SD 13, after the first treatment, the mean value was 1,257 CPG. In Group M, on SD1, the mean value of Giardia CPG was 2,427 and after the first treatment with Metro, on SD 13, the mean value was reduced to 227 CPG. However, after the second treatment in both groups, by using Metro in Group F and Fenb in Group B, the mean of CPG decreased significantly, in particular in Group M (Tables 4, 5). Moreover, the results of the mean CPG of Giardia for each cage after 6 days of treatment with every single drug used in the study were the following: post-treatment with Fenb/Metro in Group F (n = 261/6 mean CPG in the CG1, n = 752/0 mean CPG in the CG2, and n = 5,200/945 mean CPG in the CG3) and post-treatment with Metro/Fenb in Group M (n = 368/1 mean CPG in the CG4, n = 162/15 mean CPG in the CG5, and n = 334/11 mean CPG in the CG6). The efficacy of Fenb was ranged from 30 to 67% on SDs 7–12 and from 52 to 90% on SDs 19–24 after the second treatment with Metro. In Group M, the efficacy of Metro ranged between 82 and 96% on SDs 7–12 and 98 and 100% on SDs 19–24 after the second treatment with Fenb. Overall, the synergistic effects of Fenb and Metro against the Giardia infection in Group M showed a statistically significant difference (p = 0.001) as compared to the Group F. All the subjects included in both groups F and M continued to eliminate Giardia cysts after the first treatment with either Fenb or Metro. Instead, all the subjects from the cages CG1 and CG3 remained Giardia negative and the only subjects from CG2 remained Giardia positive after the second treatment with Metro in Group F. Furthermore, all the subjects from Group M remained Giardia negative after the second treatment with Fenb, at the end of the study (SD 24). In addition, the subjects from CG2 and CG6 (Cercopithecus mona and Semnopithecus entellus) presented co-infections with other parasites, such as Trichuris sp. and Blastocystis sp.
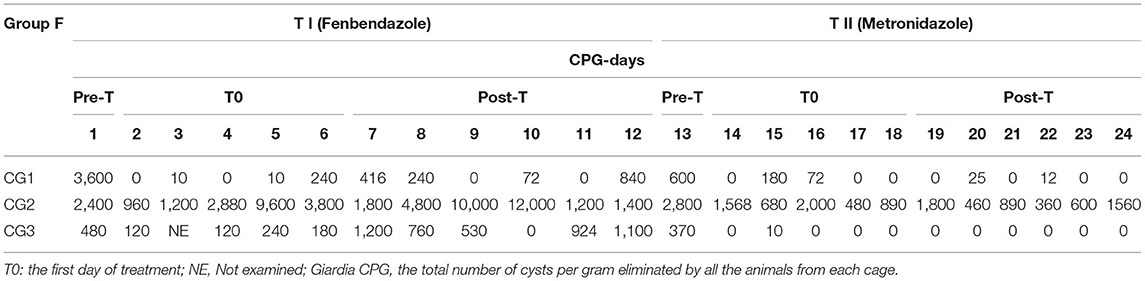
Table 2. Results of Giardia cyst per gram (CPG) for all the animals in each cage (CG1, CG2, and CG3) during the entire study (pre-treatment and post-treatments T1 and T2) for Group F.
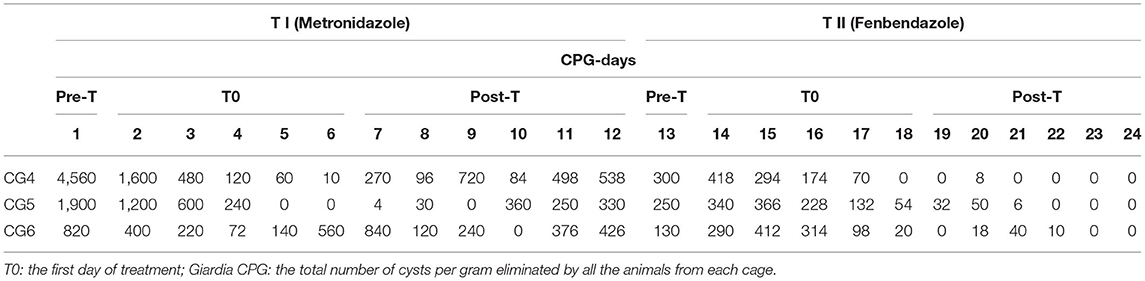
Table 3. Results of Giardia cyst per gram (CPG) for all the animals in each cage (CG4, CG5, and CG6) during the entire study (pre-treatment and post-treatments T1 and T2) for Group M.
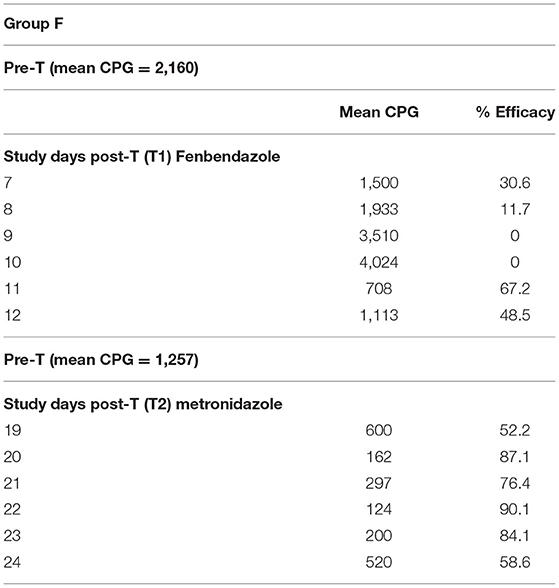
Table 4. Results of mean Giardia cyst per gram (CPG) and efficacy (%) of the treatments performed in Group F, on study days (SDs) 7–12 and SDs 19–20.
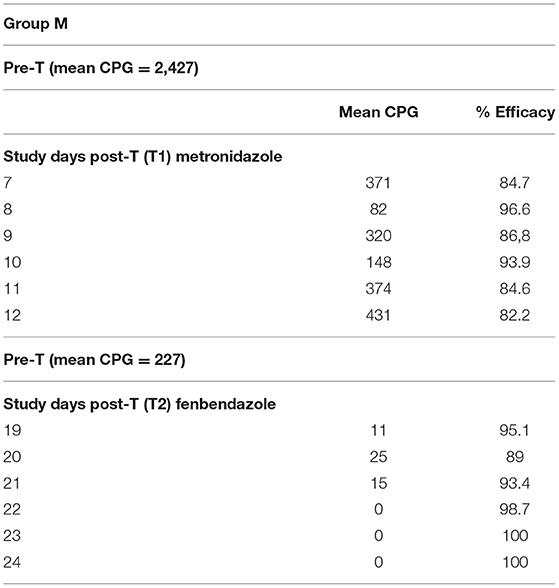
Table 5. Results of mean Giardia cyst per gram (CPG) and efficacy (%) of the treatments performed in Group M, on study days (SDs) 7–12 and SDs 19–24.
Moreover, all the fecal samples from each sampling point resulted in negative at the antigen detection of Giardia using the rapid enzyme immunoassay when compared with direct IFA, which resulted in all positive. The overall k agreement between FLOTAC and IFA was 0.858 (p = 0.0001). Analyses were not carried out when comparing FLOTAC with the rapid ELISA, given that no sample resulted positive with the ELISA test.
All six samples were confirmed positive for Giardia by nested PCR. Only two positive samples (CG1 and CG2) were successfully sequenced that showed 100% of identity (498/498; 100% query coverage) with assemblage B (Accession number: MF095053). Cercopithecus mona from CG2 also resulted positive for Blastocystis sp.; the sample was successfully sequenced showing 100% of identity (579/579; 100% query coverage) with Subtype ST1 (Accession number: MN338073).
Little is known about the occurrence and genetic diversity of Giardia duodenalis in NHPs; however, giardiosis has been described in the following species of NHPs: squirrel monkeys, rhesus macaques, lemuridae, and marmosets (3, 25, 35–37).
Though Giardia is a common parasite in zoo-housed primates, a few studies have evaluated the treatment options for giardiosis in NHP. One study reported the efficacy of tinidazole in Barbary macaque (25) and another study assessed whether oral administration of Metro dissolved in drinking water would be successful to eliminate Giardia cysts in rhesus macaques (38). Our field study evaluated the efficacy of Metro and Fenb and assessed the synergistic effect of the two drugs against G. duodenalis infection in different species of NHP housed in a zoological garden in southern Italy. This study revealed a high and persistent detection of Giardia cysts in various species of NHPs that include Lemur catta, Cercopithecus mona, Alouatta caraya, Nomascus concolor, Colobus guereza, and Semnopithecus entellus. These results obtained are consistent with other similar studies (3, 7, 8, 11). The occurrence of Giardia was reported in NPH species that were not well-documented until now, such as mona monkeys, black howler, black-crested gibbon, mantled guereza, and gray langur.
The results on the efficacy of Fenb vs. Metro and their synergistic effect on Giardia infection underlined that almost all the animals became negative when combining Fenb at 50 mg/kg sid for 5 days with Metro at 25 mg/kg bid for 5 days. The only group of animals that remained positive for Giardia cysts at the end of the study was the one that belong to the Cercopithecidae family (the pair of one female and male of mona monkeys). In addition, they had the highest infection rate due to the variation in cyst shedding per day. Our data further indicate that pooling serial fecal samples from multiple colony animals likely would identify the presence of Giardia in a colony.
In the present study, Giardia infection in NHPs was not associated with clinical signs of diarrhea. These findings contrast with several studies on humans, in which 60–70% of infected persons show signs of diarrhea (39). Instead, the condition observed in the NHPs seems to be similar to the chronic asymptomatic carrier state that occurs in companion animals (40–43). This condition of subclinical Giardia infection indicates that NHPs may represent important reservoirs and serve as a source of zoonotic infection for other animals.
This study provided an unexpected finding concerning the diagnostic tools for antigen detection of Giardia. In fact, all the samples that resulted positive with FLOTAC were confirmed with the gold standard IFA, assessing a very high agreement between the two techniques (30). However, the present study yielded discordant results when using the rapid test (Remel Giardia/Cryptosporidium) on Giardia-positive samples. In fact, all the fecal samples from each point of the study that were positive at FLOTAC and IFA had a negative antigen result with the rapid test used. These findings are not in agreement with several studies that reported the rapid enzyme immunoassays as a precise tool for detecting Giardia in fecal specimens with test sensitivities and specificities that have approached 100% (44–46). However, future work should include investigations regarding the combined factors that may result in Giardia-antigenic stimulation of the intestinal tract in NHP.
The molecular analysis revealed that assemblages A and B in all samples tested positive for Giardia-DNA. Giardia zoonotic assemblages A and B have been already described in NHP in many studies that include some species of NHPs from a zoological garden in Rome, Italy (3, 47–50). The potential zoonotic G. duodenalis assemblage B was identified in two groups of NHP (Lemur catta and Cercopithecus mona). These findings suggest that ring-tailed lemurs, as already has been reported, and mona-monkeys may be asymptomatic carriers of G. duodenalis and a higher parasitic load might occur in these species of NHP held in walk-through enclosures (4). Moreover, all the animals (Cercopithecus mona) from CG2 were the only animals that remained positive for Giardia, until the end of the study, after two treatments of 5 days each with Fenb and Metro. We cannot rule out reinfection, due to the prepatent period of Giardia, that can be as short as 5 days, but on the other side, the host susceptibility of the NHPs for Giardia infection can be as well incriminated for the persistence of the Giardia cysts. However, the increase in the number of Giardia cysts shed by the two animals from CG2, after the treatment, e.g., Day 9 (N = 10,000 CPG) and Day 10 (N = 12,000 CPG) has occurred in other studies as well (41, 51). It is important to note that the shedding of the mean CPG of Giardia from CG2 in Group F resulted in a high value (N = 5,200 CPG) post-treatment with Fenb when compared with a low value (N = 945 CPG) post-treatment with Metro in the same group.
In addition to the presence of G. duodenalis, the other parasites identified in this study were Trichuris sp. and Blastocystis sp. that could cause serious gastrointestinal enteritis in NHPs (6, 35, 36).
To our knowledge, this is the first study that showed the efficacy of Fenb that ranged from 30 to 67% on SDs 7–12 and the efficacy of Metro that ranged between 82 and 96% on SDs 7–12 against the subclinical infection by Giardia in NHPs. Moreover, the final output revealed the synergistic effects of Metro and Fenb (98–100%) over the combination of Fenb and Metro (52–90%) against the infection by Giardia in NHPs. In addition, tinidazole can be a good therapeutic strategy for giardiosis in NHPs, as it has been already demonstrated in marmosets (25); however, future work should include the evaluation of the efficacy of this drug in other species of NHP. Indeed, the evaluation of health profile markers of pre- and post-treated animals would have underlined the safety of the drugs used in this study.
The results of this study showed that G. duodenalis is a common parasite in NHP in southern Italy. The findings of this study enriched the list of host species susceptibility of NHPs to G. duodenalis from Italy, by adding other species of NHPs, such as Cercopithecus mona, Nomascus concolor, Colobus guereza, Semnopithecus entellus, and Alouatta caraya, that were either not studied or tested negative for Giardia cysts in previous studies. Furthermore, the substantial variation in the number of Giardia cysts per gram shed on various days of the study could be explained by the differences in susceptibility in the host species of NHPs or by the continuous exposure to cysts from the environment. Indeed, a more effective sampling strategy would have improved the overall output of the study regarding the epidemiological data obtained, for example, collecting individual fecal samples and not pooled samples from the cages as performed in the present study.
For Blastocystis sp. detection, as reported by Köster et al. (6), ST1 in NHPs in zoological gardens was uniquely documented in Spain, despite being ST1 the most prevalent genotype worldwide. This trend may be justified by the lack of molecular data regarding Blastocystis sp. isolated from zoological institutes (6).
Zookeepers and veterinarian staff resulted negative to the parasitological exams. However, the monitoring of a potential zoonotic transmission must continue to be a priority, possibly with a temporal continuity in sampling and through an increased number of recovered samples. This would enable to assess any potential transmission risk between staff, visitors, and NHPs.
Overall, the study emphasizes the need for traditional diagnostic tools combined with molecular characterization of the parasite and for the elimination of the infection by using an association of Metro and Fenb, in particular, for the NHPs that are asymptomatic with marked persistence of cysts, in order to better assess and reduce the zoonotic risk of Giardia infection. Regarding the detected zoonotic assemblages in the NHP from the Maitine Zoo in southern Italy, NHPs are considered as important hosts of G. duodenalis; it is therefore strongly recommended to conduct the screening of this infection within the routine examinations in zoological gardens all over the world.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, MF095053; https://www.ncbi.nlm.nih.gov/genbank/, MN338073.
Ethical approval was not provided for this study on human participants because since the study was carried out on stool samples provided voluntarily by the staff from the zoo (zookeepers and veterinarians), no ethical approval was required for our study. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. This study was performed under the annual preventive medicine plan of the facility recognized by the National/International Regulations for the EU Zoo Directive (DL73/2005 and 92/65 CEE).
LC, LR, and MC contributed to the conception and design of the study. LC and IP performed the laboratory analysis. IP, FZ, and FB organized the database and performed the statistical analysis. MC, LC, GC, FB, and LR wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ryan UM, Hijjawi N, Feng Y, Xiao L. Giardia: an under-reported foodborne parasite. Int J Parasitol. (2019) 49:1–11. doi: 10.1016/j.ijpara.2018.07.003
2. Levecke B, Geldhof P, Claerebout E, Dorny P, Vercammen F, Cacciò SM, et al. Molecular characterisation of Giardia duodenalis in captive non-human primates reveals mixed assemblage Aand B infections and novel polymorphisms. Int J Parasit. (2009) 39:1595–601. doi: 10.1016/j.ijpara.2009.05.013
3. Berrilli F, Prisco C, Friedrich KG, Di Cerbo P, Di Cave D, De Liberato C. Giardia duodenalis assemblages and Entamoeba species infecting non-human primates in an Italian zoological garden: zoonotic potential and management traits. Parasit Vectors. (2011) 4:199. doi: 10.1186/1756-3305-4-199
4. Fomsgaard AS, Bornbusch SL, Bueno GL, Noromalala E, Poulsen M, Rasmussen M, et al. Prevalence, infection intensity and genotyping of Giardia duodenalis in ring-tailed lemurs Lemur catta from European zoos and wild populations. J Zoo Aquarium Res. (2020) 8:253–8. doi: 10.19227/jzar.v8i4.509
5. Chen L, Zhao J, Li N, Guo Y, Feng Y, Feng Y, et al. Genotypes and public health potential of Enterocytozoon bieneusi and Giardia duodenalis in crab-eating macaques. Parasit Vectors. (2019) 1:254. doi: 10.1186/s13071-019-3511-y
6. Köster PC, Martínez-Nevado E, González A, Abelló-Poveda MT, Fernández-Bellon H, de la Riva-Fraga M, et al. Intestinal protists in captive non-human primates and their handlers in six European zoological gardens. Molecular evidence of zoonotic transmission. Front Vet Sci. (2022) 8:819887. doi: 10.3389/fvets.2021.819887
7. Levecke B, Dorny P, Geurden T, Vercammen F, Vercruysse J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol. (2007) 148:236–46. doi: 10.1016/j.vetpar.2007.06.020
8. Beck R, Sprong H, Bata I, Lucinger S, Pozio E, Cacciò S. Prevalence and molecular typing of Giardia spp. in captive mammals at the zoo of Zagreb, Croatia. Vet Parasitol. (2011) 175:40–6. doi: 10.1016/j.vetpar.2010.09.026
9. Maesano G, Capasso M, Ianniello D, Cringoli G, Rinaldi L. Parasitic infections detected by FLOTAC in zoo mammals from Warsaw, Poland. Acta Parasitol. (2014). 59:343–53. doi: 10.2478/s11686-014-0249-8
10. Mravcová K, Štrkolcová G, Mucha R, Goldová M. Zoonotic assemblages of Giardia duodenalis in captive non-human primates from the largest zoo in Slovakia. J Parasit Dis. (2021) 45:302–5. doi: 10.1007/s12639-020-01324-3
11. Martínez-Díaz RA, Sansano-Maestre J. del Carmen Martínez-Herrero M, Ponce-Gordo F, Gómez-Muñoz MT. Occurrence and genetic characterization of Giardia duodenalis from captive nonhuman primates by multi-locus sequence analysis. Parasitol Res. (2011) 109:539–44. doi: 10.1007/s00436-011-2281-z
12. David EB, Patti M, Coradi ST, Oliveira-Sequeira TCG, Ribolla PEM, et al. Molecular typing of Giardia duodenalis isolates from nonhuman primates housed in a brazilian zoo. Rev Inst Med Trop São Paulo. (2014) 56:49–54. doi: 10.1590/S0036-46652014000100007
13. Capasso M, Maurelli MP, Ianniello D, Alves LC, Amadesi A, Laricchiuta P, et al. Use of Mini-FLOTAC and Fill-FLOTAC for rapidly diagnosing parasitic infections in zoo mammals. Rev Bras Parasitol Vet. (2019) 28:168–71. doi: 10.1590/s1984-296120180087
14. Zanzani SA, Gazzonis AL, Epis S, Manfredi MT. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis). Parasitol Res. (2015) 115:307–12. doi: 10.1007/s00436-015-4748-9
15. Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. (2008) 160:75–80. doi: 10.1016/j.molbiopara.2008.04.006
16. Cacciò SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int J Parasitol. (2008) 38:1523–31. doi: 10.1016/j.ijpara.2008.04.008
17. Karim MR, Wang R, Yu F, Li T, Dong H, Li D, et al. Multi-locus analysis of Giardia duodenalis from nonhuman primates kept in zoos in China: geographical segregation and host adaptation of assemblage B isolates. Infect Genet Evol. (2015) 30:82–8. doi: 10.1016/j.meegid.2014.12.013
18. Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, et al. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun. (2003) 71:4079–86. doi: 10.1128/IAI.71.7.4079-4086.2003
19. Kowalewski MM, Salzer JS, Deutsch JC, Raño M, Kuhlenschmidt MS, Gillespie TR. Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: patterns of zoonotic protozoa infection relative to degree of humanprimate contact. Am J Primatol. (2011) 73:75–83. doi: 10.1002/ajp.20803
20. Ryan U, Caccio SM. Zoonotic potential of Giardia. Int J Parasitol. (2013) 43:943–56. doi: 10.1016/j.ijpara.2013.06.001
21. Einarsson E, Ma'ayeh S, Svard SG. An up-date on Giardia and giardiasis. Curr Opin Microbiol. (2016) 34:47–52. doi: 10.1016/j.mib.2016.07.019
23. Fung HB, Doan TL. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther. (2005) 27:1859–84. doi: 10.1016/j.clinthera.2005.12.012
24. Petri WA. Treatment of giardiasis. Curr Treat Options Gastroenterol. (2005) 8:13–7. doi: 10.1007/s11938-005-0047-3
25. Kramer JA, Hachey AM, Wachtman LM, Mansfield KG. Treatment of giardiasis in common marmosets (Callithrix jacchus) with tinidazole. Comp Med. (2009) 59:174–9; Erratum in: Comp Med. (2009) 59:220.
26. Manual Veterinary (MSD) by Terri Parrott DVM St. Charles Veterinary Hospital Last Full Review/Revision Jan 2020 | Content Last Modified Feb 2020MSD. Available online at: https://www.msdvetmanual.com/exotic-and-laboratory-animals/nonhuman-primates/parasitic-diseases-of-nonhuman-primates#v3304837 (accessed April 22, 2022).
27. Modry D, Pafco B, Petrzelkova KJ, Hasegawa H. Parasites of Apes, An atlas of Coproscopic Diagnostics Edition Chimaira. (2018). p. 98–102.
28. Ortega-Pierres G. Advances in Parsitology Giardia and Giardiasis, Part B, Vol 107. Ciudad de Mexico: Academic Press Elsevier (2020). p. 176–82.
29. Cringoli G, Rinaldi L, Maurelli MP, Utzinger J. FLOTAC new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. (2010) 5:503–15. doi: 10.1038/nprot.2009.235
30. Pepe P, Ianniello D, Alves LC, Morgoglione ME, Maurelli MP, Bosco A, et al. Comparative cost-effectiveness of immunoassays and FLOTAC for diagnosing Giardia spp. infection in dogs. Parasit Vectors. (2019) 12:158. doi: 10.1186/s13071-019-3425-8
31. Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, et al. Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis. (2003) 11:1444–52. doi: 10.3201/eid0911.030084
32. Scicluna SM, Tawari B, Clark CG. DNA Barcoding of Blastocystis. Protist. (2006) 157:77–85. doi: 10.1016/j.protis.2005.12.001
33. Geurden T, Olson ME, O'Handley RM, Schetters T, Bowman D, Vercruysse J. World association for the advancement of veterinary parasitology (WAAVP): guideline for the evaluation of drug efficacy againstnon-coccidial gastrointestinal protozoa in livestock and companion animals. Vet Parasitol. (2014) 204:81–6. doi: 10.1016/j.vetpar.2014.02.050
34. Altman DG. Practical Statistics for Medical Research. London, Chapman and Hall (1991). p. 277–321.
35. Hamlen HJ, Lawrence JM. Giardiasis in laboratory-housed squirrel monkeys: a retrospective study. Lab Anim Sci. (1994) 44:235–9.
36. Kalishman J, Paul-Murphy J, Scheffler J, Thomson JA. Survey of Cryptosporidium and Giardia spp in a captive population of common marmosets. Lab Anim Sci. (1996) 46:116–9.
37. Potkay S. Diseases of the Callitrichidae: a review. J Med Primatol. (1992) 21:189–236. doi: 10.1111/j.1600-0684.1992.tb00583.x
38. Labberton L, Bakker J, Klomp R, Langermans JA, van Geijlswijk IM. Challenges in oral administration of metronidazole dissolved in drinking water to rhesus monkeys (Macaca mulatta). Lab Anim. (2013) 42:213–6. doi: 10.1038/laban.264
39. Hashan MR, Elhusseiny KM, Huu-Hoai L, Tieu TM, Low SK, Minh LHN, et al. Effect of nitazoxanide on diarrhea: a systematic review and network meta-analysis of randomized controlled trials. Acta Trop. (2020) 210:105603. doi: 10.1016/j.actatropica.2020.105603
40. Chon SK, Kim NS. Evaluation of silymarin in the treatment on asymptomatic Giardia infections in dogs. Parasitol Res. (2005) 97:445–51. doi: 10.1007/s00436-005-1462-z
41. Bowman DD, Liotta JL, Ulrich M, Charles SD, Heine J, Schaper R. Treatment of naturally occurring, asymptomatic Giardia sp. in dogs with Drontal Plus flavour tablets. Parasitol Res. (2009) 105(Suppl. 1):S125–34. doi: 10.1007/s00436-009-1503-0
42. Tysnes KR, Skancke E, Robertson LJ. Subclinical Giardia in dogs: a veterinary conundrum relevant to human infection. Trends Parasitol. (2014) 11:520–7. doi: 10.1016/j.pt.2014.08.007
43. Ciuca L, Pepe P, Bosco A, Caccio SM, Maurelli MP, Sannella AR, et al. Effectiveness of fenbendazole and metronidazole against Giardia infection in dogs monitored for 50-days in home-conditions. Front Vet Sci. (2021) 8:626424. doi: 10.3389/fvets.2021.626424
44. Maraha B, Buiting AG. Evaluation of four enzyme immunoassays for the detection of Giardia lamblia antigen in stool specimens. Eur J Clin Microbiol Infect Dis. (2000) 19:485–7. doi: 10.1007/s100960000286
45. Oster N, Gehrig-Feistel H, Jung H, Kammer J, McLean JE, Lanzer M. Evaluation of the immunochromatographic CORIS Giardia-Strip test for rapid diagnosis of Giardia lamblia. Eur J Clin Microbiol Infect Dis. (2006) 25:112–5. doi: 10.1007/s10096-006-0088-0
46. Weitzel T, Dittrich S, Möhl I, Adusu E, Jelinek T. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin Microbiol Infect. (2006) 7:656–9. doi: 10.1111/j.1469-0691.2006.01457.x
47. Thompson RC. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. (2000) 30:1259–67. doi: 10.1016/S0020-7519(00)00127-2
48. Graczyk TK, Bosco-Nizeyi J, Ssebide B, Thompson RC, Read C, Cranfield MR. Anthropozoonotic Giardia duodenalis genotype (assemblage) a infections in habitats of free-ranging human-habituated gorillas, Uganda. J Parasitol. (2002) 5:905–9. doi: 10.1645/0022-3395(2002)088[0905:AGDGAA]2.0.CO;2
49. Nizeyi JB, Cranfield MR, Graczyk TK. Cattle near the Bwindi impenetrable national park, Uganda, as a reservoir of Cryptosporidium parvum and Giardia duodenalis for local community and free-ranging gorillas. Parasitol Res. (2002) 88:380–5. doi: 10.1007/s00436-001-0543-x
50. Vitazkova SK, Wade SE. Parasites of free-ranging black howler monkeys (Alouatta pigra) from Belize and Mexico. Am J Primatol. (2006) 68:1089–97. doi: 10.1002/ajp.20309
Keywords: non-human primates, metronidazole, fenbendazole, zoos, Giardia duodenalis
Citation: Capasso M, Ciuca L, Procesi IG, Zinno F, Berrilli F, Cringoli G and Rinaldi L (2022) Single and Synergistic Effects of Fenbendazole and Metronidazole Against Subclinical Infection by Giardia duodenalis in Non-Human Primates in a Zoological Garden in Southern Italy. Front. Vet. Sci. 9:929443. doi: 10.3389/fvets.2022.929443
Received: 26 April 2022; Accepted: 16 May 2022;
Published: 16 June 2022.
Edited by:
Vikrant Sudan, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Yang Zou, Lanzhou Veterinary Research Institute (CAAS), ChinaCopyright © 2022 Capasso, Ciuca, Procesi, Zinno, Berrilli, Cringoli and Rinaldi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lavinia Ciuca, bGF2aW5pYS5jaXVjYUB1bmluYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.