- 1Department of Veterinary Clinical Sciences, The Ohio State University, Columbus, OH, United States
- 2College of Veterinary Medicine Flint Animal Cancer Center, Colorado State University, Fort Collins, CO, United States
This report describes the first potential case of seeding after fine-needle aspiration (FNA) of a rib osteosarcoma in a dog. An 8-year-old, 28-kg female spayed Golden Retriever was presented to her primary veterinarian with a 3-week history of a 3-cm firm, unpainful, immobile mass arising from the 9th rib. The mass was aspirated and submitted for cytological examination. A subcutaneous nodule developed several days after the FNA was performed in a location immediately overlying but distinct from the primary rib tumor on palpation. Both the primary mass and the newly diagnosed subcutaneous nodule were biopsied and were consistent with an osteosarcoma. Although it cannot be ruled out that the subcutaneous lesion was metastatic, seeding was a reasonable explanation based on where the new mass was located and how quickly it appeared after the FNA was performed. The aim of this case report was to describe the possibility of tumor seeding during FNA for osteosarcoma. It is the authors' opinion that utility of cytological diagnosis of bone tumors outweighs the risk of possible seeding and should continue to be used as a routine diagnostic test for the diagnosis of aggressive bone lesions.
Introduction
Osteosarcoma (OSA) is an aggressive tumor accounting for 85% of primary bone tumors in dogs (1). This type of tumor usually affects large to giant breed dogs at a median age of 7 years (2). While 75 percent of OSA occurs in the appendicular skeleton, cases of OSA originating from the axial skeleton have been reported (3–6). OSA most commonly metastasizes to the lungs and other bones; however, visceral, subcutaneous, and cutaneous metastases have also been reported (7–10).
A preoperative diagnosis of bone tumors can be obtained by performing fine-needle aspiration (FNA) or incisional biopsy of the lesion. Collecting an FNA sample via ultrasound guidance has been described and may increase the ability of obtaining a diagnostic sample (11). While needle track seeding from carcinomas has been reported in dogs, cats, and humans (12–19), to the authors' knowledge, seeding from an FNA of OSA has not previously been described before in the human or veterinary literature.
Case Presentation
An 8-year-old, 28-kg female spayed Golden Retriever was presented to her primary veterinarian with a 3-week history of a 3-cm firm, unpainful, immobile, and ill-defined mass on the left lateral thorax. No other abnormalities were noted on physical examination. Radiographs of the thorax were obtained to identify the origin of the mass and revealed an expansile, lytic, and proliferative bone lesion arising from the 9th rib. A blind FNA sample was collected for cytology and evaluated by a veterinary clinical pathologist. The pathological description was consistent with a malignant neoplasia with primary differentials including osteosarcoma, chondrosarcoma, and plasma cell tumor. Three-view thoracic radiographs revealed no evidence of pulmonary metastatic disease. The dog was then referred to a specialty hospital for further diagnostics and discussion of treatment options.
Three weeks following the original presentation, the dog was assessed in our institution. On physical examination, a new 1-cm erythematous, freely movable, and subcutaneous nodule located directly over the rib mass was noted. According to the owner, the nodule developed a few days after the FNA sample of the mass was obtained. The size of the rib mass had also increased from 3 to 5 cm. The remainder of the physical examination was otherwise unremarkable. Bloodwork was performed with biochemical analysis showing a mild elevation in alkaline phosphatase of 153 IU/L (15–140 IU/L), with no other abnormalities, and complete blood cell count was within normal limits. Cytology of both the subcutaneous nodule and the rib mass was performed via blind FNA and showed the presence of a malignant neoplasia with certain cellular features suspicious for osteoblasts in both samples, but other tumor types such as histiocytic sarcoma and hemangiosarcoma could not be ruled out. A biopsy for histopathology was recommended.
Thoracic radiographs were offered to the client for staging purposes but were declined at this time because of a preference to have them performed by the primary veterinarian. Flouride-18 fludeoxy glucose positron emission tomography and computed tomography (18F-FDG PET/CT) (Phillips Gemini TF Big Bore 16-slice; Phillips North America, Cambridge, MA, United States) was also offered to more thoroughly stage the patient but was declined for financial reasons. Three-view thoracic radiographs were obtained the following day by the primary veterinarian. The images did not show evidence of pulmonary metastatic disease, intrathoracic lymphadenopathy, or mediastinal masses.
Due to the absence of lung metastasis, the owner decided to return to our institution 2 weeks later to have an 18F-FDG PET/CT performed. On physical examination, the mass appeared to be progressive in size (6.5 cm). The dog also had a new onset of exercise intolerance. A total body F-18FDG PET/CT was performed after injection of 5 microcuries of 18F-FDG. The study showed a large, aggressive, osteolytic, and osteoproductive mass, with high avidity [degree of uptake of 18F-FDG measured as maximum standardized uptake value (SUVmax) 16.3] centered around the left 9th rib. An avid subcutaneous mass separated from the primary rib lesion was identified (Figure 1). Additionally, an indistinct soft tissue nodule with a mineral center in the caudal mediastinum (SUVmax 7.4), a very avid left sternal lymph node (SUVmax 9.8) compared to the right (SUVmax 1.9), and many small soft tissue pulmonary nodules throughout the pulmonary parenchyma were identified. The remainder of the study found no significant abnormalities. During the same anesthetic event, Tru-cut biopsies with a 16-gauge needle were obtained from the mass on the 9th rib, as well as the associated subcutaneous nodule. No aspirations of the other abnormalities noted on imaging were performed. The histopathology of the subcutaneous nodule showed haired skin and subcutaneous adipose tissue with an inner neoplastic osseous core (Figure 2). Cells in the neoplastic region were stellate to spindle with eosinophilic cytoplasm. Mitotic index was moderate with 1–4 mitotic figures per high-power field. These findings were consistent with extra-skeletal osteosarcoma. The histopathology of the rib mass had similar findings, and the mass was classified as osteosarcoma.
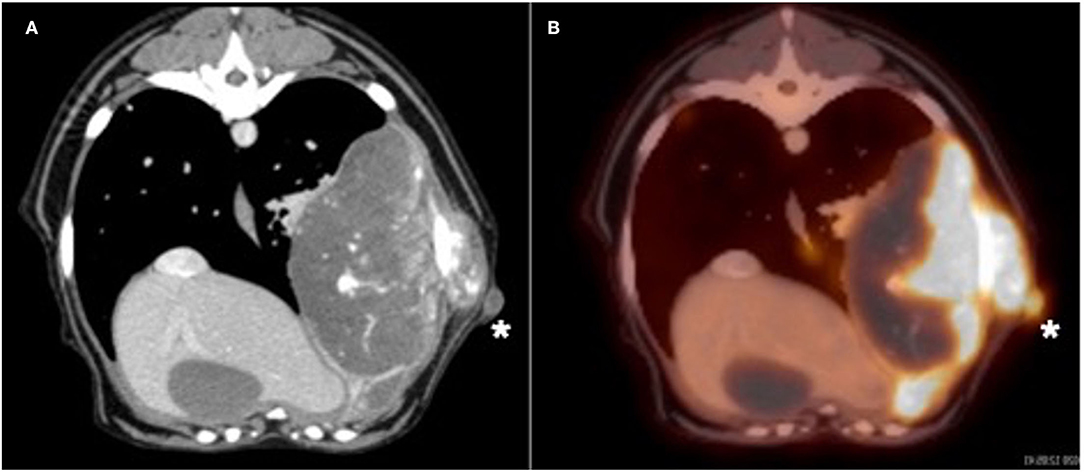
Figure 1. (A) Transverse CT and positron emission tomography (PET)/CT (B) images of the large left 9th rib lesion and the subcutaneous nodule (white asterisk). Note the marked avidity of bone associated with the primary bone lesion and the subcutaneous nodule after intravenous administration of flouride-18 fludeoxy glucose (18F-FDG) in the PET/CT image.
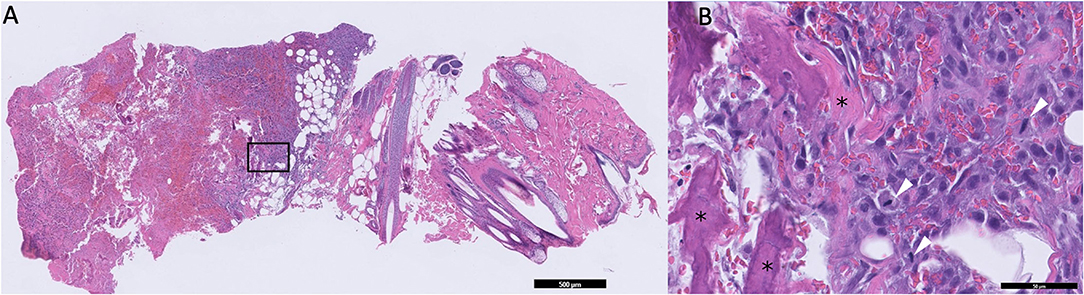
Figure 2. (A) Low magnification microphotograph of the suspected seeded lesion in the subcutaneous tissue. The black box represents the area of increased magnification in B. (B) High-magnification microphotograph of the neoplastic tissue. Areas containing osteoids are marked with asterisks. Tumor cells undergoing mitosis are marked with white arrowheads.
Due to the advanced stage of the tumor, palliative radiation therapy with or without the addition of chemotherapy was offered to the client but was declined. The patient was maintained on NSAIDs for pain control. Two weeks following the last appointment, the patient was humanely euthanized due to rapid progression of clinical signs causing decreased quality of life combined with a grave prognosis.
Discussion
Cytology is a practical and useful technique for obtaining a definitive diagnosis of many tumor types in small animals (20). Bone tumors can be accurately diagnosed using cytological evaluation, and the technique has been shown to be useful in differentiating between malignant and nonmalignant lesions (21, 22). The diagnostic accuracy of cytology compared to histology for bone tumors has been reported to be 83–92% in two studies (23, 24). Compared to incisional bone biopsy, fine needle aspiration with cytology is associated with lower risk of pathologic fracture, does not require special instrumentation, and results in faster diagnosis. To increase the chance of obtaining a diagnostic sample, FNA can be performed under ultrasound guidance (11).
The subcutaneous lesion identified in this patient cannot be ruled out as metastasis from the primary rib lesion. However, seeding was thought to be a reasonable explanation, since the lesion appeared a few days after the FNA was performed and was in a location immediately overlying but distinct from the primary rib tumor. This theory is supported by the findings from a case report where seeding was detected 2 weeks after obtaining an FNA sample in a cat with a lung carcinoma (25). Needle tract seeding is a reported complication of FNA of carcinomas in small animals and in humans (12–19, 25), but it has not been reported for sarcomas. The size of the needle used to obtain samples may play a role in the risk of seeding. Needles larger than 23-gauge are reported to increase the risk of seeding in human patients with thyroid carcinoma (26). Unfortunately, the size of the needle used in our case was not reported in the medical record for this case.
The possibility of seeding after core biopsies using Tru-cut needles in humans with sarcomas has been briefly described in case reports and small case series, but the low sample size and limited patient follow-up do not allow for definitive conclusions on the true frequency of this event (27, 28). One human study analyzed the contamination of biopsy tracts from primary malignant bone tumors. The results of this study showed 11.4% contamination and correlated with local recurrence of neoplasia (29). Another human study published in 2015 showed that 20% of patients had tumor foci around the biopsy tract line when needle core biopsy was performed for diagnosis (30). Based on these findings, it is important for clinicians to perform incisional biopsies in a location that will allow for excision of the biopsy tract during tumor removal.
The aim of this case report was to describe, for the first time in veterinary medicine, the possibility of tumor seeding during FNA for OSA. It is the authors' opinion that utility of cytological diagnosis of bone tumors outweighs the risk of possible seeding and should continue to be used as a routine diagnostic test for the diagnosis of aggressive bone lesions.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the animal study because no experimental procedures were performed. Written informed consent for participation was not obtained from the owners because no experimental procedures were performed. The owner consented to all diagnostics performed.
Author Contributions
TF wrote the first draft. GT, BS, DW, and MG managed the patient and contributed to conception of the case report. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr. Paula Schaffer for kindly providing the microphotographs of the lesions.
References
1. Brodey RS, McGrath JT, Reynolds H. A clinical and radiological study of canine bone neoplasms. J Am Vet Med Assoc. (1959) 134:53–71.
2. Tuohy JL, Shaevitz MH, Garrett LD, Ruple A, Selmic LE. Demographic characteristics, site andphylogenetic distribution of dogs with appendicular osteosarcoma: 744 dogs (2000–2015). PLoS ONE. (2019) 14:e0223243. doi: 10.1371/journal.pone.0223243
3. Pirkey-Ehrhart N, Withrow SJ, Straw RC, Ehrart EJ, Page RL, Hottinger HL, et al. Primary rib tumors in 54 dogs. J Am Anim Hosp Assoc. (1995) 31:65–9. doi: 10.5326/15473317-31-1-65
4. Dickerson ME, Page RL, LaDue TA, Hauck ML, Thrall ME, Stebbins ME, et al. Retrospective analysis of axial skeleton osteosarcoma in 22 large-breed dogs. J Vet Intern Med. (2001) 15:120–4. doi: 10.1111/j.1939-1676.2001.tb01242.x
6. Coyle VJ, Rassnick KM, Borst LB, Rodriguez CO Jr, Northup NC, Fan TM, et al. Biological behaviour of canine mandibular osteosarcoma. A retrospective study of 50 cases (1999–2007). Vet Comp Oncol. (2013) 13: 89–97. doi: 10.1111/vco.12020
7. Jankowski MK, Steyn PF, Lana SE, Dernell WS, Bloom CM, Uhrig JL, et al. Nuclear scanning with 99mTc-HDP for the initial evaluation of osseous metastasis in canine osteosarcoma. Vet Comp Oncol. (2003) 1:152–8. doi: 10.1111/j.1476-5829.2003.00021.x
8. Oblak ML, Boston SE, Woods JP, Nykamp S. Comparison of concurrent imaging modalities for staging of dogs with appendicular primary bone tumours. Vet Comp Oncol. (2013) 13:28–39. doi: 10.1111/vco.12016
9. Cesario L, Garrett LD, Barger AM, O'Brien RT, Fan TM. Diagnosis and ultrasonographic appearance of hepatic metastasis in six cases of canine appendicular osteosarcoma (2005–2013). Aust Vet J. (2016) 94:160–5. doi: 10.1111/avj.12435
10. Parachini-Winter C, Curran KM, Pellin MK, Laver T, Hanot C, Vernier TH, et al. Cutaneous and subcutaneous metastasis of appendicular osteosarcoma in dogs: 20 cases. J Vet Intern Med. (2019) 33:2200–8. doi: 10.1111/jvim.15557
11. Britt T, Clifford C, Barger A, Moroff SA, Drobatz K, Tacher S, et al. Diagnosing appendicular osteosarcoma withultrasound-guided fine-needle aspiration: 36 cases. Journal of Small Animal Practice. (2007) 48:145–50. doi: 10.1111/j.1748-5827.2006.00243.x
12. Gilson SD, Stone EA. Surgically induced tumor seeding in eight dogs and two cats. J Am Vet Med Assoc. (1990) 196:1811–5.
13. Nyland TG, Wallack ST, Wisner ER. Needle-tract implantation following us guided fine-needle aspiration biopsy of transitional cell carcinoma of the bladder, urethra, and prostate. Vet Radiol Ultrasound. (2002) 43:50–3. doi: 10.1111/j.1740-8261.2002.tb00443.x
14. Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. (2007) 33:437–47. doi: 10.1016/j.ctrv.2007.04.001
15. Warren-Smith CM, Roe K, de la Puerta B, Lamb CR. Pulmonary adenocarcinoma seedingalong a fine needle aspiration tract in a dog. Veterinary Record. (2011) 169:181. doi: 10.1136/vr.d2357
16. Matsui T, Nishikawa K, Yukimoto H, Katsuta K, Nakamura J, Tanaka S, et al. Needle tract seeding following endoscopicultrasound-guided fine-needle aspiration for pancreatic cancer: a report of two cases. World J Surg Oncol. (2019) 17:134. doi: 10.1186/s12957-019-1681-x
17. Jegatheeson S, Dandrieux JRS, Cannon CM. Suspected pancreatic carcinoma needle tract seeding in a cat. J Feline Med Surg Open Rep. (2020) 6:1–6. doi: 10.1177/2055116920918161
18. Guo Y, Koh AJ. Needle tract seeding of thyroid follicular carcinoma after fine-needle aspiration. Case Rep Otolaryngol. (2020) 1:1–4. doi: 10.1155/2020/7234864
19. Merickel JL, Lawrence J, Young SJ, Thomson CB. Cutaneous seeding of transitional cell carcinoma of the urinary bladder after placement of a subcutaneous ureteral bypass device in a dog with bilateral ureteral obstruction. J Am Vet Med Assoc. (2021) 258:877–82. doi: 10.2460/javma.258.8.877
20. Khalbuss WE, Teot LA, Monaco SE. Diagnostic accuracy and limitations of fine needle aspiration cytology of bone and soft tissue lesions. Cancer Cytopathol. (2010) 118:24–32. doi: 10.1002/cncy.20058
21. Loukopoulos P, Rozmanec M, Sutton RH. Cytological versus histopathological diagnosis in canine osteosarcoma. Veterinary Record. (2005) 157:784–784. doi: 10.1136/vr.157.24.784
22. Reinhardt S, Stockhaus C, Teske E, Rudolph R, Brunnberg L. Assessment of cytological criteria for diagnosing osteosarcoma in dogs. J Small Anim Pract. (2005) 46:65–70. doi: 10.1111/j.1748-5827.2005.tb00294.x
23. Sabattini S, Renzi A, Buracco P, Defourny S, Garnier-Moirouxet M, Capitani O, et al. Comparative assessment of the accuracy of cytological and histologic biopsies in the diagnosis of canine bone lesions. J Vet Intern Med. (2017) 31:864–71. doi: 10.1111/jvim.14696
24. Berzina I, Sharkey LC, Matise I, Kramek B. Correlation between cytologic and histopathologic diagnoses of bone lesions in dogs: a study of the diagnostic accuracy of bone cytology. Vet Clin Pathol. (2008) 37:332–8. doi: 10.1111/j.1939-165X.2008.00050.x
25. Vignoli M, Rossi F, Chierici C, Terragni R, DeLorenzi D, Stanga M, et al. Needle tract implantation after fine needle aspiration biopsy (FNAB) of transitional cell carcinoma of the urinary bladder and adenocarcinoma of the lung. Schweizer Archiv für Tierheilkunde. (2007) 149:314–8. doi: 10.1024/0036-7281.149.7.314
26. Polyzos SA, Anastasilakis AD. A systematic review of cases reporting needle tract seeding following thyroid fine needle biopsy. World J Surg. (2010) 34:844–51. doi: 10.1007/s00268-009-0362-2
27. Saghieh S, Masrouha KZ, Musallam KM, Mahfouz R, Abbous M, Khoury NJ, et al. The risk of local recurrence along the core-needle biopsy tract in patients with bone sarcomas. Iowa Orthop J. (2010) 30:80–3.
28. Berger-Richardson D, Swallow CJ. Needle tract seeding after percutaneous biopsy of sarcoma: risk/benefit considerations. Cancer. (2016) 123:560–7. doi: 10.1002/cncr.30370
29. Oliveira MP, Lima PM, de Mello RJ. Tumor contamination in the biopsy path of primary malignant bone tumors. Revista Brasileira De Ortopedia. (2012) 47:631–7. doi: 10.1016/S2255-4971(15)30015-X
Keywords: seeding, canine (dog), osteosarcoma, fine needle aspiration (FNA), oncology
Citation: Faletti T, Seguin B, Selmic LE, Lapsley J, Worley D, Griffin M and Tremolada G (2022) Potential Seeding From Fine-Needle Aspiration of an Axial Osteosarcoma: A Case Report. Front. Vet. Sci. 9:847933. doi: 10.3389/fvets.2022.847933
Received: 03 January 2022; Accepted: 24 March 2022;
Published: 29 April 2022.
Edited by:
Susanne M. Stieger-Vanegas, Oregon State University, United StatesReviewed by:
Jennifer Johns, Oregon State University, United StatesAlexandra Collins-Webb, Oregon State University, United States
Copyright © 2022 Faletti, Seguin, Selmic, Lapsley, Worley, Griffin and Tremolada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Tremolada, dHJlbW9sYWRhLjEmI3gwMDA0MDtvc3UuZWR1
 Tasha Faletti1
Tasha Faletti1 Bernard Seguin
Bernard Seguin Laura Elizabeth Selmic
Laura Elizabeth Selmic Janis Lapsley
Janis Lapsley Maureen Griffin
Maureen Griffin Giovanni Tremolada
Giovanni Tremolada