
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 12 May 2022
Sec. Animal Behavior and Welfare
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.881101
This article is part of the Research Topic Captive Animal Behavior: Individual Differences in Learning and Cognition, and Implications on Animal Welfare View all 10 articles
In humans and rats, changes in affect are known to occur during pregnancy, however it is unknown how gestation may influence mood in other non-human mammals. This study assessed changes in pigs' judgment bias as a measure of affective state throughout gestation. Pigs were trained to complete a spatial judgment bias task with reference to positive and negative locations. We tested gilts before mating, and during early and late gestation, by assessing their responses to ambiguous probe locations. Pigs responded increasingly negatively to ambiguous probes as gestation progressed and there were consistent inter-individual differences in baseline optimism. This suggests that the pigs' affective state may be altered during gestation, although as a non-pregnant control group was not tested, an effect of learning cannot be ruled out. These results suggest that judgment bias is altered during gestation in domestic pigs, consequently raising novel welfare considerations for captive multiparous species.
Research investigating the links between pregnancy, affect and cognition is most often carried out with a human-centric focus with studies typically using case studies and cohorts. In humans, changes in affective state during pregnancy are common and alterations in levels of anxiety, depression and cognitive ability have been demonstrated in humans and rodents (1–3). These changes are often linked to the large and rapid hormone fluctuations that occur during the gestational period (4, 5). Where human subjects cannot be used, rodent models are often employed to experimentally investigate how factors such as diet, enrichment or stress can influence behavior during gestation (6–8). To infer anxiety and depressive-like behaviors, lab-based behavioral tests, such as a forced swim or open-field test are often used (9). These studies are conducted under laboratory conditions and are generally aimed at modeling human gestation, rather than investigating how gestation may impact on the rodent itself. Results from both human and rodent studies are varied, however most show that affective state is altered throughout gestation [for review see (2)] and it is clear that pregnancy impacts maternal affective state.
Understanding an animals' affective state better enables us to understand their subjective experience, both positive and negative, and is a key component of animal welfare (10). Affective state can influence and alter cognitive processes, such as judgment (11, 12), which may then be used to infer and understand an animals' affective state. Cognitive bias or judgment bias is the influence of affect on information processing, with more content individuals likely to make positive assumptions about ambiguous stimuli, and vice versa (13). Judgment bias tests have been used to assess changes in affective state in a range of species, including pigs, dogs, honeybees and European starlings (14–17). Research typically focuses on the impact of external stimuli on judgment bias; this is likely to act via alteration to the internal, physiological environment ultimately resulting in changes in behavior and judgment bias (11, 18, 19). As such, we would expect internal stimuli, such as physiological changes, would also impact judgment bias directly even in the absence of external influences. Pregnancy is one of the biggest physiological changes a mammal may experience, involving major hormonal and cognitive adjustments (20, 21), yet little is known of how information processing and affective state may change in relation to gestation in animals.
The domestic pig (Sus scrofa domesticus) has been used as a human model in a wide range of medical research such as infectious disease (22), nutritional (23) and neurological studies (24). Pigs allow for longer lifespan studies and are more anatomically and physiologically similar to humans than other laboratory species, such as rodents (25, 26). More commonly, pigs are farmed around the globe for meat production. Modern intensive farming systems have been designed to produce food as quickly and cost efficiently as possible, and research is continually ongoing to understand how animal welfare can be optimized within these systems. Despite many studies on the behavioral and welfare needs of sows during gestation (27–30), only two studies used a specific judgment bias task to assess affective state in gestating sows. These studies focused on using judgment bias as a welfare indicator in gestating sows however, did not investigate how gestation itself influenced judgment bias (31, 32). More recently another study showed that gestating gilts that were classified as “friendly” visited an electronic sow feeder more often than individuals that were classified as “fearful” (33). The authors hypothesized that this feeding behavior may be similar to a judgment bias task and that the friendly individuals may have been more optimistic. However, again this study did not investigate how gestation itself influenced judgment bias.
We investigated how gestation may alter judgment, and therefore affective state, in domestic pigs. We compared within-individual affective state, as measured by a spatial judgment bias test, before mating, and during early and late gestation. We hypothesized that within-individual judgment bias would be more pessimistic during gestation than prior to mating, leading to an increase in latency to approach ambiguous cues throughout gestation. This is the first study to our knowledge to investigate the possible impact of gestation on judgment bias in domestic pigs.
This work was carried out between July and October 2015 (replicate one) and between January and July 2017 (replicate two) on a pig farm in the UK.
20 gilts (primiparous female pigs; N = 10 for each replicate) were selected based on age and time until first mating. Using gilts allowed for training time before gestation, as there is limited time between pregnancies once a sow has begun breeding. The average age of all 20 pigs on day one of training was 241.7 (SD: 15.93) days. Replicate two contained one Duroc and three Landrace pigs, the breed of all other individuals was Large White. Pigs were housed in pens of five or six animals, each pen (4.67 × 5.35 m) contained a sheltered sleeping area with straw bedding (2.70 × 4.67 m) and a run partially exposed to outdoor elements, such as wind and natural light (2.65 × 4.67 m). A standard lactating sow ration was fed once a day before mating and throughout gestation; there was continuous access to water and natural lighting. During the course of the study the animals remained within the same groups and pens to keep the external environment as controlled as possible throughout. The study pigs were able to interact with pigs in the pen next door via the gate and animals in the Neighboring pens may have been moved/changed. Due to involvement in a separate study, replicate one pigs received Regumate® (containing a steroidal progestin) orally with feed 23 days before planned estrus to allow for Synchronized farrowing. As of June 2020, no previous research was found investigating possible effects of Regumate® on affective state or behavior of pigs. Due to this research taking place on a working farm, it was not possible to test a separate non-pregnant control group and each pig was used as its own control.
The training and testing area (Figure 1) used comprised of a testing room (3.72 × 5.26 m) and a starting room (3.72 × 1.79 m). All pigs were habituated to the test area in groups for two to three sessions, and then individually for a maximum of seven sessions to habituate the pigs to eating from a bowl which was initially placed in the center of the test area. Following this, individuals were trained to associate the bowl in two opposite corner locations with a positive (P) and a negative (N) outcome. When in the P location, the bowl contained a small amount of chocolate raisins (replicate 1) or sugar-coated chocolates (replicate 2) and when it was in the N location, the bowl contained unpalatable food (bitter tasting coffee beans) to discourage the pigs from approaching this location. The pigs were trained to discriminate between these reference locations by first only receiving positive trials and then later interspersing negative trials. Latency to reach the bowl was recorded using video cameras and was then used as a metric to assess whether each individual had learned the discrimination. Each trial was 30 s in duration. Correct responses were recorded when the subject approached and touched their nose to the bowl during the positive (P) trials; during negative (N) trials, a correct response was recorded when the individual did not approach the bowl within 30 s. The location of P and N was counterbalanced across individuals. For both replicates a criterion of 70% correct responses in the last 20 trials was required before moving onto the testing phase. Per individual, forty-four training trials were conducted during replicate one and sixty-two for replicate two. Replicate two required more training trials due to the pigs being slower to differentiate between the positive and negative locations, though had a higher rate of meeting the criterion by the end of training. Five pigs from replicate one failed to meet this criterion and were removed from the study. Two pigs from replicate 2 did not meet this criterion. The analysis represents only those 13 that met the learning criterion.
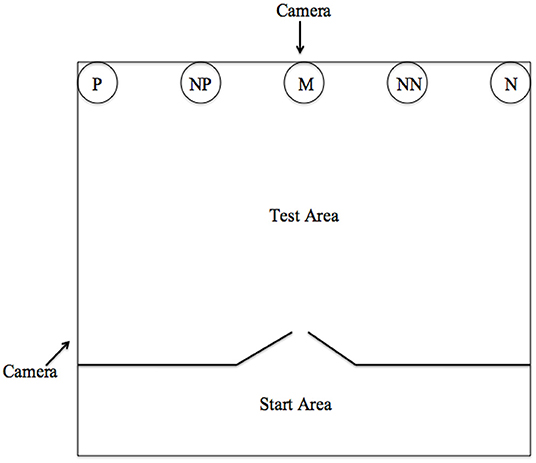
Figure 1. Experimental set up for the judgement bias test with positive (P), near positive (NP), middle (M), near negative (NN), and negative (N) locations. The figure shows the locations for an individual trained to expect a positive reward in the left corner and to avoid the right. Only one bowl was present in one location at a time.
Each testing session comprised two sets of nine trials carried out on the same day, involving five different bowl locations; the three intermediate ambiguous probes: near positive (NP), middle (M) and near negative (NN), interspersed with P and N reference locations (e.g. P, N, M, P, N, NP, P, N, NN). Only one bowl was in the arena during each trial. The ambiguous probes were placed in predetermined equidistant positions (0.74 m) and were not reinforced (i.e., they were left empty). They were presented in a pseudo-randomized order and interspersed among training trials. All “during gestation” testing sessions were preceded by five “reminder” training trials the day before testing. Each pig was tested three times: before gestation (1–2 weeks before mating); early gestation (4 weeks after mating); and late gestation (10–11 weeks after mating). One pig in replicate two was not tested before gestation and was only tested in the early and late test phases.
All data were analyzed in R version 3.4.1 using general linear mixed effects models with the lmer function in the package lme4 (34). The response variable was natural logged to ensure that the residuals conformed to the assumptions of normality. To test the effects of gestation time on judgment bias, the response variable was log time taken to approach the presented probes; fixed explanatory effects were probe location, coded as a continuous variable from positive (1) to negative (5) with ambiguous locations at points 2, 3, and 4; and gestation time coded as a factor with three levels (pre, early and late gestation). Probe location squared was included as initial data exploration suggested curvature in the fits. Interactions between gestation time and probe location and probe location squared were also included.
To find the most appropriate structure for the random model, we compared eight models: two intercept only models and six combinations of random intercept and slope models such that random intercepts were fitted for each pig at each experimental timepoint (or for each pig independent of experimental replicate), with variation allowed between gestation times and the shape of the curve was allowed to vary between pigs (Table 1).
The Akaike Information Criteria (AIC) values for all models were compared using the model.sel function in the MuMIn package (35). In each case the residuals of the final minimal model were visually assessed for deviations from normality. For the final models, predicted fits were produced using the predict function in base R. R2 values for each model were calculated using the r.squaredGLMM function in the MuMIn package (35). For every model, the general pattern of results was robust, with the different random models only affecting the predictions very slightly. The best model is reported in the main text, and the corresponding figure for the other model where AIC comparison had delta <2 is reported as supplementary information.
The pigs' responses to ambiguous locations in the judgement bias test changed throughout gestation (Tables 2, 3; Figure 2). Pigs consistently approached the positive probe quickly and the negative probe slowly (or not at all), getting generally slower during gestation (Figure 2). However, whilst the mean speed of approach was fairly linear between positive and negative pre- and early gestation (Figures 2A,B), by late gestation, pigs showed a shift toward pessimism, such that the positive probe continued to be approached quickly but ambiguous probes were approached more slowly (Figure 2C).
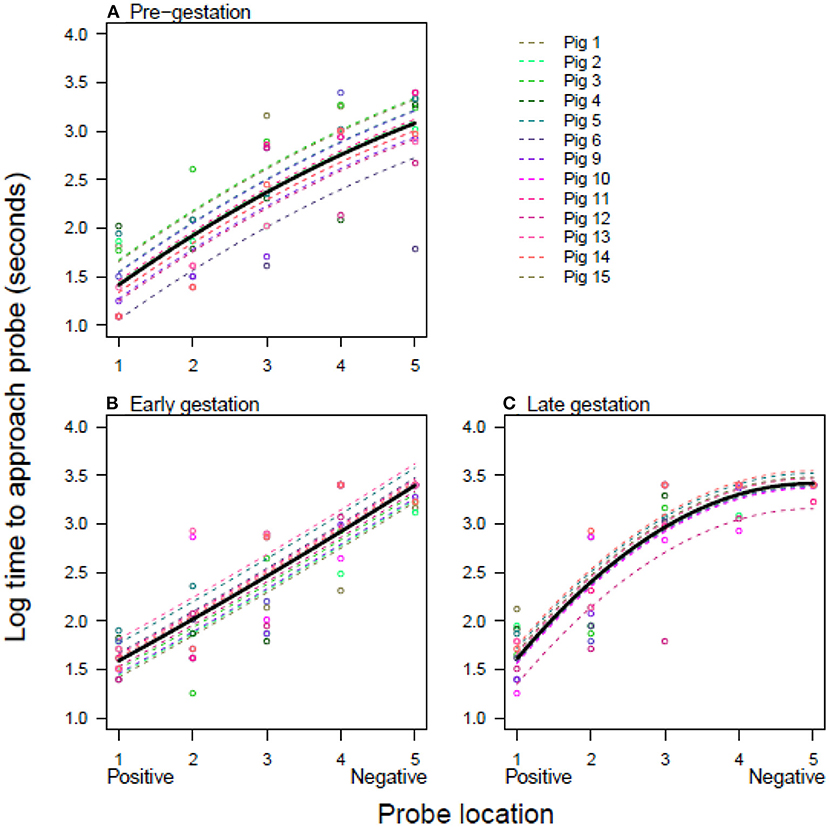
Figure 2. The time to approach each location at three stages of gestation. Log time taken to approach each location for pigs at three different stages of the pig's 16-week gestational period; (A) pre-gestation, (B) early gestation (5 weeks), and (C) late gestation (10–11 weeks). The open circles are raw data points and the lines are model predictions from the minimal adequate model fixed to the level of experimental replicate 1. Results from model 1 are shown, where the intercept is allowed to vary for each pig at each gestation time. Pigs 1–5 are from replicate 1 and pigs 6–15 are from replicate 2.
All models retained all interactions and gave qualitatively similar results. The best model was model 1, where the intercept was allowed to vary for each pig at each gestation time (Table 1). However, the result for model 2, where the intercept was allowed to vary for each pig at each gestation time, within each replicate, was equally well supported (delta AIC <2; Table 2, Supplementary Figure 1).
In livestock species, judgment bias tasks are typically used to assess the impact of external factors, for example environmental enrichment (36) or stocking density (37). However, internal factors, such as the large physiological changes associated with gestation, also have the potential to influence affective state and therefore judgment bias. The aim of this study was to assess judgment bias in domestic pigs throughout gestation. It was hypothesized that the gilts would be more pessimistic during gestation than prior to mating, as indicated by an increase in latency to approach the ambiguous cues. Our results suggest this to be the case, with the gilts taking longer to approach the ambiguous locations in the later stage of gestation than before mating which indicates that judgment bias changed as gestation progressed. This was most apparent at the middle and most ambiguous location (Figure 2) and suggests the pigs were more pessimistic during the late gestational stage. Crucially, the latency to reach the positive location did not vary markedly throughout gestation, highlighting that changes, such as impaired locomotion or an increase weight, did not affect the gilts' response latencies to the other four locations (Figure 2). This also shows that the gilts were highly motivated by the reward, even though they were not feed-restricted. Thus, these results suggest increased pessimism during the late stage of gestation, despite the fact that the immediate external environment remained constant. This may infer that, alongside external factors, internally-driven factors can also influence judgment bias and affective state in domestic pigs. Although this result should be interpreted in light of the pigs being their own control and no separate control group being tested.
In this study, a spatial go/no-go judgment bias test was used as this type of task has been successfully used with pigs previously (14, 31, 32, 36). Previous judgment bias studies with livestock species have shown that a change of bias can occur in response to a change in external factors, such as enrichment (36) or handling (38). Recent studies by Horback and Parsons (31, 32) also used a spatial go/no-go task and found that group housed gestating sows displayed both positive and negative biases despite having the same external conditions. Interestingly, the sows' behavioral traits influenced judgment bias however, these studies were not specifically focusing on the effect of gestation on judgment bias, and therefore it is unclear if the stage of gestation may also play a role in these bias differences. The possibility that pigs' judgment bias may change from a positive to a more negative state during the late stage of gestation suggests that the pigs' welfare needs may change too. This highlights the importance of considering the impact of large physiological changes, such as gestation, on animal welfare. This study may have implications not only for the welfare of farmed animals that experience gestation, but also for research into affective state during gestation in other captive multiparous mammalian species, including how this may impact cumulatively across the life course on their health and welfare. For example, in humans, multiparous women appear to be more at risk and have a different pattern of anxious or depressive symptoms compared to primiparous women (39, 40). In humans, hormone fluctuations and other physiological changes throughout pregnancy are often correlated with changes in mood and affective state (4, 5). Pigs are frequently used as models for humans in medical and pharmaceutical studies (22, 23, 41, 42), so it is possible that a change in affective state during gestation may be caused by comparative mechanisms, however, further research is required to validate this.
Alongside this interesting result, there are some limitations to take into consideration. Previous studies have shown that multiple testing time points can result in an increase in pessimistic responses (43, 44) and this increase in latencies during the later testing phases is similar to what was found in this study. As it was not possible to test a non-pregnant control group, this effect of learning cannot be ruled out. However, the effects of gestation represent a plausible driver for the changes in affect we report as previous research in rodents and humans has shown that mood and affective state can vary throughout gestation (1–3), with negative mood more likely to be present during the first and third trimester in humans (45, 46). Future studies should consider the role of learning by including a non-gestating control group, and whether ambiguous trial locations should be rewarded or un-rewarded (47). There were also some differences between replicates, such as one replicate receiving Regumate®, and different rewards being used. Despite this, the effect of replicate on the data was marginal, showing that the change in judgment bias over the course of gestation was robust and not influenced by these differences between replicates.
In conclusion, this study suggests that judgment bias in farmed domestic pigs may change with stage of gestation, inferring that internally driven stimuli may directly affect judgment bias without external influence. This study raises novel welfare considerations for captive primiparous, and possibly multiparous, species and provides a basis for future research into the effect of gestation on judgment bias in non-human animals.
The data and R code is available from the Open Science Framework database: https://osf.io/32wgy/.
The University of Lincoln, College of Science Ethics Committee approved this study (COSREC189; COSREC262). The animal owners provided written informed consent for participation of their animals in this study.
EB carried out data collection. LC conceived of the study. SC carried out statistical analysis. All authors assisted with study design and coordination, drafting the final manuscript, and gave final approval for publication.
LC and MF were funded by the Biotechnology and Biological Sciences Research Council Grant BB/K002554/2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the farm staff for their help and cooperation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.881101/full#supplementary-material
1. Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. (2008) 1241:136–47. doi: 10.1016/j.brainres.2008.09.006
2. Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. (2010) 34:452–67. doi: 10.1016/j.neubiorev.2009.08.011
3. Ferreira CR, Orsini MC, Vieira CR. do Amarante Paffaro AM, Silva RR. Prevalence of anxiety symptoms and depression in the third gestational trimester. Arch Gynecol Obstet. (2015) 291:999–1003. doi: 10.1007/s00404-014-3508-x
4. Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. (2011) 126:54–72. doi: 10.1037/a0025538
5. Uguz F, Gezginc K, Kayhan F, Sari S, Buyukoz D. Is pregnancy associated with mood and anxiety disorders? A cross-sectional study. Gen Hosp Psychiatry. (2010) 32:213–5. doi: 10.1016/j.genhosppsych.2009.11.002
6. Tang M, Liu Y, Wang L, Li H, Cai H, Zhang M, et al. An Ω-3 fatty acid-deficient diet during gestation induces depressive-like behavior in rats: the role of the hypothalamo–pituitary–adrenal (HPA) system. Food Funct. (2018) 9:3481–8. doi: 10.1039/C7FO01714F
7. Rosenfeld A. WellerA. Behavioral effects of environmental enrichment during gestation in WKY and Wistar rats. Behav Brain Res. (2012) 233:245–55. doi: 10.1016/j.bbr.2012.05.006
8. de Brito Guzzo SFC, Rafael C, Fitipaldi BM, Garcia AA, Dias KV, Luiz YJ, et al. Impact of chronic stressors on the anxiety profile of pregnant rats. Physiol Behav. (2015) 142:137–45. doi: 10.1016/j.physbeh.2015.02.014
9. Belovicova K, Bogi E, Csatlosova K, Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip Toxicol. (2017) 10:40–3. doi: 10.1515/intox-2017-0006
10. Mendl M, Burman OH, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc R Soc B Biol Sci. (2010) 277:2895–904. doi: 10.1098/rspb.2010.0303
11. Mendl M, Burman OH, Parker RM, Paul ES. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl Anim Behav Sci. (2009) 118:161–81. doi: 10.1016/j.applanim.2009.02.023
12. Boissy A, Arnould C, Chaillou E, Désiré L, Duvaux-Ponter C, Greiveldinger L, et al. Emotions and cognition: a new approach to animal welfare. Anim Welf. (2007) 16:37–43.
13. Bethell EJ A. “how-to” guide for designing judgment bias studies to assess captive animal welfare. J Appl Anim Welf Sci. (2015) 18:18–42. doi: 10.1080/10888705.2015.1075833
14. Asher L, Friel M, Griffin K, Collins LM. Mood and personality interact to determine cognitive biases in pigs. Biol Lett. (2016) 12:20160402. doi: 10.1098/rsbl.2016.0402
15. Bateson M, Desire S, Gartside SE, Wright GA. Agitated honeybees exhibit pessimistic cognitive biases. Curr Biol. (2011) 21:1070–3. doi: 10.1016/j.cub.2011.05.017
16. Mendl M, Brooks J, Basse C, Burman O, Paul E, Blackwell E, et al. Dogs showing separation-related behaviour exhibit a “pessimistic” cognitive bias. Curr Biol. (2010) 20:R839–40. doi: 10.1016/j.cub.2010.08.030
17. Matheson SM, Asher L, Bateson M. Larger, enriched cages are associated with “optimistic” response biases in captive European starlings (Sturnus vulgaris). Appl Anim Behav Sci. (2008) 109:374–83. doi: 10.1016/j.applanim.2007.03.007
18. Verbeek E, Ferguson D, Lee C. Are hungry sheep more pessimistic? The effects of food restriction on cognitive bias and the involvement of ghrelin in its regulation. Physiol Behav. (2014) 123:7–75. doi: 10.1016/j.physbeh.2013.09.017
19. Oliveira FR, Nogueira-Filho SL, Sousa MB, Dias CT, Mendl M, Nogueira SS. Measurement of cognitive bias and cortisol levels to evaluate the effects of space restriction on captive collared peccary (Mammalia, Tayassuidae). Appl Anim Behav Sci. (2016) 181:76–82. doi: 10.1016/j.applanim.2016.05.021
20. Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. (2005) 84:701–10. doi: 10.1016/j.fertnstert.2005.02.045
21. Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. (2003) 74:67–83. doi: 10.1016/S0165-0327(02)00432-9
22. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. (2012) 20:50–7. doi: 10.1016/j.tim.2011.11.002
23. Roura E, Koopmans SJ, Lallès JP, Le Huerou-Luron I, De Jager N, Schuurman T, et al. Critical review evaluating the pig as a model for human nutritional physiology. Nutr Res Rev. (2016) 29:60–90. doi: 10.1017/S0954422416000020
24. Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. Animal. (2009) 3:1138–51. doi: 10.1017/S1751731109004649
25. Flisikowska T, Kind A, Schnieke A. Genetically modified pigs to model human diseases. J Appl Genet. (2014) 55:53–64. doi: 10.1007/s13353-013-0182-9
26. Fan N, Lai L. Genetically modified pig models for human diseases. J Genet Genomics. (2013) 40:67–73. doi: 10.1016/j.jgg.2012.07.014
27. Algers B, Uvnäs-Moberg K. Maternal behaviour in pigs. Livest Sci. (2007) 52:78–85. doi: 10.1016/j.yhbeh.2007.03.022
28. Boyle LA, Leonard FC, Lynch PB, Brophy P. Effect of gestation housing on behaviour and skin lesions of sows in farrowing crates. Appl Anim Behav Sci. (2002) 76:119–34. doi: 10.1016/S0168-1591(01)00211-8
29. Damm BI, Lisborg L, Vestergaard KS, Vanicek J. Nest-building, behavioural disturbances and heart rate in farrowing sows kept in crates and schmid pens. Livest Sci. (2003) 80:175–87. doi: 10.1016/S0301-6226(02)00186-0
30. Wischner D, Kemper N, Krieter J. Nest-building behaviour in sows and consequences for pig husbandry. Livest Sci. (2009) 124:1–8. doi: 10.1016/j.livsci.2009.01.015
31. Horback KM, Parsons TD. Judgement bias testing in group-housed gestating sows. Behav Processes. (2019) 159:86–92. doi: 10.1016/j.beproc.2018.12.021
32. Horback KM, Parsons TD. Judgement bias of group housed gestating sows predicted by behavioral traits, but not physical measures of welfare. PLoS ONE. (2022) 17:e0264258. doi: 10.1371/journal.pone.0264258
33. Rooney HB, Schmitt O, Courty A, Lawlor PG, O'Driscoll K. Like mother like child: do fearful sows have fearful piglets? Animals. (2021) 11:1232. doi: 10.3390/ani11051232
34. R Core Team. R: A Language Environment for Statistical Computing. (2017). R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/ (accessed April 20, 2022).
35. Bartoń K. MuMIn: Multi-Model Inference. R Package Version 1421. (2018). Available online at: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf
36. Douglas C, Bateson M, Walsh C, Bédué A, Edwards SA. Environmental enrichment induces optimistic cognitive biases in pigs. Appl Anim Behav Sci. (2012) 139:65–73. doi: 10.1016/j.applanim.2012.02.018
37. Scollo A, Gottardo F, Contiero B, Edwards SA. Does stocking density modify affective state in pigs as assessed by cognitive bias, behavioural and physiological parameters? Appl Anim Behav Sci. (2014) 153:26–35. doi: 10.1016/j.applanim.2014.01.006
38. Doyle RE, Fisher AD, Hinch GN, Boissy A, Lee C. Release from restraint generates a positive judgement bias in sheep. Appl Anim Behav Sci. (2010) 122:28–34. doi: 10.1016/j.applanim.2009.11.003
39. Canário C, Figueiredo B. Anxiety and depressive symptoms in women and men from early pregnancy to 30 months postpartum. J Reprod Infant Psychol. (2017) 35:431–49. doi: 10.1080/02646838.2017.1368464
40. Dipietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynecol. (2008) 29:115–24. doi: 10.1080/01674820701701546
41. Flisikowska T, Kind A, Schnieke A. The new pig on the block: modelling cancer in pigs. Transgenic Res. (2013) 22:673–80. doi: 10.1007/s11248-013-9720-9
42. Verma N, Rettenmeier AW, Schmitz-Spanke S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics. (2011) 11:776–93. doi: 10.1002/pmic.201000320
43. Doyle RE, Vidal S, Hinch GN, Fisher AD, Boissy A, Lee C. The effect of repeated testing on judgement biases in sheep. Behav Process. (2010) 83:349–52. doi: 10.1016/j.beproc.2010.01.019
44. Murphy E, Nordquist RE, van der Staay FJ. Responses of conventional pigs and Göttingen miniature pigs in an active choice judgement bias task. Appl Anim Behav Sci. (2013) 148:64–76. doi: 10.1016/j.applanim.2013.07.011
45. Markon KE, Brunette CA, Whitney BM, O'Hara MW. Mood during pregnancy: Trends, structure, and invariance by gestational day. J Psychiatr Res. (2021) 140:260–6. doi: 10.1016/j.jpsychires.2021.06.006
46. Rallis S, Skouteris H, McCabe M, Milgrom J A. prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. (2014) 27:36–e42. doi: 10.1016/j.wombi.2014.08.002
Keywords: pregnancy, gestation, cognitive bias, affective state, information processing, pig
Citation: Bushby EV, Cotter SC, Wilkinson A, Friel M and Collins LM (2022) Judgment Bias During Gestation in Domestic Pigs. Front. Vet. Sci. 9:881101. doi: 10.3389/fvets.2022.881101
Received: 22 February 2022; Accepted: 12 April 2022;
Published: 12 May 2022.
Edited by:
Christian Nawroth, Leibniz Institute for Farm Animal Biology (FBN), GermanyReviewed by:
Inonge Reimert, Wageningen University and Research, NetherlandsCopyright © 2022 Bushby, Cotter, Wilkinson, Friel and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa M. Collins, bC5jb2xsaW5zQGxlZWRzLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.