
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci. , 17 June 2022
Sec. Animal Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.882102
This article is part of the Research Topic Functions of Liver and Adipose Tissue in Metabolic Disorder Diseases of Ruminants View all 8 articles
 Vera Stiensmeier
Vera Stiensmeier Marion Schmicke*†
Marion Schmicke*†In previous studies, triiodothyronine (T3) was found to be lower in cows with ketosis and an effect of T3 on Growth Hormone Receptor (GHR) expression is described, e. g., in a human hepatoma cell line. Therefore, this study aimed to test whether T3 affects GHR messenger RNA (mRNA) expression in a well-established bovine hepatocyte model. Hepatocytes were kept in a sandwich culture and stimulated for 6 days with constant (10 μg/ml) or decreasing (from 10 to 5 μg/ml) T3 concentrations, and GHR, as well as IGF-1 mRNA expression, was measured using real-time polymerase chain reaction (RT-PCR). We could confirm in vitro that T3 has a stimulatory effect on GHR1A mRNA expression.
At the onset of lactation in dairy cows, the somatotropic axis becomes uncoupled due to a hepatic Growth Hormone (GH)-resistance by decreasing Growth Hormone Receptor (GHR) expression, resulting in an increase of GH and a decline of IGF-1 (1–3). This mediates a metabolic adaption as high GH levels stimulate lipolysis and gluconeogenesis, thereby supporting milk yield. Insufficient adaption can lead to metabolic disorders like ketosis. Also, within the thyrotropic axis changes occur, which lead to an adjustment of metabolism in the transition period (4). The onset of lactation results in the systemic decline of thyroxine (T4) and metabolically active triiodothyronine (T3) (5), but increasing concentrations of 5′deidonisase and with that T3 in the mammary gland (6, 7). That results in a metabolic prioritization of the mammary gland (6), but downregulation of metabolic demands in the periportal period (8). In ketotic cows, a decline of thyrotropic hormones, as well as hepatic GHR expression, was previously detected (9, 10). A direct stimulatory effect of T3 on GHR expression was previously demonstrated in, for example, human hepatoma cells (11). The effect of T3 is mediated by various intracellular pathways and directly at nuclear thyroid hormone receptors. This study aimed to test whether the active form of thyroid hormones (T3) may have an additive and direct effect on hepatic GHR mRNA expression in vitro in bovine primary hepatocytes.
Hepatic tissue used in this study came from a donor animal (Deutsche Holstein, female, 2 years old, non-lactating) that was euthanized in the Clinic for Cattle, University of Veterinary Medicine, Hanover, Germany because of an unfavorable prognosis due to lead contamination. A clinical chemistry revealed that liver values were normal (bilirubin 5.5 μmol/l reference of <7; aspartate aminotransferase 59 U/l reference <100; gamma-glutamyl transferase 29 U/L reference <33) or slightly elevated (glutamate dehydrogenase 25.8 U/L reference <14). Primary bovine hepatocytes were isolated and cultivated according to an established protocol by Witte et al. (12), with the following slight modifications: For purification of hepatocytes, Percoll® (REF P4937; Sigma-Aldrich Merck KGaA, Darmstadt, Germany) was applied. The viability of cells was tested prior to and after purifying, using trypan blue exclusion. The viability was 93%. Culture medium was William's E medium, hormone-free without dexamethasone and insulin, according to Witte et al. (12). To investigate the effect of T3, the hepatocytes were incubated for 6 days in three experimental setups: 1. invariant high hormone concentrations (INVARIANT; day 1–6: 10 μg/ml); 2. variant hormone concentrations according to physiological blood levels antepartum (VARIANT; day 1–6: 10, 9, 8, 7, 6, and 5 ng/ml); 3. control without any hormonal additive (CONTROL [CTRL]; day 1–6: 0 ng/ml). T3 (3,3″,5-Triiodo-L-Thyronine Sodium, T6397, Sigma-Aldrich Merck KGaA, Darmstadt, Deutschland) was added to the culture medium, which was changed every 24 h.
Cultured hepatocytes were daily morphologically evaluated by brightfield microscopy. Cells and supernatant a were collected on days 1, 4, and 6 and stored at −80°C until further analysis. Day 0 is defined as the day of isolation of the hepatocytes. Expression of GHR and IGF 1 mRNA was determined using a quantitative reverse transcriptase PCR. Total RNA was extracted using TRIzolTM Reagent (REF 15596018; Life Technologies Corporation, Carlsbad, USA), as previously published by Ehrhardt and Schmicke (13). The concentration and purity of extracted RNA were measured using a NanoDrop (NanoDrop 2000c, Thermo Fisher Scientific, Waltham, Massachusetts, USA) before transcription of RNA into complementary DNA (cDNA). PCR was performed using the Rotor-Gene Q (QUIAGEN GmbH, Hilden, Germany). Primers for GHR1A and IGF-1 were previously used and validated (12, 13).
In primary bovine hepatocytes (Figure 1) gathered from an adult dairy cow, T3 leads to an increase in GHR-mRNA expression, as well as IGF-1-mRNA expression, compared to the control (Figure 2). The changes in GHR expression after adding T3 were irrespective, if T3 was added in constant concentrations over 6 days (INVARIANT) or if T3 was added in decreasing concentrations (VARIANT) mirroring the decrease in T3 antepartum. Cell morphology of the hepatocytes did not change after T3 addition to the culture media (Figure 2).
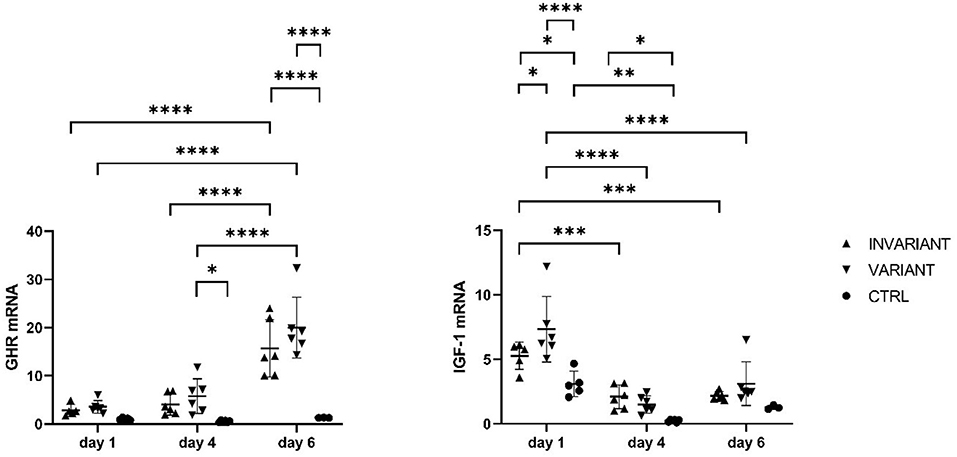
Figure 2. Influence of T3 on GHR and IGF-1 mRNA expression in primary bovine hepatocytes. Relative mRNA expression in adult primary bovine hepatocytes after culturing 1, 4, and 6 days with high (INVARIANT) or varied (VARIANT) concentrations of triiodthyronine and no hormone substitute (CTRL). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We could confirm that there is an additive effect of T3 on the expression of GHR and IGF 1 mRNA in primary bovine hepatocytes, using a well-established cell culture model (12–14) with primary hepatocytes derived from an adult dairy cow. A pivotal question is how T3 can induce IGF-1 production in primary hepatocytes without growth hormone (GH) being added to the cell culture medium. An unaware substitution of GH to the cell culture by FBS can be ruled out because of CTRL that was treated equally while cultivation, afterward no FBS was added to the culture medium. Also, a cellular stress-induced alteration of mRNA expression can be ruled out because of CTRL. We hypothesize there is another unknown stimulus in the cell culture that mediates alterations of basal gene expression because similar effects occurred in Endriß et al., who also did not add GH to the medium (15). Over time IGF-1 mRNA expression increased and GHR expression decreased in T3-stimulated cells. This might be because GHR expression is not further stimulated due to the lack of GH in the medium. It also can be a specific effect of T3 on GHR and IGF-1 mRNA expression at nuclear thyroid hormone receptors to modulate transcriptional activities via thyroid hormone response elements in the regulatory region of target genes. The mechanism behind this should be deeply studied further. From that, in vitro data, it can be speculated that in cows with lower T3 less stimulatory effect on the GHR is present and this may further uncouple the somatotropic axis. This effect was previously indicated also in hypothyroid rats, which had a distinct lower binding capacity to GH in the liver (16). In chickens, the treatment with propylthiouracil leads to hypothyroidism (T4 and T3 low) and this leads to lower GHR and IGF-1 mRNA expression in the liver (17). It must be critically discussed that the lead exposure of the animal may influence the pituitary-thyroid hormone axis as was shown in men. Lead exposure alter TSH levels (18), but no hint was found that mechanisms at GHR or IGF-1 expression might be affected. As our results were in strong agreement with data from mice, humans, pig, and other species the effect of lead contamination might be insignificant. However, further studies are needed to reveal the impact and pathomechanism of T3 in the context of ketosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
VS performed the experiments and wrote the manuscript. MS conducted the study, manuscript writing, and manuscript correction. All authors contributed to the article and approved the submitted version.
This research received funding from the H. Wilhelm Schaumann Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge Martina Hoedemaker and Kai Endriß from Clinic for Cattle, University of Veterinary Medicine, Hanover, Germany for supporting liver tissue.
1. Kobayashi Y, Vandehaar MJ, Tucker HA, Sharma BK, Lucy MC. Expression of growth hormone receptor 1A messenger ribonucleic acid in liver of dairy cows during lactation and after administration of recombinant bovine somatotropin. J Dairy Sci. (1999) 82:1910–6. doi: 10.3168/jds.S0022-0302(99)75426-3
2. Lucy MC, Jiang H, Kobayashi Y. Changes in the somatotrophic axis associated with the initiation of actation. J Dairy Sci. (2001) 84:E113–9. doi: 10.3168/jds.S0022-0302(01)70205-6
3. Radcliff RP, McCormack BL, Crooker BA, Lucy MC. Plasma hormones and expression of growth hormone receptor and insulin-like growth factor-I mRNA in hepatic tissue of periparturient dairy cows. J Dairy Sci. (2003) 86:3920–6. doi: 10.3168/jds.S0022-0302(03)74000-4
4. Steinhoff L, Jung K, Meyerholz MM, Heidekorn-Dettmer J, Hoedemaker M, Schmicke M. Thyroid hormone profiles and TSH evaluation during early pregnancy and the transition period in dairy cows. Theriogenology. (2019) 129:23. doi: 10.1016/j.theriogenology.2019.01.023
5. Refsal KR, Nachreiner RF, Anderson CR. Relationship of season, herd, lactation, age, and pregnancy with serum thyroxine and triiodothyronine in holstein cows. Domest Anim Endocrinol. (1984) 1:225–34. doi: 10.1016/0739-7240(84)90003-1
6. Capuco A V, Connor EE, Wood DL. Regulation of mammary gland sensitivity to thyroid hormones during the transition from pregnancy to lactation. Exp Biol Med. (2008) 233:1309–14. doi: 10.3181/0803-RM-85
7. Pezzi C, Accorsi PA, Vigo D, Govoni N, Gaiani R. 5′-deiodinase activity and circulating thyronines in lactating cows. J Dairy Sci. (2003) 86:152–8. doi: 10.3168/jds.S0022-0302(03)73595-4
8. Tiirats T. Thyroxine, triiodothyronine and reverse-triiodothyronine concentrations in blood plasma in relation to lactational stage, milk yield, energy and dietary protein intake in Estonian dairy cows. Acta Vet Scand. (1997) 38:339–48. doi: 10.1186/BF03548480
9. Ropstad E, Halse K, Refsdal AO. Thyroxine in blood plasma related to plasma levels of acetoacetate and glucose in ketotic and healthy cows. Acta Vet Scand. (1989) 30:175–83. doi: 10.1186/BF03548054
10. Piechotta M, Kedves K, Araujo M, Hoeflich A, Metzger F, Heppelmann M, et al. Hepatic mRNA expression of acid labile subunit and deiodinase 1 differs between cows selected for high versus low concentrations of insulin-like growth factor 1 in late pregnancy. J Dairy Sci. (2013) 96:6341. doi: 10.3168/jds.2012-6341
11. Mullis PE, Eblé A, Marti U, Bürgi U, Postel-Vinay M-C. Regulation of human growth hormone receptor gene transcription by triiodothyronine (T3). Mol Cell Endocrinol. (1999) 147:17–25. doi: 10.1016/S0303-7207(98)00232-9
12. Witte S, Brockelmann Y, Haeger JD, Schmicke M. Establishing a model of primary bovine hepatocytes with responsive growth hormone receptor expression. J Dairy Sci. (2019) 102:7522–35. doi: 10.3168/jds.2018-15873
13. Ehrhardt S, Schmicke M. Isolation and cultivation of adult primary bovine hepatocytes from abattoir derived liver. Excli J. (2016) 15:858–66. doi: 10.17179/excli2016-794
14. Ruhmann B, Giller K, Hankele AK, Ulbrich SE, Schmicke M. Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy. Theriogenology. (2017) 104:198–204. doi: 10.1016/j.theriogenology.2017.07.051
15. Endriß KJ, Meyerholz MM, Fischbach T, Brimmers L, Pfarrer C, Marth CD, et al. In vitro effects of Type i interferons (IFNτ and IFNα) on bovine hepatocytes cultured with or without Kupffer cells. Reprod Fertil Dev. (2021) 33:305–17. doi: 10.1071/RD20278
16. Hochberg Z, Bick T, Harel Z, Harel Z. Alterations of human growth hormone binding by rat liver membranes during hypo- and hyperthyroidism. Endocrinology. (1990) 126:325–9. doi: 10.1210/endo-126-1-325
17. Tsukada A, Ohkubo T, Sakaguchi K, Tanaka M, Nakashima K, Hayashida Y, et al. Thyroid hormones are involved in insulin-like growth factor-I (IGF-I) production by stimulating hepatic growth hormone receptor (GHR) gene expression in the chicken. Growth Horm IGF Res. (1998) 8:235–42. doi: 10.1016/S1096-6374(98)80116-0
Keywords: hepatocytes, Cattle, growth hormone, growth hormone receptor, triiodothyronine
Citation: Stiensmeier V and Schmicke M (2022) Brief Research Report: Effect of Triiodothyronine on Hepatic Growth Hormone Receptor Expression in Primary Bovine Hepatocytes. Front. Vet. Sci. 9:882102. doi: 10.3389/fvets.2022.882102
Received: 23 February 2022; Accepted: 17 May 2022;
Published: 17 June 2022.
Edited by:
Xiliang Du, Jilin University, ChinaReviewed by:
Yuxiang Song, Jilin University, ChinaCopyright © 2022 Stiensmeier and Schmicke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marion Schmicke, bWFyaW9uLnNjaG1pY2tlQHRpaG8taGFubm92ZXIuZGU=
†Present address: Marion Schmicke, Clinic for Cattle, Veterinary-Endocrinology and Laboratory Diagnostic, University of Veterinary Medicine Hannover, Hannover, Germany
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.