- 1Inner Mongolia Key Laboratory of Animal Nutrition and Feed Science, College of Animal Science, Inner Mongolia Agricultural University, Hohhot, China
- 2Animal Husbandry Service Center for Bayannaoer, Bayannaoer, China
The objectives of this research were to investigate the effects of Allium mongolicum Regel essential oil on growth performance, nutrient digestibility, rumen fermentation, and bacterial communities in sheep Twenty sheep were randomly divided into two dietary groups with 10 replicates each: (1) a basal diet without AMO as the control group (n = 10) and (2) a basal diet supplemented with 40 mg/kg AMO as the AMO group (n = 10). The average daily gain (ADG) was increased (P < 0.05), and the feed conversion ratio (FCR) was reduced (P < 0.05) in the AMO group compared with the control. The ruminal acetate, propionate, total volatile fatty acids (TVFA), and microbial protein (MCP) were higher (P < 0.05) in the AMO group than in the control. Moreover, ruminal pH and ammonia nitrogen (NH3-N) were lower (P < 0.05) in the AMO group than in the control. The relative abundances of the phylum levels of Firmicutes, Actinobacteriota, and Verrucomicrobiota were higher (P < 0.05) in the AMO group than in the control, and the relative abundances of Bacteroidetes and Spirochaetota were lower (P < 0.05) in the AMO group than in the control. The relative abundance of Prevotella and Prevotellaceae_UCG-003 at the genus level was increased (P < 0.05) in the AMO group compared with the control; however, the relative abundance of Succiniclasticum, Norank_f__F082, Christensenellaceae_R-7_group, and Norank_f__Muribaculaceae was decreased (P < 0.05) in the AMO group compared with the control. The activities of cellulase, α-amylase, and proteinase were higher (P < 0.05) in the AMO group than in the control. The apparent digestibility of dry matter (DM) and crude protein (CP) was increased (P < 0.05) in the AMO group compared with the control. In conclusion, AMO supplementation has the potential to improve growth performance. Moreover, supplementation with AMO improved nutrient digestibility, rumen fermentation, and bacterial communities in the rumen of sheep.
Introduction
Ionophores (monensin) have been extensively used in ruminant production to reduce energy and protein losses in the rumen (1). The appearance of residues in products and the spread of antibiotic-resistant microbes have led to restrictions in the European Union and a ban in China on their use (2, 3). Consequently, the feed industry has investigated safe and effective alternatives that can functionally replace antibiotics, such as plant extracts (phytobiotics) and essential oils (EOs), which provide similar results as antibiotics in terms of feed efficiency and rumen fermentation (4).
EOs are natural bioactive compounds extracted from plants via distillation methods, or they can be obtained by chemical extraction (1). EOs as alternatives to antibiotics have the potential to be used in livestock feed. EOs were found to improve nutrient digestibility and increase growth performance in animals (5, 6). Laura et al. noted that a blend of EO dietary supplementation decreased the concentration of acetate and ammonia nitrogen (NH3-N) in the rumen of finishing beef cattle (7). Moreover, oregano EO was shown to increase the relative abundance of Prevotella and Dialister in ruminal fluids in vitro (8). This capacity has become a promising research field for investigating the potential of EOs to alter rumen fermentation, resulting in improved feed efficiency in ruminants, but studies have mostly been conducted in vitro (9). In small ruminants, only a few in vivo studies have been performed to appraise the effects of EOs on animal growth performance, nutrient digestibility, rumen fermentation, and bacterial communities.
The Allium genus consists of many species, including shallot (Allium ascalonicum), leek (Allium ampeloprasum), garlic (Allium sativum), onion (Allium cepa), and chive (Allium schoenoprasum) (10). Allium mongolicum Regel (AM) is an Allium plant that grows widely in northern China (11). AM is a precious source of forage as it offers extensive nutrients and the contents of protein, ether extract (EE), flavonoids, minerals, amino acids, essential trace elements, and other components (12). A recent study noted that the use of AM water extract could increase the average daily gain (ADG) and decrease the feed conversion ratio (FCR) in sheep (13). In addition, Zhao et al. found that AM ethanol extract at doses of 2.8 g with diets lowered ruminal NH3-N values after feeding in sheep (14). AM could increase the dry matter digestibility (DMD) and acid detergent fiber digestibility (ADFD) of Simmental calves (15). According to our previous literature, the use of polysaccharides from AM could decrease the abundances of f-Prevotellaceae and g-Prevotellaceae_UCG-001 in the Bacteroidetes of sheep (16).
To date, no study has assessed the possible impact of Allium mongolicum Regel essential oil (AMO) on growth performance, nutrient digestibility, rumen fermentation, and bacterial communities in sheep. The purpose of this study, therefore, was to fill the gap in knowledge in this field and explore the potential of AMO as an antibiotic substitute for sheep.
Materials and methods
Ethics approval and consent to participate
All the animal procedures were carried out according to the protocols approved by the College of Animal Science, Inner Mongolia Agricultural University, China. We confirm that the authors complied with the Animal Research Reporting of in vivo Experiments (ARRIVE) guidelines.
Extraction process of AMO
Dried powder derived from AM leaves was purchased from Hao Hai Biological Co., Ltd. (Alashan League, Inner Mongolia, China). AMO was processed from the AM by hydrodistillation (1 kg in 5 L of distilled water) using a Clevenger-type apparatus for 3 h in line with the procedure noted in the European Pharmacopeia (17). The oil acquired above the aqueous distillate was dried with anhydrous sodium sulfate (1 mg/2 mL). The AMO was kept in a stainless sealed container at 4°C until use. AMO was analyzed for its chemical compounds using a GC/MS apparatus at the Atomic and Molecular Physics Unit. The major components of AMO are anethole (37.63%), aromatic hydrocarbon (30.52%), and gingerol (12.60%).
Animals, diets, and experimental design
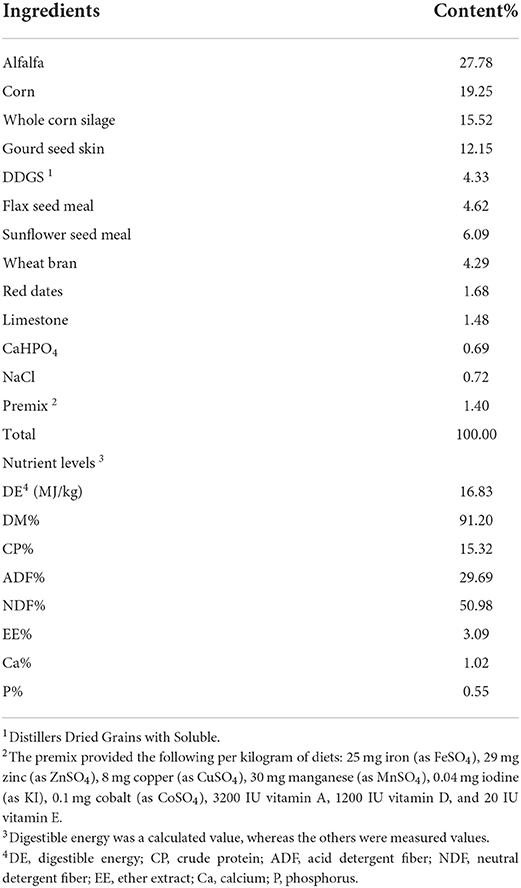
Twenty healthy male Dorper sheep with a mean BW (± SD) of 34.5 kg (± 2.5 kg) and an average age of 6 months were used. The sheep were randomly divided into two dietary groups with 10 replicates each: (1) a basal diet without AMO as the control group (n = 10) and (2) a basal diet supplemented with 40 mg/kg AMO as the AMO group (n = 10). The doses of the AMO were determined based on rumen fermentation using in vitro 24-h batch incubation (18). The basal diet was formulated to meet the standard nutrient requirements of sheep according to NRC recommendations (19). The dietary ingredients and nutrient levels of the diet are presented in Table 1. The AMO was completely mixed with the concentrate portion of the TMR before being mixed with the forage component. The diets were provided ad libitum twice per day at 07:00 and 18:00. Fresh water was available for the sheep during the whole feeding trial. The experiment lasted 75 days and comprised 15 days for the adaptation period and 60 days for the data and sample collection period.
Data and sample collection
During the trial, sheep were measured for health status, and their body weight was measured at 15-day intervals. Feed was suspended before initial and final weighing. The ADG and dry matter intake (DMI) of sheep were recorded, and the FCR was computed. FCR was determined as average daily DMI/ADG.
The rumen fluid was sampled at 2 h after the morning feeding via a stomach tube linked to an Erlenmeyer flask and a vacuum pump on the 60th day of the experimental period. The first 100 mL rumen fluid collected was discarded to prevent saliva contamination, and 100 mL rumen fluid was acquired. pH was immediately determined using an electric handheld pH meter (PHS-3C, Beijing Huoze Experimental Instrument Co., Ltd., Beijing, China). After that, the rumen fluid was then strained immediately through four layers of cheesecloth. One tube of the filtrate with 250 g/L (w/v) metaphosphoric acid (8: 2, v/v) was stored (−20°C) to detect the volatile fatty acids (VFA), and another tube of 10 mL filtrate was preserved by adding 2 mL of 20 g/L (w/v) H2SO4 for NH3-N determination in another tube. The third tube of the filtrate was kept at −20°C to determine the microbial protein (MCP), cellulase, β-glucanase, α-amylase, lipase, and proteinase contents. The fourth tube of the filtrate was stored at – 80°C for analysis of the bacterial composition.
Approximately 100 g of fecal samples was collected from the rectum at 08:00, 11:00, 14:00, and 17:00 on the 58th, 59th, and 60th days of the experimental period to estimate the DMD, organic matter digestibility (OMD), crude protein digestibility (CPD), EE digestibility (EED), neutral detergent fiber digestibility (NDFD), and ADFD. Fecal yield was determined using acid-insoluble ash as an internal indicator (20).
Sample analysis
Ruminal NH3-N, MCP, and VFA
The VFA contents were measured using gas chromatography (Trace 1100; Thermo Fisher Scientific Co., Ltd., Beijing, China) as described by Zhou et al. (21). The NH3-N contents were determined using the method described by the Kjeldahl assay (22). The concentration of MCP yield was determined by urinary purine derivative excretion according to the method described by Hu et al. (23). The cellulase, β-glucanase, α-amylase, lipase, and proteinase activities were analyzed using kits (Nanjing Jiancheng Biological Technology Co. Ltd., Nanjing, China) according to the method of Laura et al. (7).
DNA extraction and PCR amplification
Microbial community genomic DNA was extracted from ruminal samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, Norcross, GA, U.S.) according to the manufacturer's instructions. The DNA was extracted according to Jiang (24). The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with the primer pairs 338F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA) (8). The PCR amplification of the 16S rRNA gene was performed according to Liu (25). The PCR mixtures refer to Wang (26). The PCR product was extracted from a 2% agarose gel, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's instructions, and quantified using a Quantus™ Fluorometer (Promega, USA) (26).
Illumina MiSeq sequencing
Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Processing of sequencing data
The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0 (27), and merged by FLASH version 1.2.7 (28).
Feed and fecal chemical analyses
The fecal samples and diets were dried in a forced-air oven at 55°C for 72 h and ground using a 1 mm screen. All feeds were analyzed in duplicate for DM, calcium (Ca), phosphorus (P), EE, CP, and ADF (29). The NDF content was analyzed with sodium sulfite and α-amylase (30). The total fecal output for each sheep was measured based on the content of indigestible neutral detergent fiber (iNDF) and used as an endogenous indicator. The iNDF content was calculated to determine in vivo digestibility using the following equation from Menezes et al. (31).
iNDF (% DM) = iNDF (g)/DM (%) × 100
Statistical analysis
The data were analyzed using SAS software (version 9.0, SAS Inst. Inc., Cary, NC). Before analysis, all data were examined for normality using the UNIVARIATE procedure. Data on growth performance, rumen fermentation parameters, rumen enzyme activities, bacterial relative abundance, and nutrient digestibility were analyzed separately using a one-way analysis of variance (ANOVA) with dietary treatments used as the main factor.
Statistically significant differences among treatments were evaluated using Tukey's multiple range test. Probability values of P ≤ 0.05 were declared significant, and probability values were declared statistical trends when 0.05 < P ≤ 0.10.
Results
Effects of AMO on growth performance and feed efficiency
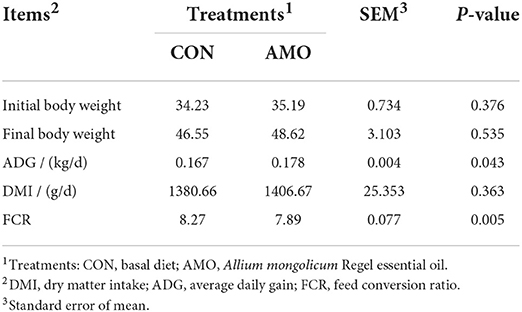
The effects of AMO on the growth performance and feed efficiency of sheep are shown in Table 2. Dietary supplementation with AMO increased (P < 0.05) ADG compared with the control. Dietary supplementation with AMO decreased (P < 0.05) FCR compared with the control. There was no effect of AMO inclusion in the diet on DMI (P > 0.05).
Effects of AMO on rumen fermentation parameters
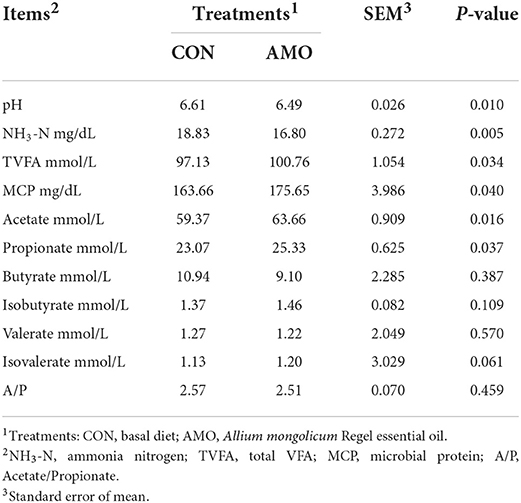
The effects of AMO on the rumen fermentation parameters of sheep are shown in Table 3. Dietary supplementation with AMO increased (P < 0.05) the concentrations of MCP, TVFA, acetate, and propionate compared with the control. Moreover, a lower rumen pH (P < 0.05) and a lower NH3-N (P < 0.05) concentration were noted in the AMO group. There were no effects of AMO inclusion in the diet on the acetate-to-propionate ratio or the concentrations of butyrate, isobutyrate, valerate, and isovalerate (P > 0.05).
Effects of AMO on the rumen bacterial community
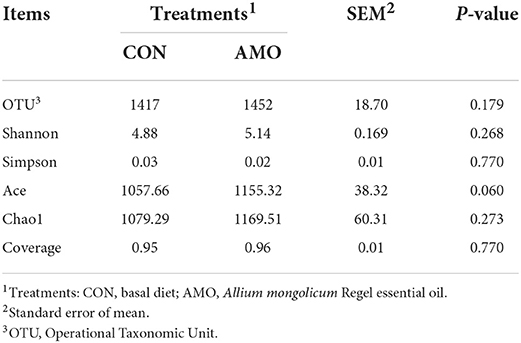
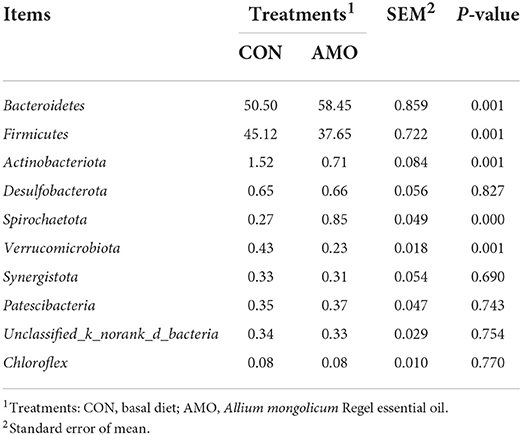
Table 4 shows that the OTU (Operational Taxonomic Unit), Shannon, Simpson, Ace, Chao1, and coverage indices were not affected (P > 0.05) by AMO. Table 5 shows that the relative abundance of the phylum level Bacteroidetes and Spirochaetota in the AMO group was lower than that in the control. Conversely, the relative abundances of Firmicutes, Actinobacteriota, and Verrucomicrobiota of sheep fed the AMO diet were higher (P < 0.05) than those fed the control diet. Furthermore, no differences were noted for the relative abundances of other rumen bacterial phyla between treatments.
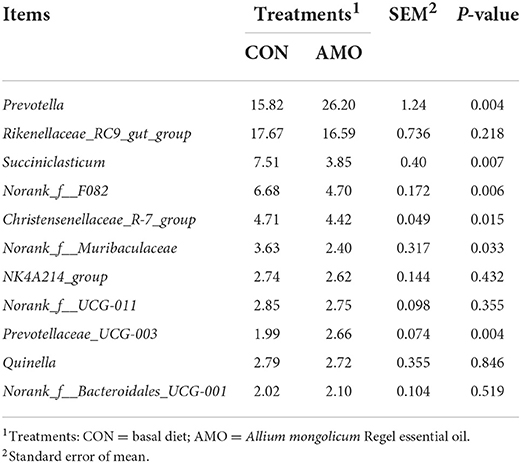
Table 6 shows that the relative abundances of the genera Prevotella (P < 0.05) and Prevotellaceae_UCG-003 (P < 0.05) were increased in the AMO group compared with those in the control. In contrast, sheep fed the AMO diet decreased the relative abundance of Succiniclasticum, norank_f__F082, Christensenellaceae_R-7_group, and norank_f__Muribaculaceae (P < 0.05). Moreover, no differences were noted for the relative abundances of other rumen bacterial genera between treatments.
Effects of AMO on rumen enzyme activities
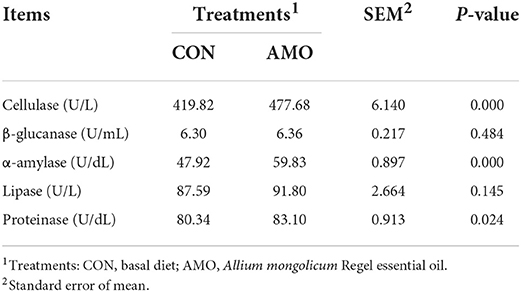
The effects of AMO on the rumen enzyme activities of sheep are shown in Table 7. Dietary supplementation with AMO increased (P < 0.05) the activities of cellulase, α-amylase, and proteinase. In addition, no difference was found for the activities of rumen lipase and β-glucanase (P > 0.05).
Effects of AMO on nutrient digestibility
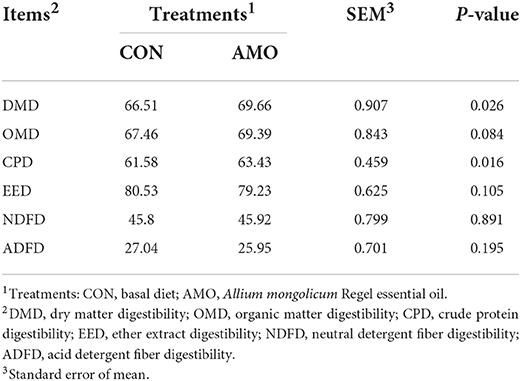
The effects of AMO on the apparent digestibility of sheep are shown in Table 8. Dietary supplementation with AMO increased (P < 0.05) the apparent digestibility of DM and CP. No difference was found for the apparent digestibility of OM, EE, ADF, and NDF (P > 0.05).
Discussion
Effects of AMO on growth performance and feed efficiency
As one of the main components of AM, AM flavonoids have been used as feed additives to increase the growth hormone (GH) level in the serum and ADG of sheep (32). Du et al. reported an improvement in the growth performance of sheep with dietary inclusion of AM extract (3.4 g/sheep/day) (16). However, scarce literature is available with regard to the effects of dietary supplementation with AMO on the DMI and ADG of sheep. The present research investigated whether sheep-fed AMO had a change in DMI compared with the control group. However, in contrast to this finding, an enhancement in ADG was found for sheep fed the diet supplemented with AMO. In the current study, the addition of AMO to the diet of sheep clearly decreased FCR, resulting from the increased ADG and similar DMI. These results are consistent with Zhao et al., who stated that diets supplemented with AM water extract in sheep resulted in changes in ADG and FCR (14). Such favorable effects on ADG and FCR were likely due to an increase in the relative abundance of bacteria involved in carbohydrate metabolism in the rumen, thereby enhancing the digestibility of DM and CP. Furthermore, the increases in growth performance may be partly related to the immunoenhancement and antioxidative effects of AMO (33).
Effects of AMO on rumen fermentation parameters
The decrease in ruminal Ph following AMO supplementation was in accordance with the increase in TVFA content (34). A lower ruminal pH was reported in sheep receiving AMO addition and was 6.49, which was suitable for rumen microbial growth and nutrient degradation. The major products of carbohydrate fermentation in the rumen are VFAs, which are the primary energy sources of ruminants (24). The total and molar proportions of VFA generated in the rumen depend on the substrate composition, nutrient availability, ruminal microbial community, and rumen pH reached (35). These alterations in TVFA proportions could be due to the stimulating action of AMO on propionate- and acetate-producing bacteria, indicating that AMO increased the fermentation of carbohydrates. It was also noted that AM water extract and AM ethanol extract could increase the quantity of amylolytic bacteria and cellulolytic bacteria in the rumen (14). This could be used for carbohydrate fermentation, generating high VFA. These current results differ in part from those of Ding et al., who did not observe a difference in the ruminal concentrations of TVFA or acetic and propionic acids in the rumen fluid of sheep-fed AM diets (36). Zhao et al. also found that feeding sheep with AM water extract increased the content of butyric acid and isobutyric acid (14). The difference between studies could be related to the source of AMO and AM water extract, the amount consumed, and the basic diet.
Our previous research found that the use of AMO decreases the content of NH3-N in in vitro rumen fermentation (18). Consistent with the above study, the addition of AMO decreased the NH3-N content in the rumen of sheep. Wallace et al. affirmed that a rumen NH3-N content between 15 and 30 mg/dL was more reasonable (37). Consequently, we reported that using AMO as a dietary additive could accelerate nitrogen metabolism in the rumen within a legitimate range (16.80 mg/dL) in the present study. MCP is synthesized by rumen microorganisms using NH3-N, peptide, and amino acids in the rumen, and energy is necessary during the whole process of MCP synthesis (24). The contents of MCP and NH3-N maintain a balance in the rumen, and excessive NH3-N will increase the loss of nitrogen in the nitrogen cycle, while less NH3-N will influence the production of MCP (24). The present research found that the output of MCP in the rumen was increased by feeding a diet with AMO to sheep, which indicates that AMO addition supplies an adequate nitrogen source, carbon skeleton, and energy for MCP synthesis. A similar consequence was acquired with dietary supplementation with different levels of AMO in the diet, which enhanced the rumen MCP yield in vitro (18).
Effects of AMO on the rumen bacterial community
The rumen is colonized by some microorganism communities (38), which play significant roles in fermenting and digesting nutrients to supply protein and energy resources (39), improving resistance to external stress, and enhancing growth and performance (40). The most significant microorganisms in the rumen were bacteria, as were also mentioned in previous literature (41). These bacterial communities live in dynamic conditions that could be influenced by several factors, such as diet, animal age, and feed additives (42). In the current study, there was no significant difference in OTU, Ace, Chao1, Simpson, Shannon, and coverage, which suggested that the addition of AMO had no negative effect on the alpha diversity of rumen microbes.
In the present research, Bacteroidetes and Firmicutes were the superior phyla in the ruminal microbial community, which is in accordance with previous literature in sheep (43, 44). In the current study, AMO addition in diets increased the relative abundance of the phylum Firmicutes and decreased the relative abundance of the phylum Bacteroidetes, consequently increasing the Firmicutes to Bacteroidetes (F:B) ratio, which could be beneficial for energy utilization and growth performance (45). The increased F:B ratio might be due to the acceleration of growth of Firmicutes by the antimicrobial function of the active ingredient in AMO.
In ruminants, Prevotella is the genus of the phylum Bacteroidetes with the highest relative abundance (26). Prevotella plays an important role in secreting the enzyme xylanase, which contributes to polysaccharide degradation (46). Some species of Prevotella are involved in the degradation and utilization processes of fiber, hemicellulose, pectin, and protein (47). In agreement with these results, our study found that the relative abundance of Prevotella seemed to be the most abundant in all rumen fluid samples at the genus level. Furthermore, the current work also found that the use of AMO in the sheep diet could promote the relative abundance of Prevotellaceae_UCG-003. The connection between the substrate and growth of Prevotella is not apparent. Previous studies have confirmed that Prevotellaceae UCG-003 participates in glucose metabolism, generating acetic acids as the primary fermentation final products (25). This explains why acetic acid was present at a higher level in the AMO group.
Effects of AMO on enzyme activity
Meanwhile, the rumen cellulase, α-amylase, and proteinase activity apparently increased in the AMO group compared with the control. This could be sensed by a higher rumen Prevotella and Prevotellaceae_UCG-003 in the AMO group, which contribute to the production of cellulase, α-amylase, and proteinase. Similar to the results observed in this study by Zhao et al., AM extracts could enhance the activities of cellulase, α-amylase, and proteinase in the rumen (14).
Effects of AMO on nutrient digestibility
Because AMO has been observed to decrease NH3-N accumulation and change microbial community structure, we supposed that AMO would enhance nutrient digestibility in sheep by improving rumen microbial synthesis efficiency. Qiao et al. suggested that dietary AMO addition promoted the apparent total tract digestibility of CP in sheep (48). In addition, Yaxing et al. (33) noted increased DM digestibility in in vitro batch cultures with rumen fluid of sheep supplemented with increasing levels of AMO. Data from our research found that the digestibility of DM and CP in sheep with AMO supplementation was increased, which was similar to what we hypothesized. Similarly, several studies have reported that nutrient digestibility is enhanced by cinnamon oil in chickens (49), by oregano essential oil in lambs (8), and by essential oil in beef cattle (50). There are three reasons for the results of the present study. First, the decreased ruminal NH3-N with sheep AMO inclusion suggested that the synthesis of MCP had accelerated and led to the increased CP digestibility. Second, Dowd et al. observed that Prevotella was very significant for the degradation and absorption of proteins as well as for the fermentation of peptides in the rumen (51). Based on the above findings, our present research detected that the relative abundance of Prevotella was most abundant in the rumen fluid of sheep in the two treatments at the genus level. Moreover, the current research also demonstrated that supplementation with AMO in the diet could increase the relative abundance of Prevotella and Prevotellaceae_UCG-003. These results revealed that dietary addition of AMO had a favorable effect on enhancing the degradation of CP in the rumen, thereby boosting rumen fermentation. Third, we surmised that the improved digestibility of DM and CP was partially due to the elevated ruminal proteinase enzyme activity.
Conclusion
In general, supplementation with AMO in sheep diets increased growth performance by increasing ADG and decreasing FCR, promoted rumen fermentation, diversified the bacterial community composition in the rumen, enhanced the apparent digestibility of DM and CP, and improved rumen enzyme activities.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI; PRJNA833585.
Ethics statement
The animal study was reviewed and approved by College of Animal Science, Inner Mongolia Agricultural University, China. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
ZY wrote the main manuscript. KE and BZ prepared Tables 1–4, and BC prepared Tables 5–8. AC edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the landmark achievement of Inner Mongolia Agricultural University (BZCG202102).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.926721/full#supplementary-material
References
1. EI-essawy AM, Anele UY, Abdel-Wahed AM, Abdou AR, Khattab IM. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Animal Feed Sci Technol. (2020) 10:114760. doi: 10.1016/j.anifeedsci.2020.114760
2. Magnusson U. Prudent and effective antimicrobial use in adiverse livestock and consumer's world. J. Anim. Sci. (2020) 98:S4–S8. doi: 10.1093/jas/skaa148
3. Guo S, Lei J, Liu L, Qu X, Li P, Liu X, et al. Effects of Macleaya cordata extract on laying performance, egg quality and serum indices in Xuefeng Black-Bone Chicken. Poult Sci. (2021) 100[4]:101031. doi: 10.1016/j.psj.2021.101031
4. Aziz MS. Karboune S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: a review. Crit Rev Food Sci Nutr. (2018) 58:486–511. doi: 10.1080/10408398.2016.1194256
5. Ruan D, Fan Q, Fouad AM, Sun Y, Huang S, Wu A, et al. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity and intestinal microbiota in yellow-feathered chickens. J Animal Sci. (2021) 99:1–11. doi: 10.1093/jas/skab033
6. Parvar R, Ghoorchi T, Kashfi H, Parvar K. Effect of Ferulago angulata (Chavil) essential oil supplementation on lamb growth performance and meat quality characteristics. Small Ruminant Res. (2018) 7:26. doi: 10.1016/j.smallrumres.2018.07.026
7. Toseti LB, Goulart RS, Gouvêa VN, Acedo TS, Vasconcellos GS, Pires AV, et al. Effects of a blend of essential oils and exogenous α- amylase in diets containing different roughage sources for finishing beef cattle. Animal Feed Sci Technol. (2020) 269:114643. doi: 10.1016/j.anifeedsci.2020.114643
8. Zhou R, Wu J, Lang X, Liu L, Casper DP, Wang C, et al. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J Dairy Sci. (2020) 103:2303–14. doi: 10.3168/jds.2019-16611
9. Joch M, Kudrna V, Hakl J, Božik M, Homolka P, Illek J, et al. In vitro and in vivo potential of a blend of essential oil compounds to improve rumen fermentation and performance of dairy cows. Animal Feed Sci Technol. (2019) 3:009. doi: 10.1016/j.anifeedsci.2019.03.009
10. Zeng Y, Li Y, Yang J, Pu X, Du J, Yang X, et al. Therapeutic role of functional components in alliums for preventive chronic disease in human being. Evid. Based Complem. Altern. Med. (2017) 94:02849. doi: 10.1155/2017/9402849
11. Schmitt B, Schulz H, Storsberg, J, Keusgen M. Chemical characterization of Allium ursinum L. depending on harvesting time. J Agric Food Chem. (2005) 53:e94. doi: 10.1021/jf0504768
12. Huang QC, Chu YL, Zhao YH, Zhao HT, Liu JM, Zhou RY. Status and prospect of plant fiber industry in Guangxi province. Plan Fib Sci. (2011) 33:202–5. doi: 10.3969/j.issn.1671-3532.2011.04.010
13. Yaxing Z, Erdene K, Changjin A, Zhibi B, Hongxi D, Zejun F, et al. Effects of Allium mongolicum Regel and its extracts supplementation on the growth performance, carcass parameters and meat quality of sheep. Italian J Animal Sci. (2021) 20:1899–1908. doi: 10.1080/1828051X.2021.1971572
14. Zhao Y, Ao C, Bao Z, Fan Z, Liu W, Ding H, et al. Effects of Allium monogolium Regel and its extracts on rumen fermentation and microflora of sheep. Anim Nutr. (2019) 31:2313–22. doi: 10.3969/j.issn.1006-267x.2019.05.038
15. Xie K, Wang Z, Wang Y, Wang C, Chang S, Zhang C, et al. Effects of Allium mongolicum Regel supplementation on the digestibility, methane production, and antioxidant capacity of Simmental calves in northwest China. Animal Sci J. (2020) 91:e13392. doi: 10.1111/asj.13392
16. Du H, Khas E, Chen S, Qi S, Bao Z, Zhao Y. Correlation of the rumen fluid microbiome and the average daily gain with a dietary supplementation of allium mongolicum regel extracts in sheep. J Anim Sci. (2019) 97:2865–77. doi: 10.1093/jas/skz139
17. Egyptian Pharmacopoeia (EP) General Organization for Governmental Printing Affairs Cairo Egypt (2005).
18. Mu CT, Ding N, Hao XY, Zhao YB, Wang PJ, Zhao JX, et al. Effects of Allium mongolicum Regel essential oil on in vitro rumen fermentation and substrate degradability of sheep. Anim Nutr. (2021) 33:4740–47. doi: 10.3969/j.issn.1006-267x.2021.08.052
19. NRC. Nutrient requirements of sheep. 11th rev. edn. Washington, DC: National Academic Press (2012).
20. Van Keulen J, Young BA. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. (1977) 44:282–289. doi: 10.2527/jas1977.442282x
21. Zhou JW, Mi JD, Degen AA, Guo XS, Wang HC, Ding LM, et al. Apparent digestibility, rumen fermentation and nitrogen balance in Tibetan and fine-wool sheep offered forage-concentrate diets differing in nitrogen concentration. J. Agric. Sci. (2015) 153:1135–45. doi: 10.1017/S0021859615000465
22. Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. (1980) 63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
23. Hu WL, Liu JX, Ye JA, Wu YM, Guo YQ. Effect of tea saponin on rumen fermentation in vitro. Anim Feed Sci Technol. (2005) 120:333–9. doi: 10.1016/j.anifeedsci.2005.02.029
24. Jiang X, Liu X, Liu S, Li Y, Zhao HB, Zhang YG. Growth, rumen fermentation and plasma metabolites of Holstein male calves fed fermented corn gluten meal during the postweaning stage. Animal Feed Sci Technol. (2019) 249:1–9. doi: 10.1016/j.anifeedsci.2019.01.012
25. Liu YZ, Chen X, Zhao W, Lang M, Zhang XF, Wang T, et al. Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on rumen fermentation parameters and bacterial-community composition in sheep. Animal Feed Sci Technol. (2019) 2:3. doi: 10.1016/j.anifeedsci.2019.02.003
26. Wang T, Jiao J, Wang H, Degen AA, Gou N, Li S, et al. The effects of supplementing sweet sorghum with grapeseeds on dry matter intake, average daily gain, feed digestibility and rumen parameters and microbiota in lambs. Animal Feed Sci Technol. (2020) 14:114750. doi: 10.1016/j.anifeedsci.2020.114750
27. Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ. Preprocessor. Bioinformatics. (2018) 34:i884–90. doi: 10.1093/bioinformatics/bty560
28. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. (2011) 27:2957–63. doi: 10.1093/bioinformatics/btr507
29. Association of Official Analytical Chemists (AOAC). Changes in official methods of analysis of the Association of Official Analytical Chemists. Arlington. (1990) 110:123–35.
30. Van Soest PJ, Robertson JB. Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
31. Menezes AC, Valadares Filho SC, e Silva LC, Pacheco MV, Pereira JM, Rotta PP, et al. Does a reduction in dietary crude protein content affect performance, nutrient requirements, nitrogen losses, and methane emissions in finishing Nellore bulls? Agric Ecosyst Environ. (2016) 3:015. doi: 10.1016/j.agee.2016.03.015
32. Ren WC, Cui FW. Chang JA. Effects of flavonoids from Allium mongolicum Regel on growth performance and growth-related hormones in meat sheep. Anim Nutr. (2017) 3:33–8. doi: 10.1016/j.aninu.2017.01.003
33. Yaxing Z, Erdene K, Changjin A, Zhibi B, Hongxi D, Zejun F, et al. Effects of essential oil from Allium mongolicum Regel on slaughter performance, physicochemical property of meat and Antioxidant activity in mutton sheep. Feed Ind. (2021) 42:60–64. doi: 10.13302/j.cnki.fi.2021.17.011
34. Dijkstra J, Ellis JL, Kebreab, E, Strathe AB, L'opez S, France J, Bannink A. Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed. Sci. Tech. (2012) 172:22–33. doi: 10.1016/j.anifeedsci.2011.12.005
35. Ceconi I, Ruiz-Moreno MJ, DiLorenzo N, DiCostanzo A, Crawford GI. Effect of urea inclusion in diets containing corn dried distillers grains on feedlot cattle performance, carcass characteristics, ruminal fermentation, total tract digestibility, and purine derivatives-to-creatinine index. J. Anim. Sci. (2015) 93:357–369. doi: 10.2527/jas.2014-8214
36. Ding H, Liu W, Ao C, Li S, Li Y, Zhang Z. Effects of dietary allium mongolicum regel powder or probiotic complex preparation on ruminal fermentation parameters and rumen fluid bacterial diversity of dorper × thin-tailed han crossbred sheep. Anim Nutr. (2018) 31:324–33. doi: 10.3969/j.issn.1006-267x.2019.01.039
37. Wallace RJ, Rooke JA, McKain N, Duthie CA, Hyslop JJ, Ross DW, et al. Effect of ruminal NH [[sb]]3[[/s]] -N levels on ruminal fermentation, purine derivatives, digestibility and rice straw intake in swamp buffaloes. Asian-Australas. J. Anim. Sci. (1999) 12:904–907. doi: 10.5713/ajas.1999.904
38. Zhang RY, Jin W, Feng PF, Liu JH, Mao SY. High-grain diet feeding altered the composition and functions of the rumen bacterial community and caused the damage to the laminar tissues of goats. Animal. (2018) 12:1–10. doi: 10.1017/S175173111800040X
39. Vymazal J, Kröpfelová L. Ruminal microbiota developing in different in vitro simulation systems inoculated with goats' rumen liquor. Anim. Feed Sci. Technol. (2013) 185:9–18. doi: 10.1016/j.anifeedsci.2013.06.003
40. Cani P D, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Design. (2009) 15:1546–58. doi: 10.2174/138161209788168164
41. Wang YY, Cao PH, Wang L, Zhao ZY, Chen YL, Yang YX. Bacterial community diversity associated with different levels of dietary nutrition in the rumen of sheep. Appl. Microbiol. Biot. (2017) 101:3717–28. doi: 10.1007/s00253-017-8144-5
42. O'Hara E, Kenny DA, McGovern E, Byrne CJ, McCabe MS, Guan LL, et al. Investigating temporal microbial dynamics in the rumen of beef calves raised on two farms during early life. FEMS Microbiol. Ecol. (2020) 96:114802. doi: 10.1093/femsec/fiz203
43. Trabi EB, Seddik H, Xie F, Wang X, Liu J, Mao S. Effect of pelleted high-grain total mixed ration on rumen morphology, epithelium-associated microbiota and gene expression of proinflammatory cytokines and tight junction proteins in Hu sheep. Anim. Feed Sci. Technol. (2020) 263:114453. doi: 10.1016/j.anifeedsci.2020.114453
44. Xia G, Sun J, Fan J, Zhao F, Ahmed G, Jin Y, Zhang Y, Wang H. β-sitosterol attenuates high grain diet-induced inflammatory stress and modifies rumen fermentation and microbiota in Sheep. Animals. (2020) 10:171. doi: 10.3390/ani10010171
45. Thomas M, Webb M, Ghimire S, Blair A, Olson K, Fenske GJ, et al. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. (2017) 7:12257. doi: 10.1038/s41598-017-12481-6
46. Kim M, Morrison M, Yu ZT. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. (2011) 76:49–63. doi: 10.1111/j.1574-6941.2010.01029.x
47. Wei Y, Zhao CX, Li XW, Cong LX, Zhang H, Liu GW. Effect of different crude fiber source diets on rumen microflora of sika deer. Chin. J. Vet. Sci. (2018) 38:217–21. doi: 10.16303/j.cnki.1005-4545.2018.01.35
48. Qiao YJ. Effects of adding allium mongolicum regel essential oil in diets on production performance, apparent digestibility of nutrients and small intestine morphology and structure of mutton sheep. Master's Thesis. (2021) 43:24–29. doi: 10.13302/j.cnki.fi.2021.11.001
49. Chowdhury, Subrata, Mandal, Guru P, Patra, Amlan K. Different essential oils in diets of chickens: 1.Growth performance, nutrient utilization, nitrogen excretion, carcass traits and chemical composition of meat. Animal Feed Sci Technol. (2017) 12:2. doi: 10.1016/j.anifeedsci.2017.12.002
50. Torres RN, Paschoaloto JR, Ezequiel JM, da Silva DA, Almeida MT, et al. Meta-analysis of the effects of essential oil as an alternative to monensin in diets for beef cattle. The Veterinary Journal. (2021) 272:105659. doi: 10.1016/j.tvjl.2021.105659
Keywords: allium mongolicum regel, essential oil, growth performance, rumen fermentation, bacterial communities
Citation: Yaxing Z, Erdene K, Zhibi B, Changjin A and Chen B (2022) Effects of Allium mongolicum regel essential oil supplementation on growth performance, nutrient digestibility, rumen fermentation, and bacterial communities in sheep. Front. Vet. Sci. 9:926721. doi: 10.3389/fvets.2022.926721
Received: 23 April 2022; Accepted: 03 October 2022;
Published: 31 October 2022.
Edited by:
Rajib Deb, National Research Center on Pig (ICAR), IndiaReviewed by:
Yang Li, Northeast Agricultural University, ChinaAnusorn Cherdthong, Khon Kaen University, Thailand
Copyright © 2022 Yaxing, Erdene, Zhibi, Changjin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ao Changjin, Y2hhbmdqaW5hb0BhbGl5dW4uY29t
†These authors have contributed equally to this work
 Zhao Yaxing
Zhao Yaxing Khas Erdene1†
Khas Erdene1†