- 1Cellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
- 2Department of Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3School of Paramedical Sciences, Behbahan Faculty of Medical Sciences, Behbahan, Iran
- 4Shoushtar Faculty of Medical Sciences, Shoushtar, Iran
Brucellosis is a bacterial zoonosis caused by Brucella spp. which can lead to heavy economic losses and severe human diseases. Thus, controlling brucellosis is very important. Due to humans easily gaining brucellosis from animals, animal brucellosis control programs can help the eradication of human brucellosis. There are two popular vaccines against animal brucellosis. Live attenuated Brucella abortus strain 19 (S19 vaccine) is the first effective and most extensively used vaccine for the prevention of brucellosis in cattle. Live attenuated Brucella melitensis strain Rev.1 (Rev.1 vaccine) is the most effective vaccine against caprine and ovine brucellosis. Although these two vaccines provide good immunity for animals against brucellosis, the expense of persistent serological responses is one of the main problems of both vaccines. The advantages and limitations of Brucella vaccines, especially new vaccine candidates, have been less studied. In addition, there is an urgent need for new strategies to control and eradicate this disease. Therefore, this narrative review aims to present an updated overview of the available different types of brucellosis vaccines.
Introduction
Despite many studies conducted to eradicate brucellosis infection worldwide, the episodic situation of brucellosis is still worrying and ambiguous (1). Brucellosis is a bacterial zoonosis caused by microorganisms belonging to the genus Brucella. They are various pathogens of domestic and wild mammals, found inside the host. Brucella could multiply in professional and non-professional phagocytes and cause heavy economic losses and many diseases in humans. Controlling brucellosis is of great importance (2). Human brucellosis is caused by direct or indirect contact with various species of infected animals, notably cattle, sheep, goats, and swine. Thus, the wipeout of the illness in animals causes the eradication of human sickness (3). Since the late 1980's, the brucellosis epidemic has been growing rapidly in some countries and parts of the world, infecting over 60 species of wildlife, causing disease worldwide, and causing great economic damage to livestock (4). Humans could easily gain brucellosis through animals and their products, even though humans are not carriers of the disease. Brucellosis is a complex disease due to the diversity of Brucella active species that, despite causing species-specific disease syndromes, could sometimes cause cross-infection (5). From the beginning of the twentieth century, the study and research on the production of brucellosis vaccines have begun. The development of brucellosis vaccines has experienced inactivated, live-attenuated, and rough-attenuated vaccines. Inactivated vaccines were first developed to prevent the disease, then live-attenuated vaccines, which are more effective in terms of immunogenicity, were superseded to control brucellosis (6). Existing vaccines that are currently used could cause problems. For example, some of these vaccines could cause human infection and abortion in pregnant cows; however, despite some shortcomings, they play an essential role in preventing and controlling brucellosis. These vaccines are used all over the world. With the development of precise molecular techniques and an accurate understanding of the mechanism of Brucella pathogenesis, new genetically-engineered vaccines have been developed and replaced traditional vaccines to prevent and control brucellosis (7, 8). In this review, different types of brucellosis vaccines and their advances evaluated.
Live-Attenuated Vaccines
In recent decades, the most effective way to control brucellosis has been to vaccinate animals. Although vaccination of individuals living in brucellosis endemic areas, veterinarians, livestock, and laboratory personnel is essential, human vaccines have not yet been developed (9). Live-attenuated vaccines are the most effective vaccines used to control animal brucellosis (10). Due to the lower efficacy of inactivated and subunit brucellosis vaccines, multiple doses should be administered, whilst live-attenuated vaccines are less expensive and more effective and induce immunity through humoral and cell-mediated responses (9, 11). However, some drawbacks have been reported to the administration of live-attenuated brucellosis vaccines, including antibiotic resistance, interference with serological diagnostic tests, and residual virulence in animals and humans (10–12).
Live-attenuated vaccines have been broadly used against brucellosis, such as B. abortus strains S19, B. melitensis strain Rev1, and M5, and B. suis strain S2 derived as an attenuated phenotype by repeated in vitro passage of strain 2308. Numerous research on the effectiveness of these vaccines has been carried out in experimental animals and proven that vaccinated animals are effectively protected against wild-type (WT) bacteria. The main disadvantage of vaccine strains S19 and Rev1 is that the agglutinins induced by these vaccines persist in immunized animals for a long time and interfere with the standard serodiagnostic tests, even if the antibodies are produced by these two vaccines are durable. Therefore, it is difficult to distinguish between infected and vaccinated animals with the vaccine strain S19 or Rev1. Although the incidence rate of abortion is low, to overcome these defects, a safe and effective vaccine is needed (13–15). Another vaccine in this category is Brucella suis S2 vaccine, which is one of the brucellosis control programs in China. Studies show that this vaccine provides a good humoral and cellular immune response and protects against Brucella heterologous species (16), but has a limited host range (17).
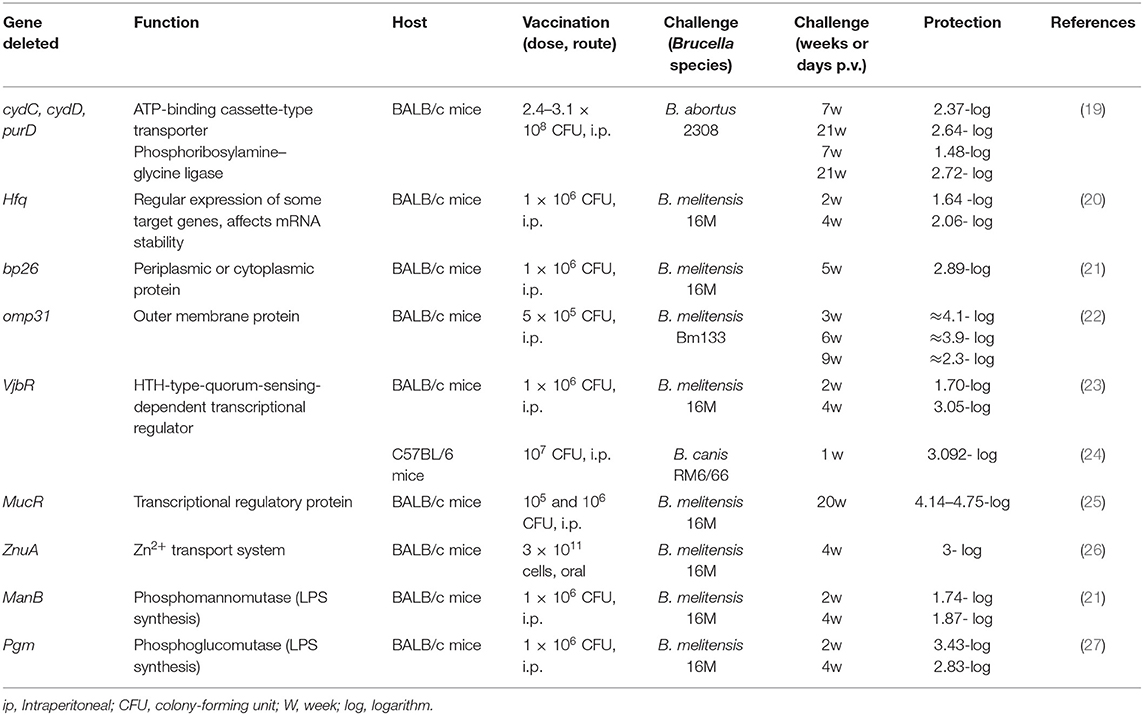
Identification of genes linked to virulence or survival of organism's aids to develop new vaccines that are both safe and protective. The best approach to developing new vaccines with minimal residual virulence is currently engineered live-attenuated vaccines based on deletions in virulence genes, which induce high safety levels compared to classical live-attenuated vaccines (18). A variety of vaccines are under development based on different deletions in B. abortus or B. melitensis virulence genes, which eventually result in significant attenuation and increased production of T cells, pro-inflammatory cytokines, and antibodies. There are many mutants listed in Table 1, which have been generated by attenuation of genes and confer protective responses against Brucella challenge in experimental animals.
Double-deletion (ΔcydCΔcydD and ΔcydCΔpurD) mutants of virulent B. abortus induce significant attenuation of virulence and long-term protective immunity. Sera collected from immunized mice with these strains were shown in a study to be associated with significant levels of IgG1 and IgG2a antibodies as well as Th1-type IFN-γ and Th2-type IL-10 cytokines; also, cytokine production was higher in these mice compared to RB51-immunized mice (19). Zhang et al. prepared B. melitensis 16M hfq (16MΔhfq) mutant strain which induced strong protective immunity, humoral responses especially IgG1 and IgG2a, and cellular responses with IFN-γ and IL-4 cytokine profiles; however, no significant difference in the production of IFN-γ and IL-4 was reported between 16MDhfq and Rev1 (20). Another study constructed a B. melitensis TcfSR promoter mutant (16MΔTcfSR) to introduce a vaccine candidate against B. melitensis infection. TcfSR is one of the two-component regulatory systems which allow host cells to detect environmental variations and respond appropriately to Brucella. Induction of a high level of protection and no interference with serodiagnostic tests were the main features of this candidate (28). The M5-90wboA mutant derived from B. melitensis M5-90 is a potential attenuated live vaccine and induces less virulence and inflammatory responses compared to its parental strains. The safety of this mutant is evaluated by the lack of splenomegaly in the host. Compared to the original strain, a higher level of protection is provided following vaccination with this mutant (95% survival). Also, another advantage of this mutant is the elicitation of an anti-Brucella-specific IgG response following vaccination, which is a diagnostic antigen for differentiation of immunization from infection (10). 16MDwzt as a rough mutant of B. melitensis, generated by the disruption of the wzt gene, which encodes the O-polysaccharide (O-PS) export system ATP-binding protein. The level of protection induced by this mutant against B. melitensis 16M challenge is similar to that conferred by the B. melitensis M5 vaccine. The two advantages of this vaccine are its safety in pregnant animals without inducing abortion as well as its ability to synthesize O-PS without inducing detectable specific antibodies in sheep, which make this vaccine candidate suitable for the eradication of animal brucellosis. The disadvantage reported for this vaccine is its more susceptibility to polymyxin B and complement-mediated killing compared to B. melitensis 16M (29). RM57 is the other Rough attenuated mutant that is generated from B. melitensis isolate M1981 has been administered in different animal models (both mice and 186 guinea pigs) and indicated good protective efficacy, especially in guinea pig model. Another advantage of this mutant includes no interference with serological diagnosis. The drawback of this mutant, which could be associated with its reduced virulence in mice and guinea pigs, is its sensitivity to polymyxin B (30). 2308DNodVDNodW rough vaccine originated from the virulent B. abortus 2308 (S2308) by deleting genes encoding a two-component regulatory system (TCS) in chromosome II in S2308. In a study, 2308DNodVDNodW showed significantly reduced survival in murine macrophages (RAW 264.7) and BALB/c mice. In this study, the mutant conferred levels of IgG antibody similar to those conferred by S19; also, a slightly higher level of protection was reported for single- and double-mutant NodVW. This mutant induced a mix of Th1- and Th2-type immune responses as well as strong humoral and cell-mediated immunity in immunized mice. Furthermore, this mutant persisted for a short time in RAW 264.7 macrophages and BALB/c mice. Another advantage of this vaccine is the provision of an ideal diagnostic antigen that could be used to differentiate immunized animals from infected ones (12).
B. ovis ΔabcBA (BoΔabcBA) vaccine, which has been tested in two formulations (encapsulation with alginate and alginate plus vitelline protein B—VpB), is effective for immunization of mice against B. melitensis strain 16M by inducing Th1 (T helper1)-mediated immune responses. Due to its efficacy, the hypothesis of conferring protection against virulent B. melitensis in small ruminants could be supported. Also, this vaccine could be administrated for caprine and ovine brucellosis due to B. melitensis infection. In rams, this vaccine has an additional advantage, including conferring protection against B. ovis, which is another Brucella species that commonly infects sheep; immunization with BoΔabcBA against B. ovis is highly protective (31). In another study ΔabcBA vaccine could prevent the infection, the secretion of wild-type B. ovis in semen and urine of rams, the shedding of neutrophils in semen, and the development of clinical changes and gross lesions induced by wild-type B. ovis. This vaccine could induce both humoral and cellular immune responses (32). In a study conducted by Sancho et al., administration of B. ovis attenuated mutants (Δomp25d and Δomp22) and B. melitensis Rev1 vaccines were compared in mice. The study indicated that mice vaccinated with B. ovis mutants developed higher serum levels of anti-B. ovis antibodies of IgG1, IgG2a, and IgG2b subclasses as well as IL-1α, as an enhancer of T cell responses to antigen, compared to Rev1-vaccinated mice. Immunization with B. ovis mutants indicates appropriate persistence, limited splenomegaly, and protective efficacy against B. ovis. Also, B. ovis mutants vaccine candidates would likely be the most appropriate vaccines against ram contagious epididymitis (33).
VTRS2 is the other type of rough vaccine which is originated from B. suis. This vaccine was constructed by deletion mutations in genes wboA (encoding glycosyltransferase) and leuB (encoding isopropyl malate dehydrogenase). The strain VTRS2 expressing mGnRH can elicit a significant IgG immune response against the mGnRH antigen at 4 and 6 weeks post-inoculation. The rough B. suis strain is an effective vaccine candidate in swine (34). B. suis Δ pgm could stimulate cellular immune responses and induce good levels of protection against the virulent B. suis strain, abortion, heifer colonization, and bacterial excretion in milk. Also, using this strain, immunized animals could be differentiated from infected ones. Due to the lack of lipopolysaccharide and the inability to synthesize cyclic beta-glucans, this strain is sensitive to detergents and polymyxin B (35). Compared to the smooth vaccine, the rough mutant strain of B. neotomae stimulates further activation of dendritic cells in vitro and confers protection against the heterologous challenge by B. suis in mice (36).
B. abortus 2308 ery promoter mutant (Δery) safety is evaluated by the lack of splenomegaly in inoculated mice. This vaccine has good protective efficacy and could induce the secretion of higher levels of IFN-γ and IL-4 compared to S19. Post-vaccination humoral responses provide an ideal diagnostic EryA antigen for the differentiation of immunization from infection using EryA-iELISA. Also, sensitivity to erythritol and reduced survival in macrophages and BALB/c mice could be observed in this vaccine (37). IVK15ΔcydD and IVK15ΔcydC mutants are created by deleting only cydD and cydC genes, encoding ATP-binding cassette transporter proteins, from the chromosome of the virulent B. abortus strain isolated from Korean cow (referred to as IVK15). Mice immunization with these mutants could protect them against the virulent B. abortus strains and S2308. Also, higher levels of anti-Brucella-specific IgG, IgG1, and IgG2a antibodies and higher levels of IgG2a than IgG1 could be observed in immunized mice compared to unvaccinated mice. Splenomegaly is a consequence of inflammatory responses is not observed in immunized mice with IVK15ΔcydD and IVK15ΔcydC. Both mutants exhibit increased sensitivity to metal ions, acidic pH, and hydrogen peroxide, which resemble the intracellular environment during host infection (11). The B. abortus S2308 mutant strain Δ22915 is constructed by deleting the putative lytic transglycosylase gene BAB_RS22915. This mutant induces an effective immune response with fewer inflammatory responses. Higher levels of antibody and better protection against B. abortus S2308 are induced by Δ22915 mutant compared with RB51 (12). Several mutants listed in Table 2, such as ΔmucR and ΔvjbR, have been studied to evaluate the level of protection and the ability to induce humoral and cellular responses (23–25). Understanding the immune responses and protective mechanisms against Brucella infection is important for the development of an effective vaccine. T-cell subsets and antibody responses are necessary to confer protection against virulent stains. Cytokine profiles, including TNF-α, IFN-γ, IL-1, and IL-12, contribute to controlling Brucella infection in its early stages. Therefore, inducing a high level of immune system responses contributes to the effectiveness of vaccines and should be considered in vaccine development (14). The main features of these vaccine candidates are mentioned in Table 2. However, in all the reviewed studies, the positive aspects of these candidates have been mentioned, but the drawbacks of these candidates must also be considered, including not complete elimination of persistent strains (44) or the risk of spreading antibiotic resistance in cloning procedures. In addition, they should be evaluated in livestock and trial studies (45).
Vector Vaccines
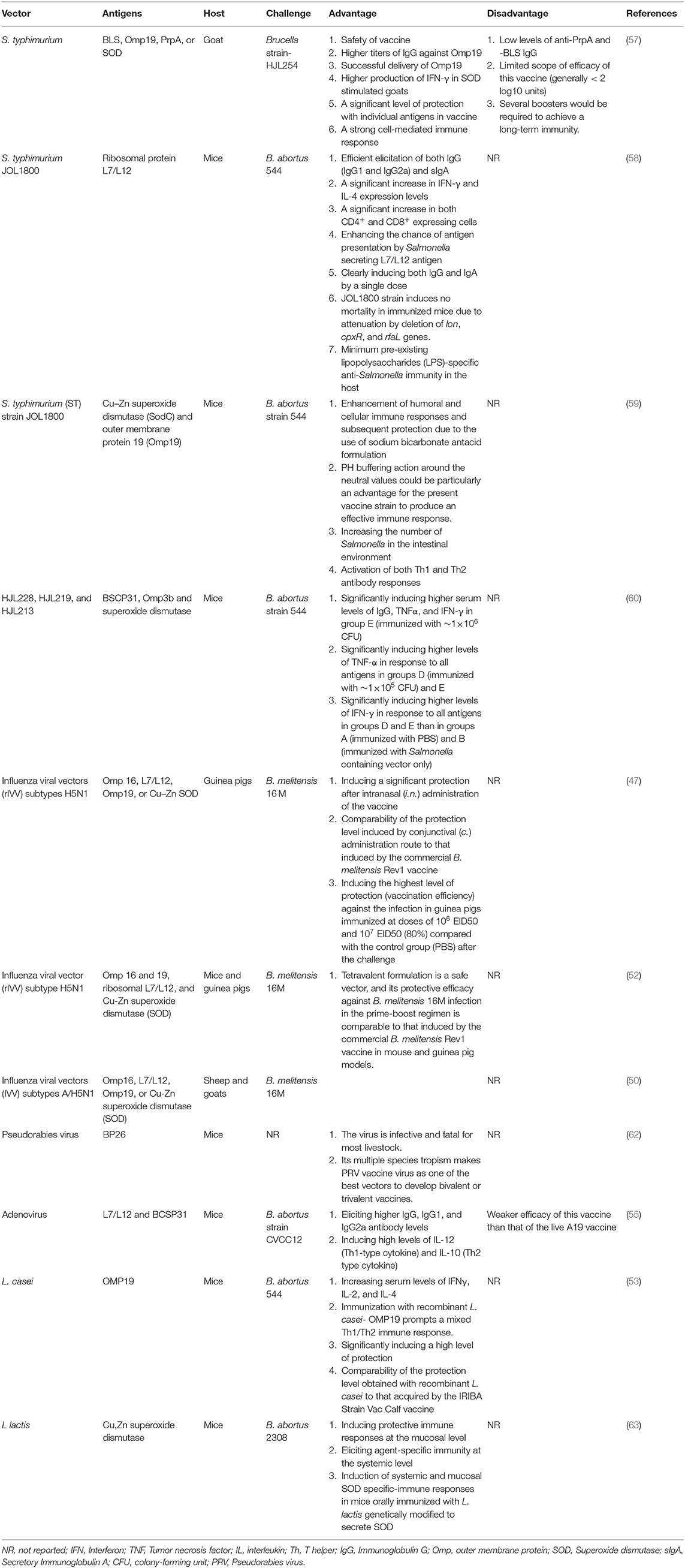
Recently, various viral or bacterial vector-based Brucella vaccines have been fostered as efficient delivery systems to deliver different heterologous or homologous antigens (46). They are live vector-based genetically modified vaccines (47). Cell-mediated immune responses induced by intracellular organisms may represent that the best choice is to present Brucella antigens to the immune system of the target host; the main goal of these candidates is to promote the formation of an antigen-specific T-cell immune response (48). These types of vaccines replicate in the host cell, producing multiple copies of the Brucella antigen (49). There are various bacterial or viral vectors for the expression of Brucella proteins, including Lactococcus Lactis, Escherichia coli, Salmonella strains, or influenza virus (47, 50). Each of these vectors has several advantages and disadvantages. Salmonella, as an intracellular pathogen, delivers antigens effectively to antigen-presenting cells such as macrophages. Other advantages of using Salmonella as a vector include inherent adjuvant effect, adequacy of a single-dose vaccination to obtain long-lasting immunity, the ability to multiply and present multiple antigens, and dynamic entrance into the natural barrier protecting antigens from host degenerative enzymes. Some research studies have indicated that multiple infections caused by Salmonella could lead to increased disease outcomes in infected animals. The potentiation of this pathogenesis may be due to the immunomodulatory effect of Salmonella, which inhibits or delays the host immune response and promotes systemic Salmonella infection. In acute conditions, salmonellosis could also cause miscarriage and death, which could lead to reduced animal productivity (51). Influenza viral vectors (IVV) have also been developed due to the lack of pre-existing immunity against H5N1 influenza virus in the human population (47). There is a confirmed IVV-based B. abortus vaccine (Flu-BA) developed in Kazakhstan for cattle vaccination; although bovines are not highly susceptible hosts to influenza A virus infection, and there is a natural immunity to influenza infection in this host. However, it could be more effective for humans because influenza A is a common human infection. There is widespread concern about the use of IVV of the H5N1 subtype, which is a pathogenic influenza virus spreading in poultry. The main concern is related to the interspecies transmission of the disease from birds to humans, which could lead to human disease. Although the replication capacity of this virus has been limited in this vaccine by eliminating the proteolytic cleavage site in HA, the risk of pandemic strains must be considered (52). Lactic acid bacteria (LAB) are also considered a desirable antigen delivery system for mucosal immunization. It has been reported that L. casei-based vaccines show a protective response against challenges (53). Recently, mucosal vaccination has been considered because the main route of natural transmission of brucellosis is usually through mucosal exposure. One of the disadvantages of using live LAB-based mucosal vaccines is related to the risk of spreading genetically engineered organisms carrying drug resistance markers to the environment and the host flora. In addition, L. lactis strains are considered to be non-colonizing bacteria that survive when passing through the gastrointestinal tract (GIT) and trigger immune responses when taken up by M cells (54). Adenovirus-based vaccines are another type of vector vaccine with several disadvantages including high levels of pre-existing immunity, transient expression of the transgene, and highly immunogenic (55). Moreover, due to the complexity of the target pathogen, multiple antigens are required to enhance effective immune responses, which incur more clinical evaluations and higher manufacturing costs (56).
Different antigens are used for developing this type of vaccine, such as proline racemase subunit A (PrpA), Cu/Zn superoxide dismutase (SOD), Brucella lumazine synthase (BLS), lipoprotein outer-membrane protein 19(Omp19) (57), and ribosomal protein L7/L12 (58). These antigens efficiently induce immune responses restricting the pathogen in the early stages of infection. The function of antigen-presenting cells such as macrophages and dendritic cells is to stimulate the production of specific antibodies, T cells responses such as CD4+ and CD8+, and the secretion of cytokines involved in bacterial resistance and elimination. BLS, Omp19, PrpA, and SOD could efficiently induce the secretion of Th1-type cytokines. PrpA also stimulates B cell responses. Omp19 induces Th1 responses and mouse dendritic cell maturation. In a study, an attenuated S. typhimurium strain expressing BLS, Omp19, PrpA, or SOD of B. abortus in goats was shown to elicit strong cell-mediated immune responses against PrpA, BLS, Omp19, and SOD, but greater humoral responses were elicited against Omp19 and SOD. This type of vaccine could provide a high level of protection for individual groups. Regardless of high protection, this type of vaccine requires multiple boosters and adjuvants to obtain long-lasting immunity, but without affecting bacterial viability (57). Brucella ribosomal protein L7/L12 has a high antigenicity due to the dominant epitopes. The combination of protein L7/L12 with Salmonella delivery system (JOL1800 strain) induces humoral and cell-mediated immune responses. High numbers of stimulated cells, including CD4+ and CD8+ T cells, and the production of IFN-γ have been reported in L7/L12-immunized mice. Besides the high antigenicity of L7/L12, the JOL1800 strain has a high level of safety, and a single dose of vaccine effectively eliminates the pathogen (58). Oral administration of attenuated Salmonella strain secreting Brucella antigens, including Cu–Zn superoxide dismutase (SodC) and outer membrane protein 19 (Omp19), with sodium bicarbonate antacid, significantly induces the secretion of a high level of systemic IgG and a mixed Th1–Th2 response. The rate of Salmonella colonization following the development of this type of vaccine has increased, stimulating protective immune responses (59). Attenuated Salmonella strains expressing B. abortus BCSP31, Omp3b, and superoxide dismutase proteins have also been investigated as a vaccine candidates (60).
Numerous recombinant viral vector vaccines have been evaluated so far. In a study, an influenza viral vector of the H5N1 subtype, as a non-replicable viral vector, expressing Brucella Omp16, L7/L12, Omp19, and Cu–Zn SOD immunodominant proteins was investigated in guinea pigs against human brucellosis. Although no immune response was reported in this study, different administration routes and vaccine doses were evaluated. To determine the best immunization route, different routes were evaluated, such as conjunctival (c.), intranasal (i.n.), and sublingual (s.l.). A significant protective effect was reported for this vaccine when administered through i.n. (2.8 log 10) and c. (2.3 log 10) administration routes, comparable to B. melitensis Rev1 vaccine results; also, the optimum dose conferring a high level of protection was determined to be 106 EID50 and 107 EID50 (47). Recently, Bugybayeva et al. suggested the tetravalent vaccine formulation Flu-NS1-80-Omp16+Flu-NS1-80-L7/L12+Flu-NS1-80-Omp19+Flu-NS1-80-SOD to develop a safe and effective human vaccine. In this study, a recombinant influenza viral vector (rIVV) of H5N1 subtype, expressing Brucella L7 / L12, Omp16, Omp19, or Cu-Zn SOD immunodominant protein containing a sequence of 80 N-terminal amino acids from the open reading frame (ORF) of the NS1 gene, was evaluated. The results of this study indicated that this formulation had a high level of safety and efficacy. This research is an important report on the development of a safe and protective vaccine against human brucellosis (52). In another study, recombinant influenza A viruses of the subtypes H5N1 and H1N1, expressing L7/L12 or Omp16, were developed and shown to elicit Th1 CD4+ and CD8+ T-cell immune responses and confer good protection against challenge (61). The expression of BP26 as a highly conserved immunogenic protein in Brucella by pseudorabies virus was also screened as a vaccine candidate in another study by Yao et al. This type of vaccine can induce humoral and cellular immune responses. The extensive tropism of this vaccine makes it a suitable vector (62). Guo-Zhen et al. reported that Adenovirus-LL/BP vaccinated mice had higher levels of IgG, IgG1, and IgG2a antibodies. Their study results indicated that this vaccine-induced primarily cellular and partially humoral immunity and provided a mild protection level against B. abortus infection. Although this type of vaccine conferred significant protection against challenge, the level of protection was lower compared to the live A19 vaccine (55). As mentioned earlier, probiotics such as L. casei are considered as a vector to elicit a good immune response and a high level of protection comparable to that induced by the IRIBA Strain Vac Calf vaccine. L. casei strains expressing the outer membrane protein OMP19 prompt Th1/Th2 immune responses and the production of IFN-γ, IL-2, and IL-4. As Brucella is an intracellular pathogen, cell-mediated immune responses are required to control the pathogen. Therefore, immunodominant antigens should be considered in developing new vaccines to stimulate cellular immune responses. In this regard, the production of cytokines such as IFN-γ, IL-2, and IL-4 is critical (53). The initial step of infection occurs in mucosal areas; thus, mucosal vaccination could be done to elicit a good response. In this context, mucosal administration of L. casei or L. lactis vector vaccines, generally regarded as safe, is a potential vaccine delivery approach. It has been suggested that the danger of eliciting immunological tolerance may also be faded compared with the persistent strains (63). Although other viral and bacterial vectors have been investigated, it should be noted that to introduce a safe vaccine, the non-pathogenicity of organisms must be proven (Table 3).
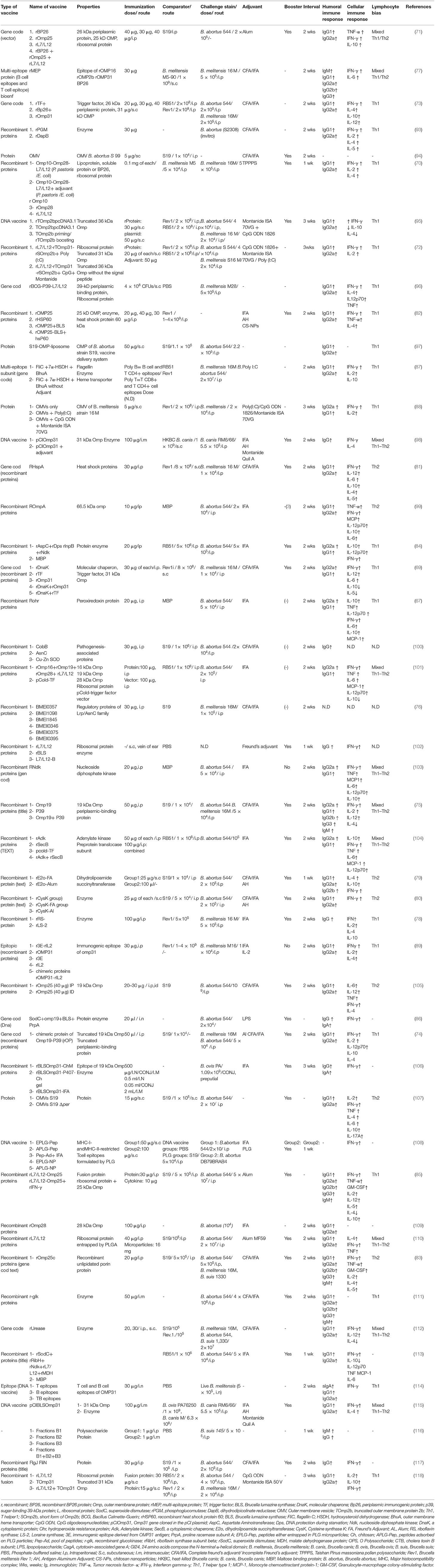
Subunit Vaccines
Brucellosis is a chronic zoonotic disease that is mainly transmitted from animals to humans and could pose significant risks to public health and safety. Brucella spp. but only is an intracellular pathogen that survives within neutrophil leukocytes without inducing significant activation, also strongly resistant to the bactericidal action of antimicrobial peptides and serum (64). Thus, the successful development of brucellosis vaccines is a major challenge. Vaccination is a major policy decision to prevent both animal and human brucellosis. The subunit vaccines are promising vaccine candidates due to their safety profile, well-defined non-infectious nature, inability to revert to a virulent strain, non-viability unlike attenuated vaccines, ability to induce the production of high levels of antibody, and capability of manipulation to maximize desirable activities. The formulation of these vaccines is the use of a recombinant highly-conserved protein that could affect multiple Brucella species. However, they could not replicate and mimic a natural Brucella infection (tissue and cell tropism) and therefore provide a lower protective efficacy compared with live-attenuated vaccines (65). The poor antigenicity, instability, and short half-life of recombinant subunit antigens are the main impediments in the design of an effective subunit vaccine against brucellosis (66). In this context, the use of adjuvants, immunomodulators, antigen delivery systems, or TLR (toll-like receptor) ligands is necessary to enhance well-balanced immune responses. The type of induced immune response depends on the type of antigen and adjuvant used in recombinant Brucella protein vaccines. Freund's adjuvant (the most commonly used adjuvant), Alum adjuvant, and aluminum hydroxide (the only adjuvant licensed for use in human vaccines) generate Th2-type immune responses, while monophosphoryl lipid A and CpG induce Th1-type responses. To screen and evaluate protective antigens, a combination of an appropriate antigen, adjuvant, booster, and delivery vehicle/vector is needed to trigger a strong protective immune response, such as the Th1 immune response as the dominant immunity against brucellosis (44). For the development of an effective vaccine against intracellular pathogen represented by Brucella, the production of Th1- derived cytokines (IL-12, TNFα, IFNγ) as well as the activation of macrophages, dendritic cells, and CD4+ and CD8+ T cells are the key factors for the clearance of infection; whereas Th2 immune responses, which are induced by the humoral immune system, have a minor role in the clearance of infection (67). Cytokines play a main role in the development, maturation, differentiation, and activation of immune cells. For instance, IL-4 (Th2 cytokines) induces IgG1 antibody formation by differentiation of naive CD4+ T cells into Th2 cells, whereas IFN-γ (Th1 cytokines) induces IgG2 antibody formation by differentiation of naive CD4+ T cells into Th1 cells (68). IL-10 is an immune-regulatory cytokine that induces the balance of Th1 or Th2 immune responses to prevent over activity of the immune system and limit further tissue damage (69). Numerous cell surface and intracellular components could be expressed by E. coli and serve as protective antigens in mouse models, such as outer Omp2b, OMP16, OMP19, L7/L12 ribosomal protein (70–72), Omp31 (73), outer membrane protein Omp25 (71), p39 (a putative periplasmic binding protein) (74, 75), AsnC (76), Omp16 (77), lumazine synthase (78), rE2o (79), rCysK (80), DnaK (81), chimeric protein from OMP19 and p39 domains (75), OMP25-BLS fusion protein (82), OMP25c protein mixed with freund's adjuvant (83), and AspC, Dps, lnpB, and Ndk (84); however, none of them have shown a successful clearance. Previous studies have shown that combining several recombinant proteins which generate a wide array of immunogenicity could induce stronger immune responses and better protection against Brucella than their univalent counterparts (74, 85, 86). Also, several studies have shown that subunit vaccines could induce protection levels and immune responses similar to those induced by live or attenuated vaccine strains (69, 72, 73, 83, 84, 87–89). At the same time, other studies have not observed such findings (90). There is a wide range of factors influencing immune responses and protection induced by vaccination in the mouse model, including intrinsic host factors (sex, age, and type of mice), vaccine factors (such as vaccine type, adjuvant type, number and dose of vaccination), administration factors (schedule, site, route, time of vaccination), and challenge factors (challenge pathogen strain, route, challenge-killing interval, time interval between vaccination and challenge and/or between challenge and assessment of splenic bacterial loads) (91, 92). Although subunit vaccines have the advantage of safety, they require multiple boosters and a combination of several antigens, adjuvant, and delivery vehicle/vector to induce an effective immunity and protection against brucellosis in cattle, which isn't economically viable (44). Moreover, it is important to consider those immune responses elicited in mice may not accurately reflect the protection and immune responses elicited in natural hosts after vaccination. Therefore, more extensive studies are needed to identify new recombinant vaccines containing more than one Brucella antigen. Unfortunately, no successful subunit vaccine for brucellosis has been developed so far despite many efforts (Table 4).
DNA Vaccines
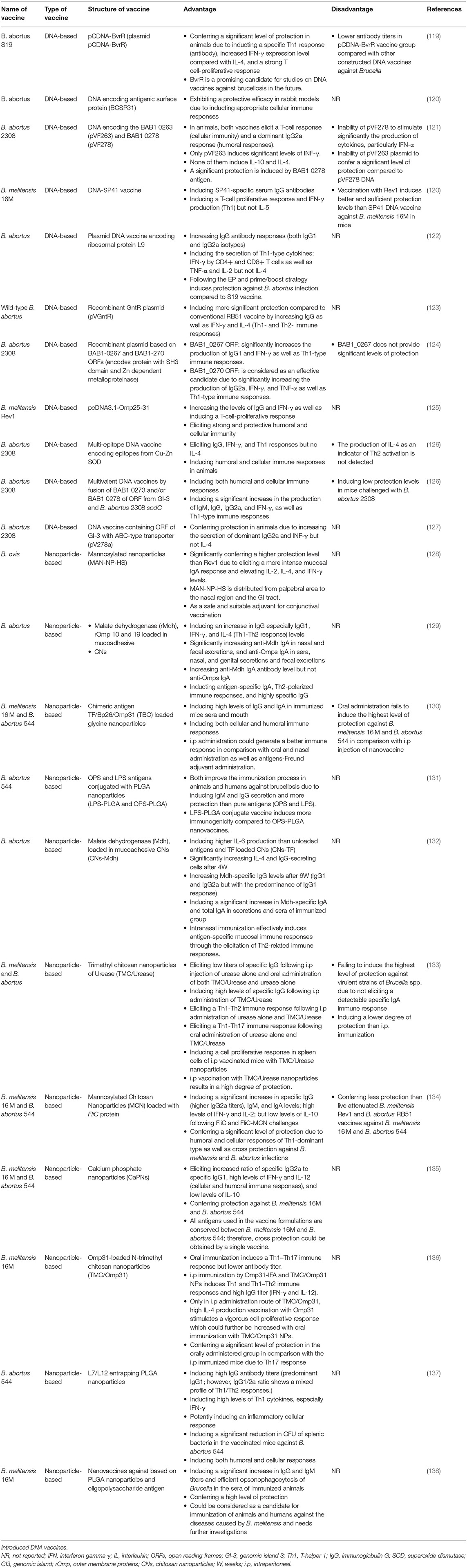
DNA-based Brucella vaccines are a kind of subunit vaccine which stimulated immune responses following multiple doses (Table 5) (18). These vaccines are safe and efficient brucellosis vaccines due to the stimulation of strong cellular immune responses, expression of several antigens, the existence of CpG motifs, and simple storage conditions (139). DNA-based vaccines contain gene sequences of pathogens, which are essential for intracellular survival of Brucella spp. The immunogenicity and efficacy of these virulence genes used in DNA vaccines have been demonstrated in animal studies, including the two-component BvrR/BvrS system (119), Cu-Zn superoxide dismutase (SOD) (126, 140), ribosomal L7/L12 or Brucella lumazine synthase (BLS) (139, 141), B. melitensis omp31 and omp25 genes (125, 142), antigenic surface protein (BCSP31) gene (120), SP41 (143), and ribosomal protein L9 (rL9) (122). According to the studies that have been done, DNA vaccines may have the ability to resolve the disadvantages of other brucellosis vaccines (119, 120, 144). In most studies, animals vaccinated with different types of DNA vaccines have shown full protection against virulent strains (e.g., B. abortus S19, B. abortus 2308, B. melitensis 16M, and B. melitensis Rev1) (120, 143).
Intramuscular (i.m.) administration of DNA-based vaccine has been shown to induce a protective immune response as similar as Rev1 in different animal model studies (125, 143, 145, 146). Jain et al. demonstrated that the electroporation (EP) approach induced further protective responses than the i.m. route (122). A combination of several suitable antigens, such as L7/L12, BCSP31, SOD, P39, and omp16, could be used to make a “divalent or poly-antigenic DNA vaccine,” which has been reported to be effective due to more antigenic components, induction of a wide range of humoral and cellular immune responses, and simulation of the most similar status to Brucella infection (78, 126, 143, 147–149).
Same as subunit vaccines, DNA-based brucellosis vaccines can stimulate both humoral and cellular immune system arms, TCD4 and TCD8 helper cells, as well as a significant increase in IFNγ, TNF-α, and IL-12 levels (122), which IFNγ exerts a protective effect by boosting macrophage activity (150).
However, several publications have shown no changes in the expression of IL-4, IL-10, and IFN-γ following DNA vaccine administration which may be related to the suppressive function of Treg in preventing IFN-γ development (151–154). DNA-based vaccines do not provide significant levels of protection compared to live-attenuated vaccines. This is consistent with the finding of Kurar et al., Leclerq et al., and Schurig et al. studies which indicated that a lower immune response and no protective response was observed following the administration of DNA based vaccine against Brucella challenge (7, 155, 156). It may happen due to the inability of the vaccine to express specific antigens such as GroEL-Hsp antigen in PcDNA3-DNA vaccine (7). The need for repeated booster doses administration in response to the rapid silencing of genes, is the main reason for the loss of long-term protective response which could be improved using an adjuvant. This result is in line with the finding of a study by Velikovsky et al., demonstrating that following repeated vaccination with PcDNA3 containing the BLS gene, a protective response was induced in mice in addition to the production of IgG2a (157). Therefore, despite the expression of protective antigens, DNA-based vaccines may unable to express antigens in high amounts, and today efforts are made to delay gene silencing for a longer time.
Nanoparticle Based Vaccines
Nanoparticles (NPs) containing Brucella vaccine induce antibody responses (IgM, mucosal IgA, and IgG) (129, 130), increase IFN-γ, IL-12, IL-4, and IL-6 levels, and decrease IL-10 levels (134, 135) in animal model studies (Table 5). Most studies have reported increased IgG1 level linked to the Th2 response, compared to IgG2 level (129, 134, 135, 137) which is linked to the Th1 response (135). Nanoparticle-based vaccines cannot be used to vaccinate humans against brucellosis due to the risk of disease (138), however, oral vaccines show more benefits in an animal model study (133). In addition to a Th1-Th2 response (129, 130, 132), oral administration of NP-based vaccines induces a Th1-Th17 response which is stronger and suitable for controlling brucellosis. Despite many advantages of oral vaccines over intraperitoneal (i.p) vaccines, including ease of preparation, painless administration, and a stronger Th1-Th17 response induction (133, 158), they are less effective in inducing antibody responses, especially IgA. Relative toxicity, limitation in both antigen loading and vaccine production as well as weak stimulation of the immune system are the most disadvantages of nanoparticle-based vaccines (159, 160). According to animal model studies, a decrease in the number of CFUs of splenic bacteria is observed following NP-based vaccines administration, indicating that these vaccine have the potential to induce protection against brucellosis. The immune response induced by NPs depends on their uptake by antigen-presenting cells (APCs) and their particle size and charge (137). A powerful protective response needs a combination of Th1 and IgA responses (135).
Mannosylation of nanoparticles in the MAN-NP-HS vaccine candidate aids nanoparticles to reach directly mannose receptors that are abundantly expressed on the surface of immune cells and are important in antigen uptake. Following administration of the MAN-NP-HS vaccine, a mixture of mucosal IgA antibodies and Th1-Th2 cytokines including IL-12, IL-4, and IFN-γ is produced, of which IFN-γ plays a critical role in inducing cellular immunity. According to these findings, MAN-NP-HS provides even more protection than Rev1 due to the induction of more specific IgA (131). This vaccine, which is administered through the eye (palpebral), shows no side effects or inflammation. Moreover, the release of the MAN-NP-HS vaccine from the palpebral to the nasal mucosa and GI tract leads to greater protection (36). Another candidate is a combination of LPS and OPS antigens with PLGA nanoparticles, which has the potential to induce strong protection in animals and humans by producing IgM and IgG antibodies. These antigens alone are not effective in inducing immunity, but when combined with nanoparticles, they produce more antibodies (156). Most effective nanovaccine candidates induce a significant reduction in bacterial load in splenocytes, Th1 response, and antibody response, especially mucosal IgA. Choosing an antigen that is protected between two pathogenic strains is critical because it contributes to the induction of cross-protection against both strains following vaccination (135).
Other Vaccines
Brucella dual vaccine is a new approach to the development of a Brucella-based vaccine platform of immunogenic antigens, oriented to simultaneously control the transmission of two important bacterial pathogens from cattle to humans. In a study by Abedi et al., the use of a total TN-OMP (outer membrane vesicles of Brucella) conjugated with rCagA (recombinant protein of Helicobacter pylori) was evaluated, and the results revealed that rCagA as an adjuvant increased the immune response against TN-OMP. Thus, this combination vaccine was effective in inducing simultaneously serum bactericidal and splenic activities of B. abortus and H. pylori in BALB/c mouse model (161). Similarly, Bahador et al. showed that subcutaneous immunization of mice by conjugated rCagA with Brucella LPS (rCagA+ LPS) induced protective effects. Iannino et al. designed the Bab-pgm strain (genetically engineered live B. abortus vaccine) as a heterologous carrier for the recombinant chimeric antigen to deliver Shiga toxin-producing E. coli (STEC) in a mouse model, which resulted in the induction of a protective immune response against two very different pathogens (162). Another approach to vaccine development is the use of a modified Brucella immunodominant antigen instead of deleting Brucella antigens or epitopes or introducing a foreign antigen, which could induce differential antibodies against B. ovis (163). Another approach to vaccine production is polysaccharide conjugate vaccines which are produced via the covalent glycan-protein conjugation of bacterial surface; they have been proven to be cost-effective tools to prevent dramatic infectious diseases. It has been demonstrated that OPS of B. abortus could be expressed in Yersinia enterocolitica O:9 and displayed on CTB via glycosylation, eliciting an antigen-specific immune response and a significant protection level against brucellosis (164). Antigen-delivery systems, such as attenuated viruses or bacteria, are essential for presenting B. abortus immunogenic antigens to the immune system cells. Recently, Lin et al. designed an adenoviral vector expressing both p39 and lumazine synthase proteins of B. abortus, which elicited significant humoral and cellular immune responses, although pre-existing immunity against adenovirus could prevent a vaccine from working (165). There are several studies using liposomes as Brucella antigen-delivery systems. Liposomes are not only widely used as a carrier to improve vaccine efficacy and efficiency in the transport of antigens to appropriate sites but also possess adjuvant properties against bacteria (166). Goel et al. showed that liposome-encapsulated recombinant Omp25 induced a protective immune response comparable to that induced by S19 in a mouse model (167). In another study, subcutaneous co-administration of Brucella Cu-Zn SOD recombinant protein with recombinant IL-18, encapsulated in E. coli lipid liposome (escheriosome), demonstrated a stronger IgG2a-type antibody response in immunized mice compared with free BaSOD DNA. Another approach to vaccine development against Brucella infection includes lysed B. abortus (168, 169) or whole organism of Brucella spp. without cytoplasmic contents. The bacterial-ghost (BG) technology is the use of biologically killed Gram-negative bacterial cells produced via controlled expression of the cloned lysis gene E of X174 bacteriophage. BGs are potential envelope structures lacking cytoplasmic contents, which act as a delivery system and an efficient adjuvant for DNA- and protein-based vaccines. In the case of Brucella, it has been reported that Brucella S2 ghosts effectively elicit a pathogen-specific antibody response, enhancing IgG antibody and T cell responses in mice compared to inactivated bacteria (170). Kwon et al. used a fragment of PMPA-36 (porcine myeloid antimicrobial peptide 36), named GI24, for B. abortus lysis and produced B. abortus ghosts, termed as B. abortus lysed cells (171). Due to the lack of genetic materials in BG vaccines, the horizontal transfer of antibiotic resistance genes or pathogenic islands to the resident gut flora does not occur.
Future Trends of Brucellosis Vaccines
There are many efforts for the development of new vaccines, safer and more effective based on new technologies such as the engineered live-attenuated vaccines based on deletions in virulence genes, and viral or bacterial vector-based Brucella vaccines, subunit vaccines, DNA vaccines, Nanoparticle-based vaccines. The majority of these vaccines designed with regard to new technologies showed the enhanced immune responses and protective immunity against brucellosis in mice, livestock, and guinea pig models. For example, Bugybayeva et al. (52) indicated that the tetravalent vaccine formulation Flu-NS1-80-Omp16+Flu-NS1-80-L7/L12+Flu-NS1-80-Omp19+Flu-NS1-80-SOD protected guinea pigs from B. melitensis 16M infection at a significant level (P < 0.05). Thus, this vaccine can be chosen as a potential vaccine candidate for further development of an effective human vaccine against brucellosis.
The subunit vaccines are promising vaccine candidates due to their safety profile, well-defined non-infectious nature, inability to revert to a virulent strain, non-viability unlike attenuated vaccines, and capability of manipulation to maximize desirable activities. However, they indicated some disadvantages such as the poor antigenicity, instability, and short half-life of recombinant subunit antigens. Hence, the use of adjuvants, immunomodulators, and antigen delivery systems, or is necessary to enhance immune responses. For these reasons, already despite many efforts, no successful subunit vaccine has been developed for brucellosis livestock (172). On the other hand, DNA vaccines are one of the methods employed for developing a safe and efficient brucellosis vaccine due to stimulation of cellular immune responses and expression of several antigens; however, they do not induce significant levels of protection due to the lack of a long-term protective response (157).
Out of vaccines with new technologies, the engineered live-attenuated vaccines based on deletions in virulence genes have accounted as the best approach for developing new vaccines with minimal residual virulence, due to the induction of high safety levels compared to classical live-attenuated vaccines. A variety of these vaccine types are under development based on different deletions in B. abortus or B. melitensis virulence genes, which eventually result in significant attenuation and increased production of T cells, pro-inflammatory cytokines, and antibodies (19). Hence, they can be considered a promising vaccine candidate for human use.
Conclusion
To date, no vaccine licensed against human brucellosis is available. Hence, the control of human brucellosis has relied heavily on the control of animal brucellosis by vaccination. Live-attenuated vaccines such as B. abortus strains S19 and B. melitensis strain Rev1 as the two most common anti-brucellosis vaccines have been widely used in the world for the immunization of animals. However, they had some drawbacks, such as the induction of abortion in pregnant animals, the virulence for humans, the production of anti-Brucella antibodies interfering with serodiagnosis, and the antibiotic resistance against brucellosis treatment. Two factors should be considered in designing an effective brucellosis vaccine: the route of vaccine administration and the design of the vaccine to induce cell-mediated immunity which is the most important component of the immune system in inducing defense. It appears that of the brucellosis vaccines, the live attenuated vaccines that some of their genes have been deleted are more effective. They can increase the production of T cells, pro-inflammatory cytokines, and antibodies. Therefore, they can be considered a promising brucellosis vaccines.
Author Contributions
MH, SD, RG, MM, NB, AD, TN, and MT contributed in revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Boschiroli M-L, Foulongne V, O'Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol. (2001) 4:58–64. doi: 10.1016/S1369-5274(00)00165-X
3. Vershilova P. The use of live vaccine for vaccination of human beings against brucellosis in the USSR. Bull World Health Organ. (1961) 24:85.
4. Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents. (2010) 36:S8–11. doi: 10.1016/j.ijantimicag.2010.06.013
5. Stear MJ. OIE manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees) 5th edn. Volumes 1 & 2. World Organization for Animal Health 2004. ISBN 92 9044 622 6.80 140. Parasitology. (2005) 130:727. doi: 10.1017/S0031182005007699
6. Montaraz J, Winter A. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. (1986) 53:245–51. doi: 10.1128/iai.53.2.245-251.1986
7. Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. (2002) 90:479–96. doi: 10.1016/S0378-1135(02)00255-9
8. Yang X, Skyberg JA, Cao L, Clapp B, Thornburg T, Pascual DW. Progress in Brucella vaccine development. Front Biol. (2013) 8:60–77. doi: 10.1007/s11515-012-1196-0
9. Mansoori N, Pourmand MR. Vaccines and vaccine candidates against brucellosis. Infect Epidemiol Microbiol. (2016) 2:32–6. doi: 10.18869/modares.iem.2.4.32
10. Li Z-Q, Shi J-X, Fu W-D, Zhang Y, Zhang J, Wang Z, et al. A Brucella melitensis M5-90 wboA deletion strain is attenuated and enhances vaccine efficacy. Mol Immunol. (2015) 66:276–83. doi: 10.1016/j.molimm.2015.04.004
11. Truong QL, Cho Y, Park S, Park B-K, Hahn T-W. Brucella abortus mutants lacking ATP-binding cassette transporter proteins are highly attenuated in virulence and confer protective immunity against virulent B. abortus challenge in BALB/c mice. Microbial Pathogenesis. (2016) 95:175–85. doi: 10.1016/j.micpath.2016.04.009
12. Bao Y, Tian M, Li P, Liu J, Ding C, Yu S. Characterization of Brucella abortus mutant strain Δ22915, a potential vaccine candidate. Vet Res. (2017) 48:1–13. doi: 10.1186/s13567-017-0422-9
13. Truong QL, Cho Y, Kim K, Park B-K, Hahn T-W. Booster vaccination with safe, modified, live-attenuated mutants of Brucella abortus strain RB51 vaccine confers protective immunity against virulent strains of B. abortus and Brucella canis in BALB/c mice. Microbiology. (2015) 161:2137–48. doi: 10.1099/mic.0.000170
14. Wang Z, Wu Q. Research progress in live attenuated Brucella vaccine development. Curr Pharm Biotechnol. (2013) 14:887–96. doi: 10.2174/1389201014666131226123016
15. Horwell F, Van Drimmelen G. Brucella melitensis strain Rev I as a vaccine for cattle. J S Afr Vet Assoc. (1971) 42:233–5.
16. Zhu L, Feng Y, Zhang G, Jiang H, Zhang Z, Wang N, et al. Brucella suis strain 2 vaccine is safe and protective against heterologous Brucella spp. infections. Vaccine. (2016) 34:395–400. doi: 10.1016/j.vaccine.2015.09.116
17. Verger J-M, Grayon M, Zundel E, Lechopier P, Olivier-Bernardin V. Comparison of the efficacy of Brucella suis strain 2 and Brucella melitensis Rev. 1 live vaccines against a Brucella melitensis experimental infection in pregnant ewes. Vaccine. (1995) 13:191–6. doi: 10.1016/0264-410X(95)93135-V
18. Gheibi A, Khanahmad H, Kashfi K, Sarmadi M, Khorramizadeh MR. Development of new generation of vaccines for Brucella abortus. Heliyon. (2018) 4:e01079. doi: 10.1016/j.heliyon.2018.e01079
19. Truong QL, Cho Y, Park S, Kim K, Hahn T-W. Brucella abortus ΔcydCΔcydD and ΔcydCΔpurD double-mutants are highly attenuated and confer long-term protective immunity against virulent Brucella abortus. Vaccine. (2016) 34:237–44. doi: 10.1016/j.vaccine.2015.11.030
20. Zhang J, Guo F, Chen C, Li Z, Zhang H, Wang Y, et al. Brucella melitensis 16 M Δhfq attenuation confers protection against wild-type challenge in BALB/c mice. Microbiol Immunol. (2013) 57:502–10. doi: 10.1111/1348-0421.12065
21. Zhang J, Yin S, Yi D, Zhang H, Li Z, Guo F, et al. The Brucella melitensis M5-90ΔmanB live vaccine candidate is safer than M5-90 and confers protection against wild-type challenge in BALB/c mice. Microb Pathog. (2017) 112:148–55. doi: 10.1016/j.micpath.2017.09.016
22. Verdiguel-Fernández L, Oropeza-Navarro R, Ortiz A, Robles-Pesina M, Ramírez-Lezama J, Castañeda-Ramírez A, et al. Brucella melitensis omp31 mutant is attenuated and confers protection against virulent Brucella melitensis challenge in BALB/c mice. J Microbiol Biotechnol. (2020) 30:497–504. doi: 10.4014/jmb.1908.08056
23. Li Z, Wang S, Zhang H, Xi L, Zhang J, Zhang X, et al. Development and evaluation of in murine model, of an improved live-vaccine candidate against brucellosis from to Brucella melitensis vjbR deletion mutant. Microb Pathog. (2018) 124:250–7. doi: 10.1016/j.micpath.2018.08.052
24. Stranahan LW, Chaki SP, Garcia-Gonzalez DG, Khalaf OH, Arenas-Gamboa AM. Evaluation of the efficacy of the Brucella canis RM6/66 ΔvjbR vaccine candidate for protection against B. canis infection in mice. Msphere. (2020) 5:20. doi: 10.1128/mSphere.00172-20
25. Arenas-Gamboa A, Rice-Ficht A, Kahl-McDonagh M, Ficht T. Protective efficacy and safety of Brucella melitensis 16MΔmucR against intraperitoneal and aerosol challenge in BALB/c mice. Infect Immun. (2011) 79:3653–8. doi: 10.1128/IAI.05330-11
26. Clapp B, Skyberg JA, Yang X, Thornburg T, Walters N, Pascual DW. Protective live oral brucellosis vaccines stimulate Th1 and Th17 cell responses. Infect Immun. (2011) 79:4165–74. doi: 10.1128/IAI.05080-11
27. Zhang Y, Li T, Zhang J, Li Z, Zhang Y, Wang Z, et al. The Brucella melitensis M5-90 phosphoglucomutase (PGM) mutant is attenuated and confers protection against wild-type challenge in BALB/c mice. World J Microbiol Biotechnol. (2016) 32:58. doi: 10.1007/s11274-016-2015-6
28. Li Z, Zhang J, Zhang K, Fu Q, Wang Z, Li T, et al. Brucella melitensis 16MΔTcfSR as a potential live vaccine allows for the differentiation between natural and vaccinated infection. Exp Ther Med. (2015) 10:1182–8. doi: 10.3892/etm.2015.2619
29. Wang Z, Niu JR, Wang XL, Wu TL, Cheng J, Lu L, et al. Evaluation of a Brucella melitensis mutant deficient in O-polysaccharide export system ATP-binding protein as a rough vaccine candidate. Microbes Infection. (2014) 16:633–9. doi: 10.1016/j.micinf.2014.06.013
30. Feng Y, Peng X, Jiang H, Peng Y, Zhu L, Ding J. Rough Brucella strain RM57 is attenuated and confers protection against Brucella melitensis. Microb Pathog. (2017) 107:270–5. doi: 10.1016/j.micpath.2017.03.045
31. Costa LF, Cabello AL, Batista DFA, Chaki SP, de Figueiredo P, da Paixão TA, et al. The candidate vaccine strain Brucella ovis Δ abcBA is protective against Brucella melitensis infection in mice. Microbiol Immunol. (2020) 64:730–6. doi: 10.1111/1348-0421.12850
32. Silva APC, Macêdo AA, Costa LF, Rocha CE, Garcia LN, Farias JR, et al. Encapsulated Brucella ovis lacking a putative ATP-binding cassette transporter (Δ abcBA) protects against wild type Brucella ovis in rams. PLoS ONE. (2015) 10:e0136865. doi: 10.1371/journal.pone.0136865
33. Sancho P, Tejedor C, Sidhu-Muñoz RS, Fernández-Lago L, Vizcaíno N. Evaluation in mice of Brucella ovis attenuated mutants for use as live vaccines against B. ovis infection. Vet Res. (2014) 45:1–10. doi: 10.1186/1297-9716-45-61
34. Smith G, Jain-Gupta N, Alqublan H, Dorneles E, Boyle S, Sriranganathan N. Development of an auxotrophic, live-attenuated Brucella suis vaccine strain capable of expressing multimeric GnRH. Vaccine. (2019) 37:910–4. doi: 10.1016/j.vaccine.2018.12.070
35. Czibener C, Del Giudice MG, Spera JM, Fulgenzi FR, Ugalde JE. Delta-pgm, a new live-attenuated vaccine against Brucella suis. Vaccine. (2016) 34:1524–30. doi: 10.1016/j.vaccine.2016.02.025
36. Jain-Gupta N, Waldrop SG, Tenpenny NM, Witonsky SG, Boyle SM, Sriranganathan N. Rough Brucella neotomae provides protection against Brucella suis challenge in mice. Vet Microbiol. (2019) 239:108447. doi: 10.1016/j.vetmic.2019.108447
37. Zhang J, Yin S, Guo F, Meng R, Chen C, Zhang H, et al. A potent Brucella abortus 2308 Δery live vaccine allows for the differentiation between natural and vaccinated infection. J Microbiol. (2014) 52:681–8. doi: 10.1007/s12275-014-3689-9
38. Truong QL, Cho Y, Barate AK, Kim S, Hahn T-W. Characterization and protective property of Brucella abortus cydC and looP mutants. Clin Vaccine Immunol. (2014) 21:1573–80. doi: 10.1128/CVI.00164-14
39. Lei S, Zhong Z, Ke Y, Yang M, Xu X, Ren H, et al. Deletion of the small RNA chaperone protein Hfq down regulates genes related to virulence and confers protection against wild-type Brucella challenge in mice. Front Microbiol. (2016) 6:1570. doi: 10.3389/fmicb.2015.01570
40. Yang X, Clapp B, Thornburg T, Hoffman C, Pascual DW. Vaccination with a ΔnorD ΔznuA Brucella abortus mutant confers potent protection against virulent challenge. Vaccine. (2016) 34:5290–7. doi: 10.1016/j.vaccine.2016.09.004
41. Li Z, Wang S, Zhang J, Yang G, Yuan B, Huang J, et al. Brucella abortus 2308ΔNodVΔNodW double-mutant is highly attenuated and confers protection against wild-type challenge in BALB/c mice. Microb Pathog. (2017) 106:30–9. doi: 10.1016/j.micpath.2017.01.043
42. Liu Y, Sun J, Peng X, Dong H, Qin Y, Shen Q, et al. Deletion of the LuxR-type regulator VjbR in Brucella canis affects expression of type IV secretion system and bacterial virulence, and the mutant strain confers protection against Brucella canis challenge in mice. Microb Pathog. (2020) 139:103865. doi: 10.1016/j.micpath.2019.103865
43. Li Z-Q, Zhang J-L, Xi L, Yang G-L, Wang S-L, Zhang X-G, et al. Deletion of the transcriptional regulator GntR down regulated the expression of genes related to virulence and conferred protection against wild-type Brucella challenge in BALB/c mice. Mol Immunol. (2017) 92:99–105. doi: 10.1016/j.molimm.2017.10.011
44. Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. (2010) 34:379–94. doi: 10.1111/j.1574-6976.2010.00211.x
45. Dorneles EM, Sriranganathan N, Lage AP. Recent advances in Brucella abortus vaccines. Vet Res. (2015) 46:1–10. doi: 10.1186/s13567-015-0199-7
46. Al-Mariri A, Mahmoud NH, Hammoud R. Efficacy evaluation of live Escherichia coli expression Brucella P39 protein combined with CpG oligodeoxynucleotides vaccine against Brucella melitensis 16M, in BALB/c mice. Biologicals. (2012) 40:140–5. doi: 10.1016/j.biologicals.2012.01.002
47. Bugybayeva D, Kydyrbayev Z, Zinina N, Assanzhanova N, Yespembetov B, Kozhamkulov Y, et al. A new candidate vaccine for human brucellosis based on influenza viral vectors: a preliminary investigation for the development of an immunization schedule in a guinea pig model. Infect Dis Poverty. (2021) 10:1–10. doi: 10.1186/s40249-021-00801-y
48. Tabynov K. Influenza viral vector based Brucella abortus vaccine: a novel vaccine candidate for veterinary practice. Expert Rev Vaccines. (2016) 15:1237–9. doi: 10.1080/14760584.2016.1208089
49. Al-Mariri A, Tibor A, Lestrate P, Mertens P, De Bolle X, Letesson J-J. Yersinia enterocolitica as a vehicle for a naked DNA vaccine encoding Brucella abortus bacterioferritin or P39 antigen. Infect Immun. (2002) 70:1915–23. doi: 10.1128/IAI.70.4.1915-1923.2002
50. Mailybayeva A, Ryskeldinova S, Zinina N, Zhou E-M, Renukaradhya GJ, Tabynov K. Evaluation of duration of immunogenicity and protective efficacy of improved influenza viral vector–based Brucella abortus vaccine against Brucella melitensis infection in sheep and goats. Front Vet Sci. (2020) 7:58. doi: 10.3389/fvets.2020.00058
51. Senevirathne A, Hewawaduge C, Lee JH. Attenuated Salmonella secreting Brucella protective antigens confer dual-faceted protection against brucellosis and salmonellosis in a mouse model. Vet Immunol Immunopathol. (2019) 209:31–6. doi: 10.1016/j.vetimm.2019.02.001
52. Bugybayeva D, Ryskeldinova S, Zinina N, Sarmykova M, Assanzhanova N, Kydyrbayev Z, et al. Development of human vectored brucellosis vaccine formulation: assessment of safety and protectiveness of influenza viral vectors expressing Brucella immunodominant proteins in mice and guinea pigs. BioMed Res Int. (2020) 2020:1438929. doi: 10.1155/2020/1438928
53. Mohammadi E, Golchin M. High protection of mice against Brucella abortus by oral immunization with recombinant probiotic Lactobacillus casei vector vaccine, expressing the outer membrane protein OMP19 of Brucella species. Comp Immunol Microbiol Infect Dis. (2020) 70:101470. doi: 10.1016/j.cimid.2020.101470
54. Rezaei M, Rabbani Khorasgani M, Zarkesh Esfahani SH, Emamzadeh R, Abtahi H. Production of Brucella melitensis Omp16 protein fused to the human interleukin 2 in Lactococcus lactis MG1363 toward developing a Lactococcus-based vaccine against brucellosis. Can J Microbiol. (2020) 66:39–45. doi: 10.1139/cjm-2019-0261
55. Guo-Zhen L, Yi-Zhong L, Kui-Zheng C, Jun-Lin L, Zhong-Ren M. Immunogenicity of recombinant adenovirus co-expressing the L7/L12 and BCSP31 proteins of Brucella abortus. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. (2018) 24:211–7. doi: 10.9775/kvfd.2017.18644
56. Vellinga J, Smith JP, Lipiec A, Majhen D, Lemckert A, van Ooij M, et al. Challenges in manufacturing adenoviral vectors for global vaccine product deployment. Hum Gene Ther. (2014) 25:318–27. doi: 10.1089/hum.2014.007
57. Leya M, Kim WK, Ochirkhuyag E, Yu E-C, Kim Y-J, Yeo Y, et al. Protective efficacy of attenuated Salmonella Typhimurium strain expressing BLS, Omp19, PrpA, or SOD of Brucella abortus in goats. J Vet Sci. (2021) 22:e15. doi: 10.4142/jvs.2021.22.e15
58. Senevirathne A, Hewawaduge C, Lee JH. Live vaccine consisting of attenuated Salmonella secreting and delivering Brucella ribosomal protein L7/L12 induces humoral and cellular immune responses and protects mice against virulent Brucella abortus 544 challenge. Vet Res. (2020) 51:1–10. doi: 10.1186/s13567-020-0735-y
59. Hewawaduge C, Senevirathne A, Lee JH. Enhancement of host infectivity, immunity, and protective efficacy by addition of sodium bicarbonate antacid to oral vaccine formulation of live attenuated Salmonella secreting Brucella antigens. Microb Pathog. (2020) 138:103857. doi: 10.1016/j.micpath.2019.103857
60. Kim WK, Moon JY, Cho JS, Hur J. Protective efficacy by various doses of a new brucellosis vaccine candidate based on Salmonella strains expressing Brucella abortus BSCP31, Omp3b and superoxide dismutase against brucellosis in murine model. Pathogens Dis. (2017) 75:ftx094. doi: 10.1093/femspd/ftx094
61. Tabynov K, Sansyzbay A, Kydyrbayev Z, Yespembetov B, Ryskeldinova S, Zinina N, et al. Influenza viral vectors expressing the Brucella OMP16 or L7/L12 proteins as vaccines against B. abortus infection. Virol J. (2014) 11:1–13. doi: 10.1186/1743-422X-11-69
62. Yao L, Wu C-X, Zheng K, Xu X-J, Zhang H, Chen C-F, et al. Immunogenic response to a recombinant pseudorabies virus carrying bp26 gene of Brucella melitensis in mice. Res Vet Sci. (2015) 100:61–7. doi: 10.1016/j.rvsc.2015.03.030
63. Sáez D, Fernández P, Rivera A, Andrews E, Oñate A. Oral immunization of mice with recombinant Lactococcus lactis expressing Cu, Zn superoxide dismutase of Brucella abortus triggers protective immunity. Vaccine. (2012) 30:1283–90. doi: 10.1016/j.vaccine.2011.12.088
64. Moreno E, Barquero-Calvo E. The role of neutrophils in brucellosis. Microbiol Mol Biol Rev. (2020) 84:e00048–20. doi: 10.1128/MMBR.00048-20
65. Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM, Rice-Ficht AC. Brucellosis: the case for live, attenuated vaccines. Vaccine. (2009) 27:D40–3. doi: 10.1016/j.vaccine.2009.08.058
66. Singha H, Mallick AI, Jana C, Fatima N, Owais M, Chaudhuri P. Co-immunization with interlukin-18 enhances the protective efficacy of liposomes encapsulated recombinant Cu–Zn superoxide dismutase protein against Brucella abortus. Vaccine. (2011) 29:4720–7. doi: 10.1016/j.vaccine.2011.04.088
67. Hop HT, Reyes AWB, Simborio HLT, Arayan LT, Min WG, Lee HJ, et al. Immunization of mice with recombinant Brucella abortus organic hydroperoxide resistance (Ohr) protein protects against a virulent Brucella abortus 544 Infection. J Microbiol Biotechnol. (2016) 26:190–6. doi: 10.4014/jmb.1505.05028
68. Pasquevich KA, Estein SM, Samartino CG, Zwerdling A, Coria LM, Barrionuevo P, et al. Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect Immun. (2009) 77:436–45. doi: 10.1128/IAI.00123-09
69. Ghasemi A, Jeddi-Tehrani M, Mautner J, Salari MH, Zarnani A-H. Immunization of mice with a novel recombinant molecular chaperon confers protection against Brucella melitensis infection. Vaccine. (2014) 32:6659–66. doi: 10.1016/j.vaccine.2014.09.013
70. Zhu L, Wang Q, Wang Y, Xu Y, Peng D, Huang H, et al. Comparison of immune effects between Brucella recombinant Omp10-Omp28-L7/L12 proteins expressed in eukaryotic and prokaryotic systems. Front Vet Sci. (2020) 7:576. doi: 10.3389/fvets.2020.00576
71. Gupta S, Singh D, Gupta M, Bhatnagar R. A combined subunit vaccine comprising BP26, Omp25 and L7/L12 against brucellosis. Pathogens Dis. (2019) 77:ftaa002. doi: 10.1093/femspd/ftaa002
72. Golshani M, Amani M, Siadat SD, Nejati-Moheimani M, Arsang A, Bouzari S. Comparison of the protective immunity elicited by a Brucella cocktail protein vaccine (rL7/L12+ rTOmp31+ rSOmp2b) in two different adjuvant formulations in BALB/c mice. Mol Immunol. (2018) 103:306–11. doi: 10.1016/j.molimm.2018.10.002
73. Abdollahi A, Mansouri S, Amani J, Fasihi-Ramandi M, Ranjbar R, Ghasemi A, et al. A recombinant chimera protein as a novel Brucella subunit vaccine: protective efficacy and induced immune response in BALB/c mice. Jundishapur J Microbiol. (2018) 11.
74. Tadepalli G, Konduru B, Murali HS, Batra HV. Intraperitoneal administration of a novel chimeric immunogen (rOP) elicits IFN-γ and IL-12p70 protective immune response in BALB/c mice against virulent Brucella. Immunol Lett. (2017) 192:79–87. doi: 10.1016/j.imlet.2017.10.013
75. Tadepalli G, Singh AK, Balakrishna K, Murali HS, Batra HV. Immunogenicity and protective efficacy of Brucella abortus recombinant protein cocktail (rOmp19+ rP39) against B. abortus 544 and B. melitensis 16M infection in murine model. Mol Immunol. (2016) 71:34–41. doi: 10.1016/j.molimm.2016.01.001
76. Wang X, An C, Yang M, Li X, Ke Y, Lei S, et al. Immunization with individual proteins of the Lrp/AsnC family induces protection against Brucella melitensis 16M challenges in mice. Front Microbiol. (2015) 6:1193. doi: 10.3389/fmicb.2015.01193
77. Yin D, Li L, Song D, Liu Y, Ju W, Song X, et al. A novel recombinant multi-epitope protein against Brucella melitensis infection. Immunol Lett. (2016) 175:1–7. doi: 10.1016/j.imlet.2016.04.016
78. Yang Y, Wang L, Yin J, Wang X, Cheng S, Lang X, et al. Immunoproteomic analysis of Brucella melitensis and identification of a new immunogenic candidate protein for the development of brucellosis subunit vaccine. Mol Immunol. (2011) 49:175–84. doi: 10.1016/j.molimm.2011.08.009
79. Verma SK, Jain S, Kumar S. Immunogenicity and protective potential of a bacterially expressed recombinant dihydrolipoamide succinyltransferase (rE2o) of Brucella abortus in BALB/c mice. World J Microbiol Biotechnol. (2012) 28:2487–95. doi: 10.1007/s11274-012-1056-8
80. Jain S, Afley P, Kumar S. Immunological responses to recombinant cysteine synthase A of Brucella abortus in BALB/c mice. World J Microbiol Biotechnol. (2013) 29:907–13. doi: 10.1007/s11274-012-1247-3
81. Ghasemi A, Zarnani A-H, Ghoodjani A, Rezania S, Salari MH, Jeddi-Tehrani M. Identification of a new immunogenic candidate conferring protection against Brucella melitensis infection in mice. Mol Immunol. (2014) 62:142–9. doi: 10.1016/j.molimm.2014.06.017
82. Yousefi S, Abbassi-Daloii T, Sekhavati MH, Tahmoorespur M. Evaluation of immune responses induced by polymeric OMP25-BLS Brucella antigen. Microb Pathog. (2018) 115:50–6. doi: 10.1016/j.micpath.2017.12.045
83. Paul S, Peddayelachagiri BV, Nagaraj S, Kingston JJ, Batra HV. Recombinant outer membrane protein 25c from Brucella abortus induces Th1 and Th2 mediated protection against Brucella abortus infection in mouse model. Mol Immunol. (2018) 99:9–18. doi: 10.1016/j.molimm.2018.04.002
84. Hop HT, Arayan LT, Huy TXN, Reyes AWB, Min W, Lee HJ, et al. Immunization of BALB/c mice with a combination of four recombinant Brucella abortus proteins, AspC, Dps, InpB and Ndk, confers a marked protection against a virulent strain of Brucella abortus. Vaccine. (2018) 36:3027–33. doi: 10.1016/j.vaccine.2018.04.019
85. Paul S, Peddayelachagiri BV, Nagaraj S, Konduru B, Batra HV. Protective and therapeutic efficacy study of divalent fusion protein rL7/L12-Omp25 against B. abortus 544 in presence of IFNγ. Appl Microbiol Biotechnol. (2018) 102:8895–907. doi: 10.1007/s00253-018-9314-9
86. Senevirathne A, Hewawaduge C, Hajam IA, Lalsiamthara J, Lee JH. Intranasally administered anti-Brucella subunit vaccine formulation induces protective immune responses against nasal Brucella challenge. Vet Microbiol. (2019) 228:112–8. doi: 10.1016/j.vetmic.2018.11.022
87. Sadeghi Z, Fasihi-Ramandi M, Bouzari S. Evaluation of immunogenicity of novel multi-epitope subunit vaccines in combination with poly I: C against Brucella melitensis and Brucella abortus infection. Int Immunopharmacol. (2019) 75:105829. doi: 10.1016/j.intimp.2019.105829
88. Golshani M, Amani M, Amirzadeh F, Nazeri E, Siadat SD, Nejati-Moheimani M, et al. Evaluation of Poly (I: C) and combination of CpG ODN plus Montanide ISA adjuvants to enhance the efficacy of outer membrane vesicles as an acellular vaccine against Brucella melitensis infection in mice. Int Immunopharmacol. (2020) 84:106573. doi: 10.1016/j.intimp.2020.106573
89. Nazifi N, Tahmoorespur M, Sekhavati MH, Haghparast A, Behroozikhah AM. In vivo immunogenicity assessment and vaccine efficacy evaluation of a chimeric tandem repeat of epitopic region of OMP31 antigen fused to interleukin 2 (IL-2) against Brucella melitensis in BALB/c mice. BMC Vet Res. (2019) 15:402. doi: 10.1186/s12917-019-2074-7
90. Delpino MV, Estein SM, Fossati CA, Baldi PC, Cassataro J. Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine. (2007) 25:6721–9. doi: 10.1016/j.vaccine.2007.07.002
91. Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. (2019) 32:e00084–18. doi: 10.1128/CMR.00084-18
92. Oliveira SC, Splitter GA. Immunization of mice with recombinant L7L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine. (1996) 14:959–62. doi: 10.1016/0264-410X(96)00018-7
93. Li Z, Zhang H, Zhang J, Xi L, Yang G, Wang S, et al. Brucella abortus phosphoglyceromutase and dihydrodipicolinate reductase induce Th1 and Th2-related immune responses. World J Microbiol Biotechnol. (2018) 34:1–8. doi: 10.1007/s11274-017-2405-4
94. Kaur G, Singh S, Sunil Kumar B, Mahajan K, Verma R. Characterization and immunogenicity of outer membrane vesicles from Brucella abortus. J Immunoassay Immunochem. (2016) 37:261–72. doi: 10.1080/15321819.2015.1132231
95. Golshani M, Rafati S, Nejati-Moheimani M, Ghasemian M, Bouzari S. Comparison of potential protection conferred by three immunization strategies (protein/protein, DNA/DNA, and DNA/protein) against Brucella infection using Omp2b in BALB/c Mice. Vet Microbiol. (2016) 197:47–52. doi: 10.1016/j.vetmic.2016.10.027
96. Zhou Y, Zheng Y, Chen Y, Li Y, Sun X, Huo Y, et al. Evaluation of a recombinant bacillus calmette-guérin vaccine expressing P39-L7/L12 of Brucella melitensis: an immunization strategy against brucellosis in BALB/c mice. Materials Express. (2020) 10:350–62. doi: 10.1166/mex.2020.1645
97. Mukherjee F, Prasad A, Bahekar VS, Rana SK, Rajendra L, Sharma GK, et al. Evaluation of immunogenicity and protective efficacy of a liposome containing Brucella abortus S19 outer membrane protein in BALB/c mice. Iran J Vet Res. (2016) 17:1–7.
98. Clausse M, Díaz AG, Ibañez AE, Cassataro J, Giambartolomei GH, Estein SM. Evaluation of the efficacy of outer membrane protein 31 vaccine formulations for protection against Brucella canis in BALB/c mice. Clin Vaccine Immunol. (2014) 21:1689–94. doi: 10.1128/CVI.00527-14
99. Simborio HLT, Reyes AWB, Hop HT, Arayan LT, Min W, Lee HJ, et al. Immune modulation of recombinant OmpA against Brucella abortus 544 infection in mice. J Microbiol Biotechnol. (2016) 26:603–9. doi: 10.4014/jmb.1509.09061
100. Fu S, Xu J, Li X, Xie Y, Qiu Y, Du X, et al. Immunization of mice with recombinant protein CobB or AsnC confers protection against Brucella abortus infection. PLoS ONE. (2012) 7:e29552. doi: 10.1371/journal.pone.0029552
101. Huy TXN, Nguyen TT, Reyes AWB, Vu SH, Min W, Lee HJ, et al. Immunization with a combination of four recombinant Brucella abortus Proteins Omp16, Omp19, Omp28, and L7/L12 induces T helper 1 immune response against virulent B. abortus 544 infection in BALB/c mice. Front Vet Sci. (2021) 7:1221. doi: 10.3389/fvets.2020.577026
102. Du Z-Q, Li X, Wang J-Y. Immunogenicity analysis of a novel subunit vaccine candidate molecule—recombinant L7/L12 ribosomal protein of Brucella suis. Appl Biochem Biotechnol. (2016) 179:1445–55. doi: 10.1007/s12010-016-2076-x
103. Hop HT, Simborio HL, Reyes AWB, Arayan LT, Min W, Lee HJ, et al. Immunogenicity and protective effect of recombinant Brucella abortus Ndk (rNdk) against a virulent strain B. abortus 544 infection in BALB/c mice. FEMS Microbiol Lett. (2015) 362:1–6. doi: 10.1093/femsle/fnv003
104. Huy TXN, Reyes AWB, Vu SH, Arayan LT, Hop HT, Min W, et al. Immunogenicity and protective response induced by recombinant Brucella abortus proteins Adk, SecB and combination of these two recombinant proteins against a virulent strain B. abortus 544 infection in BALB/c mice. Microbial Pathogen. (2020) 143:104137. doi: 10.1016/j.micpath.2020.104137
105. Goel D, Bhatnagar R. Intradermal immunization with outer membrane protein 25 protects Balb/c mice from virulent B. abortus 544. Mol Immunol. (2012) 51:159–68. doi: 10.1016/j.molimm.2012.02.126
106. Díaz AG, Quinteros DA, Paolicchi FA, Rivero MA, Palma SD, Pardo RP, et al. Mucosal immunization with polymeric antigen BLSOmp31 using alternative delivery systems against Brucella ovis in rams. Vet Immunol Immunopathol. (2019) 209:70–7. doi: 10.1016/j.vetimm.2019.02.005
107. Solanki KS, Varshney R, Qureshi S, Thomas P, Singh R, Agrawal A, et al. Non-infectious outer membrane vesicles derived from Brucella abortus S19Δper as an alternative acellular vaccine protects mice against virulent challenge. Int Immunopharmacol. (2021) 90:107148. doi: 10.1016/j.intimp.2020.107148
108. Afley P, Dohre SK, Prasad G, Kumar S. Prediction of T cell epitopes of Brucella abortus and evaluation of their protective role in mice. Appl Microbiol Biotechnol. (2015) 99:7625–37. doi: 10.1007/s00253-015-6787-7
109. Lim JJ, Kim DH, Lee JJ, Kim DG, Min W, Lee HJ, et al. Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice. J Vet Sci. (2012) 13:287. doi: 10.4142/jvs.2012.13.3.287
110. Singh D, Goel D, Bhatnagar R. Recombinant L7/L12 protein entrapping PLGA (poly lactide-co-glycolide) micro particles protect BALB/c mice against the virulent B. abortus 544 infection. Vaccine. (2015) 33:2786–92. doi: 10.1016/j.vaccine.2015.04.030
111. Singh AK, Balakrishna K, Sripathy MH, Batra HV. Studies on recombinant glucokinase (r-glk) protein of Brucella abortus as a candidate vaccine molecule for brucellosis. Vaccine. (2014) 32:5600–6. doi: 10.1016/j.vaccine.2014.07.106
112. Abkar M, Amani J, Sahebghadam Lotfi A, Nikbakht Brujeni G, Alamian S, Kamali M. Subcutaneous immunization with a novel immunogenic candidate (urease) confers protection against Brucella abortus and Brucella melitensis infections. Apmis. (2015) 123:667–75. doi: 10.1111/apm.12400
113. Arayan LT, Huy TXN, Reyes AWB, Hop HT, Son VH, Min W, et al. Substantial protective immunity conferred by a combination of Brucella abortus recombinant proteins against Brucella abortus 544 infection in BALB/c mice. J Microbiol Biotechnol. (2019) 29:330–8. doi: 10.4014/jmb.1811.10066
114. Zhang F, Li Z, Jia B, Zhu Y, Pang P, Zhang C, et al. The immunogenicity of OMP31 peptides and its protection against Brucella melitensis infection in mice. Sci Rep. (2019) 9:1–7. doi: 10.1038/s41598-019-40084-w
115. Clausse M, Díaz AG, Ghersi G, Zylberman V, Cassataro J, Giambartolomei GH, et al. The vaccine candidate BLSOmp31 protects mice against Brucella canis infection. Vaccine. (2013) 31:6129–35. doi: 10.1016/j.vaccine.2013.07.041
116. Cherwonogrodzky JW, Barabé ND, Grigat ML, Lee WE, Poirier RT, Jager SJ, et al. Thermostable cross-protective subunit vaccine against Brucella species. Clin Vaccine Immunol. (2014) 21:1681–8. doi: 10.1128/CVI.00447-14
117. Li X, Xu J, Xie Y, Qiu Y, Fu S, Yuan X, et al. Vaccination with recombinant flagellar proteins FlgJ and FliN induce protection against Brucella abortus 544 infection in BALB/c mice. Vet Microbiol. (2012) 161:137–44. doi: 10.1016/j.vetmic.2012.07.016
118. Golshani M, Rafati S, Dashti A, Gholami E, Siadat SD, Oloomi M, et al. Vaccination with recombinant L7/L12-truncated Omp31 protein induces protection against Brucella infection in BALB/c mice. Mol Immunol. (2015) 65:287–92. doi: 10.1016/j.molimm.2015.01.009
119. Chen B, Liu B, Zhao Z, Wang G. Evaluation of a DNA vaccine encoding Brucella BvrR in BALB/c mice. Mol Med Rep. (2019) 19:1302–8. doi: 10.3892/mmr.2018.9735
120. Imtiaz W, Khan A, Gul ST, Saqib M, Saleemi MK, Shahzad A, et al. Evaluation of DNA vaccine encoding BCSP31 surface protein of Brucella abortus for protective immunity. Microb Pathog. (2018) 125:514–20. doi: 10.1016/j.micpath.2018.10.016
121. Riquelme-Neira R, Retamal-Díaz A, Acuña F, Riquelme P, Rivera A, Sáez D, et al. Protective effect of a DNA vaccine containing an open reading frame with homology to an ABC-type transporter present in the genomic island 3 of Brucella abortus in BALB/c mice. Vaccine. (2013) 31:3663–7. doi: 10.1016/j.vaccine.2013.06.013
122. Jain S, Afley P, Dohre SK, Saxena N, Kumar S. Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine. (2014) 32:4537–42. doi: 10.1016/j.vaccine.2014.06.012
123. Li Z, Wang S, Zhang H, Xi L, Zhang J, Zhang X, et al. Immunization with recombinant GntR plasmid confers protection against Brucella challenge in BALB/c mice. Microb Pathog. (2017) 111:357–61. doi: 10.1016/j.micpath.2017.09.010
124. Gómez LA, Alvarez FI, Fernández PA, Flores MR, Molina RE, Coloma RF, et al. Immunogenicity and protective response induced by recombinant plasmids based on the BAB1_0267 and BAB1_0270 open reading frames of Brucella abortus 2308 in BALB/c mice. Front Cell Infect Microbiol. (2016) 6:117. doi: 10.3389/fcimb.2016.00117
125. Shojaei M, Tahmoorespur M, Soltani M, Sekhavati MH. Immunogenicity evaluation of plasmids encoding Brucella melitensis Omp25 and Omp31 antigens in BALB/c mice. Iran J Basic Med Sci. (2018) 21:957. doi: 10.22038/IJBMS.2018.27540.6722
126. Escalona E, Sáez D, Oñate A. Immunogenicity of a multi-epitope dna vaccine encoding epitopes from Cu–Zn superoxide dismutase and open reading Frames of Brucella abortus in mice. Front Immunol. (2017) 8:125. doi: 10.3389/fimmu.2017.00125
127. Sislema-Egas F, Céspedes S, Fernández P, Retamal-Díaz A, Sáez D, Oñate A. Evaluation of protective effect of DNA vaccines encoding the BAB1_0263 and BAB1_0278 open reading frames of Brucella abortus in BALB/c mice. Vaccine. (2012) 30:7286–91. doi: 10.1016/j.vaccine.2012.09.039
128. Da Costa Martins R, Gamazo C, Sánchez-Martínez M, Barberán M, Peñuelas I, Irache JM. Conjunctival vaccination against Brucella ovis in mice with mannosylated nanoparticles. J Control Release. (2012) 162:553–60. doi: 10.1016/j.jconrel.2012.07.030
129. Shim S, Soh SH, Im YB, Park H-E, Cho C-S, Kim S, et al. Elicitation of Th1/Th2 related responses in mice by chitosan nanoparticles loaded with Brucella abortus malate dehydrogenase, outer membrane proteins 10 and 19. Int J Medical Microbiol. (2020) 310:151362. doi: 10.1016/j.ijmm.2019.151362
130. Karevan G, Ahmadi K, Taheri RA, Fasihi-Ramandi M. Immunogenicity of glycine nanoparticles containing a chimeric antigen as Brucella vaccine candidate. Clin Exp Vaccine Res. (2021) 10:35. doi: 10.7774/cevr.2021.10.1.35
131. Afshari H, Maleki M, Salouti M. Immunological effects of two new nanovaccines against Brucella based on OPS and LPS antigens conjugated with PLGA nanoparticles. Eur Polym J. (2020) 139:110021. doi: 10.1016/j.eurpolymj.2020.110021
132. Soh SH, Shim S, Im YB, Park H-T, Cho C-S, Yoo HS. Induction of Th2-related immune responses and production of systemic IgA in mice intranasally immunized with Brucella abortus malate dehydrogenase loaded chitosan nanoparticles. Vaccine. (2019) 37:1554–64. doi: 10.1016/j.vaccine.2019.02.005
133. Abkar M, Fasihi-Ramandi M, Kooshki H, Lotfi AS. Oral immunization of mice with Omp31-loaded N-trimethyl chitosan nanoparticles induces high protection against Brucella melitensis infection. Int J Nanomed. (2017) 12:8769. doi: 10.2147/IJN.S149774
134. Sadeghi Z, Fasihi-Ramandi M, Azizi M, Bouzari S. Mannosylated chitosan nanoparticles loaded with FliC antigen as a novel vaccine candidate against Brucella melitensis and Brucella abortus infection. J Biotechnol. (2020) 310:89–96. doi: 10.1016/j.jbiotec.2020.01.016
135. Sadeghi Z, Fasihi-Ramandi M, Bouzari S. Nanoparticle-based vaccines for brucellosis: calcium phosphate nanoparticles-adsorbed antigens induce cross protective response in mice. Int J Nanomed. (2020) 15:3877. doi: 10.2147/IJN.S249942
136. Abkar M, Fasihi-Ramandi M, Kooshki H, Lotfi AS. Intraperitoneal immunization with Urease loaded N-trimethyl Chitosan nanoparticles elicits high protection against Brucella melitensis and Brucella abortus infections. Immunol Lett. (2018) 199:53–60. doi: 10.1016/j.imlet.2018.03.004
137. Singh D, Somani VK, Aggarwal S, Bhatnagar R. PLGA (85: 15) nanoparticle based delivery of rL7/L12 ribosomal protein in mice protects against Brucella abortus 544 infection: a promising alternate to traditional adjuvants. Mol Immunol. (2015) 68:272–9. doi: 10.1016/j.molimm.2015.09.011
138. Maleki M, Salouti M, Shafiee Ardestani M, Talebzadeh A. Preparation of a nanovaccine against Brucella melitensis M16 based on PLGA nanoparticles and oligopolysaccharide antigen. Artif Cells Nanomed Biotechnol. (2019) 47:4248–56. doi: 10.1080/21691401.2019.1687490
139. Hu X-D, Yu D-H, Chen S-T, Li S-X, Cai H. A combined DNA vaccine provides protective immunity against Mycobacterium bovis and Brucella abortus in cattle. DNA Cell Biol. (2009) 28:191–9. doi: 10.1089/dna.2008.0790
140. González-Smith A, Vemulapalli R, Andrews E, Oñate A. Evaluation of Brucella abortus DNA vaccine by expression of Cu–Zn superoxide dismutase antigen fused to IL-2. Immunobiology. (2006) 211:65–74. doi: 10.1016/j.imbio.2005.09.004
141. Velikovsky CA, Cassataro J, Giambartolomei GH, Goldbaum FA, Estein S, Bowden RA, et al. A DNA vaccine encoding lumazine synthase from Brucella abortus induces protective immunity in BALB/c mice. Infect Immun. (2002) 70:2507–11. doi: 10.1128/IAI.70.5.2507-2511.2002
142. Ranjbar R, Sharifimoghadam S, Saeidi E, Jonaidi N, Sheikhshahrokh A. Induction of protective immune responses in mice by double DNA vaccine encoding of Brucella melitensis Omp31 and Escherichia coli Eae genes. Trop J Pharmaceut Res. (2016) 15:2077–83. doi: 10.4314/tjpr.v15i10.4
143. Al-Mariri A, Abbady AQ. Evaluation of the immunogenicity and the protective efficacy in mice of a DNA vaccine encoding SP41 from Brucella melitensis. J Infect Develop Countr. (2013) 7:329–37. doi: 10.3855/jidc.2296
144. Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. (2005) 175:633–9. doi: 10.4049/jimmunol.175.2.633
145. Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, et al. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immunity. (2005) 73:6537–46. doi: 10.1128/IAI.73.10.6537-6546.2005
146. Onate AA, Céspedes S, Cabrera A, Rivers R, González A, Muñoz C, et al. A DNA vaccine encoding Cu, Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect Immun. (2003) 71:4857–61. doi: 10.1128/IAI.71.9.4857-4861.2003
147. Luo D, Ni B, Li P, Shi W, Zhang S, Han Y, et al. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun. (2006) 74:2734–41. doi: 10.1128/IAI.74.5.2734-2741.2006
148. Yu D-H, Hu X-D, Cai H, Li M. A combined DNA vaccine encoding BCSP31, SOD, and L7/L12 confers high protection against Brucella abortus 2308 by inducing specific CTL responses. DNA Cell Biol. (2007) 26:435–43. doi: 10.1089/dna.2006.0552
149. Zhao C, Sun Y, Zhao Y, Wang S, Yu T, Du F, et al. Immunogenicity of a multi-epitope DNA vaccine against hantavirus. Hum Vaccin Immunother. (2012) 8:208–15. doi: 10.4161/hv.18389
150. Paranavitana C, Zelazowska E, Izadjoo M, Hoover D. Interferon-γ associated cytokines and chemokines produced by spleen cells from Brucella-immune mice. Cytokine. (2005) 30:86–92. doi: 10.1016/j.cyto.2004.12.009
151. Cassataro J, Velikovsky CA, Bruno L, Estein SM, de la Barrera S, Bowden R, et al. Improved immunogenicity of a vaccination regimen combining a DNA vaccine encoding Brucella melitensis outer membrane protein 31 (Omp31) and recombinant Omp31 boosting. Clin Vaccine Immunol. (2007) 14:869–74. doi: 10.1128/CVI.00472-06
152. Vemulapalli R, He Y, Boyle SM, Sriranganathan N, Schurig GG. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1 type immune responses. Infect Immun. (2000) 68:3290–6. doi: 10.1128/IAI.68.6.3290-3296.2000
153. Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-γ synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Nat Acad Sci USA. (2011) 108:18336–41. doi: 10.1073/pnas.1110566108
154. Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. (2005) 22:93–104. doi: 10.1016/j.immuni.2004.11.016
155. Choi DS, Seo SI, Shin WG, Park CH. Risk for colorectal neoplasia in patients with Helicobacter pylori infection: a systematic review and meta-analysis. Clin Transl Gastroenterol. (2020) 11:e00127. doi: 10.14309/ctg.0000000000000127
156. Leclerq S, Harms JS, Rosinha GMS, Azevedo V, Oliveira SC. Induction of a th1-type of immune response but not protective immunity by intramuscular DNA immunisation with Brucella abortus GroEL heat-shock gene. J Med Microbiol. (2002) 51:20–6. doi: 10.1099/0022-1317-51-1-20
157. Velikovsky CA, Cassataro J, Sanchez M, Fossati CA, Fainboim L, Spitz M. Single-shot plasmid DNA intrasplenic immunization for the production of monoclonal antibodies. Persistent expression of DNA. J Immunol Methods. (2000) 244:1–7. doi: 10.1016/S0022-1759(00)00244-1
158. Abkar M, Fasihi-Ramandi M, Kooshki H, Lotfi AS. Intraperitoneal immunization with Urease loaded N-trimethyl Chitosan nanoparticles elicits high protection against Brucella melitensis and Brucella abortus infections. Immunol Lett. (2018) 199:53–60.
159. Al-Halifa S, Gauthier L, Arpin D, Bourgault S, Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. (2019) 10:22. doi: 10.3389/fimmu.2019.00022
160. Gregory AE, Titball R, Williamson D. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. (2013) 3:13. doi: 10.3389/fcimb.2013.00013
161. Abadi AH, Mahdavi M, Khaledi A, Esmaeili S-A, Esmaeili D, Sahebkar A. Study of serum bactericidal and splenic activity of Total-OMP-CagA combination from Brucella abortus and Helicobacter pylori in BALB/c mouse model. Microb Pathog. (2018) 121:100–5. doi: 10.1016/j.micpath.2018.04.050
162. Iannino F, Herrmann CK, Roset MS, Briones G. Development of a dual vaccine for prevention of Brucella abortus infection and Escherichia coli O157: H7 intestinal colonization. Vaccine. (2015) 33:2248–53. doi: 10.1016/j.vaccine.2015.03.033
163. Aragón-Aranda B, de Miguel MJ, Martínez-Gómez E, Zúñiga-Ripa A, Salvador-Bescós M, Moriyón I, et al. Rev1 wbdR tagged vaccines against Brucella ovis. Vet Res. (2019) 50:1–9. doi: 10.1186/s13567-019-0714-3
164. Huang J, Pan C, Sun P, Feng E, Wu J, Zhu L, et al. Application of an O-linked glycosylation system in Yersinia enterocolitica serotype O: 9 to generate a new candidate vaccine against Brucella abortus. Microorganisms. (2020) 8:436. doi: 10.3390/microorganisms8030436
165. Lin G-Z, Yang J-T, Wei S-C, Chen S-E, Huo S-D, Ma Z-R. Immunogenicity of adenovirus and DNA vaccines co-expressing P39 and lumazine synthase proteins of Brucella abortus in BALB/c mice. Trop Anim Health Prod. (2018) 50:957–63. doi: 10.1007/s11250-018-1517-7
166. Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Rel. (2019) 303:130–50. doi: 10.1016/j.jconrel.2019.04.025
167. Goel D, Rajendran V, Ghosh PC, Bhatnagar R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine. (2013) 31:1231–7. doi: 10.1016/j.vaccine.2012.12.043
168. Kim WK, Moon JY, Cho JS, Akanda MR, Park BY, Kug Eo S, et al. Brucella abortus lysed cells using GI24 induce robust immune response and provide effective protection in Beagles. Pathogens Dis. (2018) 76:ftx124. doi: 10.1093/femspd/ftx124
169. Kim W-K, Moon J-Y, Cho J-S, Ochirkhuyag E, Akanda MR, Park B-Y, et al. Protective efficacy of an inactivated Brucella abortus vaccine candidate lysed by GI24 against brucellosis in Korean black goats. Can J Vet Res. (2019) 83:68–74.
170. Liu J, Li Y, Sun Y, Ji X, Zhu L, Guo X, et al. Immune responses and protection induced by Brucella suis S2 bacterial ghosts in mice. Vet Immunol Immunopathol. (2015) 166:138–44. doi: 10.1016/j.vetimm.2015.04.008
171. Kwon AJ, Moon JY, Kim WK, Kim S, Hur J. Protection efficacy of the Brucella abortus ghost vaccine candidate lysed by the N-terminal 24-amino acid fragment (GI24) of the 36-amino acid peptide PMAP-36 (porcine myeloid antimicrobial peptide 36) in murine models. J Vet Medical Sci. (2016) 2016:16–0036. doi: 10.1292/jvms.16-0036
Keywords: brucellosis, vaccine, Brucella, review, Brucella abortus, Brucella melitensis
Citation: Heidary M, Dashtbin S, Ghanavati R, Mahdizade Ari M, Bostanghadiri N, Darbandi A, Navidifar T and Talebi M (2022) Evaluation of Brucellosis Vaccines: A Comprehensive Review. Front. Vet. Sci. 9:925773. doi: 10.3389/fvets.2022.925773
Received: 21 April 2022; Accepted: 03 June 2022;
Published: 18 July 2022.
Edited by:
Jiabo Ding, Institute of Animal Sciences (CAAS), ChinaReviewed by:
Qisheng Peng, Jilin University, ChinaHai Jiang, Chinese Center for Disease Control and Prevention, China
Copyright © 2022 Heidary, Dashtbin, Ghanavati, Mahdizade Ari, Bostanghadiri, Darbandi, Navidifar and Talebi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malihe Talebi, dGFsZWJpXzI1QHlhaG9vLmNvbQ==
 Mohsen Heidary
Mohsen Heidary Shirin Dashtbin2
Shirin Dashtbin2 Malihe Talebi
Malihe Talebi