- 1Jiangxi Provincial Key Laboratory for Animal Health, College of Animal Science and Technology, Institute of Animal Population Health, Jiangxi Agricultural University, Nanchang, China
- 2Department of Animal Science and Technology, Jiangxi Biotech Vocational College, Nanchang, China
- 3Department of Theriogenology, Faculty of Veterinary & Animal Sciences, The Islamia University of Bahawalpur, Pakistan, Bahawalpur, Pakistan
- 4China Institute of Veterinary Drug Control, MOA Center for Veterinary Drug Evaluation, Beijing, China
- 5Jian City Livestock and Veterinary Bureau, Jiangxi, China
- 6Jiangxi Agricultural Technology Extension Center, Nanchang, China
- 7Agricultural Technology Extension Center, Jinxi County Agriculture and Rural Bureau, Fuzhou, China
- 8Animal Epidemic Prevention and Quarantine Unit, Fengcheng Agricultural and Rural Bureau, Fengcheng, China
- 9Yichun Agriculture and Rural Affairs Bureau, Yichun, China
In animal husbandry, traditional Chinese medicine (TCM) as a reasonable alternative to antibiotics has attracted more and more concerns to reduce microbial resistance. This study was aimed to investigate the effects of dietary supplementation with TCM prescriptions on serum parameters and thymus inflammation responses in finishing pigs. Thirty finishing pigs were randomly divided into three groups, which included the Con group (basal diet), the TCM1 group (basal diet supplemented with Xiao Jian Zhong prescriptions), and the TCM2 group (basal diet supplemented with Jingsananli-sepsis). The results showed that the contents of C3 and C4 in the serum were significantly increased in both the TCM1 and TCM2 groups compared to the Con group on day 30. Similarly, the levels of IgA, IgG, and IgM were increased in the TCM2 group, and only the level of IgM in TCM1 was increased on day 30. Meanwhile, the levels of classical swine fever virus (CSFV) and respiratory syndrome virus (PRRSV) antibodies had a notable increase in the TCM1 and TCM2 groups. Both TCM1 and TCM2 inhibited the levels of TLR4/MyD88/NF-κB signaling pathway-related mRNA (TLR4, MyD88, NF-κB, IL6, IL8, and TNF-α) and protein (p-IκBα and p-P65) expression levels in the thymus. In conclusion, dietary supplementation with TCM could reduce thymic inflammation levels and improve humoral immunity of finishing pigs.
Introduction
As we all know, piglet breeding is the most critical period in the process of pig breeding. To safeguard the health of finishing pigs during the nursery period, the use of antimicrobials either in feed or in water has been an essential tool. Antibiotics are used in swine feed as a growth promoter, to improve feed efficiency, and to reduce susceptibility to bacterial infections (1, 2). Nevertheless, abuse of antibiotic feed additives not only leads to excessive antibiotic residues in animals but also endangers human food safety and health. The China Ministry of Agriculture (MOA) gradually reduced the types and doses of antibiotics allowed in feeds in 2017 with the intent to ban the usage of antibiotics in feed additives by 2020 (3). Thus, it is necessary to develop new alternatives instead of using antibiotics to control diseases.
Traditional Chinese medicine (TCM) has lately received greater attention from researchers because of its natural structure and biological activity that can enhance or restore the immune system (4, 5). A recent study has shown that some phytochemicals were beneficial to health by promoting immune function and reducing inflammation responses (6). According to the theory of TCM, prescriptions are usually composed of several herbs or minerals, one of which represents the principal component and the other serves as an auxiliary component to assist or promote the transmission of the principal component. In most cases, the mixture of a variety of TCM will have a synergistic therapeutic effect (7).
The TLR4/MyD88/NF-κB pathway is a classical toll-like receptor activation pathway that plays an essential role in anti-inflammatory and immune regulations. A large number of studies have reported that inhibition of the TLR4/MyD88/NF-κB pathway could effectively inhibit the activation of NF-κB and reduce the secretion of pro-inflammatory factors such as TNF-a and IL-6, thereby inhibiting the occurrence of inflammation (8, 9). A previous study demonstrated that cinnamaldehyde has anti-inflammatory effects that can prevent inflammation-related health problems associated with over-activation of the TLR4 signaling pathway (10). Meanwhile, Luo et al. (11) found that pachymaran enhanced the immune function of mice by regulating genes associated with T and B cell functions. Additionally, it is reported that saikosaponin-a strongly inhibited pro-inflammatory mediators (12). Thus, dietary supplementation with TCM may be a feasible strategy to replace or reduce the use of antibiotics by enhancing immune responses, consequently promoting growth performance in finishing pigs (13). In conclusion, natural plants with pharmacological activities are recommended as dietary supplements or therapeutic agents to effectively care for the organism.
There is little research on the effects of TCM prescriptions on the immunity of finishing pigs. Therefore, this study was conducted to assess the effects of TCM1 and TCM2 on serum parameters and thymus inflammation responses in finishing pigs, and aimed to provide valuable insights for improving the immune performance of finishing pigs.
Materials and methods
Animals and treatments
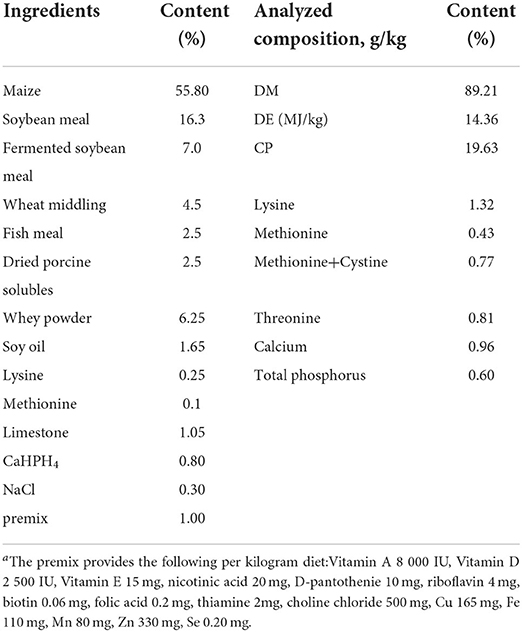
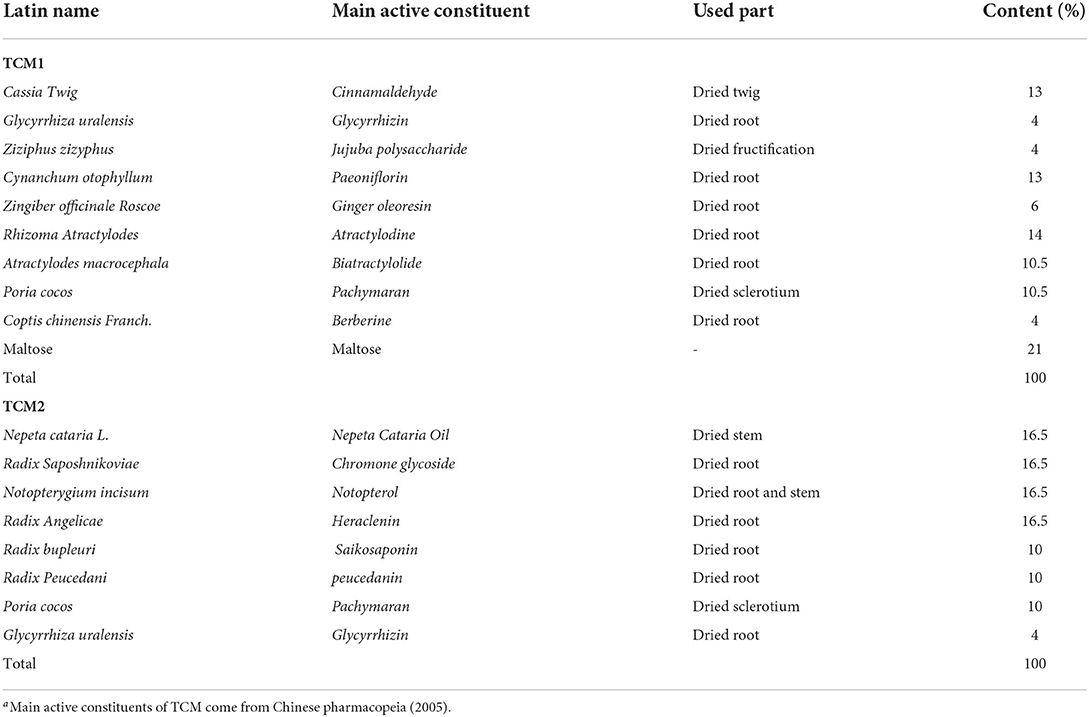
This trial was performed in the Researching and Teaching Base of Jiangxi Agricultural University and treated for 60 days. Thirty finishing pigs (white × landrace × Duroc) weighing 21.43 ± 2.86 kg were allowed ad libitum access to feed and water throughout the experimental period. Experimental animals of all three treatment groups were fed with the same basal diet, which was formulated to meet the nutrient requirements of finishing pigs (14). The composition and nutrient levels of basal diet are shown in Table 1. The finishing pigs were randomly allotted to three treatment groups (n = 10): The Con group (basal diet), the TCM1 group (basal diet + 10 g/kg Xiao Jian Zhong, XJZ) and the TCM2 group (basal diet + 3 g/kg Jingsananli-sepsis, JSS). The doses of TCM1 and TCM2 were evaluated according to our preliminary study and traditional Chinese pharmacopeia (2005). All raw materials of TCM1 were bought from Chinese Traditional Medicine Chang Sheng (Jiangxi, China), and TCM2 was provided by Jiangxi Chuang Dao Animal Health Products Co., Ltd. (Jiangxi, China). The composition and main active constituents of TCM1 and TCM2 are presented in Table 2. All experimental protocols were approved by the Committee for the Care and Use of Experimental Animals, Jiangxi Agricultural University, Jiangxi, China.
Sample collection
The experimental period lasted 60 days, and blood samples were collected on days 0, 30, and 60 and centrifuged (3,000 × g, 10 min) to obtain the serum which was then stored at −20°C for follow-up studies. All the finishing pigs were killed by euthanasia with an intravenous injection of sodium pentobarbital (40 mg/kg body weight), and the thymus of the finishing pigs was collected immediately after euthanasia, rapidly frozen in liquid nitrogen, and stored at −80°C for various analyses on day 60.
Serum parameter analysis
IgA, IgM, IgG, C3, and C4 were assayed following the manufacturer's instructions and using porcine-specific immunoturbidimetry kits (Nanjing Jiancheng Bioengineering Institute, China).
Blocking enzyme-linked immunosorbent assay (ELISA)
The serum samples were assayed following the manufacturer's instructions and using the IDEXX classical swine fever virus (CSFV) antibody test kit and IDEXX porcine reproductive and respiratory syndrome virus (PRRSV) antibody test kit (IDEXX Laboratories, Netherlands).
Real-time quantitative polymerase chain reaction (qRT-PCR)
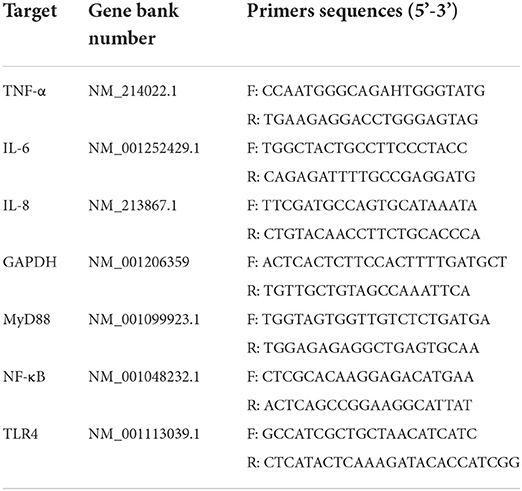
The mRNA transcription levels of TLR4/MyD88/NF-κB signal pathway-related genes (TLR4, MyD88, NF-κB, IL-6, IL-8, and TNF-α) in the thymus were determined by qRT-PCR assay according to the manufacturer's instructions. Briefly, total RNA was isolated using the TransZol Up Reagent (TransGen Biotech, Beijing, China). Then, cDNA was synthesized using a TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix reagent kit (TransGen Biotech, Beijing, China) according to the kit's instructions and stored at −20°C for SYBR Green qRT-PCR. Gene-specific primers of all the genes were designed using the Primer Premier software (PREMIER Biosoft International, CA, United States). The GAPDH gene was used as an internal reference, and the primer sequences are shown in Table 3. The qRT-PCR profiles were as follows: 95°C for 10 min, 40 cycles at 95°C for 15 s, 60°C for 60 s, and extension at 95°C for 15 s. All the reactions were carried out using an ABI 7900HT machine (Applied Biosystems, United States).
Western blot
The total protein from about 0.1g thymus of each piglet was extracted using a RIPA lysis buffer (APPLYGEN, Beijing, China) supplemented with 1 mM phenylmethanesulfonyl fluoride and phosphatase inhibitor, and the concentrations of total proteins were measured using a BCA (bicinchoninic acid) protein assay kit (Solarbio, China). Protein supernatant was separated by 10% SDS-PAGE and transferred into PVDF membranes. After blocking with 5% skimmed milk powder, the membranes were incubated with appropriate primary antibodies overnight at 4°C, followed by incubation with corresponding secondary antibodies for 1 h at room temperature. The membranes were washed thrice for 10 min each, incubated with a SuperSignal chemiluminescent substrate (Pierce), and imaged with ChemiDoc XRS+ Imaging System (Bio-Rad). Blots were semi-quantified using the ImageJ software. Primary antibodies for NF-κB p65 (Bioss, 1:1,000) and p-NF-κB p65, p-IκB-α (Bioss, 1:800) and phospho-IκB-α were used in this study.
Statistical analysis
A statistical analysis was performed using the SPSS 17.0 software (SPSS Inc., Chicago, United States). All the results are expressed in the format of mean ± standard deviation (SD). Comparisons between two or multiple groups were made by the Student's t-test or one-way ANOVA. A P-value of <0.05 was significant.
Results
Effects of TCM1 and TCM2 on immunoglobulins in serum
The effects of TCM1 and TCM2 on serum immunoglobulins are shown in Figure 1. Compared to the Con group, the TCM2 group showed a higher concentration of IgA (P < 0.01) on day 30 and a higher concentration of IgG (P < 0.05) on day 60. Furthermore, the TCM1 group showed higher serum levels of IgM (P < 0.05) on day 30, and the TCM2 group showed a higher concentration of IgM (P < 0.01) on day 30.
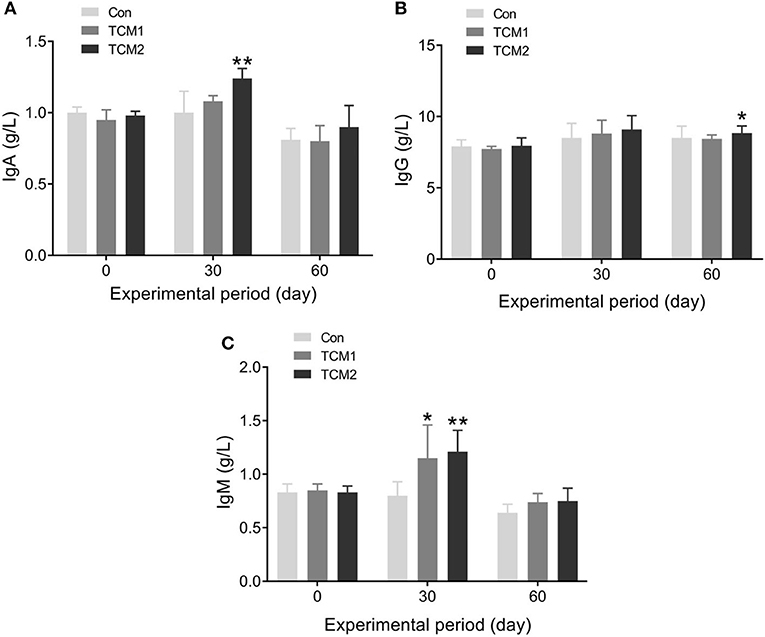
Figure 1. Effect of TCM1 and TCM2 on serums IgA, IgG, and IgM in finishing swine. “*” indicates significant difference compared with the control group (*P < 0.05, **P < 0.01). Below is the same. (A) IgA level. (B) IgG level. (C) IgM level.
Effects of TCM1 and TCM2 on complement in serum
The effects of TCM1 and TCM2 on serum complement are shown in Figure 2. Compared to the Con group, the TCM1 and TCM2 groups showed the highest concentration of C3 (P < 0.01) on day 30. No dramatic difference was observed in the serum concentrations of C3 among the three groups on day 60. The TCM2 group showed the highest concentration of C4 (P < 0.01) on day 30, and the TCM1 group showed a higher concentration of C4 (P < 0.05) on day 30. Furthermore, the TCM1 group showed highest serum levels of C4 (P < 0.01) on day 60.
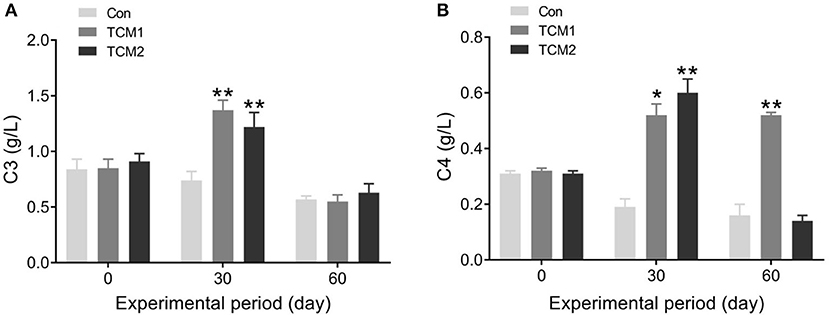
Figure 2. Effect of TCM1 and TCM2 on the levels of serums C3 and C4 in finishing swine. (A) The serum C3 level. (B) The serum C4 level. “*” indicates significant difference compared with control group (*P < 0.05 and **P < 0.01).
CSFV and PRRSV antibodies in finishing pigs
CSFV and PRRSV antibodies in the finishing pigs are evaluated in Figure 3. Compared to the Con group, the TCM1 and TCM2 groups showed the highest concentration of CSFV and PRRSV antibodies (P < 0.01) on day 30.
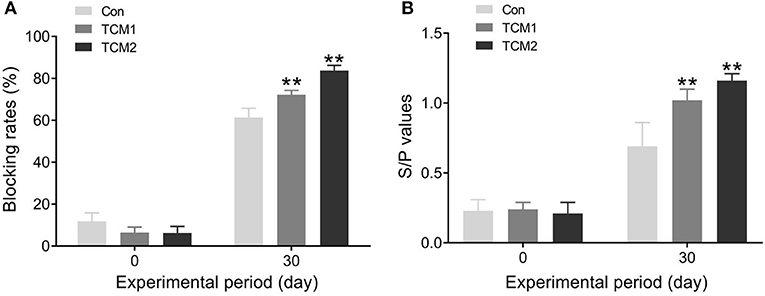
Figure 3. Effect of TCM1 and TCM2 on CSFV and PRRSV antibodies in finishing swine. (A) The concentration of CSFV antibody. (B) The concentration of PRRSV antibody. “*” indicates a significant difference compared with control group (*P < 0.05 and **P < 0.01).
Effects of TCM1 and TCM2 on TLR4/MyD88/NF-κB pathway-related mRNA and protein levels
The effects of TCM1 and TCM2 treatments on mRNA expression levels of the TLR4/MyD88/NF-κB signaling pathway in thymus tissues of the finishing pigs are shown in Figures 4A,B. Compared to the Con group, the mRNA expression levels of MyD88 were decreased in the TCM1 and TCM2 (P < 0.01) treatments; NF–κB levels in all the treatments group were decreased and significant in TCM1 (P < 0.05) and TCM2 (P < 0.01). Similarly, the levels of TLR4 were downregulated in the TCM1 (P < 0.05) and TCM2 (P < 0.01) treatments. The protein levels of P65, p-P65, IκBα, and p-IκBα were notably increased (P < 0.01 or P < 0.001) in the TCM1 and TCM2 groups (Figures 5A–C). Additionally, the p-P65/P65 ratio and the p-IκBα/IκBα ratio in both TCM1 and TCM2 groups was down-regulated (P < 0.01 or P < 0.001) in comparison with the control group.
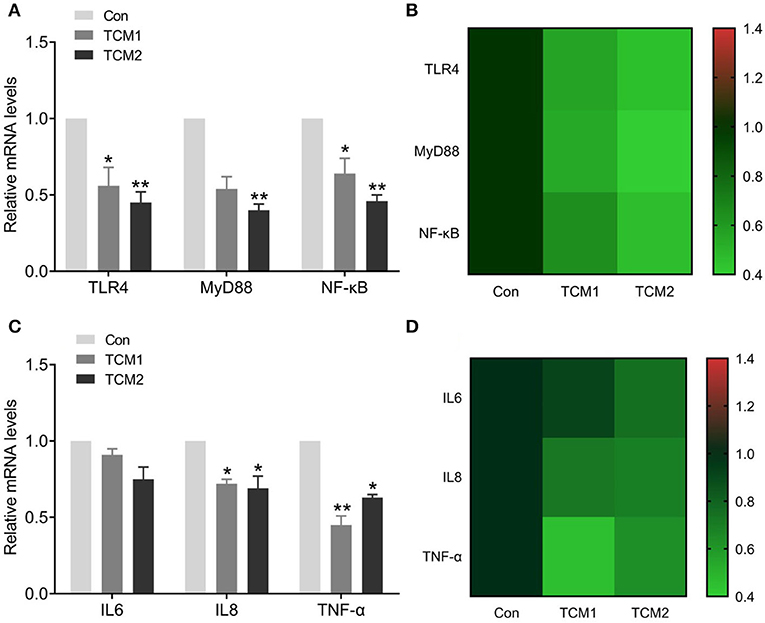
Figure 4. Effects of TCM supplement on mRNA levels of the TLR4/MyD88/NF-κB signaling pathway and inflammatory cytokine genes. (A) Effects of TCM supplement on mRNA levels of TLR4/MyD88/NF-κB pathway-related genes. (B) Heat map shows the mRNA levels of TLR4/MyD88/NF-κB signaling pathway-related genes. (C) Effects of TCM supplement on mRNA levels of inflammatory cytokine genes. (D) Heat map shows the mRNA levels of inflammatory cytokines genes. “*” indicates a significant difference compared with control group (*P < 0.05 and **P < 0.01).
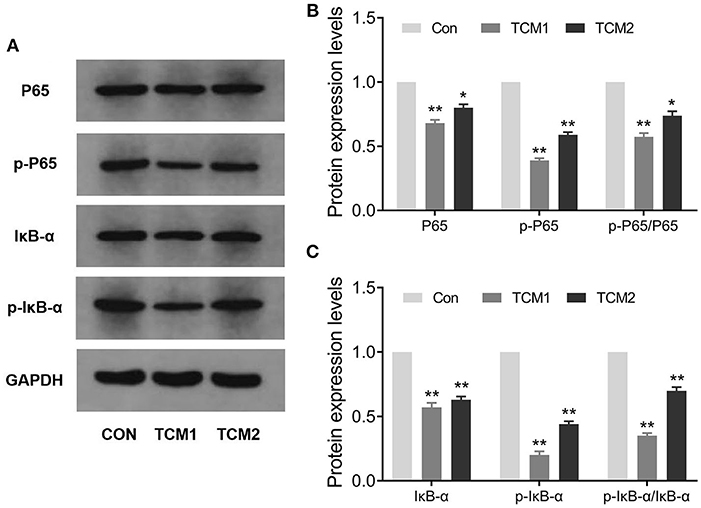
Figure 5. Effects of TCM supplement on key proteins levels of the NF-κB signaling pathway. (A,B) Immunoblot analysis of P65 and p-P65 proteins. (C) Immunoblot analysis of IκBα and p-IκBα proteins. “*” indicates a significant difference compared with control group (*P < 0.05 and **P < 0.01).
Effects of TCM1 and TCM2 on inflammatory cytokine mRNA levels
The effects of TCM supplementation on thymus tissue mRNA expression levels of IL-6, IL-8 and TNF-α are shown in Figures 4C,D. Compared to the Con group, the mRNA expression levels of IL-8 and TNF-α declined. IL8 levels in all the treatment groups were decreased and significant in TCM1 and TCM2 (P < 0.05). Similarly, the levels of TNF-α were downregulated in the TCM1 (P < 0.01) and TCM2 (P < 0.05) treatments.
Discussion
In recent decades, medicinal plants have attracted much attention because of their significant bioactivities such as increasing growth performance, antioxidant activity, antiviral activity, and immunomodulatory activity, which make them suitable as antibiotic replacements (15). TCM as an alternative to antibiotics is receiving more and more attention. Numerous studies have been demonstrated that TCM poses lots of function to livestock and poultry breeding, such as anti-inflammatory, oxidation resistance, resistance to allergy, protect the cardiovascular and antitumor (16, 17). Animal immune response to infection is closely related to immunoglobulin levels. Humoral immunity could help the body prevent infections and diseases, which are mediated by the IgA, IgM, and IgG antibodies (18, 19). It has been found that polysaccharides of traditional Chinese medicine alleviated the decrease of serum IgG concentration in broilers treated with cyclophosphamide (20). Similarly, polysaccharides of traditional Chinese medicine significantly increased the values of immune organ indices and serum IgM and IgG in mice and rats (21, 22). The polysaccharides of traditional Chinese medicine injection increased the content of IgG, IgM, and IgA in weaned piglets (15). We found that adding TCM1 and TCM2 as feed additives in the finishing pigs increased IgA, IgG, and IgM levels compared to the Con group. The complement system restricts viral infections through both classical and alternative pathways (23, 24). They contain numerous small proteins that enhance the ability of antibodies and phagocytic cells to clear microbes and damaged cells in the blood (25, 26). In addition, they also can facilitate inflammation and attack cells infected with pathogens. C3, the body's most abundant complement, is the body's innate immune system central link. It can interact with at least 25 soluble or membrane-bound proteins to activate the complement system through classical pathways, bypass pathways, and lectin pathways (14). In our study, the C3 and C4 in the TCM1 and TCM2 groups were significantly higher than those in the Con group on day 30. The results indicated that TCM1 and TCM2 could enhance immune function in finishing pigs.
Vaccination is often conducted in the pig industry to prevent CSF and PRRS, but there are many factors that can affect its effectiveness. It was described that astragalus polysaccharide and oxymatrine can synergistically improve the immune efficacy of Newcastle disease vaccine in chicken (27). Additionally, Chinese herbal medicine additives can improve the level of antibody against classic swine fever (28). We found that adding TCM1 and TCM2 could increase the levels of antibody against CSFV and PRRSV compared to the Con group. These results indicated that TCM1 and TCM2 could enhance the immune response of finishing pigs by increasing the levels of CSFV and PRRSV antibodies.
In the past few years, a study has shown that many kinds of polysaccharides of TCM have immunomodulatory effects through the TLR4/MyD88/NF-κB signaling pathway (29). TNF-α has the characteristics of a multifunctional pro-inflammatory cytokine with an important role in the pathogenesis of inflammatory diseases in this pathway (30). TNF-α activated phosphorylate IκB and induced its degradation, in parallel with leading to the liberation of NF-κB, and evoking the expression of a variety of genes, which participate in inflammatory responses (31). Our results showed that TCM1 and TCM2 could reduce the expression levels of genes (TLR-4, MyD88, NF-κB, TNF-α, IL6, and IL8) and proteins (IκBα, p-IκBα, p65, and p-p65) to different degrees. Furthermore, this activation was significantly inhibited by TCM1 and TCM2, suggesting that TCM1 and TCM2 exerted important effects resulting in reduced inflammation. Our results showed that TCM1 and TCM2 could act on the NF-κB signaling pathway and reduce the body's inflammatory response, thereby improving the immune performance.
Conclusion
In summary, the results showed that TCM1 and TCM2 had a significant inhibition of inflammation and low toxicity. These positive effects indicate that TCM1 and TCM2 can be dietary additives for animals to enhance humoral immunity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by the Committee for the Care and Use of Experimental Animals, Jiangxi Agricultural University, Jiangxi, China. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
XW, JC, and FY contributed to conception and design of the study. YM, AH, and TX organized the database. FW, GZ, and YY performed the statistical analysis. HC wrote the first draft of the manuscript. XG, AH, and TX wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The authors declare that this study received funding from Spirit Jinyu Biological Pharmaceutical Co. Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou X, Zhang Y, Wu X, Wan D, Yin Y. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early-weaned piglets. Cell Physiol Biochem. (2018) 48:993–1002. doi: 10.1159/000491967
2. Wang L, Zhu F, Yang H, Li J, Li Y, Ding X, et al. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J Anim Physiol Anim Nutr. (2019) 103:618–25. doi: 10.1111/jpn.13059
3. Qin L, Ji W, Wang J, Li B, Hu J, Wu X, et al. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food Funct. (2019) 10:2359–71. doi: 10.1039/C8FO02327A
4. Kamatou GPP, Makunga NP, Ramogola WPN, Viljoen AM. South African Salvia species: a review of biological activities and phytochemistry. J Ethnopharmacol. (2008) 119:664–72. doi: 10.1016/j.jep.2008.06.030
5. Zhao LM, Jia YL, Ma M, Duan YQ, Liu LH. Prevention effects of Schisandra polysaccharide on radiation-induced immune system dysfunction. Int J Biol Macromol. (2015) 76:63–9. doi: 10.1016/j.ijbiomac.2015.02.020
6. Shin HJ, Hwang KA, Choi KC. Antitumor effect of various phytochemicals on diverse types of thyroid cancers. Nutrients. (2019) 11:125. doi: 10.3390/nu11010125
7. Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, et al. Dissection of mechanisms of Chinese medicinal formula realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. (2008) 105:4826–31. doi: 10.1073/pnas.0712365105
8. Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J, et al. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem. (2018) 47:1497–508. doi: 10.1159/000490866
9. Wang L, Li N, Lin D, Zang Y. Curcumin protects against hepatic ischemia/reperfusion induced injury through inhibiting TLR4/NF-κB pathway. Oncotarget. (2017) 8:65414–20. doi: 10.18632/oncotarget.18676
10. Lee SC, Wang SY, Li CC, Liu CT. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J Food Drug Anal. (2018) 26:211–20. doi: 10.1016/j.jfda.2017.03.006
11. Luo X, Huang S, Luo S, Liao H, Wang Y, Deng X, et al. Identification of genes underlying the enhancement of immunity by a formula of lentinan, pachymaran and tremelia polysaccharides in immunosuppressive mice. Sci Rep. (2018) 8:10082. doi: 10.1038/s41598-018-28414-w
12. Ali J, Khan AU, Shah FA, Ali H, Islam SU, Kim YS, et al. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. (2019) 239:116888. doi: 10.1016/j.lfs.2019.116888
13. Li LL, Yin FG, Zhang B, Peng HZ, Li FN, Zhu NS, et al. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest Sci. (2011) 142:33–41. doi: 10.1016/j.livsci.2011.06.013
14. National Research Council. Nutrient Requirements of Swine. Washington, DC: National Academy Press (2012).
15. Xie JH, Jin ML, Morris GA, Zha XQ, Xie M. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr. (2016) 56(Suppl 1):S60. doi: 10.1080/10408398.2015.1069255
16. Kong XF, Wu GY, Liao YP, Hou ZP, Liu HJ, Yin FG, et al. Effects of Chinese herbal ultra-fine powder as a dietary additive on growth performance, serum metabolites and intestinal health in early-weaned piglets. Livestock Sci. (2007) 108:272–5. doi: 10.1016/j.livsci.2007.01.079
17. Kommera SK, Mateo RD, Neher FJ, Kim SW. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian-Au J Animal Sci. (2006) 19:1784–9. doi: 10.5713/ajas.2006.1784
18. Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Develop Compar Immunol. (2009) 33:384–93. doi: 10.1016/j.dci.2008.07.007
19. Rahe MC, Murtaugh MP. Effector mechanisms of humoral immunity to porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. (2017) 186:15–8. doi: 10.1016/j.vetimm.2017.02.002
20. Li J, Li R, Li N, Zheng F, Dai Y, Ge Y, et al. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J Pharm Biomed Anal. (2018) 158:451–60. doi: 10.1016/j.jpba.2018.06.024
21. Rui L, Chen W, Wang W, Tian W, Zhang X. Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohyd Polym. (2009) 78:738–42. doi: 10.1016/j.carbpol.2009.06.005
22. Meng F, Xu P, Wang X, Huang Y, Wu L, Chen Y, et al. Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saudi Pharm J. (2017) 25:460–3. doi: 10.1016/j.jsps.2017.04.006
23. Gialeli C, Gungor B, Blom AM. Novel potential inhibitors of complement system and their roles in complement regulation and beyond. Mol Immunol. (2018) 102:73–83. doi: 10.1016/j.molimm.2018.05.023
24. Moreno-Navarrete JM, Fernandez-Real JM. The complement system is dysfunctional in metabolic disease: Evidences in plasma and adipose tissue from obese and insulin resistant subjects. Semin Cell Dev Biol. (2019) 85:164–72. doi: 10.1016/j.semcdb.2017.10.025
25. Liao Z, Wan Q, Yuan G, Su J. The systematic identification and mRNA expression profiles post viral or bacterial challenge of complement system in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. (2019) 86:107–15. doi: 10.1016/j.fsi.2018.11.032
26. Zhao P, Wu J, Lu F, Peng X, Liu C, Zhou N, et al. The imbalance in the complement system and its possible physiological mechanisms in patients with lung cancer. Bmc Cancer. (2019) 19:201. doi: 10.1186/s12885-019-5422-x
27. Chen Y, Wang D, Hu Y, Guo Z, Wang J, Zhao X, et al. Astragalus polysaccharide and oxymatrine can synergistically improve the immune efficacy of Newcastle disease vaccine in chicken. Int J Biol Macromol. (2010) 46:428. doi: 10.1016/j.ijbiomac.2010.02.004
28. Fu-Zhuang LU, Wang ZG, Yuan FU, Shi TY, Hua WD, Zhang XJ, et al. Effects of Chinese herbal medicine additives on productive performances and levels of antibody against classical swine fever (CSF) for swine. Acta Agriculturae Zhejiangensis. (2011) 23:56.
29. Shan Y, Jiang B, Yu J, Wang J, Wang X, Li H, et al. Protective effect of Schisandra Chinesis polysaccharides against the immunological liver injury in mice based on Nrf2/ARE and TLR4/NF-kappaB signaling Pathway. J Med Food. (2019). doi: 10.1089/jmf.2018.4377
30. Du L, Li J, Zhang X, Wang L, Zhang W, Yang M, et al. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264, 7. macrophages via the suppression of TLR4/NF-kappaB pathway activation. Food Nutr Res. (2019) 63:3392. doi: 10.29219/fnr.v63.3392
Keywords: traditional Chinese medicine, finishing pigs, immune function, inflammation, thymus
Citation: Wang X, Chen J, Yang F, Ali F, Mao Y, Hu A, Xu T, Yang Y, Wang F, Zhou G, Guo X and Cao H (2022) Two kinds of traditional Chinese medicine prescriptions reduce thymic inflammation levels and improve humoral immunity of finishing pigs. Front. Vet. Sci. 9:929112. doi: 10.3389/fvets.2022.929112
Received: 26 April 2022; Accepted: 04 July 2022;
Published: 06 September 2022.
Edited by:
Dayou Shi, South China Agricultural University, ChinaReviewed by:
Xiaolong Gu, Yunnan Agricultural University, ChinaHaichong Wu, Zhejiang University, China
Copyright © 2022 Wang, Chen, Yang, Ali, Mao, Hu, Xu, Yang, Wang, Zhou, Guo and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huabin Cao, Y2hiaW4yMDAyMDgwNEBqeGF1LmVkdS5jbg==
†These authors share first authorship
 Xiaoyu Wang1†
Xiaoyu Wang1† Fan Yang
Fan Yang Huabin Cao
Huabin Cao