- 1Department of Animal Science, College of Coastal Agricultural Sciences, Guangdong Ocean University, Zhanjiang, China
- 2Department of Food Science and Biotechnology, College of Life Science, Sejong University, Seoul, South Korea
Heat stress has become a widespread concern in the world, which is one of the major environmental stressors and causes substantial economic loss in the rabbit industry. Heat stress leads to multiple damages to the health of rabbits, such as organ damage, oxidative stress, disordered endocrine regulation, suppressed immune function and reproductive disorders, ultimately, induces the decreased production performance and increased mortality. Nutritional approaches, including feeding strategies, adjusting feed formula, and supplementing vitamins, minerals, electrolytes, Chinese herbal medicines, and functional active substances to the feed, were reported to mitigate the detrimental effects of heat stress in rabbits. Therefore, elucidating the damage of heat stress to rabbits; proper management and nutritional approaches should be considered to solve the heat stress issue in rabbits. This review highlights the scientific evidence regarding the effects of heat stress on rabbit's immune function, endocrine, blood biochemical changes, antioxidant capacity and production performance, and the potential mitigation strategies of nutritional intervention to alleviate heat stress in rabbits; which could contribute to develop nutritional strategies in relieving heat stress of rabbits.
Introduction
During recent years, the rabbit meat production is growing in China and European countries to meet the increasing demands of diverse meat product, which has become a highly specialized industry (1). There are about 1.4 million tons of rabbit meat produced worldwide each year, of which China is the largest producer, and the Europe is the second largest producing region (2). Rabbit meat is considered to have good sensory properties, it is tender, lean and flavored (3). Rabbit meat contains less fat than other meat (e.g., pork and chicken) and rich in protein, unsaturated fatty acids, conjugated linoleic acid and minerals, which are easy for humans to digest. Also, the rabbit meat contains a lot of selenium and antioxidant vitamins, and rich in polyamine (4). Dietary polyamines can heal the wound in intestinal mucosa growth, maturation and regeneration (5). Considering these facts, many researchers and farmers have focused on the productive performance and carcass yield of rabbit meat during the last decades (3). The genetically improved rabbits in recent years have higher metabolic rates and production performances, making them sensitive to environmental stress, such as high temperature, transportation and changes in feed composition and intensive farming (6). These stressors affect the health and production performance of rabbits, and the negative impact of high temperature on production is prominent due to the thick villi and lack of sweat glands in rabbits; facing with the high ambient temperature, rabbits stretch out to lose heat by radiation and convection and raise their ear temperature, stretch the ear pinnae and spread them far from the body to expose the surface to the surroundings (7, 8).
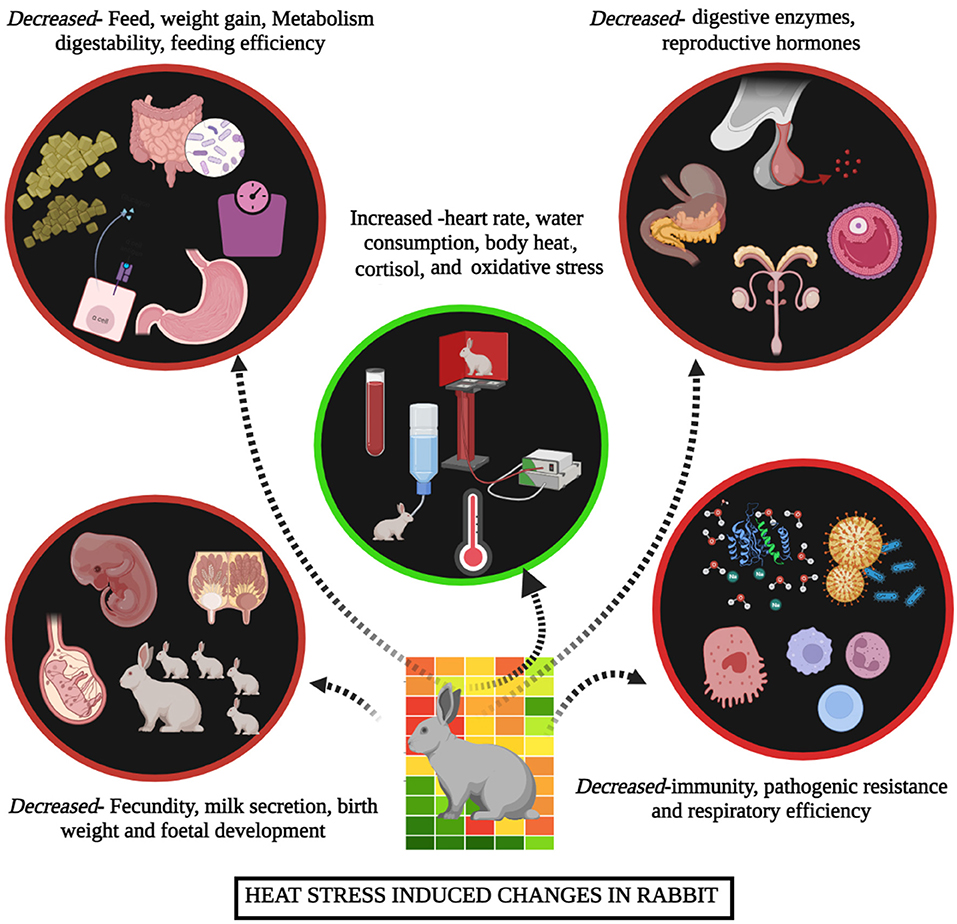
Heat stress is a condition where rabbits are unable to maintain a balance between heat production and emission. High ambient temperature in summer is easy to cause heat stress in rabbits, which brings a series of adverse effects to rabbit production (6). Heat stress results from the interaction of different factors, such as high temperature, humidity, radiant heat and air speed. Of these, high ambient temperature plays a major role in leading to heat stress (9). The normal body temperature of rabbit ranges from 38.5 to 39.5°C, and the individual difference ranges from 0.5 to 1.2°C. The optimal temperature range of rabbits is 15–25°C, and the optimal humidity is 55–65%, heat stress occurs when the ambient temperature is higher than 30°C, when the temperature is higher than 35°C, rabbits cannot regulate body temperature, resulting in heat failure (7, 10). As known, heat stress has multiple unfavorable impacts on rabbit health and production performance, it has been suggested that heat stress causes 20–25% reduction in daily weight gain, 8–15% decrease in feed conversion ratio and 9–12% increase in mortality rate; and the reproductive performance decreased by 6–10%, as well as negatively influences the meat quality and carcass traits (6, 11, 12). Thus, heat stress causes great challenge for rabbit industry, especially with global warming (Figure 1). The potential mitigation strategies have been studied in the past, and the nutritional intervention proven to be effective mitigation approach (11). It is important to summarize these findings for rabbit researchers and industry. Therefore, this review focuses on the effect of heat stress on rabbit production and the potential mitigation strategies of nutritional intervention in rabbits.
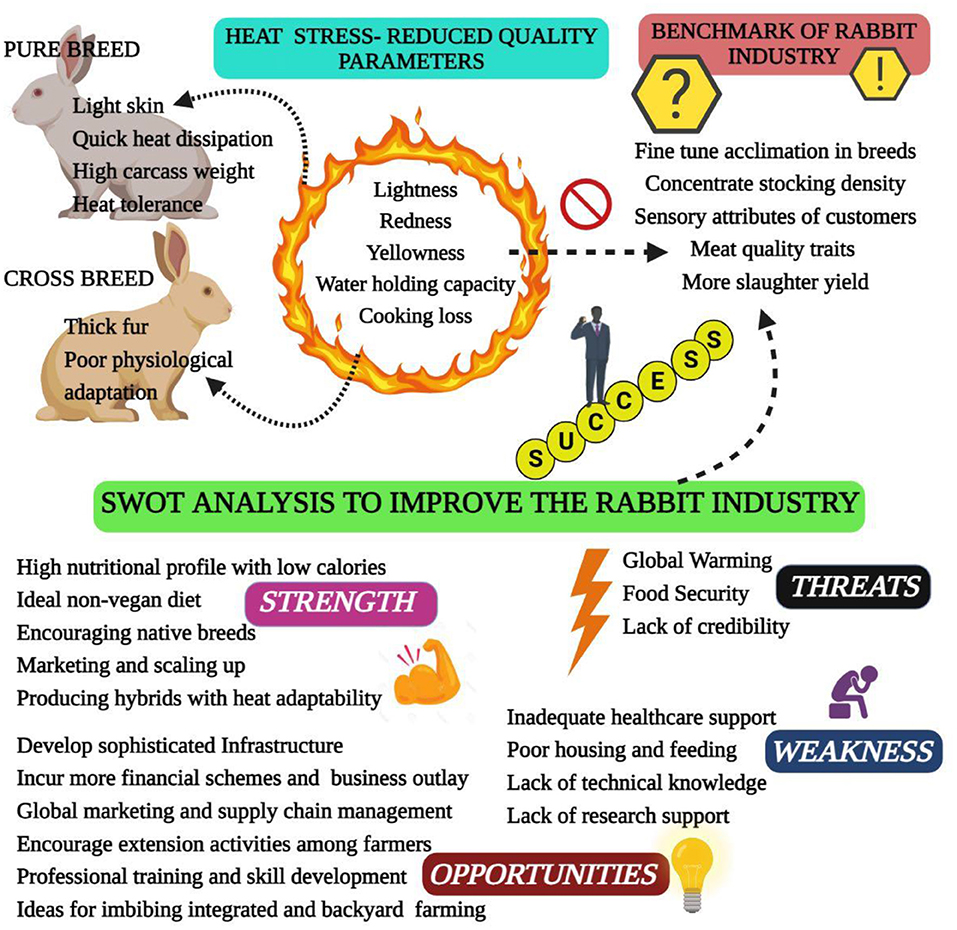
Figure 1. Challenges and opportunities for the rabbit industry in the context of global warming-induced heat stress.
Impacts of Heat Stress on Rabbits
Impacts on Immune Function and Endocrine
The immune dysfunction in rabbits caused by heat stress through the regulation of brain, sympathetic nerve and adrenal cortical hormone (11). The sympathetic adrenal medulla (SAM) axis is activated, which regulates the homeostasis in the early period of heat stress in rabbits, increased ambient temperature is perceived by the sympathetic nerves, transmitting the impulse to the adrenal medulla. The adrenal medulla increases the secretion of catecholamines, releasing glucose in the blood, depleting liver glycogen, reducing muscle glycogen, increasing respiration rate, vasodilating the peripheral blood vessels and increasing neural sensitivity to cope with the stress (13). When rabbits are exposed to a higher environmental temperature than their thermoneutral zone, the hypothalamic-pituitary-adrenal (HPA) axis is activated. The synthesis and secretion of hypothalamic adrenocorticotropic hormone releasing hormone (CRH) are increased significantly; CRH is used to act on the anterior pituitary to evoke the release of adrenocorticotropic hormone (ACTH) and it acts on the adrenal gland to promote the synthesis and secretion of glucocorticoid (14). Glucocorticoid provides the function of anti-immune response (15). The increased glucocorticoid inhibits cellular and humoral immunities, the protein synthesis in the lymphoid tissue and immune organs can be decreased, which eventually leads to a significant decline in overall immune function, and the weight of the thymus and spleen also decreases in heat stress treatment (16). Thus, heat stress reduces the immune function and causes rabbits are vulnerable to pathogens, bringing serious losses to rabbit production (17–19).
Under high ambient temperature, the synthesis of hypothalamic thyroid stimulating hormone (TSH) is highly reduced, resulting in a decrease of TSH in the anterior lobe of the pituitary gland and a reduction of thyroid hormone in rabbits; the decreased thyroid hormone could lower the metabolic rate and heat production of rabbits (20). In the early stage of heat stress, the body of rabbits accelerates the oxidation rate and peripheral circulation to resist the heat stress, and the levels of triiodothyronine (T3) and thyroxine (T4) were increased significantly, thus increasing the body's heat dissipation (20). With the extension of heat stress, the thyroid hormone level is gradually decreased (21). Reduced thyroid hormone affects the synthesis of protein (e.g., total blood protein, albumin and globulin) and causes the metabolic disorders of carbohydrates, fat and minerals in rabbits (22, 23).
Impacts on Blood Biochemical Indexes
The blood biochemical index plays a crucial role in reflecting the metabolic changes and organ damage in rabbits under heat stress situation. The concentrations of total protein, blood glucose and triglyceride are decreased, whereas cholesterol concentrations are markedly increased during heat stress in rabbits (16, 24). These results can be attributed to the increased glucocorticoid secretion, which promotes gluconeogenesis process (25–27). In a previous study, immune cell proliferation and immunoglobulins synthesis were reduced, indicating the negative effect of heat stress on immune cell proliferation and differentiation (28). Yang et al. (29) showed that the differentially expressed genes in immune cells was involved in the cellular stress response, apoptosis, oxidative stress and glucose metabolism during heat stress. The concentrations of creatine phosphokinase (CPK), lactic dehydrogenase (LDH) and alkaline phosphatase (ALP) were decreased, and the glutamic pyruvic transaminase (GPT) and aspartate aminotransferase (AST) were increased in rabbits exposed to heat stress (30). The changes of these enzymes show significant liver damage and inflammation by heat stress in rabbits. In addition, heat stress causes alterations in minerals of rabbit plasma. Due to the increased ACTH by endocrine regulation, it promotes the glomeruli to retain sodium and excrete potassium so that the blood potassium level is reduced to maintain body fluid balance. Retaining sodium (Na) and removing potassium (K) increase the concentration of Na+ and decrease the concentration of K+ in serum, besides the concentration of copper ion (Cu2+) in plasma is decreased, while Zn2+ is increased during heat stress (14).
Impacts on Antioxidant Capacity in vivo
The produced free radicals are removed to maintain a dynamic balance in the body during thermoneutral temperature. As summarized in Figure 2, the redox balance is broken by heat stress, and a large number of reactive oxygen species (ROS) and their metabolites (ROS-M) are released to the blood of rabbits, which are prone to oxidative stress (31). The nuclear factor erythroid 2-related factor 2 (Nrf2) is a key factor in cellular oxidative stress response and a central regulator of cellular antioxidants (32). The expression of Nrf2 is parallel to the degree of oxidative stress to maintain the balance of the redox state in the body (33). The increased levels of ROS by heat stress, and the downstream target genes of Nrf2, including catalase enzyme (CAT), superoxide dismutase (SOD), glutathione sulfurtransferase (GST) and heme oxygenase (HO-1), are expressed to against oxidative stress (34–38). These antioxidant enzymes, such as SOD and CAT, can remove substances with strong oxidative activity, such as oxygen ion (O2−) and hydrogen peroxide (H2O2), through the synergistic effect in the body to maintain the physiological function and prevent cell damage (39). However, the concentration and activity of SOD and CAT in rabbits are decreased when rabbits exposed to heat stress (30, 40, 41). For instance, the activities of SOD and CAT in the pituitary and hypothalamus of New Zealand rabbits were decreased along with the higher temperature and longer exposure time (42). Interestingly, the antioxidant enzymes activities in the body temporarily increase in the early stage of heat stress by accelerating the clearance of oxygen free radicals and reducing the production of lipid peroxidation products; however, long-term and/or high-intensity of heat stress causes the increase of oxygen free radicals, and an excessive load of the Nrf2 pathway leads to a decrease in antioxidase activity and induces oxidative stress (43), thereby inducing an excessive accumulation of free radicals in rabbits and damaging all the components of the cell, including proteins, lipids and DNA (39). Then the concentration of serum metabolites (Malondialdehyde, MDA) causing organ damage had also been changed by heat stress in rabbits (44). The oxidative stress in rabbits is associated with lower production performance, severe health disorders and biological damage (45, 46). Hence, reducing oxidative stress is key means for alleviating the heat stress in rabbits.
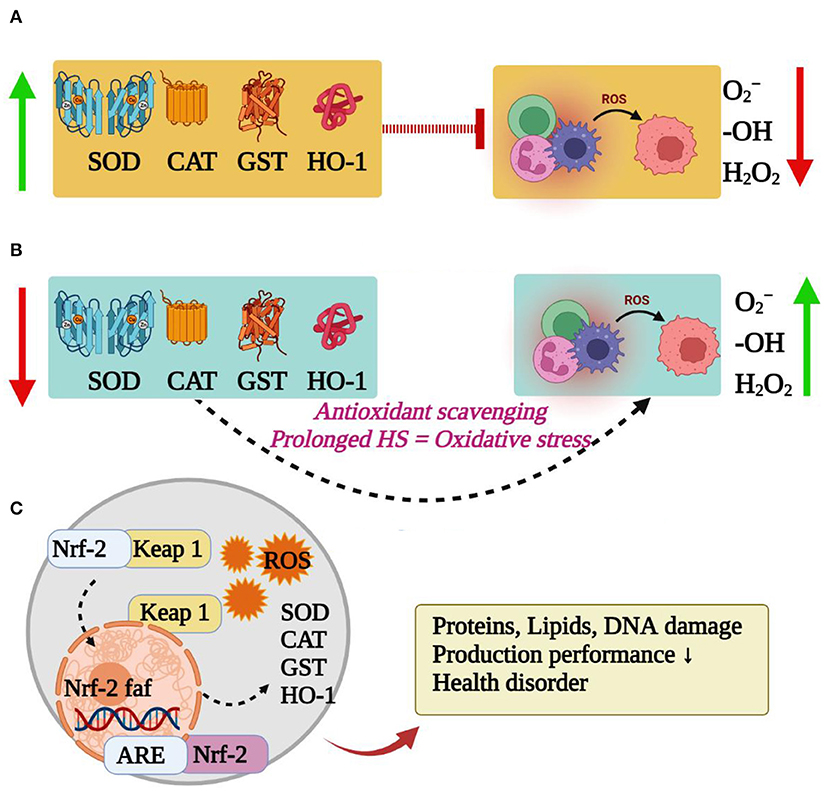
Figure 2. Effects of heat stress on antioxidant capacity and related signaling pathway of rabbits. (A) Thermoneutral temperature. (B) Heat stress. (C) Nrf pathway-Induction of oxidative stress.
Impacts on Reproductive Performance
The reproductive performance of rabbits is an important trait that affecting the economic benefits of rabbit production. The high reproductive performance leads to short generation interval in rabbit which provide the needed animal protein with low capital outlay and time (47). The most suitable temperature for rabbit reproduction ranges from 15 to 20°C. If the ambient temperature exceeds this range, rabbits are prone to stress and diseases, affecting their reproductive performance negatively (48).
Female Rabbits
The growth of embryos, pregnancy rate, litter size, litter weight and milk yield of female rabbits are affected by heat stress (49). The high temperature significantly reduces estrogen secretion and causes irregular estrus, it can cause abnormal morphology of egg cells, such as cytoplasmic shrinkage and rupture of the transparent membrane, making egg cells unable to fertilize and affecting the reproduction of female rabbits (20). In order to increase heat dissipation, pregnant rabbits flow a large amount of blood to the skin, the blood in the uterus and umbilical cord is greatly reduced during heat stress, which causes the serious insufficient blood supply to the fetus and embryo sac, and then the embryo becomes smaller, affecting the fetus growth and leading to a high mortality rate (48). The weight of pregnant rabbits and litter is significantly decreased during heat stress (49). Pregnant rabbits are sensitive to mutant heat stress, high temperatures above 35°C can cause salivation and neurological symptoms, and even abortion in pregnant rabbits (11). In addition, the lactation of female rabbits was adversely affected by high ambient temperature compared with the thermoneutral temperature during late pregnancy (24, 50).
Male Rabbits
Male rabbits are more sensitive to high temperatures than female rabbits. The synthesis and secretion of hypothalamic gonadotropin-releasing hormone (GnRH) are inhibited under high temperature, which significantly affects the function of testis and decreases the semen quality in male rabbits (51, 52). The ejaculation volume of young male rabbits decreased by 80%, the sperm vitality decreased by 75%, and the number of sperm per mL of semen decreased by 92% during heat stress due to the 51 days sperm production cycle of male rabbits and the 8–13 days sperm storage time in the epididymis (30). The temporary infertility of male rabbits lasts 45–70 days during heat stress, which is one of the reasons for the reproductive difficulties of male rabbits in autumn (53). Heat stress can affect the semen quality due to the accumulation of free radicals in the gonad of male rabbits, resulting in the damage of the antioxidant system (41). The semen quality decreases due to the heat stress that leads to a series of physiological and biochemical reactions in the testis, which change the internal microenvironment of the testis, the structural abnormalities of ROS, heat stress protein (HSP), mitochondrial, smooth endoplasmic reticulum etc. (52, 54). The integrity of DNA is destroyed, which induces the changes of sperm chromatin conformation and DNA methylation, thus damaging the spermatogenesis system and affecting the reproductive performance of male rabbits (54).
Impacts on Production, Carcass and Meat Quality
As summarized in Figure 3, when rabbits are exposed to heat stress, they dissipate excess heat produced inside the body manifested by specific behavioral and physiological changes in rabbits. Heat stress severely reduces the feed intake in rabbits due to the effect on the feeding center of the lower thalamus and heat increment (55). The sympathetic nerve resulting in reduced gastrointestinal function can decrease food intake during heat stress (21). Decreased feed intake results in a lower supply of nutrients, thus reducing the weight and growth rate in rabbits; besides, decreased endocrine and antioxidant capacity can lead to reduced immune function, decreased fat and protein deposits, organ and cell inflammation, growth rate and reproduction and increased mortality that can significantly decrease the heath, meat and hair production in rabbits (56–60).
Carcass and meat quality traits of rabbits, such as tenderness and color, are crucial to consumer acceptance (1). To strengthen the knowledge of how heat stress affects the carcass and meat quality traits is important to promote rabbit meat production. Heat stress affects the maintenance of heat balance in rabbits, resulting in the change of physiological adjustment and biochemical profile (61), which may decrease the production performance and affect the carcass and meat quality traits (62). Previous study has shown that the redness and yellowness of meat are decreased along with the increment of ambient temperature, which also increase the cooking loss and lead to the reduction of meat juiciness (63). Excessive ambient temperature depressed slaughter and carcass weight at the fixed market age, it can be explained that heat stress reduce the feed intake and performance of growing rabbits, and cause the dysplasia of gastrointestinal tract (64). On the contrary, the reference carcass percentage has a positive linear relationship with ambient temperature, which can be attributed to the decreased relative portion of metabolic active organs (chest, liver and kidney, etc.) along with the aggravation of heat stress (63). In summary, heat stress causes profound impact on production performance, carcass and meat quality of rabbits. Therefore, mitigation measures should be taken to minimize the negative impact of heat stress on rabbit production.
Potential Mitigation Strategies of Nutritional Intervention
Feeding Strategies to Prevent Heat Stress
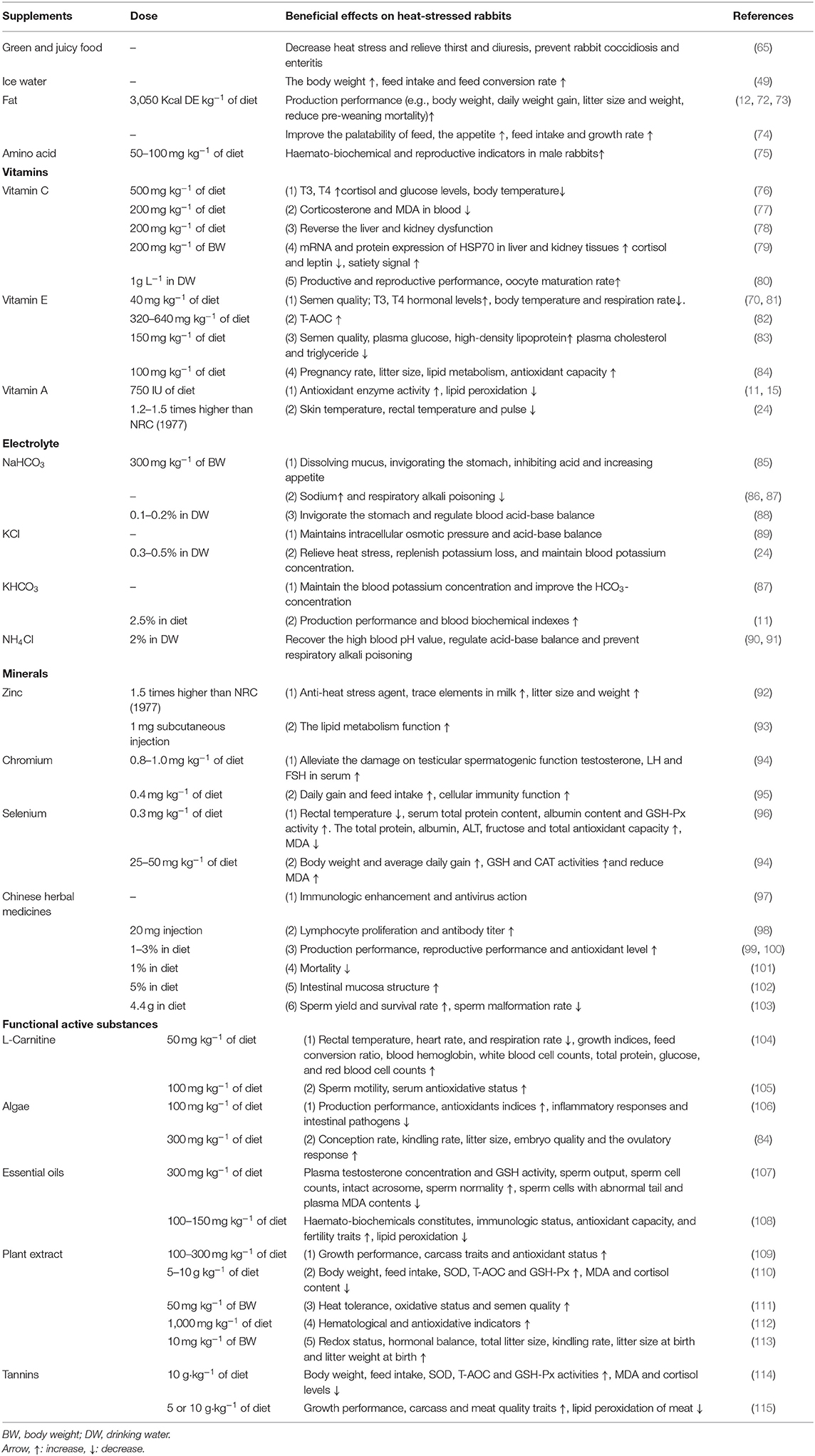
Compared with morning and evening, the appetite of rabbits is decreased in the summer noon. Therefore, the feeding strategy of rabbits needs to be combined with its physiological status to adjust the feeding time and amount. The specific strategies are to appropriately increase the feeding in the morning and evening, less fed or feeding of the green and juicy feeds are used at noon (40, 65–67). The green and juicy feeds can alleviate heat stress in rabbits. Feeding dandelion, Ixeris sonchifolia, watermelon peel and fruit peel can decrease heat stress and relieve thirst and diuresis, adding an appropriate amount of welsh onion and garlic to the rabbit diet can prevent rabbit coccidiosis and enteritis (65). Evaporative heat dissipation by increasing the breathing rate is an important way to dissipate heat during heat stress for the rabbit, which can cause water loss in vivo (68). Studies have shown that an adequate supply of drinking water can alleviate heat stress in rabbits. Stephan reported that when the temperature increased from 18 to 38°C, and the water requirement of rabbits increased by 50% (69). The earlier study found that the water intake of female rabbits at 30°C was 10.7% higher than the temperature of the control group (20°C) (70). It is better to provide low-temperature drinking water and add an appropriate amount of salt for rabbits, which can make up for the consumption of electrolytes in vivo and reduce heat stress (71). According to Marai et al. (49), the water intake, respiratory rate and rectal temperature of rabbits were decreased, but the body weight, feed intake and feed conversion rate were increased in rabbits after drinking cold water at 10–15°C during heat stress. However, excessive drinking water is unnecessary after the evaporation reaches its maximum level. Drinking too much water leads to increased urination, washing away the nutrients in the digestive tract, resulting in a decrease in nutrient retention and hindering the growth of rabbits (11). Taken together, the possible feeding and nutritional strategies in relieving heat stress impacts on rabbits have been shown in Table 1.
Adjusting Feed Formula
The feed intake of rabbits is significantly decreased during heat stress, resulting in insufficient nutrients and energy intake (12). Thus, increasing the dietary energy levels can be used to alleviate the adverse effects of heat stress, it has been found that the energy level in the rabbit diet should be 0.95 MJ·kg−1 higher than the recommended value of National Research Council (NRC) (1977) during summer (70). The energy level in the diet is improved by adding fat in the rabbit industry usually (116). Previous studies indicated that using high energy level feed can significantly improve the production performance (e.g., body weight, daily weight gain, litter size and weight, reduce pre-weaning mortality) and the serum albumin in rabbit during heat stress (16, 72, 73). Supplementing fat to the diet could also improve the palatability of feed, thereby increasing the appetite of rabbits (74). Increased appetite could ameliorate the decreased feed intake by heat stress and enhance the growth rate of rabbits. In a recent study, high energy feed was used to improve the heat stress, results showed that the rectal temperature, heart rate, and respiration rate decreased significantly in rabbits during heat stress (104). Moreover, due to the high unsaturated fatty acid content in vegetable fat helps rabbits to against heat stress, adding 3% vegetable fat to the diet can reportedly relieve the rabbit from heat stress (12).
During protein digestion, heat is produced in the metabolic process due to the serious heat increment, and the heat dissipation is greatly decreased during heat stress (117). Therefore, feeding a low protein diet and balanced amino acid pattern could be used to alleviate the heat stress of rabbits (118). Abdelnour et al. (75) suggested that increased the proline levels in feed could improve the haemato-biochemical and reproductive indicators in male rabbits during heat stress. The phycocyanin (100 mg/kg) which rich in amino acids was used to enhance the performance, antioxidants indices, decrease inflammatory responses and intestinal pathogens of growing rabbits during heat stress (106). Besides, it has been demonstrated that using plant protein in diet is better than that of animal protein under heat stress. When rabbits are subjected to heat stress, plant proteins could be selected according to the amino acid standard to prepare diets for reducing the adverse effects of heat stress (119).
In addition to adjusting the energy and protein concentrations in the feed formula, it has been found that the pellet feed has high nutrient density and ideal palatability, which can also improve the production performance of heat-stressed rabbits (11). Under heat stress situation, there was weak digestive capacity for rabbits, supplementing green forage in the feed formula can increase the cellulose and vitamins in digestive tract, improving appetite and digestion of rabbits (12). Therefore, an appropriate feed formula and form are beneficial for heat-exposed rabbits and relieving the detrimental effects of heat stress.
Application of Feed Additives
Vitamins
Vitamin C, known as ascorbic acid, provides anti-stress and antioxidant function (106). It has been wildly known that vitamin C supplementation can mitigate heat stress and increase the growth performance in rabbits (120). Vitamin C participates in the redox reaction and eliminates the free radicals produced by peroxidation in vivo to prevent tissue cells from oxidative damage (121). Vitamin C promotes the synthesis of antibodies, enhances the phagocytosis of leukocytes, and improves the detoxification ability of the liver (122). Studies have shown that dietary inclusion of vitamin C inhibits the increased body temperature in heat-stressed rabbits, increases the T3 and T4 levels, and decreases the concentration of cortisol and glucose, corticosterone and MDA in blood, thereby reducing the detrimental impact of heat stress in vivo (76, 77, 123). Based on previous report, adding 150–200 mg·kg-1 vitamin C to the diet improved the production performance of rabbits and reversed the liver and kidney dysfunction caused by heat stress (78). It has also been reported that vitamin C improves the mRNA and protein expression of heat shock protein (HSP70) in liver and kidney tissues, and reduces cortisol and leptin, improving the satiety signal in heat-stressed rabbits (79). On the other hand, adding fresh tap water supplemented daily with vitamin C (1 g/L) improves the productive and reproductive performance, and also the oocyte maturation rate (66–80%) in rabbit exposed to heat stress conditions (80).
Vitamin E is an intracellular antioxidant, and its lipid solubility makes it a suitable membrane antioxidant against oxidative damage, maintaining the function of the cell membrane system and reducing the release of creatine kinase in muscle cells during stress, thereby preventing excessive calcium influx and interfering with normal cell metabolism (124). Vitamin E can alleviate the immunosuppression caused by the release of adrenal cortex hormone at high temperatures and promote the synthesis of immunoglobulin to improve the body's disease resistance and reduce mortality (125), vitamin E can also reduce the serum cortisol levels, thereby reducing rabbit's adverse reactions to heat stress (126). Studies showed that adding vitamin E to the water increased the normal sperm, volume, count, motility, live sperm and T3, T4 hormonal levels, then decreased the rectal, skin, ear temperature and respiration rate, thus improving the fecundity of male rabbits (81). However, vitamin E supplementation had no significant effect on reducing the body temperature of rabbits under heat stress (24). Zhang et al. (82) found that vitamin E improved the total antioxidant capacity (T-AOC) of rabbits under acute heat stress. Similarly, Hashem et al. (83) indicated that inclusion of 150 mg·kg−1 vitamin E alleviated the negative impact of heat stress on semen quality, and increased the plasma glucose and high-density lipoprotein concentrations of male rabbits, but the plasma cholesterol and triglyceride concentration have been decreased during heat stress. It has been suggested that 100 mg·kg−1 vitamin E supplementation in female rabbits' diet could improve the pregnancy rate and litter size of heat-stressed rabbits, and increase the lipid metabolism and antioxidant capacity (84). However, feeding high doses of vitamin E causes the lack of other fat-soluble vitamins (117).
Vitamin A is associated with innate immunity, T cell proliferation and antibody production (127, 128). According to previous studies, vitamin A improves the body's antioxidant enzyme activity, reduces the degree of lipid peroxidation caused by free radicals, and enhances the rabbit's resistance during heat stress (11, 15). It has been found that feeding vitamin A to 3-month-old rabbits with 750 IU could decrease the skin temperature, rectal temperature and pulse of heat-stressed rabbits (24). Suggesting that the use of vitamin A could improve the growth performance and health of rabbits under heat stress. In addition, vitamin D, vitamin K and nicotinic acid also play an important role in regulating heat stress response and preventing high body temperature (129, 130), but more researches about these vitamins are needed.
Electrolyte
Because of the dense hair and absence of sweat glands, rabbits use their ears and respite to decrease the body temperature during heat stress (6). The dramatically increased respiration rate in response to heat stress causes a serious acid-base imbalance in rabbits by discharging excess CO2 (85, 117). Therefore, the appropriate addition of sodium bicarbonate (NaHCO3), potassium chloride (KCl), potassium bicarbonate (KHCO3) and ammonium chloride (NH4Cl) to the diet or drinking water of rabbits plays an important role in restoring the acid-base balance of rabbits under heat stress. The NaHCO3 is a kind of electrolyte additive and acid-base regulator, which equips the function of dissolving mucus, invigorating the stomach, inhibiting acid and increasing appetite (131, 132). It can be used to supply sodium and can work as the main buffer material in blood and tissue to decrease respiratory alkali poisoning and improve the ability to resist heat stress (86, 87). According to Zhou (88), NaHCO3 can be used to invigorate the stomach and regulate blood acid-base balance, adding 0.1–0.2% NaHCO3 to drinking water could reduce the loss of heat stress. KCl maintains intracellular osmotic pressure and acid-base balance (89). The secretion of the adrenocorticotropic hormone is increased in rabbits, thereby promoting the excretion of potassium by glomerular, resulting in decreased blood potassium levels (133, 134). Previous study has shown that supplementation of 0.3–0.5% KCl to drinking water could relieve heat stress, replenish potassium loss, and maintain blood potassium concentration (24). The KHCO3 can be used to maintain the blood potassium concentration and alleviate the HCO3- concentration caused by heat stress (87). It had been observed that dietary inclusion of KHCO3 (2.5%) improved the production performance and blood biochemical indexes of heat-stressed rabbits (11). NH4Cl was also reported to recover the high blood pH value caused by heat stress, which regulates acid-base balance and prevents respiratory alkalosis in rabbits (90, 91).
Zinc
The activity of more than 300 enzymes (e.g., oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases) requires zinc as substrates (135). It is also involved in the metabolism of enzymes in the body (136, 137). Zinc is related to the antioxidant defense system, immune function and skeletal development, a low level of zinc increases the oxidative damage of membrane caused by free radicals (138). Zinc supplementation inhibits free radicals because it is a part of SOD, GSH, GST and HO-1 (139). Moreover, zinc plays an essential role in the synthesis of metallothionein, which acts as a free radical scavenger (140, 141). Accordingly, zinc is an important element that needs to be added to feed when heat stress occurs in livestock and poultry (142). Zinc as an anti-heat stress agent mainly exists in the form of zinc monocarbonate (ZnCO3), zinc sulfate monohydrate (ZnSO4·H2O), zinc pyridine acid (ZnPic), bacitracin zinc, granular coated bacitracin zinc, amino acid zinc, and so on. They can act alone or interact with each other and are combined with vitamins for anti-heat stress. It is noted that the use of bacitracin zinc should be permitted by the local law. The release of trace elements to milk was increased when zinc was upregulated by 50% in the rabbit diet under heat stress, and the litter size and weight were significantly affected by Zn addition (92). Because of high temperatures, the lipid metabolism of animals is disordered, resulting in fat deposition; dietary supplementation of zinc could improve the lipid metabolism function in rabbits under heat stress, this may be related to the fact that zinc is involved in the synthesis of lipid metabolism enzymes (93).
Chromium
Chromium is an essential mineral, which is an integral component of chromodulin and also necessary for insulin functioning (143). The chromium is able to promote glucose transporter 4 (GLUT4) to transfer from cytoplasm to the cell membrane via activating insulin receptors, which in turn facilitates the entry of glucose into cells (144). Besides, chromium is an important component of the glucose tolerance factor (GTF), which affects the metabolism of glucose, lipids, proteins and nucleic acids by enhancing insulin activity (145). Supplementing 0.8–1.0 mg·kg−1 yeast chromium to the rabbits' diet alleviated the negative effects of heat stress on testicular spermatogenic function, luteinizing hormone (LH) and follicle stimulating hormone (FSH) in the serum of male rabbits (94). Huang et al. (95) demonstrated that organic chromium could significantly increase daily gain and feed intake of heat-stressed rabbits, and supplementation of 0.4 mg·kg−1 organic chromium to feed could improve the production performance, and inclusion of 1.6 mg·kg−1 organic chromium could be used to improve the cellular immunity function.
Selenium
Selenium is involved in many key physiological processes, such as reproduction, immunity and growth, and is an essential trace element for mammals (146). Selenium is an important component of at least 25 different selenium proteins, such as glutathione peroxidase (GSH-Px) and thioredoxin reductase (TrxRs) (147, 148), and the GSH-Px is a major phase II detoxification enzyme and can reduce the lipid peroxide level. Therefore, an appropriate amount of selenium in rabbit diets is crucial for the anti-oxidation and immune function of rabbits (149). Previous report suggested that inclusion of 0.034 mg·kg−1 organic selenium to the diet of heat-stressed rabbits decreases their rectal temperature by about 0.5°C. On the contrary, the serum total protein content, albumin content and GSH-Px activity were increased. Also, the total protein, albumin, alanine aminotransferase (ALT), fructose content and total antioxidant capacity in seminal plasma were increased, while the MDA content was significantly decreased (96). Adding biosynthesized nano-selenium (25 or 50 mg·kg−1) to the feed could increase the body weight and average daily gain of the rabbit under heat stress, improve GSH and CAT activities and reduce the serum MDA content (150). Considering the higher biological activity of nano selenium, future researches should focus more on the relieving effect of nano-selenium on heat-stressed rabbits.
Compound Chinese Herbal Medicines
Chinese herbal medicines and their ingredients equip immunologic enhancement and antivirus action, which can be used as immunopotentiator or anti-infection drugs (97). Chinese compound herbs have been suggested to promote lymphocyte proliferation and enhance antibody titer in rabbits (98). Numerous studies have indicated that the addition of some heat-clearing, detoxicating, bactericidal and disease-resistant Chinese herbal medicines, such as Radix Bupleuri, Rhizoma Coptidis and Artemisia annua, to the diet of rabbits can reduce the problems associated with heat stress and improve the production performance, reproductive performance and antioxidant level of heat-stressed rabbits (99, 100). The traditional Chinese medicine formula (including Radix Rehmanniae, Rhizoma Coptidis, mulberry bark, etc.) was reported to decrease the rabbit's mortality during heat stress (101). Wang et al. (102) found that using 5% Chinese herbal compounds (e.g., Huoxiang, Atractylodes, Rhizoma Coptidis, etc.) to the feed of New Zealand rabbits reduced the damage of rabbit intestinal mucosa structure during heat stress. In addition, Li et al. (103) have found that Wuzi Yanzong Pill could increase the reproductive capacity of male rabbits in summer, thus improving the sperm yield and survival rate, and reducing the sperm malformation rate. Chinese herbal medicines contain active ingredients, such as flavonoids, polysaccharides, polyphenols, alkaloids, etc., which exhibits antioxidant, anti-inflammatory, antibacterial and antiviral properties (97). This could explain the role of Chinese herbal medicines in relieving heat stress in rabbits. However, due to the composition of Chinese herbal medicines is complex, which may lead to inconsistent conclusions in the application. Further researches on the use of Chinese herbal medicines to ameliorate heat stress in rabbits are still required; in particular, there is a need to elucidate the active ingredients in Chinese herbal medicines.
Functional Active Substances
At present, the functional active substances, including L-carnitine, algae, essential oils, plant extract, tannins etc., which have been got growing attentions and were also used to improve the health and production in rabbit during heat stress.
L-carnitine (LC) is a functional additive which work as an important role in fatty acid metabolism and energy production (151). It plays an essential role in the oxidation of mitochondrial fatty acids by transporting long-chain fatty acids like acylcarnitine esters through the inner mitochondrial membrane (152). Supplementation of 50 mg of LC kg−1 in basal diet could decrease the rectal temperature, heart rate, and respiration rate, whereas enhance the growth rate and feed conversion ratio in heat-stressed rabbit; rabbit fed LC contained diet also showed higher blood hemoglobin, white blood cell counts, total protein, glucose and red blood cell counts, compared with those fed basal diet (104). Adding 100 mg·kg−1 LC to male rabbit diet was observed to improve heat stress-induced impairment of semen quality (105). These beneficial effects may be related to LC improving the antioxidant capacity and up-regulating HSP expression (153).
Algae have been exhausted for several years as nourishment for people and animals owing to the outstanding nutritious profile and great carotenoid substance (154). The algae like spirulina platensis usually employed as a food complement and a good source of vitamins and proteins (155). It has been reported that the phycocyanin (the main active components of spirulina platensis) ameliorates lipid peroxidation and inhibits the generation of pro-inflammatory cytokines (IL-1, IL-6, and TNF-a) and the activities of inducible nitric oxide synthase (iNOS) as well as cyclooxygenase 2 (COX-2) enzymes (156). Phycocyanin (100 mg·kg−1 in basal diet) was also reported to enhance the growth performance, antioxidants indices and decrease the inflammatory responses and intestinal pathogens in heat-stressed rabbit (106). A recent study showed that Spirulina platensis (300 mg·kg−1 in basal diet) increases the conception rate, kindling rate, litter size, embryo quality and the ovulatory response (corpora lutea number and ovulation rate) in rabbit during heat stress (84). Therefore, the mode of action of algae in alleviating heat stress impacts of rabbits was possibly due to its anti-inflammatory and antioxidant activity (155).
The essential oils have been widely used as effective feed additives for promoting the growth performance in animals, and they appear many beneficial aspects such antimicrobial and antioxidant functions (156). Adding 0.5–1.5 mL·kg−1 grape seed oil to basal diet can increase the body weight and feed intake in rabbit (157). El-Ratel et al. (107) demonstrated that extra virgin olive oil supplementation (300 mg·kg−1) increases plasma testosterone concentration and GSH activity, sperm output, sperm cell counts, intact acrosome, sperm normality, while reduces sperm cells with abnormal tail and plasma MDA contents in male rabbits under heat stress. Abdelnour et al. (108) found that inclusion of 100–150 mg·kg−1 thyme essential oil in the diet improves the haemato-biochemicals constitutes, immunologic status, antioxidant capacity, reduces the lipid peroxidation, thus enhancing the fertility traits (e.g., litter size, viability rate at birth, viability rate at weaning) and milk production. The positive effects of essential oils mainly attributed to the antioxidant activity (156). However, certain types of essential oils have a characteristic odor that may affect the rabbit's appetite, which should be avoided in application.
Plant extract has also been used to decrease the heat stress in recent year. Grape seed extract contains a lot of polyphenols, which are powerful free radical scavengers and antioxidants to decrease the oxidative stress and DNA damage (158). It has been revealed that the grape seed extract against heat stress to maintain performance, carcass traits and antioxidant status in rabbit (109). Authors attributed their findings to the presence of procyanidin from the grape seed extracts, which reduced heat stress impacts and improved rabbit's health (159). Procyanidin is a type of flavonoids and have been reported that can trap lipid peroxides and free radicals, chalate to free iron molecules (inhibit iron-induced lipid peroxidation), non-competitively inhibit xanthine oxidase (a major generator of free radicals) (110). These characteristic of procyanidin can be used to interpret how the grape seed extract improve rabbit's health from heat stress. Furthermore, El-Desoky et al. (111) reported that Moringa oleifera leaves ethanolic extract (MLEE) supplementation at a level of 50 mg·kg−1 body weight could be effectively used to enhance heat tolerance, oxidative status and semen quality of rabbit bucks under heat stress. Al-Sagheer et al. (112) suggested that addition of 1,000 mg·kg−1 in diet could improve the hematological and antioxidative indicators, and efficiently mitigate the detrimental effects of chronic temperature stress on performance, hematobiochemical features, and oxidative stability. El-Desoky et al. (113) indicated that nanoencapsulated MLEE has 30 active components, which alleviates (supplemental dose of 10 mg·kg−1 body weight) the negative impacts of heat stress by improving metabolism, redox status, and hormonal balance of rabbit does during summer, thereby increase the reproductive performance (e.g., total litter size, kindling rate, litter size at birth and litter weight at birth). The mode of action of plant extract is complex due to it contains a variety of active components, which still needs further in-depth researches.
Tannins are a group of polyphenolic compounds, which consist of aromatic rings with one or more hydroxyl groups, which can combine with free radicals to form resonance-stabilized phenoxyl radicals, this structure indicate the strong antioxidant properties of tannins (160). It has been reported that the body weight, feed intake, SOD, T-AOC and GSH-Px activities were increased, and the MDA and cortisol levels were decreased by tannins supplementation (10 g·kg−1 of diet) in heat-stressed rabbit (114). Liu et al. (115) suggested that addition of 5 or 10 g·kg−1 of tannins in diet could improve the growth performance, carcass and meat quality traits, and certain stress parameters in rabbits reared under high ambient temperature, and the tannins also showed inhibitory effects on heat stress-induced lipid peroxidation of rabbit meat.
The role of functional active substances in relieving heat stress of rabbits is due to the biological activities, such as antioxidant, anti-inflammatory, antibacterial functions, therefore, the bioactive substances have broad application prospects in rabbits under heat stress situation. However, existing reports mainly researched the protective effects of active substances on growth rate, carcass traits, meat quality, reproductive performance, redox status, and immune response of heat-stressed rabbits, the study of molecular mechanism is lacking. In future studies, it is necessary to elucidate the underlying mechanisms of the beneficial effects of active substances in rabbits under heat stress. Meanwhile, it is also worthwhile to study the mitigation effect of active substances on heat stress-induced impairment of intestinal barrier function in rabbits.
Conclusions
In summary, heat stress has been a severe challenge for modern rabbit industry, especially in the tropical and subtropical regions. Heat stress results from several factors (e.g., high environmental temperature and humidity, high stocking density), which causes a series of unfavorable changes in immune function, endocrine, blood biochemical indexes and antioxidant capacity, thus negatively affecting the production performance (e.g., growth rate, carcass and meat quality, reproductive performance) in rabbits. Therefore, researchers need to further study the physiological change caused by neuroendocrine under heat stress and figure out a holistic approach to attenuate the detrimental effect of heat stress on rabbits. The potential use of feeding strategies and nutritional regulation could be beneficial to ameliorate heat stress. Further studies should be attempted on the combination of several approaches for relieving heat stress and evaluating the efficiency and economic benefit in rabbit production.
Author Contributions
BB and W-CL: conceptualization and writing—review and editing. Z-LL, SP, and FC: selection and collection of bibliographies, and the data curation. Z-LL and FC: writing—original draft preparation. W-CL: supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cullere M, Zotte AD. Rabbit meat production and consumption: state of knowledge and future perspectives. Meat Sci. (2018) 143:137–46. doi: 10.1016/j.meatsci.2018.04.029
2. FAOSTAT. The Statistics Division of the FAO. (2020). Available online at: http://www.fao.org/faostat/en/#data (accessed February 17, 2022).
3. Zotte AD. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livestock Prod Sci. (2002) 75:11–32. doi: 10.1016/S0301-6226(01)00308-6
4. Zotte AD, Szendroe Z. The role of rabbit meat as functional food. Meat Sci. (2011) 88:319–31. doi: 10.1016/j.meatsci.2011.02.017
5. Dadáková E, Pelikánová T, Kala P. Concentration of biologically active polyamines in rabbit meat, liver and kidney after slaughter and their changes during meat storage and cooking. Meat Sci. (2012) 90:796–800. doi: 10.1016/j.meatsci.2011.11.017
6. Marai IFM, Haeeb AAM, Gad AE. Biological functions in young pregnant rabbit does as affected by heat stress and lighting regime under subtropical conditions of Egypt. Trop Subtrop Agroecosyst. (2007) 7:165–76.
7. Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, et al. Stunning methods and slaughter of rabbits for human consumption. EFSA J. (2020) 18:e05927. doi: 10.2903/j.efsa.2020.5927
8. Chiericato GM, Bailoni L, Rizzi C. The effect of environmental temperature on the performance of growing rabbits. J Appl Rabbit Res. (1992) 15:723–31.
9. Lara LJ, Rostagno MH. Impact of heat stress on poultry production. Animals. (2013) 3:356–69. doi: 10.3390/ani3020356
10. Li CY, Kuang LD, Ren YJ, Mei YL, Yang C, Lei M, et al. Preliminary observation of meat rabbit behavior under continuous heat stress. Heilongjiang Anima Husb Vet Med. (2016) 22:196–9. doi: 10.13881/j.cnki.hljxmsy.2016.2031
11. Song Z, Zhao G, Zhang Y. The effect of heat stress on rabbits and its nutrition regulation. Feed Res. (2006) 07:19–22. doi: 10.3969/j.issn.1001-0084.2006.07.007
12. Yan Y, Li M. Feeding Management Technology of Breeding Rabbit in Hot Climate. Qingdao Kanada Food Company Limited Kanada Group. (2008). p. 25–27. Available online at: http://hostcambodia.com/mekarn/prorab/yan.htm
13. Ferguson AV, Bauce L, Veale WL, Cooper KE. An investigation of the age-related deficits in the febrile response of the rabbit. Am J Physiol. (1983) 245:R379–85. doi: 10.1152/ajpregu.1983.245.3.R379
14. Harbuz MS, Jessop DS, Chowdrey HS, Blackwell JM, Larsen PJ, Lightman SL. Evidence for altered control of hypothalamic CRF in immune-mediated diseases. Ann N Y Acad Sci. (2010) 771:449–58. doi: 10.1111/j.1749-6632.1995.tb44701.x
15. Bellavance MA, Rivest S. The HPA – immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. (2014) 5:136. doi: 10.3389/fimmu.2014.00136
16. Ayyat MS, Marai IFM. Effects of heat stress on growth, carcass traits and blood components of New Zealand White rabbits fed various dietary energy–fibre levels, under Egyptian conditions. J Arid Environ. (1997) 37:557–68. doi: 10.1006/jare.1997.0308
17. Zhang FT, Zhen HJ, Qiu CZ, Liao WJ, Hua XC. Experimental study on rabbit heat stroke. Chin J Pathophysiol. (1996) 04:406–10.
18. Wen SY, Fang H. Diagnosis, treatment and precautions of a case of rabbit heatstroke. Jilin Anim Husb and Vet Med. (2010) 268:41–2. doi: 10.3969/j.issn.1672-2078.2010.07.021
19. Zhang FT, Hua XC, Yan WS, Zhen HJ. Experimental study on respiratory failure of rabbits with heat stroke. Chin J Occup Dis Labor Health. (1998) 2:27–9.
20. Garcíal ML, Argente MJ. Exposure to high ambient temperatures alters embryology in rabbits. Int J Biometeorol. (2017) 61:1555–60. doi: 10.1007/s00484-017-1334-0
21. Habeeb AAM, Aboulnaga AI, Yousef HM. Influence of exposure to high temperature on daily gain, feed efficiency and blood components of growing male Californian rabbits. Egypt J Rabbit Sci. (1993) 3:73–80.
22. Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. (2006) 281:20666–72. doi: 10.1074/jbc.M512671200
23. Abdel-Hamid TM, El-Tarabany MS. Effect of bee pollen on growth performance, carcass traits, blood parameters, and the levels of metabolic hormones in New Zealand White and Rex rabbits. Trop Anim Health Prod. (2019) 51:2421–9. doi: 10.1007/s11250-019-01961-8
24. Hen Li, Wang FY. Research progress of rabbit heat stress. Chin J Rabbit Raising. (2004) 4:28–30.
25. Gallagher ML, Mcdowell LR. Vitamins in animal nutrition: comparative aspects to human nutrition. Bioscience. (1990) 40:693–95.
26. Kumari KNR, Nath DN. Ameliorative measures to counter heat stress in poultry. Worlds Poult Sci. J. (2018) 74:117–30. doi: 10.1017/S0043933917001003
27. Siegel HV, Kampen MV. Energy relationships in growing chickens given daily injections of corticosterone. Br Poult Sci. (1984) 25:477–85. doi: 10.1080/00071668408454889
28. Amici A, Franci O, Mastroiacono P, Merendino N, Nardini M, Tomassi G. Short term acute heat stress in rabbits: Functional, metabolic and immunological effects. World Rabbit Sci. (2000) 8:11–6. doi: 10.4995/wrs.2000.412
29. Yang SH, He JB Yu LH, Li L, Xu SW. Effect of reduplicative heat stress on oxidation damage in gonad of male rabbit. Chin Vet Sci. (2012) 5:241–51. doi: 10.16656/j.issn.1673-4696.2012.01.010
30. Maria SA, Oriol TP, Maria TM, Jane MM, Manel LB. Heat stress has an effect on motility and metabolic activity of rabbit spermatozoa. Anim Reprod Sci. (2016) 173:18–23. doi: 10.1016/j.anireprosci.2016.08.004
31. Jimoh OA, Ewuola EO, Balogun AS. Oxidative stress markers in exotic breeds of rabbit during peak of heat stress in Ibadan, Nigeria. J Adv Biol Biotechnol. (2017) 12:1–9. doi: 10.9734/JABB/2017/30437
32. Cui Y, Ma HY, Kong L. Research progress of Nrf2/ARE pathway and body's antioxidant mechanism. J Jilin Univ Med Ed. (2011) 37:187–90. doi: 10.13481/j.1671-587x.2011.01.001
33. Jiang T, Huang Z, Lin Y, Zhang Z, Fang D, Zhang DD. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes. (2010) 59:850–60. doi: 10.2337/db09-1342
34. Chartoumpekis DV, Kensler TW. New player on an old field; the keap1 /Nrf2 pathway as a target for treatment of type 2 diabetes and metabolic syndrome. Curr Diabetes Rev. (2013) 9:137–45. doi: 10.2174/1573399811309020005
35. Kumar H, Kim IS, More SV, Kin BW, Choi DK. Natural productderived pharmacological modulators of Nrf2 /ARE pathway for chronic diseases. Nat Prod Rep. (2014) 31:109–39. doi: 10.1039/C3NP70065H
36. Zhuang C, Miao Z, Sheng C, Zhang W. Updated research and applications of small molecule inhibitors of Keap1-Nrf2 protein-protein interaction: a review. Curr Med Chem. (2014) 21:1861–70. doi: 10.2174/0929867321666140217104648
37. Gao P, Li L, Ji L, Wei Y, Li H, Shang G, et al. Nrf2 ameliorates diabetic nephropathy progression by transcriptional repression of TGFbeta1 through interactions with c-Jun and SP1. Biochim Biophys Acta Gene Regul Mech. (2014) 1839:1110–20. doi: 10.1016/j.bbagrm.2014.06.018
38. Vincent AM, Kato K, McLean LL, Soules ME, Feldman EL. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal. (2009) 11:425–38. doi: 10.1089/ars.2008.2235
39. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Ann Rev Pharmacol Toxicol. (2007) 47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046
40. Jimoh OA, Ayedun ES, Oyelade WA, Oloruntola OD, Daramola OT, Ayodele SO, et al. Protective effect of soursop (Annona muricata Linn) juice on oxidative stress in heat stressed rabbits. J Anim Sci Technol. (2018) 60:28. doi: 10.1186/s40781-018-0186-4
41. Kuang LD Li CY, Guo ZQ, Ren YJ, Zheng J, Mei XL, et al. Effects of heat stress on reproductive performance, serum biochemical indexes and reproductive hormones in female rabbit of Qixing. Southwest China J Agric Sci. (2021) 34:1323–9. doi: 10.16213/j.cnki.scjas.2021.6.027
42. Li DX, Yang SH, He JB. The effect of heat stress on the levels of CAT and SOD in rabbit pituitary gland and hypothalamus. Adv Vet Med. (2010) 31:77–80. doi: 10.16437/j.cnki.1007-5038.2010.06.020
43. Li CY Li J, Kuang LD, Mei XL, Guo ZQ, Lei M, et al. Study on blood indicators of meat rabbits at the initial stage of heat stress and their correlation with heat-resistant time. Chin J Anim Husb. (2017) 53:111–5. doi: 10.19556/j.0258-7033.2017-12-111
44. Garner JB, Chamberlain AJ, Jagt CV, Nguyen TTT, Mason BA, Marett LC, et al. Gene expression of the heat stress response in bovine peripheral white blood cells and milk somatic cells in vivo. Sci Rep. (2020) 10:19181. doi: 10.1038/s41598-020-75438-2
45. Lodovici M, Raimondi L, Guglielmi F, Gemignani S, Dolara P. Protection against ultraviolet B-induced oxidative DNA damage in rabbit corneal-derived cells (SIRC) by 4-coumaric acid. Toxicology. (2003) 184:141–7. doi: 10.1016/S0300-483X(02)00572-3
46. Abdel-Khalek AM. Supplemental antioxidants in rabbit nutrition: a review. Livest Sci. (2013) 158:95–105. doi: 10.1016/j.livsci.2013.10.019
47. Omeje VI. Effect of Dietary Supplementation of Organic Selenium at Different Levels on Reproductive Performance of Rabbit Does. Nsukka: University of Nigeria Virtual Library (2016). 5 p.
48. Marco-Jiménez F, García-Diego FJ, Vicente JS. Effect of gestational and lactational exposure to heat stress on performance in rabbits. World Rabbit Sci. (2017) 25:17–25. doi: 10.4995/wrs.2017.5728
49. Marai IFM, Habee AAM, Gad AE. Rabbits' productive, reproductive and physiological performance traits as affected by heat stress: a review. Livestock Prod Sci. (2002) 78:71–90. doi: 10.1016/S0301-6226(02)00091-X
50. Maertens L, De Groote G. Comparison of feed intake and milk yield of does under normal and high ambient temperature. J Appl Rabbit Res. (1990) 13:159–62.
51. Daader AH, Yousef MK, Abdel-Samee AM. Recent trends in rabbit does reproductive management: special reference to hot regions. In: Proceedings 1th World Rabbit Congress (Qingdao), (2016). p. 149–66.
52. Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online. (2015) 30:14–27. doi: 10.1016/j.rbmo.2014.09.018
53. Jie Z, Chao Y, Min L, Li T, Zhang XY, Xie XH. The effect of heat stress on the reproductive performance of rabbits and the research progress of related heat shock proteins. Rabbit Rais China. (2020) 235:19–22. doi: 10.3969/j.issn.1005-6327.2020.01.005
54. Zheng J, Xie XH, Lei M, Tang L, Zhang XY, Yang C. Research progress on the effect of heat stress on the semen quality of male rabbits and its mechanism. China Rabbit Rais. (2018) 6:24–8. doi: 10.3969/j.issn.1005-6327.2018.06.008
55. Bakr MH, TuselL L, Rafel O, Terré M, Sánchez JP, Piles M. Lactating performance, water and feed consumption of rabbit does reared under a Mediterranean summer circadian cycle of temperature v. comfort temperature conditions. Animal. (2015) 9:1203–9. doi: 10.1017/S1751731114003310
56. Yang LP, Gao SX, Bai LY, Zhang XL, Sun HT, Wang WZ, et al. Comparative study on hair production performance of long-haired rabbits in different seasons. China Rabbit Rais. (2016) 5:4–6. doi: 10.3969/j.issn.1005-6327.2016.05.001
57. Ayyat MS, Al-Sagheer AA, Abd El-Latif KM. Organic selenium, probiotics, and prebiotics effects on growth, blood biochemistry, and carcass traits of growing rabbits during summer and winter seasons. Biol Trace Elem Res. (2018) 186:162–73. doi: 10.1007/s12011-018-1293-2
58. Bahga CS, Kaur P, Handa MC. Performance of meat and wool type rabbit as affected by heat stress and microclimatic modification. Indian J Anim Res. (2010) 1:67–9. doi: 10.1016/j.domaniend.2009.07.004
59. Marongiu M L, Pinna W, Moniello G. Attard G, Floris BR. Rabbit meat production as affected by high temperatures: preliminary results. World Rabbit Sci. (2006) 14:27–8.
60. Jimoh OA, Oyeyemi BF, Oyeyemi WA. Soursop juice enhanced seminal antioxidant defence and semen quality of rabbit bucks in extremely dry climatic condition of Southwestern Nigeria. J Therm Biol. (2021) 8:103034. doi: 10.1016/j.jtherbio.2021.103034
61. Chiericato GM, Ravarotto L, Rizzi C. Study of the metabolic profile of rabbits in relation to two different environmental temperatures. World Rabbit Sci. (1994) 2:153–60. doi: 10.4995/wrs.1994.232
62. Zeferino CP, Moura ASAMT, Fernandes S, Kanayama JS, Scapinello C, Sartori JR. Genetic group 3 ambient temperature interaction effects on physiological responses and growth performance of rabbits. Livest Sci. (2011) 140:177–83. doi: 10.1016/j.livsci.2011.03.027
63. Zeferino CP, Komiyama CM, Fernandes S, Sartori JR, Teixeira PS, Moura ASA MT. Carcass and meat quality traits of rabbits under heat stress. Animal. (2013) 7:518–23. doi: 10.1017/S1751731112001838
64. Chiericato GM, Rizzi C, Rostellato V. Effect of genotype and environmental temperature on the performance of the young meat rabbit. World Rabbit Sci. (1993) 1:119–25. doi: 10.4995/wrs.1993.204
65. Li MY. New technology for breeding and management of meat breeding rabbits in summer. China Rabbit Rais. (2008) 6:8–11. doi: 10.3969/j.issn.1005-6327.2008.06.003
66. Du JH. How to raise rabbits in summer. Special Econ Anim Plants. (2006) 9:4. doi: 10.3969/j.issn.1001-4713.2006.07.004
67. Liang JM. Four measures to prevent heatstroke in domestic rabbits. Rural Breed Technol. (2009) 13:10. doi: 10.3969/j.issn.1007-0869.2009.13.005
68. Jimoh OA, Ewuola EO. Thermoregulatory response of exotic rabbit breeds during peak temperature humidity index of Ibadan. Trop Anim Prod Investigation. (2016) 19:41−7.
69. Stephan E. The influence of environmental temperatures on meat rabbits of different breeds. Commer Rabbit. (1980) 2:399–409.
70. Li QF, Han YL Li JG. Rabbit heat stress and its nutrition regulation. China Feed. (2003) 13:18–9. doi: 10.3969/j.issn.1004-3314.2003.13.009
71. Marai IFM, Habeeb AA, Gad AE. Rabbits productive, reproductive and physiological traits as affected by drinking saline water: a review. In: International Conference of Rabbit Production in Hot Climates Faculty of Agriculture (Assuit), (2010). p. 177−89.
72. El-Monem A, Mahrose KM, Khalil BA. Effects of increasing dietary energy level on the performance of adult New Zealand white and Flander rabbits under summer conditions of Egypt. Zagazig Vet J. (2009) 37:105–14.
73. El-Monem A, Kh M, Ba K. Effects of cage density and climatic conditions on the performance of growing rabbits. Zagazig Vet J. (2009) 37:198–208.
74. Montmayeur JP, Le CJ. Fat-rich food palatability and appetite regulation – fat detection: taste, texture, and post ingestive effects. Front Neurosci. (2010) 14:67767. doi: 10.1201/9781420067767
75. Abdelnour SA, Al-Gabri NA, Hashem NM, Gonzalez-Bulnes A. Supplementation with proline improves haemato-biochemical and reproductive indicators in male rabbits affected by environmental heat-stress. Animals. (2021) 11:373. doi: 10.3390/ani11020373
76. Daader AH, Al-Sagheer AA, Gabr HA, Abd El-Moniem EA, et al. Alleviation of heat-stress-related physiological perturbations in growing rabbits using natural antioxidants. Spanish J Agric Res. (2018) 16:e0610. doi: 10.5424/sjar/2018163-13184
77. Feng Q Z. Effects of vitamin C and coenzyme Q_(10) on plasma contents of nitric oxide nitric oxide synthase and malondialdehyde in rabbits fed with alcohol. Chin J Clin Rehabil. (2005) 27:62–3. doi: 10.5424/1671-5926(2005)27-0062-02
78. Wang WL, Xu YQ, Jiang BY. Research progress of rabbit heat stress. Feed Res. (2021) 44:140–143. doi: 10.13557/j.cnki.issn10022813.2021.03.033
79. Abdel-Latif M, Sakran T, Badawi YK, Abdel-Hady DS, et al. Influence of Moringa oleifera extract, vitamin C, and sodium bicarbonate on heat stress induced HSP70 expression and cellular immune response in rabbits. Cell Stress Chaperones. (2018) 23:975–84. doi: 10.1007/s12192-018-0906-1
80. Yassein S, Mahmoud KM, Maghraby N, Ezzo O. Hot climate effects and their amelioration on some productive and reproductive traits in rabbit does. World Rabbit Sci. (2008) 16:173–81. doi: 10.4995/wrs.2008.626
81. Sharaf AK, El-Darawany AA, Nasr AS, Habeeb AAM. Recent techniques for amelioration the effect of heat stress conditions on male rabbits. Zagazig J Agric Res. (2019) 46:501–14. doi: 10.21608/zjar.2019.33404
82. Zhang W, Li YQ, Qin YH Li FC. The effect of dietary supplementation of vitamin E on blood antioxidant indexes of growing rabbits under acute heat stress. Chin Rabbit Rais. (2007) 5:19–22. doi: 10.3969/j.issn.1005-6327.2007.05.010
83. Hashem NM, Abd El-Hady A, Hassan O. Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hematobiochemical changes of rabbit bucks during hot season. Livest Sci. (2013) 157:520–6. doi: 10.1016/j.livsci.2013.09.003
84. El-Ratel IT, Gabr AA. Effect of spirulina and vitamin E on reproduction and in vitro embryo production in heat-stressed rabbits. Pak J Biol Sci. (2019) 22:545–53. doi: 10.3923/pjbs.2019.545.553
85. Staten FE. Renal regulation of acid-base balance in heat stressed chickens. Austr N Z J Med. (1988) 11:1–5. doi: 10.1111/j.1445-5994.1981.tb03552.x
86. Anoh KU, Archibong EE, Nsa EE, Udoekong EC, Jimmy NP. Antioxidant activities of vitamin C and bi-carbonate buffers on hormones secretion and serum metabolites of heat-stressed rabbit buck. Ann Res Rev Biol. 37:59–68. doi: 10.9734/arrb/2022/v37i130478
87. Anoh KU, Barje PP, Erakpotobor GI, Akpa GN. Thermoregulatory response of growing rabbits fed diets supplemented with bicarbonate buffer, vitamin C and baobab fruit pulp meal. J Anim Prod Res. (2016) 28:50–4.
89. Zhu CB. Heat stress in rabbits and the mitigation measures. J Shandong Anim Husb Vet. (2010) 31:48–9. doi: 10.3969/j.issn.1007-1733.2010.08.029
90. Li Z, Li PQ. Comprehensive measures to relieve heat stress in rabbits. Rural Breed Technol. (2003) 15:9–10.
91. Zhou FS, Wang SR Li JH, Liu BL. Problems that should be paid attention to in raising rabbits in summer. China Rais Rabbits. (2008) 4:30. doi: 10.3969/j.issn.1005-6327.2008.04.023
92. Kowalska D, Bielanski P. The role of iron and zinc in the nutrition of rabbit does. Probl Notebooks Progr Agric Sci. (2009) 34:265–72.
93. Thomas JA, Thiert JP. Experimental production of liposarcomas in the rabbit by the trace elements zinc and cobalt. Compte Rendu Lacademie Sci. (1953) 236:1387–9.
94. Cheng YF, Zhao ML, Li XH, Liu LW, Wang HW, Geng GR, et al. Effects of dietary chromium levels on testicular tissue and serum reproductive hormones in heat-stressed male rabbits. Chin J Vet Med. (2015) 35:946–948, 952. doi: 10.16303/j.cnki.1005-4545.2015.06.20
95. Huang CB, Tang L, Guo ZQ, Yan JY, Xie XH, Lei M. Effects of organic chromium on the production performance and immune function of heat-stressed rabbits. Chin J Anim Husb. (2017) 53:93–5. doi: 10.19556/j.0258-7033.2017-03-093
96. Hosny NS, Hashem NM, Morsy AS, Abo-Elezz ZR. Effects of organic selenium on the physiological response, blood metabolites, redox status, semen quality, and fertility of rabbit bucks kept under natural heat stress conditions. Front Vet Sci. (2020) 7:290. doi: 10.3389/fvets.2020.00290
97. Xie XQ. Exploitation and Application of New-type Preparation of Chinese Herbal Medicine. Beijing: People Sanitary Press (2000). p. 377–83.
98. Sun JL, Hu YL, Wang DY, Zhang BK, Liu JG. Immunologic enhancement of compound Chinese herbal medicinal ingredients and their efficacy comparison with compound Chinese herbal medicines. J Nanjing Agric Univ. (2005) 24:2343–8. doi: 10.1016/j.vaccine.2005.11.053
99. Li YJ, Gu ZL, Liu YJ. Effects of adding traditional Chinese medicine residues on the production performance, blood biochemical indexes and immune function of rex rabbits under heat stress conditions. China Anim Husb Vet Med. (2011) 38:105–8. doi: 10.19556/1671-7236(2011)05-0105-04
100. Li YJ, Wen XJ, Dai FW, Gao L, Ye YJ, Huo XD. Effects of adding Chinese herbal medicine residues on the production performance, blood biochemistry and immune function of rex rabbits under heat stress conditions. China Feed. (2018) 5:53–6. doi: 10.15906/j.cnki.cn11-2975/s.20180512
101. Yang HJ, Sun FQ Li CR, Wang HJ, Feng CR. Prevention and treatment of heat stress in woolly rabbits with traditional Chinese medicine. Chin J Vet Med. (2011) 47:75–6. doi: 10.3969/j.issn.0529-6005.2011.05.034
102. Wang ZL, Zhu Z, Tang Q, Tu JP, Wang MZ, Wang CC. Effects of Chinese herbal compound on intestinal tissue structure and liver antioxidant function of heat-stressed rabbits. Chin J Vet Med. (2014) 50:48–54. doi: 10.3969/0529-6005(2014)06-0048-04
103. Li PP, Wu RZ, Xing JP, Wang XD, Gong XC. Effects of Wuzi Yanzong Pills on the reproductive ability of male rabbits in summer. Heilongjiang Anim Sci Vet Med. (2018) 2:177−8, 249. doi: 10.13881/j.cnki.hljxmsy.2016.12.0247
104. Ayyat MS, El-Latif A, Khaled M, Helal AA, Al-Sagheer AA. Interaction of supplementary L-carnitine and dietary energy levels on feed utilization and blood constituents in New Zealand White rabbits reared under summer conditions. Trop Anim Health Prod. (2021) 53:1–8. doi: 10.1007/s11250-021-02723-1
105. El-Tohamy MM, Kotp MS, El-Nattat WS, Mohamed AH. Semen characteristics and oxidative/antioxidati in semen and serum of male rabbits supplemented with antioxidants during heat stress. Iran J Appl Anim Sci. (2012) 2:175–83.
106. Abdelnour SA, Swelum AA, Salama A, Al-Ghadi MQ, Qattan SY, Abd El-Hack ME, et al. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital J Anim Sci. (2020) 19:1046–56. doi: 10.1080/1828051X.2020.1815598
107. El-Ratel IT, Attia KAH, El-Raghi AA, Fouda SF. Relief of the negative effects of heat stress on semen quality, reproductive efficiency and oxidative capacity of rabbit bucks using different natural antioxidants. Anim Biosci. (2021) 34:844. doi: 10.5713/ajas.20.0258
108. Abdelnour SA, El-Ratel IT, Peris SI, El-Raghi AA, Fouda SF. Effects of dietary thyme essential oil on blood haematobiochemical, redox status, immunological and reproductive variables of rabbit does exposed to high environmental temperature. Ital J Anim Sci. (2022) 21:51–61. doi: 10.1080/1828051X.2021.2006807
109. Hassan HA, Edrees GM, El-Gamel EM. El-sayed EA. Amelioration of cisplatin-induced nephrotoxicity by grape seed extract and fish oil is mediated by lowering oxidative stress and DNA damage. Cytotechnology. (2014) 66:419–29. doi: 10.1007/s10616-013-9589-8
110. García J, Nicodemus N, Carabaño R, De Blass JC. Effect of inclusion of defatted grape seed meal in the diet on digestion and performance of growing rabbits. J Anim Sci. (2002) 80:162–70. doi: 10.2527/2002.801162x
111. El-Desoky N I, Hashem N M, Elkomy A, Abo-Elezz ZR. Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal. (2017) 11:1549–57. doi: 10.1017/S1751731117000088
112. Al-Sagheer AA, Abdel Monem UM, Sayed-Ahmed EE, Khalil BA. Navel orange peel hydroethanolic extract as a phytogenic feed supplement: impacts on growth, feed intake, nutrient digestibility, and serum metabolites of heat stressed growing rabbits. Anim Biotechnol. (2021) 16:1–12. doi: 10.1080/10495398.2021.2011740
113. El-Desoky NI, Hashem NM, Gonzalez-Bulnes A, Elkomy AG, Abo-Elezz ZR, et al. Effects of a nanoencapsulated moringa leaf ethanolic extract on the physiology, metabolism and reproductive performance of rabbit does during summer. Antioxidants. (2021) 10:1326. doi: 10.3390/antiox10081326
114. Liu HW, Dong XF, Tong JM, Zhang Q A. comparative study of growth performance and antioxidant status of rabbits when fed with or without chestnut tannins under high ambient temperature. Anim Feed Sci Technol. (2011) 164:89–95. doi: 10.1016/j.anifeedsci.2010.09.020
115. Liu H, Zhou D, Tong J, Vaddella V. Influence of chestnut tannins on welfare, carcass characteristics, meat quality, and lipid oxidation in rabbits under high ambient temperature. Meat Sci. (2012) 90:164–9. doi: 10.1016/j.meatsci.2011.06.019
116. Cervera C, Blas E, Fernández Carmona J. Growth of rabbits under different environmental temperatures using high fat diets. World Rabbit Sci. (1997) 5:71–5. doi: 10.4995/wrs.1997.322
117. Yang FZ, Guo ZQ, Xie XH Yi J, Lei M. Research progress on nutrition regulation of rabbit heat stress. Chin Herbivores. (2011) 216:68–71. doi: 10.3969/j.issn.2095-3887.2011.04.024
118. Carabaño LRM, Villamide DMJ, García J, Nicodemus MN, Llorente A, Chamorro S, et al. New concepts and objectives for protein-amino acid nutrition in rabbits: a review. World Rabbit Sci. (2009) 17:1–14. doi: 10.4995/wrs.2009.664
119. Zhao GX, Wang YD, Li YQ, Zhang ZS, Zhu HZ, Zhang BQ. Effects of adding amino acids in low-protein diets on weight gain and slaughter performance of meat rabbits. Chin Rabbit Rais. (1997) 1:20–2.
120. Traber MG, Stevens JF. Vitamins C and E: Benefificial effffects from a mechanistic perspective. Free Radic Biol Med. (2011) 51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017
121. Anoh KU, Barje PP, Iyeghe-Erakpotobor GT, Akpa GN. Growth performance of heat stressed rabbits fed diets supplemented with synthetic and organic antioxidants. Niger J Anim Prod. (2017) 44:177–80. doi: 10.51791/njap.v44i5.1349
122. Khan R, Ali S, Mumtaz S, Andleeb S, Ulhaq M, Tahir HM, et al. Toxicological effects of toxic metals (cadmium and mercury) on blood and the thyroid gland and pharmacological intervention by vitamin C in rabbits. Environ Sci Pollut Res. (2019) 26:16727–41. doi: 10.1007/s11356-019-04886-9
123. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. (2017) 9:1211. doi: 10.3390/nu9111211
124. Packer L, Weber SU, Rimbach G. Molecular aspects of alphatocotrienol antioxidant action and cell signalling. J Nutr. (2001) 131:369S−73S. doi: 10.1093/jn/131.2.369S
125. Meydani SN, Blumberg JB. Vitamin E and the immune response. Nutrient Modul Immune Response. (1992) 16:223–228. doi: 10.1201/9781003066644-16
126. Sharaf AK, El-Darawany AA, Nasr AS, Habeeb AAM. Alleviation the negative effects of summer heat stress by adding selenium with vitamin E or AD3E vitamins mixture in drinking water of female rabbits. Biol Rhythm Res. (2021) 52:535–48. doi: 10.1080/09291016.2019.1613796
127. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of vitamin A in the immune system. J Clin Med. (2018) 7:258. doi: 10.3390/jcm7090258
128. Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. (2009) 15:401–9. doi: 10.1038/nm.1925
129. Ravid A. Vitamin D inhibits the activation of stress-activated protein kinases by physiological and environmental stresses in keratinocytes. J Endocrinol. (2002) 173:525. doi: 10.1677/joe.0.1730525
130. Lebas F. Vitamins in rabbit nutrition: literature review and recommendations. World Rabbit Sci. (2000) 8:185–92. doi: 10.4995/wrs.2000.438
131. Ansari AS, Badar A, Balasubramanian K, Lohiya NK. Contraception with RISUG® and functional reversal through DMSO and NaHCO3 in male rabbits. Asian J Androl. (2017) 19:389. doi: 10.4103/1008-682X.185000
132. Anoh KU, Ayuba D, Ozung PO, Udayi MA. Physiological performance of heat stressed growing rabbits fed diets supplemented with vitamin anti-oxidants and bicarbonate buffers. Afr J Biotechnol. (2022) 21:139–45. doi: 10.5897/AJB2021.17382
133. Besouw MTP, Bockenhauer D. Potassium Metabolism. Nephrology and Fluid/Electrolyte Physiology. Amsterdam: Elsevier (2019). p. 31–46.
134. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. (2015) 10:1050–60. doi: 10.2215/CJN.08580813
135. Wolfgang M. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutri. (2013) 4:82–91. doi: 10.3945/an.112.003038
136. Banaszak M, Górna I, Przysławski J. Zinc and the innovative zinc-α2-glycoprotein adipokine play an important role in lipid metabolism: a critical review. Nutrients. (2021) 13:2023. doi: 10.3390/nu13062023
137. Severo JS, Morais JBS, Freitas TEC, Andrade ALP, Feitosa MM, Fontenelle LC. The role of zinc in thyroid hormones metabolism. Int J Vitamin Nutr Res. (2019) 89:1–2. doi: 10.1024/0300-9831/a000262
138. Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. (2002) 21:291–5. doi: 10.1023/A:1021215111729
139. Lee SR. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longevity. (2018) 20:9156285. doi: 10.1155/2018/9156285
140. Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. (2007) 146:443–59. doi: 10.1016/j.cbpc.2007.07.010
141. Ebadi M, Leuschen MP, El Refaey H, Hamada FM, Rojas P. The antioxidant properties of zinc and metallothionein. Neurochem Int. (1996) 29:159–66. doi: 10.1016/0197-0186(95)00116-6
142. Li CX, Zhou AG, Wang ZS. Study on the anti-heat stress effect of zinc. Feed Ind. (2006) 16:56–9. doi: 10.3969/j.issn.1001-991X.2006.16.019
144. Vincent JB. Is the pharmacological mode of action of chromium (III) as a second messenger. Biol Trace Elem Res. (2015) 166:7–12. doi: 10.1007/s12011-015-0231-9
146. Qazi IH, Angel C, Yang H, Zoidis E, Pan B, Wu Z, et al. Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences. Antioxidants. (2019) 8:268–304. doi: 10.3390/antiox8080268
147. Zhou J, Huang K, Lei XG. Selenium and diabetes—Evidence from animal studies. Free Radic Biol Med. (2013) 65:1548–56. doi: 10.1016/j.freeradbiomed.2013.07.012
148. Naziroglu M, Yildiz K, Tamtürk B, Erturan I, Flores-Arce M. Selenium and psoriasis. Biol Trace Element Res. (2012) 150:3–9. doi: 10.1007/s12011-012-9479-5
149. Wang HL, Zhang JS Yu HQ. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. (2007) 42:1524–33. doi: 10.1016/j.freeradbiomed.2007.02.013
150. Sheiha AM, Abdelnour SA, Abd El-Hack ME, Khafaga AF, Metwally KA, El-Saadony MT. Effects of dietary biological or chemicalsynthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. (2020) 10:430. doi: 10.3390/ani10030430
151. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab. (2010) 7:30. doi: 10.1186/1743-7075-7-30
152. Tein I. Carnitine transport: pathophysiology and metabolism of known molecular defects. J Inherit Metab Dis. (2003) 26:147–69. doi: 10.1023/A:1024481016187
153. Qiao N, Chen H, Du P, Kang Z, Pang C, Liu B, et al. Acetyl-L-carnitine induces autophagy to promote mouse spermatogonia cell recovery after heat stress damage. Biomed Res Int. (2021) 2021:8871328. doi: 10.1155/2021/8871328
154. Hassan F, Mobarez S, Mohamed M, Attia Y, Mekawy A, Mahrose K. Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals. (2021) 11:756. doi: 10.3390/ani11030756
155. Farag MR, Alagawany M, Abd El-Hac ME, Dhama K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int J Pharmacol. (2016) 12:36–51. doi: 10.3923/ijp.2016.36.51
156. Abdel-Wareth AAA, Taha EMM, Südekum KH, Lohakare J. Thyme oil inclusion levels in a rabbit ration: evaluation of productive performance, carcass criteria and meat quality under hot environmental conditions. Anim Nutr. (2018) 4:410–6. doi: 10.1016/j.aninu.2018.02.004
157. Nasr AM, Ela SEDSA, Ismail IE, Aldhahrani A, Soliman MM, Alotaibi SS, et al. Comparative study among dietary supplementations of antibiotic, grape seed and chamomile oils on growth performance and carcass properties of growing rabbits. Saudi J Biol Sci. (2022) 29:2483–8. doi: 10.1016/j.sjbs.2021.12.016
158. Hwang JH, Chen JC, Yang SY, Wang MF, Liu TC, Chan YC. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. (2011) 121:361–4. doi: 10.1002/lary.21283
159. Hassan FA, Mahrose KM, Basyony MM. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch Anim Nutr. (2016) 70:141–54. doi: 10.1080/1745039X.2016.1139609
Keywords: rabbit, heat stress, production, mitigation strategy, nutritional intervention, immune function, redox status
Citation: Liang Z-L, Chen F, Park S, Balasubramanian B and Liu W-C (2022) Impacts of Heat Stress on Rabbit Immune Function, Endocrine, Blood Biochemical Changes, Antioxidant Capacity and Production Performance, and the Potential Mitigation Strategies of Nutritional Intervention. Front. Vet. Sci. 9:906084. doi: 10.3389/fvets.2022.906084
Received: 28 March 2022; Accepted: 02 May 2022;
Published: 26 May 2022.
Edited by:
Anusorn Cherdthong, Khon Kaen University, ThailandReviewed by:
Wuttigrai Boonkum, Khon Kaen University, ThailandAdham Al-Sagheer, Zagazig University, Egypt
Copyright © 2022 Liang, Chen, Park, Balasubramanian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balamuralikrishnan Balasubramanian, YmFsYS5tLmtAc2Vqb25nLmFjLmty; Wen-Chao Liu, bGl1d2NAZ2RvdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zi-Long Liang1†
Zi-Long Liang1† Balamuralikrishnan Balasubramanian
Balamuralikrishnan Balasubramanian Wen-Chao Liu
Wen-Chao Liu