
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 24 June 2022
Sec. Animal Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.889485
This article is part of the Research Topic Effect of Dietary Antioxidants on Gut Microbiota: Associated Role in Animal Health and Production View all 8 articles
In order to reduce the negative effects caused by oxidative stress on broilers, it is particularly important to find ways to alleviate oxidative stress. As a natural plant extract, L-theanine has a variety of biological effects, such as improving antioxidant capacity, promoting growth, and enhancing immunity and antitumor. This trial evaluated the effects of dietary supplementation of L-theanine on growth performance, antioxidation, meat quality, and intestinal microflora in 817 White Feather Broilers. A total of 108 21-day-old 817 broilers with similar body weight (BW) were randomly divided into three groups with six replicates per group and six chickens within each replicate. The three groups were corn-soybean-based diet (NC group); basal diet plus drinking water with 30 mg hydrocortisone/kg (PC group); and basal diet supplemented with 400 mg L-theanine/kg plus drinking water with 30 mg hydrocortisone/kg (LT group). Compared with the NC group, from 21 to 24 days of age, the PC and LT groups had decreased BW, average daily gain (ADG), and average daily feed intake (ADFI), and increased feed to gain ratio (F/G; p < 0.05). At 24 days of age, the LT group had improved superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in serum as compared to the NC group (p < 0.05). The LT group broilers also had significantly higher concentrations of malondialdehyde (MDA) in serum and liver (p < 0.05). On the 42nd days, the PC group had lower PH45min (p < 0.05) than the NC and LT groups and higher cooking loss and shear force (p < 0.05). Moreover, the villi height of the PC group was significantly lower in jejunum than the NC group (p < 0.05). The LT group had a higher ZO-1 content in duodenum than the NC and PC groups (p < 0.05). The activity of GSH-Px in the liver of the LT group was increased than in the PC group (p < 0.05). The relative abundance of Firmicutes in the LT group was significantly higher than in the NC and PC groups (p < 0.05). These results suggested that the effects of acute oxidative stress on growth performance and meat quality of broilers are continuous, and dietary supplementation of L-theanine could improve the growth performance and meat quality, enhance the intestinal mucosal barrier and antioxidant capacity, and improve the composition of the intestinal flora of broilers caused by acute oxidative stress.
Oxidative stress is an imbalance between the production of cell-damaging free radicals and the body's ability to neutralize them (1, 2). Oxidative stress exerts a series of adverse effects on broiler breeding, such as decreased immunity, feed intake, quality of broilers, and slower daily weight gain (3–5). For the moment, many places around the world, such as China, have forbidden to add antibiotic growth promoters in poultry diets. Therefore, it is imperative to find an effective, safe, and sustainable natural additive that can both increase the productive potential and maintain broiler health.
Previous studies have demonstrated the great potential of plant-extracted natural polysaccharides as an alternative to antibiotic additives (6, 7). L-theanine is a characteristic non-protein amino acid extracted from the tea. It is one of the flavor substances of tea, accounting for 1–2% of the weight of the dry tea, with a chemical formula C7H14N2O3. Studies have found that L-theanine has various biological activities, such as antioxidant, promoting animal growth, enhancing immunity, and protecting nerves (8, 9). Intestinal states, such as intestinal mucosal morphology, barrier integrity, and flora, are closely related to animal growth and health status (10). The intestine is an important organ for absorbing nutrients and digesting. In the state of oxidative stress, excessive accumulation of free radicals destroys the intestinal barrier. The damage in the intestine is easy to cause intestinal absorption dysfunction, bacterial community disorder, and decreased immunity (11, 12). The purpose of this experiment was to study the effects of a low dosage of L-theanine have on growth performance, antioxidant capacity, meat quality, and intestinal states of 817 White Feather Broilers that subjected to hydrocortisone-induced acute oxidative stress. It can provide theoretical guidance for practical production.
This study was approved by the Ethics Committee of Foshan University (Fosu2022024), China. All experimental procedures involving the use of animals were conducted in compliance with relevant laws and institutional guidelines. A total of 108 21-day-old 817 broilers (a hybrid of high-quality male broiler and female laying hens) with similar body weight were randomly divided into three groups with six replicates/cages per group and six chickens within each replicate (obtained from Guangdong Muyuan Ltd, China). The three groups were corn-soybean-based diet (NC group); basal diet plus drinking water with 30 mg hydrocortisone/kg (PC group); and basal diet supplemented with 400 mg L-theanine/kg plus drinking water with 30 mg hydrocortisone/kg (LT group). All chickens were raised in a wire cage of 65 × 65 × 42 cm, in an environmentally controlled room with continuous light and ad libitum access to feed and water throughout the 21 days experiment.
Hydrocortisone is a glucocorticoid. Previous studies showed that it can induce oxidative stress in rats (13). The preliminary study demonstrated that the hydrocortisone can also induce oxidative stress in broilers. Chemical formulas of hydrocortisone and L-theanine (Aladdin Biochemical Technology Co., Ltd., Shanghai, China) respectively are C21H30O5 and C7H14O3, both have a purity of 98%.
All diets were formulated to meet the National Research Council (NRC, 1994) nutrient requirements. The ingredients and chemical composition of the basal diet are shown in Table 1.
All broilers were individually weighted before the experiment, on day 3, and on day 21 after a 12 h fast. The feed intake of broilers in each replicate was recorded to determine average daily gain (ADG) and average daily feed intake (ADFI). The F/G was calculated by ADFI/ADG.
On day 3 and 21, one broiler was randomly selected from each replicate and marked. After 12 h of fasting, blood samples (3–5 ml) were collected from the wing vein. The sample was allowed to clot at 37°C for 2 h and subsequently centrifuged at 3,500 rpm for 15 min at 4°C to obtain serum and stored at −80°C refrigerator for future testing. The broiler was slaughtered by cervical dislocation after bleeding on day 21 of the experiment. Liver tissue samples were harvested, stored at −80°C, and used for antioxidant capacity assays. Then the right side of the pectoralis was collected to measure muscle PH and meat quality. The mucosa of the duodenum, jejunum, ileum, and the middle intestinal segment was collected and then preserved with 4% formaldehyde phosphate buffer saline (PBS) solution. The contents of the cecum and duodenum were collected and stored at −80°C for later testing.
The liver was isolated on an ice tray and homogenized with cold saline with a weight-to-volume ratio of 1:9. The homogenate was centrifuged at 9,000 × g for 10 min at 4°C. The supernatant was collected for measuring the indices. The total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and malondialdehyde (MDA) concentration were determined with commercially assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the method described by Yan et al. (14) and Wen et al. (15).
The intestinal tissue samples were embedded in paraffin-wax firstly, and three consecutive sects (5 μm) were stained with hematoxylin-eosin for analysis. Villi height and crypt depth were measured by using a microscope (MD50-T, OLYMPUS, China) at 40 × magnification. A total of 20–30 villi and their associated crypts per section were randomly selected, and the villus length and crypt depth were analyzed by MShot Image Analysis System. Three different intestinal villi and crypts were measured in each sample. The intestinal tight junction protein ZO-1 was measured by using a ZO-1 enzyme-linked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China) in accordance with the manufacture's instructions.
Total genome DNA was extracted from samples using cetyltrimethylammonium bromide (CTAB)/sodium dodecyl sulfate (SDS) method. DNA concentration and purity were monitored on 1% agarose gels. DNA was diluted to l μg/μl using sterile water. 16S rRNA genes of distinct regions (V3–V4) were amplified using a specific primer with the barcode. All PCR reactions were carried out with 15 μl of Phusion® High-Fidelity PCR Master Mix (New England Biolabs); 0.2 μM of forward and reverse primers and about 10 ng template DNA. Thermal cycling consisted of initial denaturation at 98°C for 1 min, followed by 30 cycles of denaturation at 98°C for 10 s, annealing at 50°C for 30 s, elongation at 72°C for 30 s, and finally, 72°C for 5 min. Sequencing libraries were generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, United States) following the recommendations of manufacturer, and index codes were added. The library quality was assessed on the Qubit@2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. At last, the library was sequenced on an Illumina NovaSeq platform and 250 bp paired-end reads were generated.
All data were analyzed using the one-way ANOVA of SPSS 20.0 (SPSS Inc., Chicago, IL, United States). The data were analyzed as a completely randomized design with the cages as the experimental unit. Orthogonal polynomial contrasts were used to determine linear and quadratic effects of the increasing L-theanine. Statistical significance was declared at p < 0.05 and trends at 0.05 < p < 0.10.
The effects of dietary L-theanine supplementation on the growth performance of broilers under the oxidative stress are shown in Table 2. From 21–24 to 21–42 days of age, when compared with the NC group, the PC and LT groups had significantly lower BW, ADG, and ADFI (p < 0.05) and higher F/G at the 21–24 days (p < 0.05). In addition, the F/G in the PC group was higher than in the other two groups at 21–42 days (p < 0.05).
Table 3 shows the results on the meat quality of the broilers, at 42 days of age, the PH45min of the PC group is significantly lower than the NC and LT groups (p < 0.05). The cooking loss and shear force of the PC group were higher than the NC and LT groups (p < 0.05).
As shown in Table 4, when compared with the NC group, the villi height of jejunum in the PC group is significantly decreased (p < 0.05).
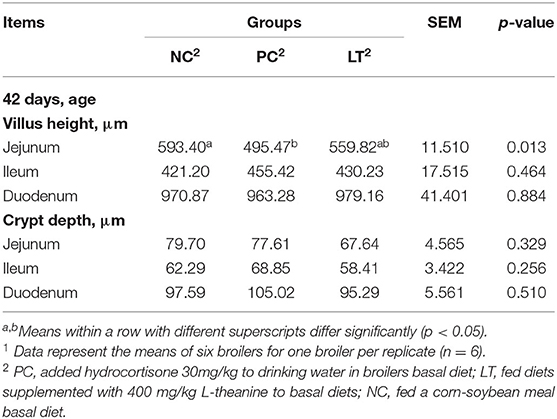
Table 4. Effects of dietary L-theanine on intestinal morphology of broilers under the oxidative stress1.
At 42 days of age, the content of ZO-1 in duodenum of the LT group was higher (p < 0.05) than in the other two groups from Table 5.
At 24 days of age, when compared to the NC group, the Table 6 result showed that the MDA concentrations in serum and liver were significantly increased in the PC and LT groups (p < 0.05). In addition, the LT group had increased serum SOD and GSH-Px activities (p < 0.05). At 42 days of age, the activity of GSH-Px in LT was higher than in the PC group (p < 0.05).
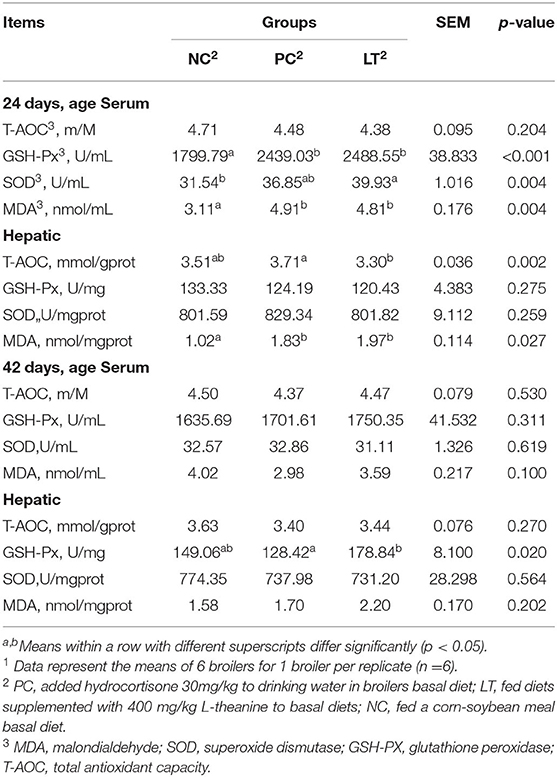
Table 6. Effects of dietary L-theanine on antioxidant indices in the serumand hepatic of broilers under the oxidative stress1.
According to the database annotation results, as shown in Figure 1, the top 10 functional pieces of information of each sample or group with the largest abundance at each annotation level were selected, and a columnar stacking bar was generated.
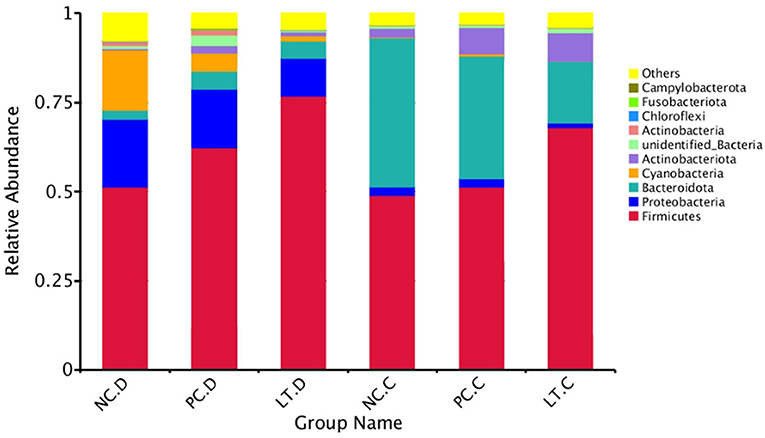
Figure 1. Histogram of phylum level species relative abundance. NC.D: the content of duodenum in NC group. PC.D, the content of duodenum in PC group. LT.D, the content of duodenum in LT group. NC.C, the content of cecum in NC group. PC.C, the content of cecum in PC group. LT.C, the content of cecum in LT group. a, bMeans within a row with different superscripts differ significantly (P < 0.05). Data represent the means of 6 bird for 1 bird per replicate (n = 6). PC, added hydrocortisone 30 mg/kg to drinking water in broilers basal diet; LT, fed diets supplemented with 400 mg/kg L-theanine to basal diets. NC, fed a corn-soybean meal basal diet.
Figure 2 shows that the Firmicutes abundance of the LT group is significantly higher than the NC and PC groups at 42 days of age (p < 0.05). Figure 3 suggests that the Bacteroidetes abundance of the LT group is lower than the PC and NC groups (p < 0.05).
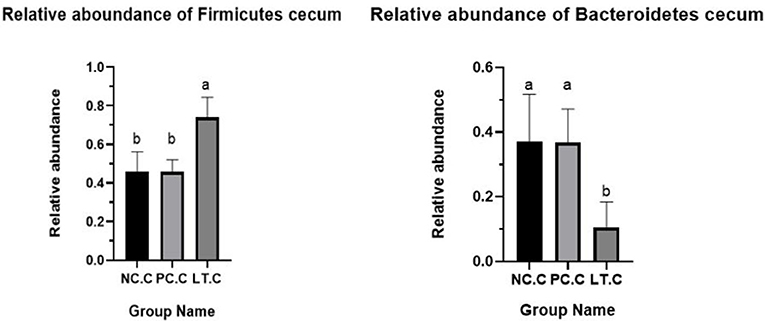
Figure 2. Abundance of firmicutes and bacteroidetes in cecum. NC.C, the content of cecum in NC group. PC.C, the content of cecum in PC group. LT.C, the content of cecum in LT group. a, bMeans within a row with different superscripts differ significantly (P < 0.05). Data represent the means of 6 bird for 1 bird per replicate (n = 6). PC, added hydrocortisone 30 mg/kg to drinking water in broilers basal diet; LT, fed diets supplemented with 400 mg/kg L-theanine to basal diets. NC, fed a corn-soybean meal basal diet.
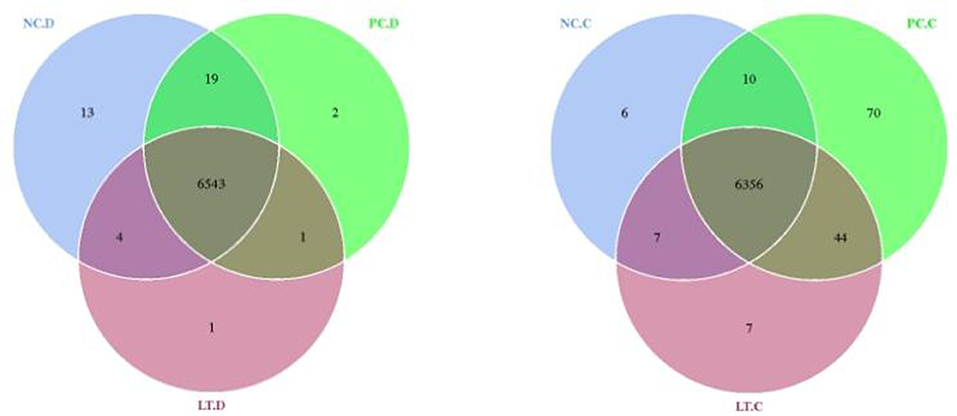
Figure 3. Venn diagram of OUT distribution between groups. NC.D, the content of duodenum in NC group. PC.D, the content of duodenum in PC group. LT.D, the content of duodenum in LT group. NC.C, the content of cecum in NC group. PC.C, the content of cecum in PC group. LT.C, the content of cecum in LT group. a, bMeans within a row with different superscripts differ significantly (P < 0.05). Data represent the means of 6 bird for 1 bird per replicate (n = 6). PC, added hydrocortisone 30 mg/kg to drinking water in broilers basal diet; LT, fed diets supplemented with 400 mg/kg L-theanine to basal diets. NC, fed a corn-soybean meal basal diet.
As shown in Figure 3, 6,379, 6,480, and 6,414 Operational Taxonomic Units (OTUs) are obtained from the cecal samples from NC, PC, and LT groups, respectively. The number of OTUs shared by the three groups was 6,356 accounting for 99.63, 98.80, and 99.09%, respectively. In total, 6,579, 6,565, and 6,549 OTUs were obtained from the duodenal contents of the NC, PC, and LT groups, respectively. The number of OTUs shared by the three groups was 6,543, accounting for 99.45, 99.66, and 99.91% separately.
Nowadays, oxidative stress is one of the research hotspots in the animal husbandry field. Under the state of oxidative stress, broilers have many problems, such as decreased growth performance and poor meat quality, which would affect economic benefits (16). Some studies have shown that it is an effective way to alleviate oxidative stress damage of animals by adding additives with antioxidant biological function (8). L-theanine is a natural plant extracted from tea with a powerful antioxidant capacity. In addition, it has other functions and can replace antibiotics (9). Due to the actual production cost of L-theanine, this experiment investigated the effects of a low dosage of L-theanine in broilers with oxidative stress induced by hydrocortisone. The results showed that 30 mg/kg of hydrocortisone in drinking water was significantly decreased ADG, ADFI, and BW and increased in F/G as compared to the NC group. Meanwhile, the LT and PC groups had significantly increased MDA concentrations in serum and liver at 24 days of age. It meant that the challenged chickens were on a state of oxidative stress (17). The LT group had a significantly lower F/G at 21–42 days of age as compared to the PC group. This revealed that it can improve the production performance of broilers with L-theanine supplementation under the oxidative stress. Other research results also indicated that the addition of L-theanine to broiler diets can improve the production performance of poultry (18).
The intestinal tract is an important organ, which is mainly responsible for digestion and absorption (19). Villus height and crypt depth are commonly used to evaluate the functional status of the small intestine (20). The crypt is considered to be a producer of villus cells, and the lower depth of crypt indicates that the intestinal villi are in good condition (21). Lower crypt depth and higher villus height mean a higher ability of the intestine to absorb nutrients (22). Some studies have proved that the intestinal absorption of nutrients mainly depended on the intestinal villi, and the intestinal villi epithelial cells absorbed nutrients from the digestive tract into the blood. At present, it is believed that there is a positive correlation between intestinal absorption and villi height (23). Therefore, parameters of intestinal mucosal morphology can explain the differences in production performance to a certain extent (24). The results of this study demonstrated that the villi height of jejunum in the PC group was significantly lower than in the NC group. This proved that oxidative stress has negative effects on the morphology of intestinal mucosa (25). However, no significant difference in villi height of jejunum between the LT and NC groups was observed. It suggested the broilers in diet with L-theanine could improve villi height of jejunum under oxidative stress. Meanwhile, the crypt depth of jejunum, ileum, and duodenum in LT group was declined than in the PC and NC groups. Some studies provided support to improve small intestinal morphology by feeding with L-theanine in poultry diets (26), which is similar to the results in our experiment. Hence, dietary L-theanine can improve intestinal mucosal morphology, so as to improve the production performance of broilers under oxidative stress.
More and more studies have demonstrated that the intestinal flora is closely related to the host (27). In addition, the number and type of intestinal flora can affect the nutrient absorption (28), health, and even behavior (29) of the host. Therefore, 16s rRNA analysis was performed to study the effects of L-theanine on the gut flora of broilers under oxidative stress. The results showed that oxidative stress decreased the number of specific genes in the duodenum of broilers, but supplementation of L-theanine did not improve this situation. This may indicate that L-theanine cannot improve the intestinal bacterial species abundance broilers under oxidative stress in this experiment. Studies have shown that L-theanine can affect the composition of gut microbiota (30). Compared with the NC and PC groups, the LT group had significantly increased the proportion of Bacteroides and Firmicutes. It proved that L-theanine could improve the intestinal flora composition. The intestinal tract was mainly dominated by Firmicutes, followed by Proteobacteria and Bacteroidetes (31). This is the same as our results. At the same time, the relative abundance of the Firmicutes in the LT group was significantly higher than the NC and PC groups. Firmicutes were considered to be able to resist harsh environments by their physiological function (32). Related studies have revealed that increasing Firmicutes bacteria is beneficial to intestinal health and can boost the production performance of broilers (33). Studies have shown that obesity is associated with a reduction in the Bacteroidetes and the ratio of Firmicutes to Bacteroidetes (34). In this experiment, the relative abundance of Bacteroides cecum was significantly lower than that in the NC and PC groups. It suggested that L-theanine can improve the production performance via modulating the composition of intestinal microflora.
The intestinal mucosal barrier is a structure, which can prevent harmful factors in the intestine from entering the lamina propria of the intestinal mucosa (35). It is an important mechanical protection for the internal and external environment of the body. Studies have reported that oxidative stress can damage the intestinal barrier function and further allow harmful factors in the intestinal tract to enter the body's internal environment, aggravating oxidative stress, and forming a vicious circle (36). Therefore, the integrity of gut barrier was important to relieve oxidative stress. ZO-1 protein is the earliest discovered intestinal tight junction protein. Some research studies have shown that the expression level of tight junction protein is positively correlated with barrier function (37). The ZO-1 activity of the LT group was significantly increased as compared to the PC and NC groups in this study. This suggested that L-theanine can improve the intestinal barrier.
Oxidative stress can damage cell by producing excess reactive oxygen species (ROS), which affects animal health (38). There are many findings that showed that dietary supplementation of natural antioxidants can eliminate ROS and improve oxidative damage and production performance (39, 40). Zhang et al. (41) found that L-theanine can ameliorate oxidative stress caused by transportation in animals. In this study, the concentrations of MDA, SOD, T-AOC, and GSH-Px in serum and liver were measured to investigate the effects of L-theanine on the antioxidant capacity of broilers under oxidative stress. The results showed that L-theanine significantly increased the GSH-Px activity in the liver of 42-days-old broilers, indicating that L-theanine has the effect of enhancing antioxidant capacity. It may partially explain why L-theanine can improve jejunum morphology. It should be noted that the results of other researchers suggested that L-theanine can not only increase the activity of GSH-Px but also improve other antioxidant indicators, such as T-AOC and SOD (42). In this experiment, only the activity of GSH-Px was increased. This might be due to the low dosage used in our study. People have increasingly focused on meat quality recently. Not only the meat quality is required to be safe, hygienic, and nutritious but also the meat color freshness by eyes and good taste was required. Previous research showed that shear force, PH value, and cooking loss can affect the taste of meat (43). Compared with the PC group, the PH was increased and shear force and cooking loss were decreased in LT group, which was consistent with other studies (41, 44). This indicated that L-theanine can improve meat quality, which may be related to the improvement of antioxidant capacity of oxidative stress broilers.
Adding 30 mg/kg of hydrocortisone to drinking water can significantly increase the MDA concentrations and decrease the F/G and ADG in the serum and liver. This experiment verified the feasibility of using hydrocortisone to establish an acute oxidative stress model in broilers. Meanwhile, dietary supplementation with 400 mg/kg of L-theanine from days 21, the results of this study proved that L-theanine can improve the F/G ratio under acute oxidative stress in broilers. F/G ratio is a key indicator to measure the performance of broilers. This suggested that L-theanine had positive effects on the production performance of the chicken under oxidative stress. It provides certain theoretical guidance for practical production.
In conclusion, this study demonstrated that dietary supplementation of 400 mg/kg L-theanine could improve the production performance of broilers under oxidative stress. This may be achieved via improving gut morphology, enhancing antioxidant capacity, and modulating the intestinal microflora.
The data presented in the study are deposited in the NCBI repository, accession number PRJNA814510, https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA814510.
The animal study was reviewed and approved by Animal Care and Use Committee of Foshan University.
ZW and YT trial conduction, data analysis, and draft of the manuscript. LL and HZ draft and revision of the protocol, revision of the manuscript, and final approval for paper publication. All authors contributed to the article and approved the submitted version.
This work was supported by Discipline Construction Program of Foshan University (CGZ0400162), the Teachers' Characteristic Innovation Research Project of Guangdong University Research Findings Commercialization Center in Guangdong Province, China (2020SWYY02), the Open fund of Key Laboratory of Animal Nutritional Physiology and Metabolic Processes of Hunan Province, Institute of Subtropical Agriculture, Chinese Academy of Sciences in Hunan Province, China (ISA2020112), and the Scientific Research Foundation in the Higher Education Institutions of Educational Commission of Guangdong Province (2017GCZX006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. (2019) 8:235. doi: 10.3390/antiox8070235
2. Del Vesco AP, Khatlab AS, Goes ESR, Utsunomiya KS, Vieira JS, Oliveira Neto AR, et al. Age-related oxidative stress and antioxidant capacity in heat-stressed broilers. Animal. (2017) 11:1783–90. doi: 10.1017/S1751731117000386
3. Chen Z, Xing T, Li J, Zhang L, Jiang Y, Gao F. Hydrogen peroxide-induced oxidative stress impairs redox status and damages aerobic metabolism of breast muscle in broilers. Poult Sci. (2021) 100:918–25. doi: 10.1016/j.psj.2020.11.029
4. De Grande A, Leleu S, Delezie E, Rapp C, De Smet S, Goossens E, et al. Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult Sci. (2020) 99:441–53. doi: 10.3382/ps/pez525
5. Zeitz JO, Fleischmann A, Ehbrecht T, Most E, Friedrichs S, Whelan R, et al. Effects of supplementation of DL-methionine on tissue and plasma antioxidant status during heat-induced oxidative stress in broilers. Poult Sci. (2020) 99:6837–47. doi: 10.1016/j.psj.2020.08.082
6. Mishra B, Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci. (2019) 6:e60. doi: 10.3389/fvets.2019.00060
7. Qiu Y, Liu S, Hou L, Li K, Wang L, Gao K, et al. Supplemental choline modulates growth performance and gut inflammation by altering the gut microbiota and lipid metabolism in weaned piglets. J Nutr. (2021) 151:20–9. doi: 10.1093/jn/nxaa331
8. Saeed M, Khan MS, Kamboh AA, Alagawany M, Khafaga AF, Noreldin AE, et al. L-theanine: an astounding sui generis amino acid in poultry nutrition. Poult Sci. (2020) 99:5625–36. doi: 10.1016/j.psj.2020.07.016
9. Alagawany M, Abd El-Hack ME, Saeed M, Naveed M, Arain MA, Arif M, et al. Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: A review. J Anim Physiol Anim Nutr. (2020) 104:245–56. doi: 10.1111/jpn.13219
10. Diaz de Barboza G, Guizzardi S, Moine L, Tolosa de Talamoni N. Oxidative stress, antioxidants and intestinal calcium absorption. World J Gastroenterol. (2017) 23:2841–53. doi: 10.3748/wjg.v23.i16.2841
11. Righi F, Pitino R, Manuelian CL, Simoni M, Quarantelli A, De Marchi M, et al. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on poultry performances, health, and oxidative status: a review of the literature in the last 20 years. Antioxidants. (2021) 10:659. doi: 10.3390/antiox10050659
12. Lee MT, Lin WC, Lee TT. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals - a review. Asian-Australas J Anim Sci. (2019) 32:309–19. doi: 10.5713/ajas.18.0538
13. Aboelwafa HR, Yousef HN. The ameliorative effect of thymol against hydrocortisone-induced hepatic oxidative stress injury in adult male rats. Biochem Cell Biol. (2015) 93:282–9. doi: 10.1139/bcb-2014-0154
14. Yan L, An S, Lv ZZ, Wang ZG, Wu YM, Zhu YT, et al. Effects of phytonutrients on growth performance, antioxidative status, and energy utilization of broilers fed low energy diets. Anim Nutr. (2019) 5:270–7. doi: 10.1016/j.aninu.2019.03.004
15. Wen C, Chen R, Chen YP, Ding LR, Wang T, Zhou YM. Betaine improves growth performance, liver health, antioxidant status, breast meat yield, and quality in broilers fed a mold-contaminated corn-based diet. Anim Nutr. (2021) 7:661–6. doi: 10.1016/j.aninu.2020.11.014
16. Chen YP, Gu YF, Zhao HR, Zhou YM. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult Sci. (2021) 100:100919. doi: 10.1016/j.psj.2020.12.017
17. Deng Y, Xiao W, Chen L, Liu Q, Liu Z, Gong Z. In vivo antioxidative effects of l-theanine in the presence or absence of Escherichia coli-induced oxidative stress. Front Immunol. (2016) 24:e527–36. doi: 10.1016/j.jff.2016.04.029
18. Zhang C, Wang C, Chen K, Zhao X, Geng Z. Effect of L-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J Sci Food Agric. (2020) 100:1718–25. doi: 10.1002/jsfa.10192
19. Zhang H, Zheng P, Chen D, Yu B, He J, Mao X, et al. Dietary arginine supplementation improves intestinal mitochondrial functions in low-birth-weight piglets but not in normal-birth-weight piglets. Antioxidants. (2021) 10:1995. doi: 10.3390/antiox10121995
20. Yin L, Yang Q, Zhang Y, Wan D, Yin Y, Wang Q, et al. Dietary copper improves intestinal morphology via modulating intestinal stem cell activity in pigs. Animals. (2021) 11:2513. doi: 10.3390/ani11092513
21. Aguzey HA, Gao ZH, Wu HH, Cheng GL, Wu ZM, Chen JH. The effects of deoxynivalenol (don) on the gut microbiota, morphology and immune system of chicken - a review. Ann Anim Sci. (2019) 19:305–18. doi: 10.2478/aoas-2019-0013
22. Mohammadsadeghi F, Afsharmanesh M, Ebrahimnejad H. The substitution of humic material complex with mineral premix in diet and interaction of that with probiotic on performance, intestinal morphology and microflora of chickens. Livest Sci. (2019) 228:1–4. doi: 10.1016/j.livsci.2019.07.010
23. Ming DX, Wang WH, Huang CY, Wang ZJ, Shi CY, Ding J, et al. Effects of weaning age at 21 and 28 days on growth performance, intestinal morphology and redox status in piglets. Animals. (2021) 11:2169. doi: 10.3390/ani11082169
24. Salavati ME, Rezaeipour V, Abdullahpour R, Mousavi N. Effects of graded inclusion of bioactive peptides derived from sesame meal on the growth performance, internal organs, gut microbiota and intestinal morphology of broiler chickens. Int J Pept Res Ther. (2020) 26:1541–48. doi: 10.1007/s10989-019-09947-8
25. Song J, Lei X, Luo JX, Everaert N, Zhao GP, Wen J, et al. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. J Anim Physiol Anim Nutr. (2019) 103:1030–38. doi: 10.1111/jpn.13062
26. Liu ZQ, Li ZQ, Zheng Z, Li N, Mu SQ, Ma Y, et al. Effects of L-theanine on intestinal morphology, barrier function, and MAPK signaling pathways in diquat-challenged piglets. Anim Biotechnol. (2021):1-8. doi: 10.1080/10495398.2021.2013857
27. Rao ZB, Li JL, Shi BS, Zeng Y, Liu YB, Sun ZH, et al. Dietary tryptophan levels impact growth performance and intestinal microbial ecology in weaned piglets via tryptophan metabolites and intestinal antimicrobial peptides. Animals. (2021) 11:817. doi: 10.3390/ani11030817
28. Deng L, Xu HC, Liu P, Wu SZ, Shi YC, Lv YW, et al. Prolonged exposure to high humidity and high temperature environment can aggravate influenza virus infection through intestinal flora and Nod/RIP2/NF-kappa B signaling pathway. Vet Microbiol. (2020) 251:108896. doi: 10.1016/j.vetmic.2020.108896
29. Watanabe N, Mikami K, Hata T, Kimoto K, Nishino R, Akama F, et al. Effect of gut microbiota early in life on aggressive behavior in mice. Neurosci Res. (2021) 168:95–9. doi: 10.1016/j.neures.2021.01.005
30. Saeed M, Xu YT, Zhang TT, Qian R, Chao S. 16S ribosomal RNA sequencing reveals a modulation of intestinal microbiome and immune response by dietary L-theanine supplementation in broiler chickens. Poult Sci. (2019) 98:842–54. doi: 10.3382/ps/pey394
31. Gong JH, Si WD, Forster RJ, Huang RL, Yu H, Yin Y, et al. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol Ecol. (2007) 59:147–57. doi: 10.1111/j.1574-6941.2006.00193.x
32. Filippidou S, Wunderlin T, Junier T, Jeanneret N, Dorador C, Molina V, et al. A combination of extreme environmental conditions favor the prevalence of endospore-forming firmicutes. Front Microbiol. (2016) 7:e1707. doi: 10.3389/fmicb.2016.01707
33. Wu S, Li T, Niu H, Zhu Y, Liu Y, Duan Y, et al. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult Sci. (2019) 98:828–41. doi: 10.3382/ps/pey393
34. Zhang T, Ding H, Chen L, Lin Y, Gong Y, Pan Z, et al. Antibiotic-induced dysbiosis of microbiota promotes chicken lipogenesis by altering metabolomics in the cecum. Metabolites. (2021) 11:487. doi: 10.3390/metabo11080487
35. Farkas AE, Nusrat A. Pharmacological targeting of the inflamed intestinal barrier. Curr Pharm Des. (2016) 22:5400–14. doi: 10.2174/1381612822666160726123857
36. Gao Z, Wu H, Zhang K, Hossen I, Wang J, Wang C, et al. Protective effects of grape seed procyanidin extract on intestinal barrier dysfunction induced by a long-term high-fat diet. J Funct Foods. (2020) 64:92420–30. doi: 10.1016/j.jff.2019.103663
37. Mao XB, Xiao XJ, Chen DW, Yu B, He J, Chen H, et al. Dietary apple pectic oligosaccharide improves gut barrier function of rotavirus-challenged weaned pigs by increasing antioxidant capacity of enterocytes. Oncotarget. (2017) 8:92420–30. doi: 10.18632/oncotarget.21367
38. Emami NK, Jung U, Voy B, Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. (2021) 10:35. doi: 10.3390/antiox10010035
39. Sumathi T, Asha D, Nagarajan G, Sreenivas A, Nivedha R. L-Theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting upregulation of inflammatory cytokines and oxidative stress in rat brain. Environ Toxicol Pharmacol. (2016) 42:99–117. doi: 10.1016/j.etap.2016.01.008
40. Li R, Song Z, Zhao J, Huo D, Fan Z, Hou DX, et al. Dietary L-theanine alleviated lipopolysaccharide-induced immunological stress in yellow-feathered broilers. Anim Nutr. (2018) 4:265–72. doi: 10.1016/j.aninu.2018.05.002
41. Zhang C, Geng ZY, Chen KK, Zhao XH, Wang C. L-theanine attenuates transport stress-induced impairment of meat quality of broilers through improving muscle antioxidant status. Poult Sci. (2019) 98:4648–55. doi: 10.3382/ps/pez164
42. Li G, Ye Y, Kang J, Yao X, Zhang Y, Jiang W, et al. l-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes. Food Chem Toxicol. (2012) 50:363–72. doi: 10.1016/j.fct.2011.10.036
43. Saeed M, Yatao X, Hassan FU, Arain MA, Abd El-Hack ME, Noreldin AE, et al. Influence of graded levels of l-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int J Mol Sci. (2018) 19:462. doi: 10.3390/ijms19020462
Keywords: L-theanine, intestinal flora, oxidative stress, growth performance, broiler chicken
Citation: Wang Z, Tang Y, Long L and Zhang H (2022) Effects of Dietary L-Theanine on Growth Performance, Antioxidation, Meat Quality, and Intestinal Microflora in White Feather Broilers With Acute Oxidative Stress. Front. Vet. Sci. 9:889485. doi: 10.3389/fvets.2022.889485
Received: 04 March 2022; Accepted: 04 May 2022;
Published: 24 June 2022.
Edited by:
Asghar Kamboh, Sindh Agriculture University, PakistanReviewed by:
Muhammad Saeed, Northwest A&F University, ChinaCopyright © 2022 Wang, Tang, Long and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina Long, MTM5MDIyNDA4MzdAMTYzLmNvbQ==; Huihua Zhang, aGh6aGFuZzJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.