- 1UAE Biotech Research Center, Abu Dhabi, United Arab Emirates
- 2Abu Dhabi Biotech Research Foundation, Seoul, South Korea
- 3Jilin Provincial Key Laboratory of Animal Model, Jilin University, Changchun, China
- 4Hilli E.T. Cloning and Surgical Centre Presidential Camels and Camel Racing Affairs, Abu Dhabi, United Arab Emirates
- 5Department of Biology, North-Eastern Federal University, Yakutsk, Russia
The present study investigated the effect of superstimulation to improve in vitro embryo production in the Gulf area, where the temperature is high. Holstein cows were classified into the control and superstimulation groups. Superstimulation was induced with a single intramuscular injection of pregnant mare serum gonadotropin (PMSG; 2500 IU) on day 14 of the estrus cycle (day 0; estrus). The development of follicles was evaluated by ultrasonography of the ovaries daily. At 40 h after the PMSG injection, oocytes were collected by the ovum pick-up (OPU) technique. OPU was performed at the same stage of the estrus cycle in the control group as in the superstimulation group. The number of follicles with a diameter of more than 6 mm and the number of retrieved cumulus-oocyte complexes were significantly higher in the superstimulation group than in the control group. Furthermore, the maturation rate was higher in the superstimulation group than in the control group. Cloned embryos were produced by somatic cell nuclear transfer using matured oocytes. The cleavage and blastocyst formation rates were significantly higher in the superstimulation group than in the control group. In conclusion, a single injection of PMSG can facilitate the efficient production of cloned cow embryos.
Introduction
The production of animals with superior genetic value is important for the success of the livestock industry according to the economic characteristics of the breed. Somatic cell nuclear transfer (SCNT), a technique for preserving superior traits in cattle, has been studied as an important alternative to enhance reproductive efficiency (1). Various factors affect the efficiency of SCNT, including donor cell type and cycle, and oocyte activation (2). Among them, the quantity and quality of oocytes are crucial factors for improving cloning (2). Holstein cows are monotocous animals with a long gestation period and produce small numbers of calves throughout their lives. Numerous primordial follicles exist in the ovary and have the potential to produce offspring, but only a small number of oocytes that mature and ovulate give rise to calves. The potential reproductive capacity of females can be improved by artificially maturing and recovering oocytes from females with excellent genetic traits and using these oocytes to produce live offspring.
The appropriate environmental temperature for Holstein cows is 5–25°C (3). Stress occurs when the environmental temperature exceeds the appropriate temperature range, which affects the health and reproduction of Holstein cows. Furthermore, under heat stress, fertility is reduced due to hormonal changes (4). Heat stress caused by high environmental temperatures also adversely affects the efficiency of follicle generation and follicle quality (5). Several studies reported that heat stress during the estrus cycle, in which small follicles grow into pre-ovulatory follicles, decreases follicular growth (5, 6). In the Arabian Peninsula, Holstein cows have only recently been raised and milked, but reproduction is difficult due to the unique climate characteristics of this region. Therefore, the present study determined whether cloning after superstimulation would to improve reproductive efficiency in heat-stressed cows in the high temperature environment of the United Arab Emirates (UAE).
In general, pregnant mare serum gonadotropin (PMSG) and follicle-stimulating hormone (FSH) are used for superstimulation of Holstein cows. FSH must be administered twice per day for 4–5 days for hyperstimulation due to its short half-life (7, 8). This is stressful for cows because it requires frequent injections, which is also inconvenient. Additionally, FSH stimulation requires the administration of FSH every 12 h. It is consequently associated with increased amounts of labor and costs. PMSG is a complex glycoprotein containing sialic acid whose administration stimulates secondary follicles to improve the development of follicular oocytes. Furthermore, the half-life of PMSG is longer than that of FSH and thus a single intramuscular (IM) injection of PMSG can be effective (9, 10). This study was conducted to investigate the superstimulation efficiency and embryo developmental capacity of oocytes through SCNT after a single PMSG intramuscular injection in the United Arab Emirates.
Materials and Methods
Chemicals
All chemicals used were purchased from Sigma (St. Louis, MO, USA) unless otherwise noted.
Animals
This study was conducted between April 2021 and June 2021. All experiments were performed in a commercial dairy farm (Al ain dairy farm) in the UAE. During this period, the average minimum temperature was 26.32°C and the average maximum temperature was 40.90°C (https://weather.com). The control group and the superstimulation group contained 229 and 133 Holstein cows, respectively. Female Holstein cows aged between 2 and 3 years and weighing 350 kg were used. Oocytes were collected simultaneously between 10 am and 2 pm from both the control and superstimulation groups. Animal experiments were conducted according to the animal study guidelines, which were approved by the ethics committee of the UAE Biotech Research Center (Approval No.: UAEBRC-B01). These guidelines comply with the ARRIVE guidelines, the UK Animals (Scientific Procedure) Act, 1986, and EU Directive 2010/63/EU.
Superstimulation, OPU, and in vitro Maturation
On day 14 of the natural estrus cycle (estrus; day 0), oocyte donors were injected with 2500 IU PMSG (Ceva, Libourne, France) to stimulate the ovaries (Figure 1). The oocyte donors were sedated with 10 mg of xylazine (Ceva, Libourne, France). Cumulus-oocyte complexes (COCs) were obtained using an Aloka Ultrasound Unit (Aloka, Tokyo, Japan) and a needle guide (Aloka) from follicles (≥6 mm) at 40 h after PMSG injection. The control group did not receive any hormonal treatment, and COCs were collected at the same stage of the estrus cycle as in the superstimulation group (Figure 1). Lumen needle (60 cm, 18-gauge) were used for follicle oocyte retrieval, along containing 15 ml capped tubes with 2 ml OPU solution (IVF Bioscience, Falmouth, UK) using a regulated vacuum pump. The in vitro maturation of oocytes was conducted according to the instruction of the manufacturer of IVM media. For IVM, COCs were cultured at 38°C with 5% CO2 in a humidified atmosphere for 22 h with IVM media (IVF Bioscience, Falmouth, UK).
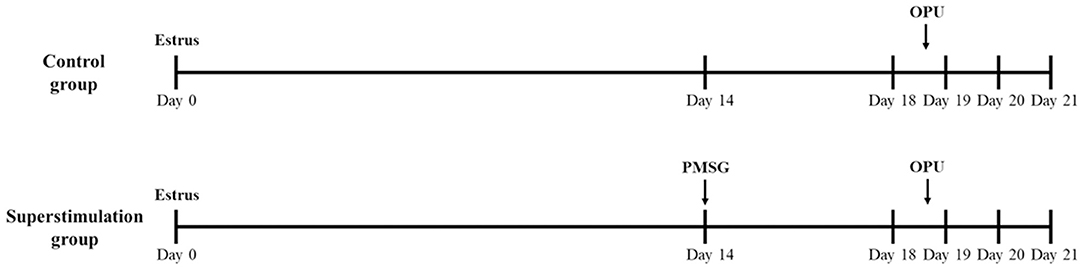
Figure 1. Superstimulation protocol to investigate the effect of PMSG injection prior to ovum pick-up (OPU) in Holstein cows. PMSG, pregnant mare serum gonadotropin; OPU, ovum pick-up.
Oocyte Nuclear Maturation Evaluation and Somatic Cell Nuclear Transfer
Donor cells were prepared using a conventional primary cell culture system as described previously (11). Evaluation of the maturation stage of the collected oocytes and SCNT were conducted as described previously with minor modifications (12). Cumulus cells were removed from oocytes with 0.1 % hyaluronidase. After denuding, metaphase II phase oocytes were stained with 5 μg/ml bisbenzimide for 3 min. The stained oocytes were enucleated by squeezing out the first polar body and the metaphase II spindle plate together with some of the surrounding cytoplasm using a glass pipette. A trypsinized fibroblast with a smooth cell surface was transferred into the perivitelline space of an enucleated oocyte. The couplets were equilibrated in 0.26 M mannitol, 0.1 mM MgSO4, 0.5 mM HEPES, and 0.05% (w/v) bovine serum albumin with two direct current pulses of 1.8 kV/cm for 15 μsec using a BTX Electro Cell Manipulator (BTX Inc., San Diego, CA, USA). After fusion, the reconstructed embryos were treated with 5 μM ionomycin for 4 min and with 2.0 mM 6-dimethylaminopurine in BO-IVC (IVF Bioscience) in a humidified incubator containing 5% CO2 at 38°C for 4 h. Following activation, 6–8 embryos were cultured in an oil-covered droplet at 38°C in a humidified atmosphere containing 5% CO2 and 5% O2.
Statistical Analysis
Between-group comparisons were performed using the independent T-test of variance with SPSS for Windows (version 23; SPSS Inc., Chicago, Il, USA). Statistical significance between mean values was assessed using Duncan's multiple range test. P-values < 0.05 were considered statistically significant. Data are presented as the mean ± standard error (SE).
Results
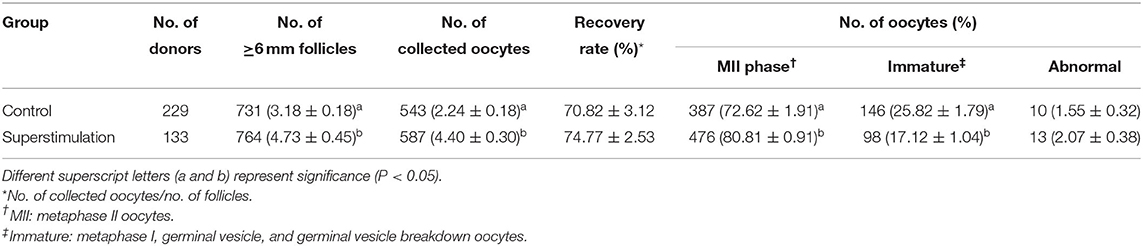
Data concerning follicular growth and collected oocytes from 362 donors in the superstimulation and control groups are presented in Table 1. The average number of follicles (≥6 mm) and collected oocytes were significantly higher in the superstimulation group than in the control group (Table 1). However, the recovery rate was similar in between the superstimulation and the control groups (Table 1). The maturation rate of oocytes was significantly higher in the superstimulation group than in the control group (Table 1). The percentage of abnormal oocytes did not differ significantly between the control and superstimulation groups. The results of embryo development are presented in Table 2. We confirmed that the fusion rates of SCNT embryos were similar between the superstimulation group and the control group (Table 2). Additionally, the cleavage and blastocyst formation rates following SCNT using these oocytes were significantly higher in the superstimulation group than in the control group (Table 2).
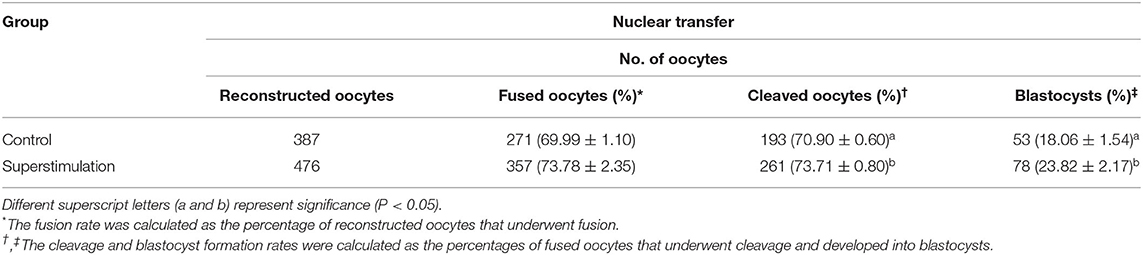
Table 2. Effects of superstimulation on the development potential of embryos generated by somatic cell nuclear transfer in Holstein cows.
Discussion
Superstimulation of oocyte donors is the first and crucial step in any in vitro embryo production system. Various hormones with stimulatory effects on the ovary, such as FSH and PMSG, are used in standard ovarian stimulation protocols for Holstein cows. However, the reproduction capacity of cloned embryos derived from oocytes collected from superstimulated cows is not evaluated in the UAE, which has a very hot climate. It is well established that high temperature has a detrimental effect on oogenesis and the quality of oocytes. Therefore, this study investigated the in vitro maturation and developmental capacity of embryos derived from oocytes obtained from PMSG-superstimulated donors cows using SCNT following OPU. Our results revealed that PMSG treatment of Holstein cows resulted in oocytes that developed into cloned blastocyst under the unique environmental conditions of the United Arab Emirates (UAE).
Environmental temperature is one of the most crucial factors according cattle reproduction (13). Several studies have reported that heat stress negatively effects reproduction in Holstein cows (14–16). Heat stress affects estrus duration, uterine function, and endocrine status (14–16). Furthermore, prolonged heat stress can affect early embryonic development and survival (17, 18). Heat stress during the estrus cycle of Holstein cows reduces the activity of granulosa cells and hinders follicular development (7). Furthermore, it has been reported that heat-stressed Holstein cows have a decreased follicle diameter and reduced fertility (19), and that heat stress during the early estrus cycle results in reduced follicular growth (5). The appropriate environmental temperature for Holstein cow is 5 to 15°C, and the temperature at which cows can maintain fertility without additional physiological changes is 5 to 25°C (3, 20) after they have been adapted to a high temperature environment. Furthermore, cows are subject to severe heat stress when the daily minimum temperature does not fall below 20°C (21). The present study demonstrated that the numbers of follicles (≥6 mm), collected oocytes, and in vitro maturation capacity increased following PMSG treatment. However, the number of follicles (≥6 mm), oocytes were lower in both the control and superstimulation groups than in a previous study, and this difference was thought to be due to heat stress (22–26). As described above, the minimum temperature during the period of this study was over 20°C, and the maximum temperature approached 40°C.
Oocyte developmental capacity gradually increases during follicular growth, and the capacity to develop to the blastocyst stage correlates with gene expression profiles during follicular growth (27, 28). Normal ovaries are strong enough to withstand superstimulation for the development of many follicles rather than a single follicle during the estrus cycle. Furthermore, ovary superstimulation increases the quality of the collected oocytes, thereby increasing the in vitro embryonic developmental capacity (27). Several studies using genetic profiling have revealed that oocytes collected from superstimulated ovaries differed from those from non-stimulated ovaries in mouse and cows (29, 30). In vitro blastocyst formation is also more efficient in superstimulated cows than in non-stimulated cows (29, 30). This study demonstrated that oocytes collected from superstimulated ovaries showed enhanced in vitro maturation and blastocyst formation capacity (29, 30). Therefore, we hypothesize that superstimulation using PMSG before OPU affects the developmental potential of oocytes. The results of our study are consistent with those of previous studies (7). Oocytes in the superstimulation group exhibited enhanced IVM and a better potential to develop to the cleavage and blastocyst stages following SCNT than oocytes in the control group (Tables 1, 2).
Although further research will be necessary to determine the effect of superstimulation on heat stress, our results showed that, follicle maturation, oocyte in vitro maturation, and embryonic development following somatic nuclear transfer were more efficient after superstimulation of Holstein cows in the UAE. In conclusion, the present study demonstrated that a single IM injection of PMSG, which is less stressful for Holstein cows, cheaper, and less labor intensive than injection of other hormones, is a useful method for superstimulation of Holstein cows in the UAE.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the Ethics Committee of the UAE Biotech Research Center (Approval No.: UAEBRC-B01). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
Y-BS and WH: conceptualization. Y-BS, YJ, XY, PO, MK, HK, YB, AT, KS, SR, AN, and WH: methodology. Y-BS, YJ, MH, MK, and AT: investigation. Y-BS, PO, and AN: validation. Y-BS and KS: formal analysis. YJ, HK, YB, AT, KS, and SR: resources. Y-BS: writing—original draft preparation. Y-BS, MH, PO, and WH: writing—review and editing. WH: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This project was supported by the Patronage of H.H. Sheikh Mansour bin Zayed Al Nahyan, Deputy Prime Minister of the UAE and the Minister of Presidential Affairs. We acknowledge his support and inspiration in the initiation and mentoring of this project, without which this project would not have been possible.
References
1. Son YB, Jeong YI, Hwang KC, Jeong YW, Hwang WS. Mitochondrial metabolism assessment of lycaon-dog fetuses in interspecies somatic cell nuclear transfer. Theriogenology. (2021) 165:18–27. doi: 10.1016/j.theriogenology.2021.01.010
2. Son YB, Jeong YI, Jeong YW, Olsson PO, Hossein MS, Cai L, et al. Development and pregnancy rates of Camelus dromedarius-cloned embryos derived from in vivo- and in vitro-matured oocytes. Anim Biosci. (2022) 35:177–83. doi: 10.5713/ab.21.0131
3. McDowell RE. Improvement of Livestock Production in Warm Climates. New York, NY: Cornell University Press (1972). p. 66–110.
4. Wolfenson D, Roth Z. Impact of heat stress on cow reproduction and fertility. Anim Front. (2018) 9:32–8. doi: 10.1093/af/vfy027
5. Badinga L, Thatcher WW, Diaz T, Drost M, Wolfenson D. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology. (1993) 39:797–810. doi: 10.1016/0093-691X(93)90419-6
6. Morton JM, Tranter WP, Mayer DG, Jonsson NN. Effects of environmental heat on conception rates in lactating dairy cows: critical periods of exposure. J Dairy Sci. (2007) 90:2271–8. doi: 10.3168/jds.2006-574
7. Vieira LM, Rodrigues CA, Castro Netto A, Guerreiro BM, Silveira CR, Moreira RJ, et al. Superstimulation prior to the ovum pick-up to improve in vitro embryo production in lactating and non-lactating Holstein cows. Theriogenology. (2014) 82:318–24. doi: 10.1016/j.theriogenology.2014.04.013
8. Oliveira LH, Sanches CP, Seddon AS, Veras MB, Lima FA, Monteiro PLJ Jr, et al. Short communication: Follicle superstimulation before ovum pick-up for in vitro embryo production in Holstein cows. J Dairy Sci. (2016) 99:9307–12. doi: 10.3168/jds.2016-11306
9. Mapletoft RJ, Steward KB, Adams GP. Recent advances in the superovulation in cattle. Reprod Nutr Dev. (2002) 42:601–11. doi: 10.1051/rnd:2002046
10. Popova E, Krivokharchenko A, Ganten D, Bader M. Comparison between PMSG- and FSH-induced superovulation for the generation of transgenic rats. Mol Reprod Dev. (2002) 63:177–82. doi: 10.1002/mrd.10173
11. Son YB, Jeong YI, Jeong YW Yu X, Olsson PO, Cai L, Choi EJ, et al. Comparison of pregnancy outcomes following the transfer of early-developmental stage embryos and blastocysts produced by somatic cell nuclear transfer in Camelus dromedarius. Anim Reprod Sci. (2021) 233:106842. doi: 10.1016/j.anireprosci.2021.106842
12. Kim HS, Lee JY, Jeong EJ, Yang CJ, Hyun SH, Shin T, et al. Effects of repetitive ionomycin treatment on in vitro development of bovine somatic cell nuclear transfer embryos. J Reprod Dev. (2012) 58:132–9. doi: 10.1262/jrd.11-040H
13. Dash S, Chakravarty AK, Singh A, Upadhyay A, Singh M, Yousuf S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: a review. Vet World. (2016) 9:235–44. doi: 10.14202/vetworld.2016.235-244
14. Gangwar PC, Branton C, Evans DL. Reproductive and physiological responses of Holstein Heifers to controlled and natural climatic conditions. J Dairy Sci. (1965) 48:222–7. doi: 10.3168/jds.S0022-0302(65)88200-5
15. Collier RJ, Beede DK, Thatcher WW, Israel LA, Wilcox CJ. Influences of environment and its modification on dairy animal health and production. J Dairy Sci. (1982) 65:2213–27. doi: 10.3168/jds.S0022-0302(82)82484-3
16. Wise ME, Armstrong DV, Huber JT, Hunter R, Wiersma F. Hormonal alterations in the lactating dairy cow in response to thermal stress. J Dairy Sci. (1988) 71:2480–5. doi: 10.3168/jds.S0022-0302(88)79834-3
17. Wolfenson D, Roth Z, Meidan R. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci. (2000) 60, 61:535–47. doi: 10.1016/S0378-4320(00)00102-0
18. De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow–a review. Theriogenology. (2003) 60:1139–51. doi: 10.1016/S0093-691X(03)00126-2
19. Wolfenson D, Thatcher WW, Badinga L, Savio JD, Meidan R, Lew BJ, et al. Effect of heat stress on follicular development during the estrous cycle in lactating dairy cattle. Biol Reprod. (1995) 52:1106–13. doi: 10.1095/biolreprod52.5.1106
20. Sirohi S, Michaelowa A. Sufferer and cause: Indian livestock and climate change. Clim Change. (2007) 85:285–98. doi: 10.1007/s10584-007-9241-8
21. NRC Subcommittee Subcommittee on Environmental Stress. Effect of Environment on Nutrient Requirements of Domestic Animals. Washington, DC: National Academies Press (US) (1981).
22. Gonzalez A, Wang H, Carruthers TD, Murphy BD, Mapletoft RJ. Superovulation in the cow with pregnant mare serum gonadotrophin: effects of dose and antipregnant mare serum gonadotrophin serum. Can Vet J. (1994) 35:158–62.
23. Lee BC, Yoon KY, Kim HI, Roh SH, Lee KN, Hwang WS. Transvaginal ultrasound-guided ovum pick-up in cattle I. Effects of estrus cycle, season and bST treatment on ovum pick-up in cattle Korean. J Vet Res. (1997) 37:917–24.
24. Pontes JH, Melo Sterza FA, Basso AC, Ferreira CR, Sanches BV, Rubin KC, et al. Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nelore cattle (Bos indicus) donors. Theriogenology. (2011) 75:1640–6. doi: 10.1016/j.theriogenology.2010.12.026
25. Jin JI, Choi BH, Kim SS, Jo HT, Sun DW, Lim HT, et al. Transplantation and production of OPU derived hanwoo IVP embryos. J. Embryo Transf. (2014) 29:273–81. doi: 10.12750/JET.2014.29.3.281
26. Borş SI, Ibanescu I, Creangǎ, Dascǎlu L, Borş A. Follicle deviation and oocyte recovering by ovum pick-up from endangered Romanian Grey cows. Rom Biotechnol Lett. (2018) 23:13918–26. doi: 10.26327/RBL2018.202
27. Labrecque R, Sirard MA. The study of mammalian oocyte competence by transcriptome analysis: progress and challenges. Mol Hum Reprod. (2014) 20:103–16. doi: 10.1093/molehr/gat082
28. Mourot M, Dufort I, Gravel C, Algriany O, Dieleman S, Sirard MA. The influence of follicle size, FSH-enriched maturation medium, and early cleavage on bovine oocyte maternal mRNA levels. Mol Reprod Dev. (2006) 73:1367–79. doi: 10.1002/mrd.20585
29. Pan H. O'brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol. (2005) 286:493–506. doi: 10.1016/j.ydbio.2005.08.023
Keywords: Holstein cows, PMSG, superstimulation, ovum pick-up, somatic cell nuclear transfer
Citation: Son Y-B, Jeong YI, Hossein MS, Yu X, Olsson PO, Kang M, Kim H, Bae Y, Tinson A, Singh KK, Rajesh S, Noura AS and Hwang WS (2022) Influence of PMSG on Superstimulation and Embryo Development Following Somatic Cell Nuclear Transfer in Holstein Cows in the United Arab Emirates. Front. Vet. Sci. 9:895325. doi: 10.3389/fvets.2022.895325
Received: 13 March 2022; Accepted: 04 April 2022;
Published: 26 April 2022.
Edited by:
Alejandro Vicente-Carrillo, ciencIAnova, Investigación y Desarrollo Magapor A.I.E., SpainReviewed by:
Juan Mateo Anchordoquy, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaJaime Palomino, Universidad Bernardo O'Higgins, Chile
Copyright © 2022 Son, Jeong, Hossein, Yu, Olsson, Kang, Kim, Bae, Tinson, Singh, Rajesh, Noura and Hwang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woo Suk Hwang, aHdhbmd3c0BhZGJyZi5vcmc=
 Young-Bum Son
Young-Bum Son Yeon Ik Jeong
Yeon Ik Jeong Mohammad Shamim Hossein
Mohammad Shamim Hossein Xianfeng Yu1,3
Xianfeng Yu1,3 Woo Suk Hwang
Woo Suk Hwang