- 1Animal and Poultry Production, Department of Animal Wealth Development, Faculty of Veterinary Medicine, Benha University, Benha, Egypt
- 2Department of Nutrition and Veterinary Clinical Nutrition, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 3Rabbit, Turkey and Waterfowl Animal Production Research Institute, Agricultural Research Center, Giza, Egypt
- 4Department of Biotechnology, Animal Production Research Institute, Agricultural Research Center, Giza, Egypt
- 5Department of Animal Production, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt
- 6Center for Applied Research on the Environment and Sustainability, American University in Cairo, New Cairo, Egypt
- 7Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 8Biology Department, Faculty of Science, King Khalid University, Abha, Saudi Arabia
- 9Zoology Department, College of Science, Damanhour University, Damanhour, Egypt
- 10Department of Pharmaceutical Sciences, Pharmacy Program, Batterjee Medical College, Jeddah, Saudi Arabia
- 11Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
Macleaya cordata (M. cordata) is a herbal plant that has abundant amounts of sanguinarine, which has many biomedical properties. The effects of M. cordata dietary supplementation on the productive performance, some blood constituents, and growth-related genes' expression were evaluated in turkey. M. cordata extract was dietary supplemented to turkey at levels of 25, 50, and 100 ppm and a control group. Growth performance measurements (FBW, ADG, and FCR) and production efficiency factor for turkey (BPEF) were similar (p > 0.05) in all supplemented groups. M. cordata has no adverse effects (p > 0.05) on the birds' health regarding hematological (Hb, RBCs, WBCs, and PCV) and blood biochemical indices evaluating liver function, kidney function, and lipid profile. Moreover, the mRNA expression of growth-related genes, such as growth hormone receptor (GHR), insulin-like growth factor 1 (IGF-1), cyclooxygenase 3 (COX-3), adenine nucleotide translocase (ANT), and uncoupling protein 3 (UCP-3) were upregulated (p < 0.001) in M. cordata treatments with the highest value for SG50 compared with the control group. We concluded that exogenous M. cordata dietary supplementation upregulated the expression of growth-related genes in turkey at a level of 50 ppm without adverse effects on their health status regarding hematological and biochemical indices.
Introduction
The increasing population and food demand require more food production and sustainable plans to ensure food availability for the next generations. In the poultry industry, the probability of infectious diseases is high, resulting in a substantial economic loss and antibiotic resistance and other environmental hazards. Under the United Nations and Food and Agriculture Organization regulations involved in food security and the production of safe animal protein products, it has become necessary to develop natural additives to substitute antibiotic usage. The supplementation of phytogenic feed additives is proven safe and efficient to be widely applied in fish, birds, and livestock production for their productive efficiency if used in the optimal dose and reasonable duration (1, 2).
Macleaya cordata is a herbal plant that has abundant amounts of sanguinarine, which is commercially known as Sangrovit® (3). The M. cordata plant contained in Sangrovit® (as a feed additive used in animal nutrition) is reliable as per the list of the European Food Safety Authority (4). Furthermore, Sangrovit® is not metabolized into potentially harmful metabolites and is excreted without being absorbed through the small intestine (3). Traditionally, M. cordata is used as a Chinese herbal with many biomedical properties (depurative, analgesic, carminative, antiedemic, and diuretic) (3, 5). It contains alkaloid substances (e.g., benzophenanthidrine), which are extracted from the rhizomes of the Sanguinaria canadensis plant (6). Incorporating M. cordata in birds' diet resulted in improving the feed efficiency resulting from enhanced intestinal health (7–12). M. cordata has antioxidant activity as well as enhanced immunity in livestock and birds (13).
Turkey meat is preferred by consumers as a source of protein and essential amino and fatty acids, minerals, and vitamins. Recently, expensive raw materials made it imperative to search for alternative feed ingredients and feed additives that will help to reduce the overall cost of the rations. As a result, the application of phytobiotics besides understanding their potential effect on performance and wellbeing is crucial to protect against antibiotic resistance. To the best of the author's knowledge, there are no previous trials that have investigated the effects of M. cordata dietary inclusion in turkey. Therefore, the present study aimed to evaluate the impact of M. cordata dietary supplementation on the growth measurements, hematological, and biochemical parameters, and the expression of growth-related genes in turkey.
Materials and Methods
Ethics Statement
This investigation was approved by the Committee of Local Experimental Animal Care, Damanhour University, Egypt, Faculty of Veterinary Medicine (VMD: 15/2018).
Birds and Dietary Treatments
One hundred and eighty one-day-old-Bronze turkey chicks were distributed into four experimental treatments of mixed-sex, randomly with 45 chicks in each. Each treatment was divided into 3 replicates (15 chick/replicate). The chicks were wing-banded for their identification. All chicks were maintained for 2 weeks for brooding with feeding on control (basal diet), for acclimatization at the beginning of the experiment, and the chicks were reared on a litter floor over a 20-week duration. Feeding and watering were freely accessed. All birds were reared under standard conditions. Chicks were exposed to 24-h light throughout the whole experimental period.
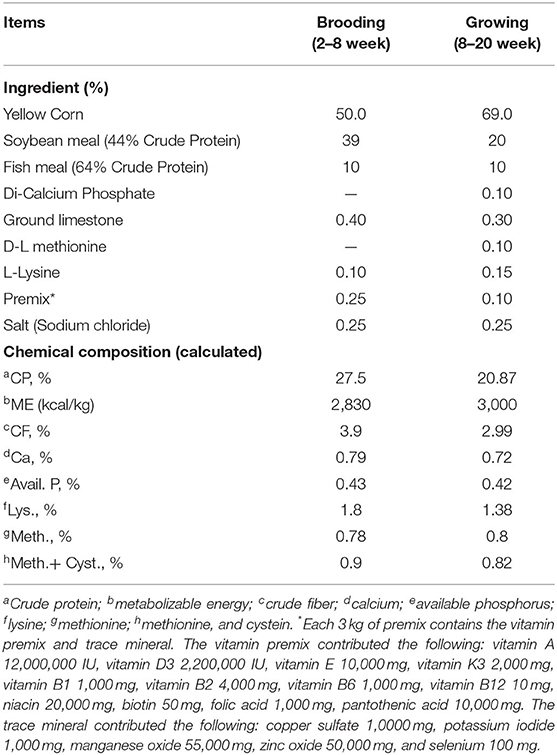
Birds were fed on a well-balanced control diet (basal diet) during the first 2 weeks, then, chicks were accessed to the experimental diets over the whole experiment (20th week of age). The four groups (control, SG25, SG50, and SG100) were treated with 0, 25, 50, and 100 ppm Sangrovit® (Germany, Registered Number: 816) and its composition was M. cordata extract 4% and powder M. cordata (carrier) 96%, with chemical analysis being crude protein 7%, crude fiber 7.2, crude ash 58.4, and moisture 4.0% (14). The basal diets (corn-soybean-based diet) were formulated according to the nutrient requirements for Bronze Turkey (Table 1).
Turkey chicks were vaccinated in all cages as follows: Hitchner 1 in water (Polimun-ND®, BioTestLab, Kiev, Ukraine) at 7 days of age. Inactivated avian influenza subtype H5N1 vaccine (MeFluvac®, MEVAC, Cairo, Egypt) at 10 and 30 days of age; live Newcastle disease (Nobilis® ND LaSota, MSD, Boxmeer, the Netherlands) vaccine in water at 16 days; inactivated Newcastle disease (MEVAC-ND®, MEVAC, Cairo, Egypt) at 45 days; inactivated bivalent (H5N1+ND) vaccine (Volvac®, Boehringer Ingelheim, Germany) at the age of 90 days and finally vaccinated with inactivated Cholera vaccine (Servac®, Abbasia, Egypt). The subcutaneous injection was used to administer all inactivated vaccines.
Growth Performance
Turkey chicks were weighed at 1 day old and then every month for 20 weeks. Bodyweight gain (BWG), relative growth rate (RGR), average feed intake (AFI), feed conversion ratio (FCR), and production efficiency factor for turkey (BPEF) (15) were evaluated throughout the whole experimental period.
Sample Collection
Blood samples (n = 16) were collected from the wing vein (2 ml) at the ages of 2nd and 20th weeks. About 1 ml of blood was collected on ethylenediaminetetraacetic acid (EDTA) to assess the blood hematology and 1 ml was centrifuged at 3,500 rpm/15 min and the extracted plasma was used for all biochemical analyses using commercial kits. Birds were euthanized using sodium pentobarbital (I/V; 50 mg/kg), immediately necropsied, and liver samples of 30 mg (n = 16; 8 males; and 8 females) were collected from each group and kept at −80°C for messenger RNA (mRNA) gene expressions.
Hematological and Biochemical Parameters
Biochemical parameters [liver function; glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), kidney function; urea, creatinine, and blood urea nitrogen (BUN), lipid profile; triglycerides, cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)] were analyzed using commercial kits (Spinreact, Barcelona, Spain) on an ultraviolet-visible spectrophotometer. Blood indices, such as white blood cells (WBCs) and red blood cells (RBCs) were counted in a Neubauer hemocytometer using a 1:200 dilution with Natt and Herrick solution. Differential leukocyte count, hemoglobin (Hb) concentration, and packed cell volume (PCV) were determined as described previously (16).
Gene Expression
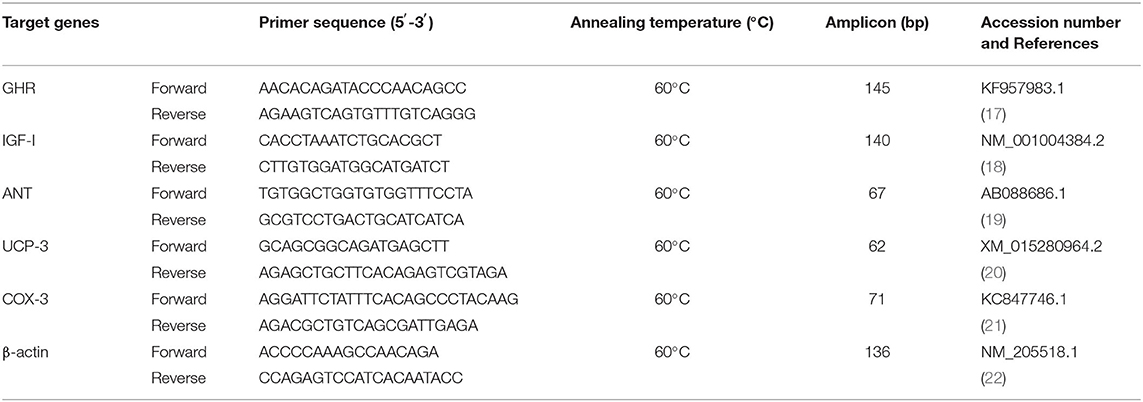
Following the manufacturer's protocol, total RNA was extracted from the tissue by TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA) and NanoDrop for quantification was utilized for RNA extraction. Single-stranded cDNA was synthesized from 1,000 ng of total RNA according to the manufacturer's High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems) protocol. Cycling conditions were as follows: 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. Then total RNA and cDNA samples were stored at −80°C till the next step. The real-time polymerase chain reaction (RT-PCR) was used to evaluate growth hormone receptor (GHR), insulin-like growth factor 1 (IGF-1), cyclooxygenase 3 (COX-3), adenine nucleotide translocase (ANT), and uncoupling protein 3 (UCP-3) expression. RT-PCR assays were performed using the fluorescent dye SYBR Green (SYBR® Green PCR Master Mix, Applied Biosystems, USA). All reactions were analyzed and normalized to the ROX Reference Dye (Invitrogen, Carlsbad, CA, USA). Amplification reactions were carried out referring to Gene Bank (http://www.ncbi.nlm.nih.gov) and β-actin was used as an endogenous control gene (Table 2). Gene expression was calculated from the obtained cycle threshold (Ct) values provided by RT-PCR instrumentation using the comparative Ct method to a reference (housekeeping) gene (β-actin), fold change = 2−ΔΔCT as previously stated (23).
Statistical Analysis
The Shapiro–Wilks test was used to examine the normal distribution of variables. The statistical software package SPSS 20 (IBM 20; SPSS Inc., Chicago, IL, USA) was used based on the analysis of variance (ANOVA). Linear and quadratic contrasts of Sangrovit® dietary inclusion were used to evaluate its effect on different measurements (growth, hematological, and biochemical parameters). Graphpad prism 5 was used to analyze the data of the RT-PCR with one-way ANOVA. Statistical significance between mean values and Tukey's post-hoc test was assessed at p < 0.05.
Results
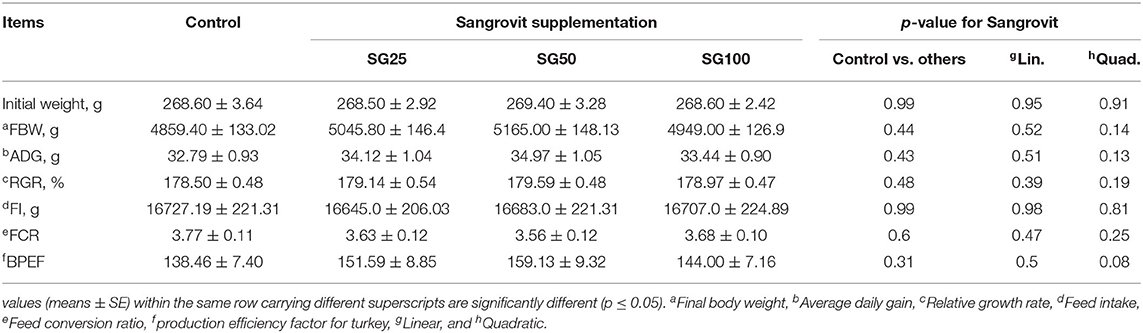
The effects of the experimental diets on performance traits in turkeys throughout the experiment are presented in Table 3. The results of linear contrast showed that growth was unaffected by Sangrovit® inclusion, however, it was marginally improved (p > 0.05) in SG50 over the whole experimental period compared with control, by 6.29, 6.6, 5.6, and 14.9% for FBW, ADG, FCR, and BPEF, respectively.
Contrast analyses of hematological and biochemical parameters are displayed in Tables 4, 5 during the experimental period at the age of 2nd and 20th weeks, respectively. No meaningful changes were recorded among the birds' groups that delivered M. cordata in terms of hematological and blood biochemical traits at the age of 2nd week, except for white blood cell (WBC), SG50 recorded the highest value (p < 0.05) compared with other supplemented groups, and triglyceride, SG50 showed the least value (p < 0.05) compared with SG100. Similarly, at 20th week among the birds' groups that delivered M. cordata, the changes recorded for mean cell volume (MCV) where SG50 was the highest value (p < 0.05) compared with SG25, WBC, SG50 recorded the highest value (p < 0.05) compared with control and other supplemented groups, GOT where SG100 showed the highest value (p < 0.05) compared with control, triglyceride where SG50 and SG100 recorded the least value (p < 0.05) compared with SG25, HDL where SG100 showed the highest value (p < 0.05) compared with SG25.
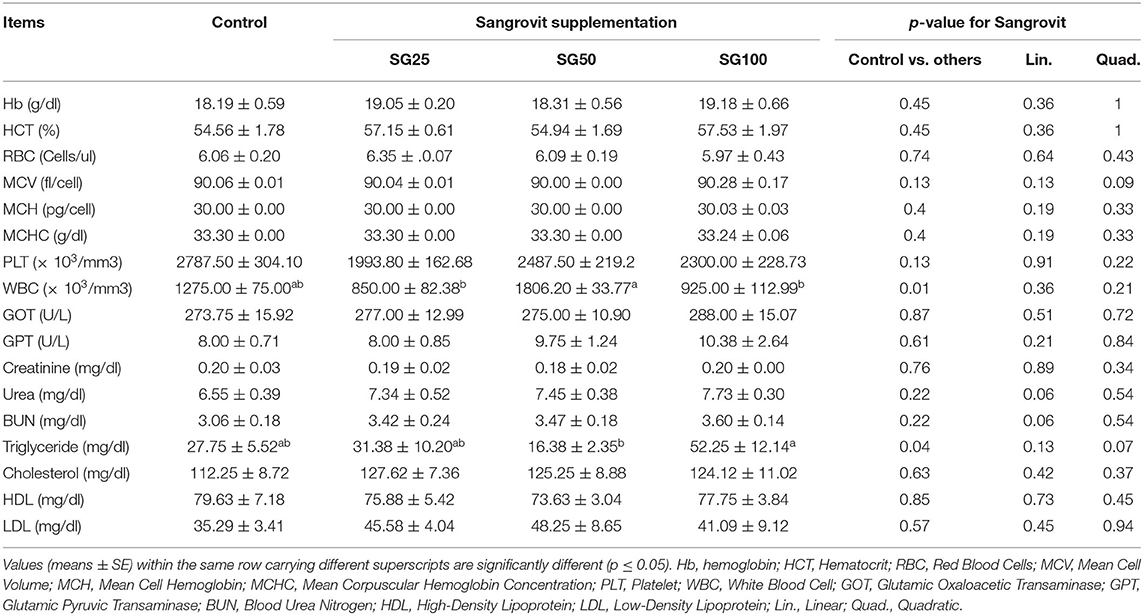
Table 4. Effect of Macleaya cordata dietary supplementation on hematological and biochemical parameters of turkey at the 2nd week of age.
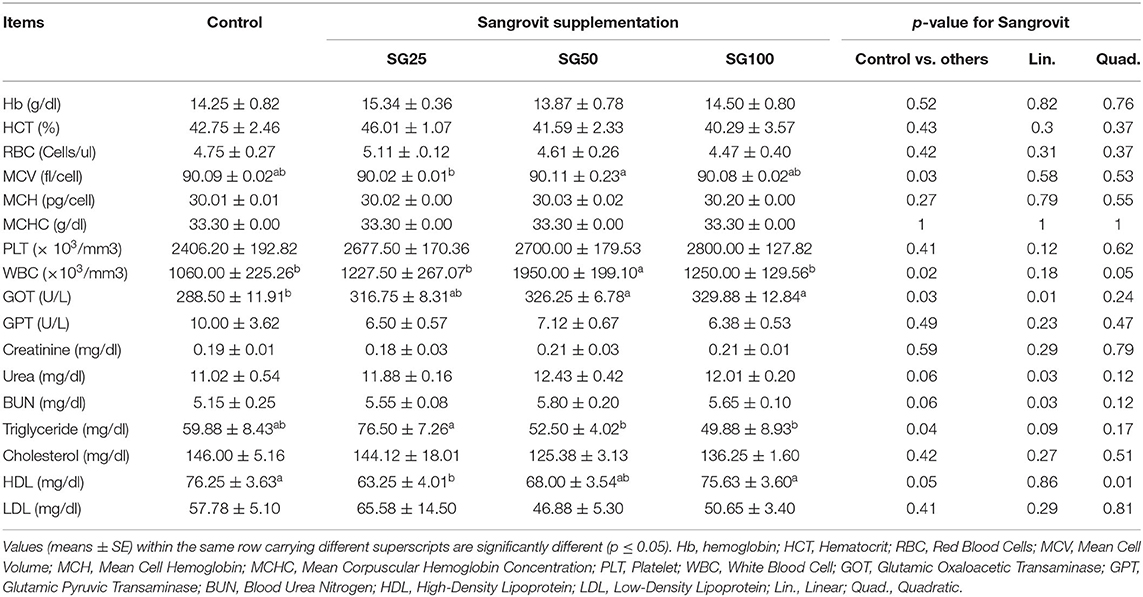
Table 5. Effect of Macleaya cordata dietary supplementation on hematological and biochemical parameters of turkey at the 20th week of age.
The results of growth gene expression are shown in Figures 1A–E. Figures 1A–C showed that the expressions of GHR, insulin-like growth factor 1 IGF-1, and COX-3 in the Sangrovit® supplemented groups were increased (p < 0.001), compared with control one, and their values upregulated (p < 0.001) in SG50 followed by SG100 and SG25, respectively. Data in Figure 1D show the mRNA expression of adenine nucleotide translocase (ANT); the SG50 group had higher (p < 0.001) level than control and SG25 groups. Moreover, its value was similar to SG100 group. As shown in Figure 1E, the fold change of uncoupling protein 3 (UCP-3) in SG50 group was upregulated (p < 0.001), compared with other groups, while their values in SG25 and SG100 groups were similar.
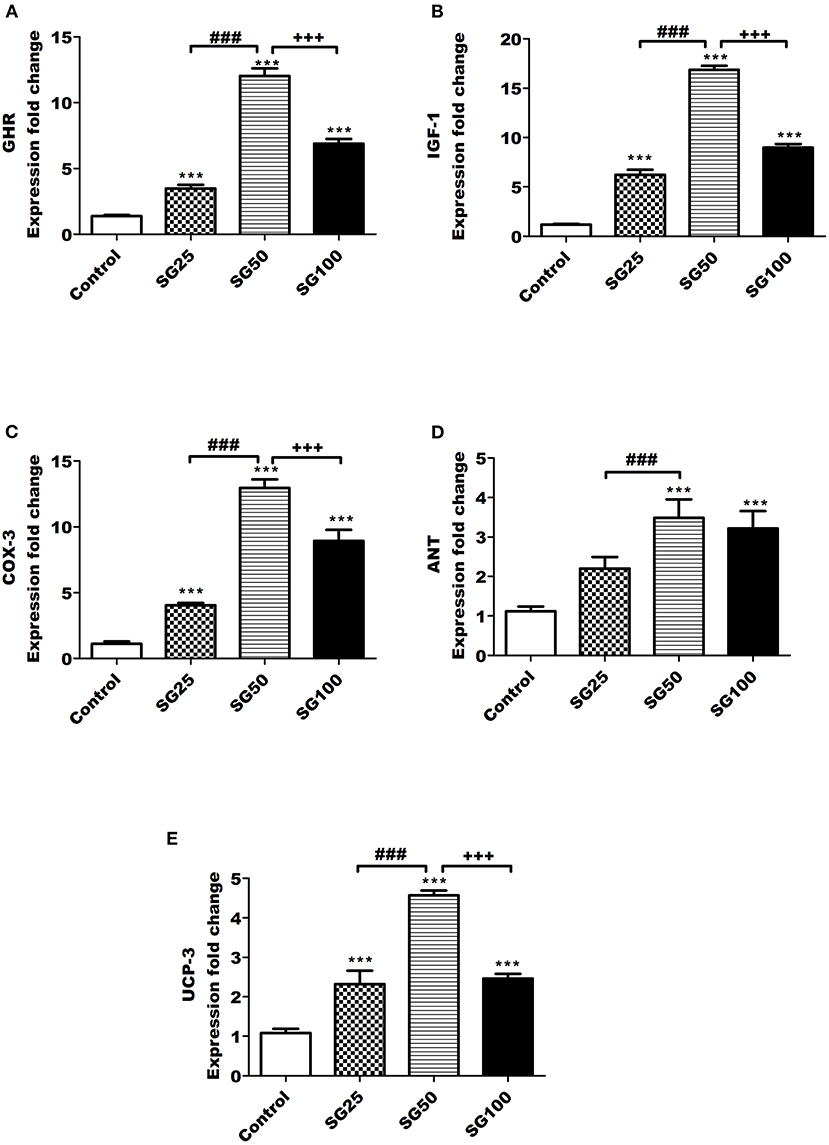
Figure 1. Real-time PCR (RT-PCR) validation of the growth hormone receptor (GHR) (A), insulin-like growth factor 1 (IGF-1) (B), cyclooxygenase 3 (COX-3) (C), adenine nucleotide translocase adenine nucleotide translocase (ANT) (D), and uncoupling protein 3 (UCP-3) (E) genes. ***p < 0.001 vs. control. ###p < 0.001 vs. SG25. +++p < 0.001 vs. SG100, SG25, birds fed 25 ppm. SG50, birds fed 50 ppm. SG100, birds fed 100 ppm Sangrovit®.
Discussion
Using antibiotics is virtually required for maintaining the performance and wellbeing of birds to resist the expected infectious diseases and stressors. However, the crucial need to reduce chemotherapies to keep food security guided the researchers' need to continue their efforts in finding more sustainable solutions for birds' production (24). M. cordata, with its abundant amounts of sanguinarine, is an effective growth promoter and anti-bacterial additive (5, 11, 12). Concurrently, this study assessed the potential effect of M. cordata on growth performance regarding some growth-related genes, hematological, and biochemical parameters of turkeys during hatching and grow-out stages. The results have clearly shown similar growth rate (p > 0.05) in turkeys delivered M. cordata during hatching and grow-out stages. These findings are similar to Kozlowski et al. (25) who stated that M. cordata supplementation 20 ppm in the diet had no significant effect on FI, BWG, or FCR. The recommended dietary level of Sangrovit® for broilers and growing turkeys was previously reported at 20–50 ppm. As a result, Zdunczyk et al. (26) investigated the dietary inclusion of Sangrovit® at 30 ppm, which did not support performance parameters and was ineffective in protein utilization. In addition, Juskiewicz et al. (27) found that the FCR in both the starter and grower period were unaffected by 15 ppm Sangrovit® supplementation. Moreover, Sangrovit® dietary supplementation at the level of 0.05 and 0.1% had no significant differences among treatments regarding FI, feed utilization rate, and small intestine morphology (28).
On the other hand, some studies approved the positive effect of M. cordata in improving the growth of chickens and livestock animals either at a level of 25–50 ppm (29) or 30 ppm (30). The enhanced feed efficiency and nutrients' digestibility are previously reported in various studies that correlated the improved feed utilization in birds treated with M. cordata at a level of 20 and 50 ppm (31). Variations between these studies may be attributed to the differences in the composition of the used diets, age, and sex of the birds, dose of M. cordata, plant extracts, and other bioactive constituents.
Evaluation of turkey's health status can be explained by testing the hematological and blood biochemical traits (32). It gives a precise diagnosis of the metabolic rates, levels of nutrients in the blood, hematic and anemic status, and immune and antioxidative responses. The present study results showed no meaningful changes (p > 0.05) among the birds' groups delivered M. cordata at the age of 2nd week in terms of hematological and biochemical indices as urea and creatinine (33). The obtained results regarding hematological and biochemical indices at the age of 20th week are in agreement with Gilani et al. (34) who examined the efficacy of organic acids and phytobiotics in poultry feed, observing significant increases in RBC and WBC counts, as well as an increase in PCV in broiler chickens. These results confirm that dietary M. cordata has no adverse effects on turkey's health status in hatching or grow-out stages. The outputs of the experiment are in accordance with previous studies (8, 35–37) that approved M. cordata in in pig and poultry feeding without impairing their health and immune status; consumption of M. cordata in the feed at up to 1,000 mg/kg had no adverse effect on blood plasma. In contrast, both (31, 38) approved the cholesterol lowering effect of M. cordata.
Interestingly, the results showed upregulated growth-related genes, such as GHR, IGF-1, ANT, COX-3, and UCP-3 were upregulated (p < 0.001) in M. cordata supplemented groups with the highest value for SG50 in birds treated with M. cordata. These genes were investigated as promising biomarkers for enhancing the growth performance of quail (39). Previous studies clarifying the impact of M. cordata on the expression of the growth-related genes were limited; however, the related anti-inflammatory gene (e.g., NF-kB) showed upregulated expression in birds delivered M. cordata (13). The upregulated NF-kB was probably linked with the growth-related genes (e.g., IGF-1) and regulated its expression as a health-involved gene (40). However, intensive research is needed to determine the possible influence of M. cordata in controlling growth related genes in turkey. Although not relevant to the present study; yet other phytogenic extracts have been confirmed to upregulate animals' growth-related genes (41). Under the current trial conditions, growth performance is not correlated with the turkey's upregulated expression of growth-related genes. This may be explained as the genotypic effect is expressed earlier than the phenotypic performance, which appears after transcription of DNA into RNA, which is measured in our study (gene expression), and then RNA is translated into proteins to exert its function on the phenotypic performance (42).
Conclusion
In conclusion, exogenous M. cordata dietary supplementation upregulated the expression of growth-related genes in turkey at a level of 50 ppm without adverse effects on their heath status regarding hematological and biochemical indices. Further research is needed to investigate the possible influence of M. cordata on gut health and in controlling growth-related genes in turkey in addition to its evaluation as an antibiotic alternative.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Ethics Statement
This investigation was approved by the Committee of Local Experimental Animal Care, Damanhour University, Egypt, Faculty of Veterinary Medicine (VMD: 15/2018).
Author Contributions
EM and MA-D: conceptualization and supervision. EM, MA-L, and MA-D: data curation. GA and AE-k: funding acquisition. SI: investigation. EM, MA-D, MA-L, AS, AE-k, and BS: methodology. AS and BS: resources. GA, AS, SI, MA-L, and BS: software. EM, MA-D, SI, GA, AS, MD, AE-k, MA-L, and BS: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This research was supported by King Khalid University, Grant number (R.G.P.2/35/43), Abha, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R30), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This research was supported by King Khalid University, Grant number (R.G.P.2/35/43), Abha, Saudi Arabia. The authors extend thanks to their respected institutes and universities.
References
1. Abdel-Latif MA, Elbestawy AR, El-Far AH, Noreldin AE, Emam M, Baty RS, et al. Quercetin dietary supplementation advances growth performance, gut microbiota, and intestinal mrna expression genes in broiler chickens. Animals. (2021) 11:2302. doi: 10.3390/ani11082302
2. Saeed M, Naveed M, Leskovec J, Kakar I, Ullah K, Ahmad F, et al. Using Guduchi (Tinospora cordifolia) as an eco-friendly feed supplement in human and poultry nutrition. Poultry Sci. (2020) 99:801–11. doi: 10.1016/j.psj.2019.10.051
3. Zdarilova A, Vrublova E, Vostalova J, Klejdus B, Stejskal D, Proskova J, et al. Natural feed additive of Macleaya cordata: safety assessment in rats a 90-day feeding experiment. Food Chem Toxicol. (2008) 46:3721–6. doi: 10.1016/j.fct.2008.09.054
4. Franz C, Bauer R, Carle R, Tedesco D, Tubaro A, Zitterl Eglseer K. Study on the assessment of plant/herb extracts and their naturally or synthetically produced components as “additives” for use in animal production (2005).
5. Chen J, Kang B, Zhao Y, Yao K, Fu C. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J Anim Physiol Anim Nutr. (2018) 102:1666–74. doi: 10.1111/jpn.12976
6. Faddeeva M, Beliaeva T. Sanguinarine and ellipticine cytotoxic alkaloids isolated from well-known antitumor plants. Intracellular targets of their action. Tsitologiia. (1997) 39:181–208.
7. Drsata J, Ulrichov, á J, Walterov, á D. Sanguinarine and chelerythrine as inhibitors of aromatic amino acid decarboxylase. J Enzyme Inhib. (1996) 10:231–7. doi: 10.3109/14756369609036530
8. Kosina P, Walterova D, Ulrichová J, Lichnovský V, Stiborová M, Rýdlová H, et al. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem Toxicol. (2004) 42:85–91. doi: 10.1016/j.fct.2003.08.007
9. Rawling MD, Merrifield DL, Davies SJ. Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture. (2009) 294:118–22. doi: 10.1016/j.aquaculture.2009.05.005
10. Kantas D, Papatsiros VG, Tassis PD, Athanasiou LV, Tzika ED. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. (2015) 86:92–8. doi: 10.1111/asj.12240
11. Li Y, Xu F, Tong X, Chen R, Shen C, Liang T, et al. Effects of Macleaya cordata extract on small intestinal morphology and gastrointestinal microbiota diversity of weaned pigs. Livestock Science. (2020) 237:104040. doi: 10.1016/j.livsci.2020.104040
12. Liu Z-Y, Wang X-L, Ou S-Q, He J-H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS ONE. (2020) 15:e0234920. doi: 10.1371/journal.pone.0234920
13. Niu X, Fan T, Li W, Xing W, Huang H. The anti-inflammatory effects of sanguinarine and its modulation of inflammatory mediators from peritoneal macrophages. Euro J Pharmacol. (2012) 689:262–9. doi: 10.1016/j.ejphar.2012.05.039
14. Chemists AOOA, Horwitz W. Official Methods of Analysis, Association of Official Analytical Chemists. Washington, DC (1975).
15. Murugan M, Ragavan A. Broiler performance efficiency factor (bpef) in commercial broiler production facilities with special reference to climate. Ind Vet J. (2017) 94:11–4.
17. Huang H, Zhao Z, Li S, Liang Z, Li C, Wang Q. Pattern of GHR mRNA expression and body growth in the S2 line of sex-linked dwarf chickens. Genet Mol Re. (2016) 15:1–7. doi: 10.4238/gmr15047416
18. Saneyasu T, Tsuchihashi T, Kitashiro A, Tsuchii N, Kimura S, Honda K, et al. The IGF-1/Akt/S6 pathway and expressions of glycolytic myosin heavy chain isoforms are upregulated in chicken skeletal muscle during the first week after hatching. Anim Sci J. (2017) 88:1779–87. doi: 10.1111/asj.12847
19. Toyomizu M, Ueda M, Sato S, Seki Y, Sato K, Akiba Y. Cold-induced mitochondrial uncoupling and expression of chicken UCP and ANT mRNA in chicken skeletal muscle. FEBS Lett. (2002) 529:313–8. doi: 10.1016/S0014-5793(02)03395-1
20. Li Q, Xu Z, Liu L, Yu H, Rong H, Tao L, et al. Effects of breeds and dietary protein levels on the growth performance, energy expenditure and expression of avUCP mRNA in chickens. Mol Biol Rep. (2013) 40:2769–79. doi: 10.1007/s11033-012-2030-0
21. Sun J, Zhong H, Chen S-Y, Yao Y-G, Liu Y-P. Association between MT-CO3 haplotypes and high-altitude adaptation in Tibetan chicken. Gene. (2013) 529:131–7. doi: 10.1016/j.gene.2013.06.075
22. De Boever S, Vangestel C, De Backer P, Croubels S, Sys S. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet Immunol Immunopathol. (2008) 122:312–7. doi: 10.1016/j.vetimm.2007.12.002
23. Gasparino E, Del Vesco A, Voltolini D, Nascimento CD, Batista E, Khatlab A, et al. The effect of heat stress on GHR, IGF-I, ANT, UCP and COXIII mRNA expression in the liver and muscle of high and low feed efficiency female quail. Brit Poultry Sci. (2014) 55:466–73. doi: 10.1080/00071668.2014.925090
24. Robinson K, Becker S, Xiao Y, Lyu W, Yang Q, Zhu H, et al. Differential impact of subtherapeutic antibiotics and ionophores on intestinal microbiota of broilers. Microorganisms. (2019) 7:282. doi: 10.3390/microorganisms7090282
25. Kozlowski K, Lecewicz A, Jeroch H, Zdunczyk Z, Jankowski J. Effect of a phytogenic feed additive from Macleaya cordata on performance and carcass parameters of broilers. Archiv fur Geflugelkunde. (2008) 72:140–2.
26. Zdunczyk Z, Gruzauskas R, Juskiewicz J, Semaskaite A, Jankowski J, Godycka-Klos I, et al. Growth performance, gastrointestinal tract responses, and meat characteristics of broiler chickens fed a diet containing the natural alkaloid sanguinarine from Macleaya cordata. J Appl Poultry Res. (2010) 19:393–400. doi: 10.3382/japr.2009-00114
27. Juskiewicz J, Gruzauskas R, Zdunczyk Z, Semaskaite A, Jankowski J, Totilas Z, et al. Raceviciute-StupelieneA: effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. J Anim Physiol Anim Nutr. (2011) 95:171–8. doi: 10.1111/j.1439-0396.2010.01037.x
28. Karimi M, Foroudi F, Abedini M. Effect of Sangrovit on performance and morphology of small intestine and immune response of broilers. Biosci Biotechnol Res Asia. (2014) 11:855–61. doi: 10.13005/bbra/1348
29. Vieira SL, Oyarzabal O, Freitas D, Berres J, Pena J, Torres C, et al. Performance of broilers fed diets supplemented with sanguinarine-like alkaloids and organic acids. J Appl Poultry Res. (2008) 17:128–33. doi: 10.3382/japr.2007-00054
30. Juskiewicz J, Zdunczyk Z, Gružauskas R, Daukšiene A, Racevičiute-Stupeliene A, Totilas ž. Comparative effects of dietary phytobiotic (Macleaya cordata alkaloid extract) and probiotic (Pediococcus acidilactici MA 18/5 M) preparations as single supplements or in combination on fermentative processes in the broiler chickens caeca. Vet Med Zootechnics. (2013) 62:50–5.
31. Lee K-W, Kim J-S, Oh S-T, An B-K. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J Poultry Sci. (2014) 0140073. doi: 10.2141/jpsa.0140073
32. Sheldon LD, Chin EH, Gill SA, Schmaltz G, Newman AE, Soma KK. Effects of blood collection on wild birds: an update. J Avian Biol. (2008) 39:369–78. doi: 10.1111/j.0908-8857.2008.04295.x
33. Alibemani A, Gheisari AA, Ebrahimnezhad Y, Rakhshandeh A, Maheri-SISN. Effects of dietary protein and macleaya cordata alkaloid extract supplementation on growth performance, apparent ileal digestibility of protein and plasma amino acid concentration in broiler chickens. Poultry Sci J. (2020) 8:233–45. doi: 10.22069/psi.2020.18260.1612
34. Gilani SMH, Zehra S, Galani S, Ashraf A. Effect of natural growth promoters on immunity, and biochemical and haematological parameters of broiler chickens. Trop J Pharmaceutical Res. (2018) 17:627–33. doi: 10.4314/tjpr.v17i4.9
35. Zhao L, Von Alvensleben S, Fusconi G, Morlacchini M. Safety evaluation of a standardized Macleaya cordata extract in a ninety day feeding study in weaned piglets. Open J Anim Sci. (2017) 7:213. doi: 10.4236/ojas.2017.72017
36. Matulka RA, Von Alvensleben S, Morlacchini M. Tolerance and residue study for standardized Macleaya cordata extract added to chicken feed. Int J Poultry Sci. (2014) 13:368. doi: 10.3923/ijps.2014.368.373
37. Matulka RA, Von Alvensleben S, Morlacchini M, Fusconi G. Tolerance study for standardized Macleaya cordata extract added to chicken layer diet. Open J Anim Sci. (2017) 8:104–17. doi: 10.4236/ojas.2018.81008
38. Lee K.-W, Everts H, Kappert H, Frehner M, Losa R, et al. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Brit Poultry Sci. (2003) 44:450–7. doi: 10.1080/0007166031000085508
39. Manaa EA, El-Attrouny MM, Baloza HS, Ramadan SI. Selection for high body weight and its association with the expression profiles of somatotropic axis and mitochondrial genes in japanese quail. (2022). doi: 10.29261/pakvetj/2022.003
40. Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. (2011) 21:146–58. doi: 10.1038/cr.2010.175
41. Liu G, Wei Y, Wang Z, Wu D, Zhou A, Liu G. Effects of herbal extract supplementation on growth performance and insulin-like growth factor (IGF)-I system in finishing pigs. J Anim Feed Sci. (2008) 17:538–47. doi: 10.22358/jafs/66681/2008
Keywords: Macleaya cordata, growth performance, gene expression, Sangrovit®, Turkey
Citation: Manaa EA, Abdel-Latif MA, Ibraheim SE, Sakr A, Dawood M, Albadrani GM, El-kott AF, Abdel-Daim MM and Shafik BM (2022) Impacts of Macleaya cordata on Productive Performance, Expression of Growth-Related Genes, Hematological, and Biochemical Parameters in Turkey. Front. Vet. Sci. 9:873951. doi: 10.3389/fvets.2022.873951
Received: 11 February 2022; Accepted: 09 May 2022;
Published: 11 July 2022.
Edited by:
Arda Yildirim, Gaziosmanpaşa University, TurkeyReviewed by:
Sahil Kalia, Cornell University, United StatesJannatara Khatun, Chattogram Veterinary and Animal Sciences University, Bangladesh
Copyright © 2022 Manaa, Abdel-Latif, Ibraheim, Sakr, Dawood, Albadrani, El-kott, Abdel-Daim and Shafik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mervat A. Abdel-Latif, bWVydmF0LmFiZGVsbGF0aWZAdmV0bWVkLmRtdS5lZHUuZWc=; Mohamed M. Abdel-Daim, YWJkZWxkYWltLm1AdmV0LnN1ZXouZWR1LmVn
 Eman A. Manaa
Eman A. Manaa Mervat A. Abdel-Latif
Mervat A. Abdel-Latif Samya E. Ibraheim
Samya E. Ibraheim Abdelaziz Sakr
Abdelaziz Sakr Mahmoud Dawood
Mahmoud Dawood Ghadeer M. Albadrani
Ghadeer M. Albadrani Attalla F. El-kott8,9
Attalla F. El-kott8,9 Mohamed M. Abdel-Daim
Mohamed M. Abdel-Daim Basant M. Shafik
Basant M. Shafik