- 1Department of Comparative Biomedicine and Food Science, University of Padua–Agripolis Campus, Legnaro, Italy
- 2Tissue Engineering Laboratories, Faculty of Dentistry, Alexandria University, Alexandria, Egypt
- 3Veterinary Practicioner, Padua, Italy
- 4Department of Animal Medicine, Production and Health, University of Padua–Agripolis Campus, Legnaro, Italy
In the present case report a show jumping 10-year-old Sella Italiano gelding, presented with severe lameness, swelling and pain at palpation of the mid-metacarpal region of the left forelimb. Clinical and ultrasound examination diagnosed a chronic tendonitis of the central region of the superficial digital flexor tendon (SDFT). The lesion was a reoccurrence since it developed from a previously healed injury. The horse had to stop competing and was unresponsive to gold-standard treatments as Non-steroidal anti-inflammatory drugs (NSAIDs) and conservative management after 6 months of therapy. The animal was subjected to repeated intralesional injections of autologous adipose-derived mesenchymal stem cells (AD-MSCs) combined with autologous platelet-rich plasma (PRP). The combined treatment was administered twice in a 1-month interval. The healing process was assessed through clinical examination, ultrasound imaging and quantification of oxidative stress products and inflammatory mediators in blood plasma. After 2 weeks from first injection, a reduction of concentration of oxidative-derived products was observed, together with an increase of anti-inflammatory cytokines and pro-mitotic growth factors. These results were reflected clinically as the horse showed a reduction of lameness along with swelling and pain after 4 weeks. At the 1-year follow-up, the horse showed no signs of lameness and swelling. The ultrasonographic examination highlighted a compact fiber alignment with a normal echogenic tendon as observed in the sound contralateral limb. Moreover, the horse went back to the previous level of competition. Our results suggest the positive effects of a repeated intralesional injection of AD-MSCs and PRP for the treatment of a chronic tendonitis with long-term effects and an improvement for both equine quality of life and athletic performance.
Introduction
Tendinopathies are one of the most common orthopedic disorders in equine and human athletes, leading to lameness and pain (1–5). Tendon injuries are responsible for approximately one third of traumas that occur during the sporting career of horses (6, 7), forcing a significant number of individuals to an early retirement from competition (8, 9). The superficial digital flexor tendon (SDFT) is frequently injured in show jumping discipline due to the repeated and excessive loading forces that the tendon has to sustain after jumping and landing (10, 11). Tendonitis affecting the SDFT have an incidence up to 43%, and most of them occur in the central tendon region (6, 12).
Tendons possess a limited regenerative capacity and usually heal by forming a fibrotic scar, but the repaired tissue possesses inferior biomechanical characteristics compared to its normal physiological counterpart (13–15). Consequently, horses that have previously sustained a tendon injury are more prone to re-injury (up to 80%) or to chronicity (9, 16).
The gold-standard treatments for tendon injuries consist of conservative therapies including administration of non-steroidal anti-inflammatory drugs (NSAIDs) and rehabilitation aiming to attenuate symptoms and to recover tendon function. Although clinical improvements might be observed (i.e., relief of symptoms), most of these options lack long-term therapeutic success (17, 18). Over the last two decades, regenerative therapies have been gaining interest because of their beneficial effects in supporting and stimulating the healing process, leading to a healed tissue that resembles healthy tendon in structure and function (19, 20).
Mesenchymal stem cells (MSCs) derived from multiple sources, as bone marrow (BM-MSCs), adipose tissue (AD-MSCs), or peripheral blood (PB-MSCs), have proved their efficacy in improving tendon healing in horses thus reducing the reoccurrence rate of injury, mainly because of their paracrine activity (21–24). Different route of administration of MSCs, including intralesional injection, have demonstrated to be a safe and effective practice to treat tendon injury in equine medicine (25, 26). Another product of interest in equine regenerative practice is platelet-rich plasma (PRP), a blood-derived product rich in growth factors and cytokines that can sustain and boost the tissue healing process (20, 27, 28). When combined, these two treatments possess a higher regenerative potential in comparison to their application alone as it has been demonstrated for treating different tissue (e.g., skin, bone, joint) in human and horses (9, 29–33). Nevertheless, only one study describes the repeated application of MSCs and PRP for the treatment of naturally occurring chronic tendonitis, which was not a re-injury, in the equine in a 16-weeks time interval (34).
In terms of risk factors (e.g., age and over-exercise) and etiology, human and horses share a similar pathophysiology of tendinopathies. For this reason, studies to test regenerative therapies in the horse including cell and cell-free treatments, or their combination, for tendon healing might be useful as preclinical data for translation purposes to human medicine (35–38).
In this case report, we describe the repeated application of autologous adipose-derived MSCs and autologous PRP for the treatment of a chronic recurrent SDFT tendonitis developed from a previous injury in a show jumping horse.
Case Description
Clinical History
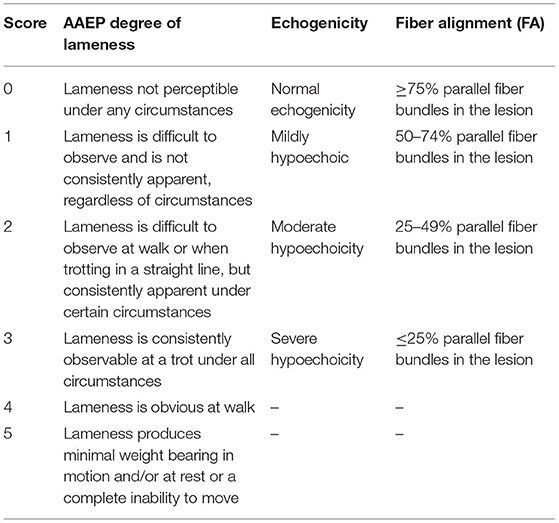
A 10-year-old Sella Italiano gelding, competing in show jumping, presented with a lesion in the SDFT of the left forelimb in the middle third of the metacarpal region. The lesion was a reoccurrence, which had developed from a previous healed injury in the same area of the SDFT. At diagnosis, the horse showed a lameness grade 2.5/5 based on the American Association of Equine Practioners (AAEP) scale (as reported in Table 1). Pain and local heat were noted at palpation along with severe swelling.
Six months ago, when diagnosed, the horse stopped competing and was treated with NSAIDs. Furthermore, a controlled rehabilitation exercise program was followed, adapted and based on the type of injury. The program started with a complete stall rest for the first 2 weeks. Then it was followed by a gradual increase of walking and trotting exercises, starting with 5 min walking a day with an increase of 5 min every 2 weeks, up to 40 min. After 20 weeks, the horse began to trot for 2 min each day with a progressive increase of trotting time, alternated with walking, every 2 weeks (up to 20 min of trot with 20 min of walking). However, the animal did not show any improvement at the clinical or ultrasonographic level.
In Figure 1 it is showed a schematic timeline of the clinical case.
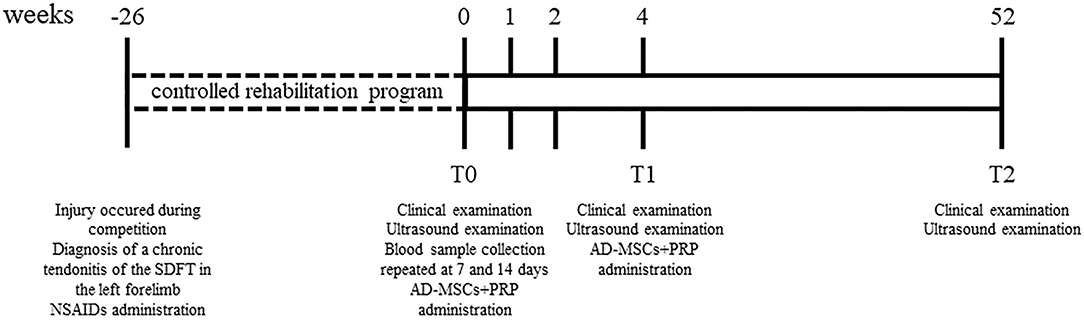
Figure 1. Schematic timeline of the clinical case reporting clinical examinations, ultrasonographic evaluations and therapy protocol.
Diagnostic Imaging: Ultrasound Evaluation
Ultrasonographic evaluations of the central metacarpal region of both forelimbs were performed using a 7.5-MHz linear transductor probe. For each assessment, a complete examination of the SDFT was conducted by means of longitudinal and transverse scans. The obtained images were evaluated and scored (from 0 to 3) at each examination for two parameters as previously described (24, 39): lesion echogenicity and lesion longitudinal fiber alignment (FA). Criteria for scoring are listed in Table 1. The contralateral healthy limb was used as comparison.
The SDFT, in the middle third of the metacarpal region, presented with a focal hypo-echogenic area together with an irregular fiber alignment, which corresponded to ~ 30% of the cross-sectional area of the tendon. The lesion presented with a proximo-distal size of 36.4 mm.
Treatment and Follow-Up
Treatment protocol consisted of ultrasound-guided intralesional injection with autologous adipose-derived MSCs (AD-MSCs) combined with PRP. AD-MSCs and PRP were isolated, characterized, and prepared for treatment as previously described (22, 40).
The adipose tissue was collected from the region above the dorsal gluteal muscle, at the base of the tail, because of the ease of access and absence of large veins. The horse was intravenously sedated with 0.01 mg/kg detomidine (Domodesan®, Orion Pharma, Italy); then the area was shaved, aseptically prepared, and locally anesthetized with 2% lidocaine (Lidor®, Richter Pharma AG, Italy). An incision of ~ 5–6 cm in length was made parallel 15 cm lateral to the spinal column, in order to allow visualization of adipose tissue between the skin and the musculature. Afterwards, ~ 4 g of subcutaneous adipose tissue was collected and stored in proper medium for transport, consisting of phosphate buffer saline (PBS) supplemented with penicillin-streptomycin (10%). Upon arrival to the laboratory, the sample was washed with PBS three times, minced and placed in a 0.01% collagenase type IA (Sigma-Aldrich, Italy) solution for 1 h at 37°C with continuous shaking. After digestion, the solution was filtered using a 100 μm cell strainer and diluted in DMEM high glucose (Sigma-Aldrich, Italy) supplemented with 10% FBS (Sigma-Aldrich, Italy). Afterwards, the solution was centrifuged twice at 300 xg for 10 min. Isolated cells were seeded in a culture flask in complete cell growth medium consisting of DMEM high glucose (Sigma-Aldrich, Italy), FBS 10% (Sigma-Aldrich, Italy), and 1% antibiotics (penicillin/streptomycin; Aurogene, Italy), maintained in culture, and expanded. Cells used for application were at passage 3 and 5. Isolated cells were characterized by flow cytometry and in vitro trilineage differentiation as previously described by (40) and stated by (41).
The PRP was obtained by following a double centrifugation tube method in sterile conditions (first centrifuge at 1300 xg for 20 min and then at 300 xg for 15 min) as described by (42).
On the day of treatment, 107 AD-MSCs were diluted in 4 mL of autologous PRP. Cell viability was assessed by trypan blue staining and more than 90% of cells were viable. Before injection, the horse was sedated with 0.01 mg/Kg detomidine (Domodesan®, Orion Pharma, Italy) and 0.1 mg/Kg butorphanol (Nargesic®, Acme Srl, Italy), and the injection area was aseptically prepared. The treatment was inoculated in the lesion site using a sterile 14G needle via ultrasonic guidance. Afterwards, protective sterile bandages were applied to the limb and the horse followed a rehabilitation protocol.
A second injection was performed 4 weeks later in order to continue the stimulation of the healing process. The applied protocol was the same as that used for the first treatment.
Blood Plasma Analysis
Blood plasma was obtained after collection of peripheral blood from the jugular vein of the horse using a lithium-heparin sterile tube (BD Vacutainer®, BD, Italy) on the day of injection, and after 1 and 2 weeks post-treatment.
The blood plasma was analyzed for assessing levels of protein related to inflammation and the relative oxidative stress such as advance oxidation protein products (AOPP), carbonyl group (CT), malondialdehyde (lipid peroxidation via thiobarbituric acid reactive substance, TBARS), and the presence of thiols (thiol/disulfide reaction of thiol); all parameters were measured by following published protocols (43–46).
In addition, the concentration of proteins involved in the inflammatory process (IL-1β, MBS2020285, MyBioSource, US; IL-10, ab155466, Abcam, UK) or tendon wound healing (PDGF, MBS907132, MyBioSource, US; IGF-1, MBS7606417, MyBioSource, US) was estimated using enzyme-linked immunosorbent assay (ELISA) commercial kits specific for the equine specie. The analyses were performed following the manufacturer's protocol.
All samples were analyzed in triplicate in a 96-well plate and absorbance was obtained by using a VICTOR multilabel plate reader (Perkin Elmer, US).
Outcomes
Clinical Evaluation
Clinical assessments were performed every 2 weeks starting from the day of the first injection. In this study we report clinical outcomes on the day of injection (T0), at 4 weeks (T1) and 52 weeks (T2) after injection. Before treatment, the horse presented pain at palpation along with local heat and severe swelling in the mid-metacarpal region of the left forelimb. In addition, on the AAEP scoring system the horse showed a Grade 2.5/5 lameness.
At T1, swelling and pain of the injured region of the left forelimb were decreased; moreover, a reduction of lameness (Grade 1.5/5) was observed. The horse was able to load more weight on the affected limb. A second injection of the combined treatment was performed on the same day. After 1 year (T2), no signs of swelling, pain at palpation and lameness of the affected limb were observed. The horse presented with a Grade 0/5 lameness as it was able to trot fine under all tested circumstances. The horse showed a complete restoration of function and returned to sport activity; currently, the horse is still competing.
Ultrasound Evaluation
Ultrasound examinations were assessed using the scoring system previously described (Figure 2). At T0, the tendon presented with a focal hypoechoic area (Grade 2.5/3) with a low FA (Grade 3/3). At T1, a slight increase in FA (Grade 2/3) was observed along with a small increase of echogenicity in the wounded area (Grade 2/3). At T2, 1 year after the first injection, echogenicity and fibers alignment were similar to the contralateral sound tendon (Grade 0/3).
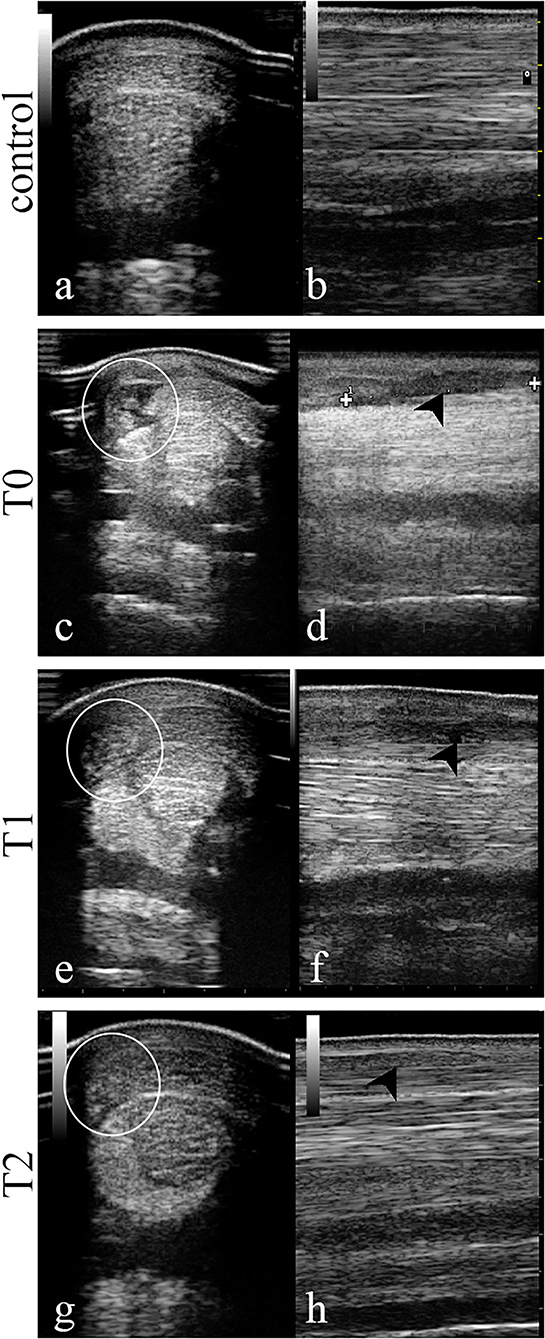
Figure 2. Transversal and longitudinal images of the central area of the superficial digital flexor tendon in the mid-metacarpal region performed at day 0 (T0, day of therapy injection), T1 (4 weeks), and T2 (52 weeks). At T0 the SDFT is characterized by a hypoechoic area (c) along with an abnormal fiber alignment (d). After 4 weeks (T1), the same area resulted less hypoechoic (e) and the fiber pattern was more aligned (f) while at T2 the area showed a normal echogenicity (g) and fiber disposition (h) as in the sound contralateral limb (a,b). White circle, corresponding injury area in transverse images; black arrow-head, injury area in longitudinal images.
Blood Plasma Analysis
The concentration level of inflammatory and growth factors present in blood plasma was assessed on the day of injection, and after 1 and 2 weeks to evaluate markers involved in the tendon healing process (Table 2).
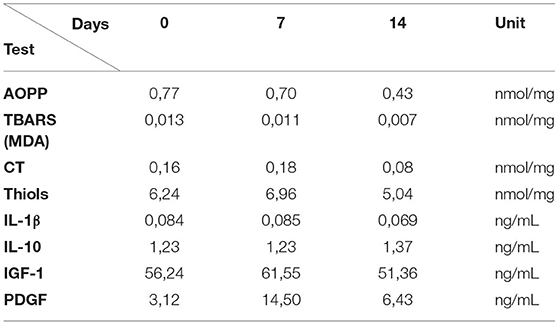
Table 2. Obtained data from analysis of blood plasma markers related to inflammation and tendon healing process.
The quantity of plasma oxidative stress products, as AOPP and TBARS, decreased gradually over time, with the lowest value at 14 days after the first treatment; CT and thiol groups showed a little increase at day 7, and then decreased at 14 days.
The pro-inflammatory cytokine IL-1β showed a decrease at 14 days while IL-10, an anti-inflammatory interleukin, showed an increase in its concentration on the same day.
The plasma concentration of growth factors, PDGF and IGF-1, showed an increase in their concentration at 7 days Post-treatment compared to the baseline values. After 14 days, a decrease of concentration of both factors was observed.
Discussion
Superficial digital flexor tendon (SDFT) injuries are a severe problem that affect a large percentage of athletic and pleasure horses; often they develop recidivisms and, in the worst scenario, have to retire from competition early (9, 47). In a One Health perspective, the equine could play an important role as a model for human musculoskeletal disorders because of their similarities (48–50), especially for investigating the regenerative efficacies of innovative treatments such as PRP, bone marrow aspirate (BMA), or MSCs. However, in human medicine there is a limited knowledge about the combinatory application of these therapies, which is restricted to the treatment of knee osteoarthritis (51, 52) and rotator cuff rupture (53).
In the present case report, repeated ultrasound-guided intralesional injections of autologous AD-MSCs combined with autologous PRP were applied for the treatment of a recurrent SDFT lesion, that had naturally occurred in the left forelimb of a show jumping horse. The horse presented with a chronic tendonitis of the SDFT of the left forelimb occurred during sporting activity, which developed from a previous injury 6 months before the treatment herein described. The horse was unresponsive to conventional treatments such as NSAIDs and a controlled rehabilitation program. Therefore, the double application of AD-MSCs with PRP for the treatment of a naturally occurring lesion in the SDFT was the novel approach chosen.
Results were beneficial for the horse as the repeated injections of AD-MSCs and PRP resulted in a positive development of a SDFT chronic tendonitis over a 1-year follow-up. In addition, during this period the horse did not suffer any re-injury. The combined treatment might be accountable for the decrease of inflammatory markers (i.e., plasma protein levels) as observed during the first 2 weeks, which might have eventually led to the slight reduction of clinical symptoms observed at T1. The ultrasonographic evaluation showed restoration in structure, echogenicity, and fiber organization of the affected tendon after 1 year. This could also suggest that the biomechanical properties of the tendon were restored to an adequate level for allowing the horse to go back to the same performance status as before the injury. The addition of PRP to MSCs for the treatment of different disorders as skin wounds (54) or bone defects (55), has demonstrated to boost the regenerative effects of MSCs, both morphologically and functionally. The same effect was also observed for treating degenerative joint disorders in equine specie (56). In all models, the observed results were achieved principally by extracellular matrix remodeling, mainly dictated by the structural action of MSCs, which is a fundamental component in tendon healing. The underlying reason may be ascribed to the soluble molecules (e.g., growth factors) present in the PRP that might stimulate MSCs proliferation and release of bioactive factors (57). Indeed, MSCs themselves are able to release a plethora of soluble bioactive molecules that possess beneficial effects (58).
During the first 2 weeks after treatment, blood plasma analysis showed a reduced concentration of inflammatory markers (AOPP, TBARS, CT, and IL-1β) along with an increase of thiols at day 7 and a reduction at day 14. The substantial increase in the concentration of thiols after 1 week may be related to their function as antioxidants (59), while reducing at day 14 as the inflammatory process started to subside. Concomitantly, the concentration of IL-10, an anti-inflammatory cytokine, increased after 2 weeks. IL-10 is known to have a pro-mitotic effect on tenocytes and tendon-derived stem cells, stimulating cell proliferation and migration (60). In addition, the growth factors IGF-1 and PDGF showed an increase 1 week post-treatment. Both growth factors have positive effects on tendon cells, inducing proliferation plus attracting them to the wound area and stimulating ECM deposition (61, 62).
These observations were reflected 4 weeks after treatment (T1) by a reduction of pain and swelling of the affected forelimb area along with a partially reduced lameness. However, at the same time point, ultrasound images still presented with a hypoechoic area in the SDFT, corresponding to the diagnosed lesion. For this reason, the same treatment was applied a second time. The double application of AD-MSCs and PRP in a 30-day interval might have provided a prolonged activation of tendon regeneration due to a more protracted exposure, at the injury site, to both constituents. Moreover, the use of autologous sources for the therapy did not provoke any immune reactions to the horse after application, confirming the safety of MSCs (63).
To conclude, a repeated injection of autologous AD-MSCs coupled with PRP over a 52-week period supported a positive progression of a chronic SDFT lesion, which developed from a previous healed injury in a show jumping horse, allowing the animal to go back to competition. Probably, the measurement of inflammatory and oxidative stress markers in blood plasma can play a pivotal role in monitoring the healing process. However, these outcomes should be confirmed in the future by large placebo-controlled studies with animals affected by SDFT chronic tendonitis.
Conclusion
To our knowledge this is the first case report of a successful treatment of a naturally occurring chronic SDFT tendonitis developed from a re-injury in a show jumping horse by a repeated and combined application of AD-MSCs with PRP. The therapy demonstrated to be safe and effective as no adverse reactions were observed; moreover, the horse was able to go back to competition. Our result might encourage the combined application of MSCs and PRP for the treatment of tendon injuries in equine clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the animal study because the owner of the horse signed a written consent, in which all treatment procedures were widely explained. All consent items were discussed in detail during the consultation visit to provide the owner with an overview regarding the benefits of the treatment and the expected results. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
AC, FB, MP, and II followed the clinical case and performed the sample collection. LM, AC, LD, and AP performed cell processing and laboratory analysis. LM, AC, and NE prepared the manuscript. LM and MP contributed to study design and supervised it. GG and MP revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Italian MIUR, Grant Number 2017FNZPNN (PRIN 2017, PI: MP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge Dr. Marta Frizzarin for helpful aid during clinical examinations and the late master and friend Professor Michael (Mike) Thorndyke (Royal Holloway, University of London, UK; University of Gothenburg, SWE).
References
1. Williams RB, Harkins LS, Hammond CJ, Wood JLN. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet J. (2001) 33:478–86. doi: 10.2746/042516401776254808
2. Lam KH, Parkin TDH, Riggs CM, Morgan KL. Descriptive analysis of retirement of thoroughbred racehorses due to tendon injuries at the Hong Kong Jockey Club (1992–2004). Equine Vet J. (2007) 39:143–8. doi: 10.2746/042516407X159132
3. O'Meara B, Bladon B, Parkin TDH, Fraser B, Lischer CJ. An investigation of the relationship between race performance and superficial digital flexor tendonitis in the thoroughbred racehorse. Equine Vet J. (2010) 42:322–6. doi: 10.1111/j.2042-3306.2009.00021.x
4. Clegg PD. Musculoskeletal disease and injury, now and in the future. Part 2: tendon and ligament injuries. Equine Vet J. (2012) 44:371–5. doi: 10.1111/j.2042-3306.2012.00563.x
5. Smith RKW, Garvican ER, Fortier LA. The current “state of play” of regenerative medicine in horses: what the horse can tell the human. Regen Med. (2014) 9:673–85. doi: 10.2217/rme.14.42
6. Dowling BA, Dart AJ, Hodgson DR, Smith RKW. Superficial digital flexor tendonitis in the horse. Equine Vet J. (2000) 32:369–78. doi: 10.2746/042516400777591138
7. Singer ER, Barnes J, Saxby F, Murray JK. Injuries in the event horse: training versus competition. Vet J. (2008) 175:76–81. doi: 10.1016/j.tvjl.2006.11.009
8. Patterson-Kane JC, Firth EC. The pathobiology of exercise-induced superficial digital flexor tendon injury in thoroughbred racehorses. Vet J. (2009) 181:79–89. doi: 10.1016/j.tvjl.2008.02.009
9. Ribitsch I, Oreff GL, Jenner F. Regenerative medicine for equine musculoskeletal diseases. Animals. (2021) 11:1–30. doi: 10.3390/ani11010234
10. Thorpe CT, Clegg PD, Birch HL. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet J. (2010) 42:174–80. doi: 10.2746/042516409X480395
11. Bradshaw H, Williams J. Evaluation of equine superficial digital flexor tendon lesions. Vet Nurse. (2012) 3:578–84. doi: 10.12968/vetn.2012.3.9.578
12. Kasashima Y, Takahashi T, Smith RKW, Goodship AE, Kuwano A, Ueno T, et al. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese thoroughbred flat racehorses in 1999. Equine Vet J. (2004) 36:346–50. doi: 10.2746/0425164044890580
13. Crevier-Denoix N, Collobert C, Pourcelot P, Denoix JM, Sanaa M, Geiger D, et al. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet J Suppl. (1997)23–26. doi: 10.1111/j.2042-3306.1997.tb05046.x
14. Dakin SG. A review of the healing processes in equine superficial digital flexor tendinopathy. Equine Vet Educ. (2017) 29:516–20. doi: 10.1111/eve.12572
15. Walden G, Liao X, Donell S, Raxworthy MJ, Riley GP, Saeed A. A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng–Part B Rev. (2017) 23:44–58. doi: 10.1089/ten.teb.2016.0181
16. Schneider M, Angele P, Järvinen TAH, Docheva D. Rescue plan for achilles: therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv Drug Deliv Rev. (2018) 129:352–75. doi: 10.1016/j.addr.2017.12.016
17. Dyson SJ. Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992–2000). Equine Vet J. (2004) 36:415–9. doi: 10.2746/0425164044868422
18. Peers KHE, Lysens RJJ. Patellar tendinopathy in athletes: current diagnostic and therapeutic recommendations. Sport Med. (2005) 35:71–87. doi: 10.2165/00007256-200535010-00006
19. Stewart MC, Stewart AA. Cell-based therapies in orthopedics. Vet Clin North Am–Equine Pract. (2011) 27:xiii–iv. doi: 10.1016/j.cveq.2011.07.002
20. Torricelli P, Fini M, Filardo G, Tschon M, Pischedda M, Pacorini A, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. (2011) 35:1569–76. doi: 10.1007/s00264-011-1237-3
21. Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. (2003) 35:99–102. doi: 10.2746/042516403775467388
22. Romero A, Barrachina L, Ranera B, Remacha AR, Moreno B, de Blas I, et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet J. (2017) 224:76–84. doi: 10.1016/j.tvjl.2017.04.005
23. Torres-Torrillas M, Rubio M, Damia E, Cuervo B, Romero A Del, Peláez P, et al. Adipose-derived mesenchymal stem cells: a promising tool in the treatment of musculoskeletal diseases. Int J Mol Sci. (2019) 20:3105. doi: 10.3390/ijms20123105
24. Depuydt E, Broeckx SY, van Hecke L, Chiers K, van Brantegem L, van Schie H, et al. The evaluation of equine allogeneic tenogenic primed mesenchymal stem cells in a surgically induced superficial digital flexor tendon lesion model. Front Vet Sci. (2021) 8: 641441. doi: 10.3389/fvets.2021.641441
25. Ricco S, Renzi S, del Bue M, Conti V, Merli E, Ramoni R, et al. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol. (2013) 26:61–8. doi: 10.1177/03946320130260S108
26. Geburek F, Roggel F, van Schie HTM, Beineke A, Estrada R, Weber K, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. (2017) 8:1–21. doi: 10.1186/s13287-017-0564-8
27. Fortier LA, Smith RKW. Regenerative medicine for tendinous and ligamentous injuries of sport horses. Vet Clin North Am-Equine Pract. (2008) 24:191–201. doi: 10.1016/j.cveq.2007.11.002
28. Geburek F, Gaus M, van Schie HTM, Rohn K, Stadler PM. Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies–a randomized prospective controlled clinical trial. BMC Vet Res. (2016) 12:1–16. doi: 10.1186/s12917-016-0826-1
29. Lopez MJ, Jarazo J. State of the art: stem cells in equine regenerative medicine. Equine Vet J. (2015) 47:145–54. doi: 10.1111/evj.12311
30. Dahlgren LA. Regenerative medicine therapies for equine wound management. Vet Clin North Am–Equine Pract. (2018) 34:605–20. doi: 10.1016/j.cveq.2018.07.009
31. Mahmoudian-Sani MR, Rafeei F, Amini R, Saidijam M. The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J Cosmet Dermatol. (2018) 17:650–9. doi: 10.1111/jocd.12512
32. Mehranfar S, Abdi Rad I, Mostafav E, Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomedicine Biotechnol. (2019) 47:882–90. doi: 10.1080/21691401.2019.1576710
33. Beerts C, Suls M, Broeckx SY, Seys B, Vandenberghe A, Declercq J, et al. Tenogenically induced allogeneic peripheral blood mesenchymal stem cells in allogeneic platelet-rich plasma: 2-year follow-up after tendon or ligament treatment in horses. Front Vet Sci. (2017) 4:158. doi: 10.3389/fvets.2017.00158
34. Vandenberghe A, Broeckx SY, Beerts C, Seys B, Zimmerman M, Verweire I, et al. Tenogenically induced allogeneic mesenchymal stem cells for the treatment of proximal suspensory ligament desmitis in a horse. Front Vet Sci. (2015) 2: 49. doi: 10.3389/fvets.2015.00049
35. Goodship AE, Birch HL, Wilson AM. The pathobiology and repair of tendon and ligament injury. Vet Clin North Am Equine Pract. (1994) 10:323–49. doi: 10.1016/S0749-0739(17)30359-0
36. Smith R, McIlwraith W, Schweitzer R, Kadler K, Cook J, Caterson B, et al. Advances in the understanding of tendinopathies: a report on the second Havemeyer workshop on equine tendon disease. Equine Vet J. (2014) 46:4–9. doi: 10.1111/evj.12128
37. Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: development, repair, regeneration, and healing. J Orthop Res. (2015) 780–4. doi: 10.1002/jor.22869
38. Oreff GL, Fenu M, Vogl C, Ribitsch I, Jenner F. Species variations in tenocytes' response to inflammation require careful selection of animal models for tendon research. Sci Rep. (2021) 11:1–14. doi: 10.1038/s41598-021-91914-9
39. Domingo RA, Riggs CM, Gardner DS, Freeman SL. Ultrasonographic scoring system for superficial digital flexor tendon injuries in horses: Intra- and inter-rater variability. Vet Rec. (2017) 181:655. doi: 10.1136/vr.104233
40. Martinello T, Bronzini I, Maccatrozzo L, Iacopetti I, Sampaolesi M, Mascarello F, et al. Cryopreservation does not affect the stem characteristics of multipotent cells isolated from equine peripheral blood. Tissue Eng-Part C Methods. (2010) 16:771–81. doi: 10.1089/ten.tec.2009.0512
41. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
42. Perazzi A, Busetto R, Martinello T, Drigo M, Pasotto D, Cian F, et al. Description of a double centrifugation tube method for concentrating canine platelets. BMC Vet Res. (2013) 9:146. doi: 10.1186/1746-6148-9-146
43. Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. (1994) 233:380–5. doi: 10.1016/S0076-6879(94)33044-1
44. Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. (1994) 233:357–63. doi: 10.1016/S0076-6879(94)33041-7
45. Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. (1996) 49:1304–13. doi: 10.1038/ki.1996.186
46. Yoshida Y, Itoh N, Hayakawa M, Piga R, Cynshi O, Jishage KI, et al. Lipid peroxidation induced by carbon tetrachloride and its inhibition by antioxidant as evaluated by an oxidative stress marker, HODE. Toxicol Appl Pharmacol. (2005) 208:87–97. doi: 10.1016/j.taap.2005.01.015
47. Murray RC, Dyson SJ, Tranquille C, Adams V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet J. (2006) 38:411–6. doi: 10.1111/j.2042-3306.2006.tb05578.x
48. Spaas JH, Guest DJ, van de Walle GR. Tendon regeneration in human and equine athletes. Sport Med. (2012) 42:871–90. doi: 10.1007/BF03262300
49. Ribitsch I, Baptista PM, Lange-Consiglio A, Melotti L, Patruno M, Jenner F, et al. Large animal models in regenerative medicine and tissue engineering: to do or not to do. Front Bioeng Biotechnol. (2020) 8:972. doi: 10.3389/fbioe.2020.00972
50. Smith RKW, McIlwraith CW. “One Health” in tendinopathy research: current concepts. J Orthop Res. (2021) 39:1596–602. doi: 10.1002/jor.25035
51. Gibbs N, Diamond R, Sekyere EO, Thomas WD. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res. (2015) 8:799–806. doi: 10.2147/JPR.S92090
52. Bastos R, Mathias M, Andrade R, Bastos R, Balduino A, Schott V, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surgery Sport Traumatol Arthrosc. (2018) 26:3342–50. doi: 10.1007/s00167-018-4883-9
53. Kim SJ, Kim EK, Kim SJ, Song DH. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cufftendon. J Orthop Surg Res. (2018) 13:1–7. doi: 10.1186/s13018-017-0693-x
54. Chicharro D, Carrillo JM, Rubio M, Cugat R, Cuervo B, Guil S, Forteza J, et al. Combined plasma rich in growth factors and adipose-derived mesenchymal stem cells promotes the cutaneous wound healing in rabbits. BMC Vet Res. (2018) 14:1–12. doi: 10.1186/s12917-018-1577-y
55. Fernandes G, Wang C, Yuan X, Liu Z, Dziak R, Yang S. Combination of controlled release platelet-rich plasma alginate beads and Bone Morphogenetic Protein-2 genetically modified mesenchymal stem cells for bone regeneration. J Periodontol. (2016) 87:470–80. doi: 10.1902/jop.2016.150487
56. Broeckx S, Suls M, Beerts C, Vandenberghe A, Seys B, Wuertz-Kozak K, et al. Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: a pilot study. Curr Stem Cell Res Ther. (2014) 9:497–503. doi: 10.2174/1574888X09666140826110601
57. Hersant B, Sid-Ahmed M, Braud L, Jourdan M, Baba-Amer Y, Meningaud JP, et al. Platelet-rich plasma improves the wound healing potential of mesenchymal stem cells through paracrine and metabolism alterations. Stem Cells Int. (2019) 2019:1234263 doi: 10.1155/2019/1234263
58. Rhatomy S, Prasetyo TE, Setyawan R, Soekarno NR, Romaniyanto FNU, Sedjati AP, et al. Prospect of stem cells conditioned medium (secretome) in ligament and tendon healing: a systematic review. Stem Cells Transl Med. (2020) 9:895–902. doi: 10.1002/sctm.19-0388
59. Ulrich K, Jakob U. The role of thiols in antioxidant systems. Free Radic Biol Med. (2019) 140:14–27. doi: 10.1016/j.freeradbiomed.2019.05.035
60. Deng G, Li K, Chen S, Chen P, Zheng H, Yu B, et al. Interleukin-10 promotes proliferation and migration, and inhibits tendon differentiation via the JAK/Stat3 pathway in tendon-derived stem cells in vitro. Mol Med Rep. (2018) 18:5044–52. doi: 10.3892/mmr.2018.9547
61. Disser NP, Sugg KB, Talarek JR, Sarver DC, Rourke BJ, Mendias CL. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J. (2019) 33:12680–95. doi: 10.1096/fj.201901503R
62. Leong NL, Kator JL, Clemens TL, James A, Enamoto-Iwamoto M, Jiang J. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J Orthop Res. (2020) 38:7–12. doi: 10.1002/jor.24475
63. van Hecke L, Magri C, Duchateau L, Beerts C, Geburek F, Suls M, et al. Repeated intra-articular administration of equine allogeneic peripheral blood-derived mesenchymal stem cells does not induce a cellular and humoral immune response in horses. Vet Immunol Immunopathol. (2021) 239:110306. doi: 10.1016/j.vetimm.2021.110306
Keywords: SDFT, platelet-rich plasma, mesenchymal stem cells, regenerative medicine, equine orthopedics, tissue regeneration, horse
Citation: Melotti L, Carolo A, Elshazly N, Boesso F, Da Dalt L, Gabai G, Perazzi A, Iacopetti I and Patruno M (2022) Case Report: Repeated Intralesional Injections of Autologous Mesenchymal Stem Cells Combined With Platelet-Rich Plasma for Superficial Digital Flexor Tendon Healing in a Show Jumping Horse. Front. Vet. Sci. 9:843131. doi: 10.3389/fvets.2022.843131
Received: 24 December 2021; Accepted: 18 January 2022;
Published: 18 February 2022.
Edited by:
Debbie Guest, Royal Veterinary College (RVC), United KingdomReviewed by:
Lynn Pezzanite, Colorado State University, United StatesIris Maria Gerner, University of Veterinary Medicine Vienna, Austria
Copyright © 2022 Melotti, Carolo, Elshazly, Boesso, Da Dalt, Gabai, Perazzi, Iacopetti and Patruno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Patruno, bWFyY28ucGF0QHVuaXBkLml0
 Luca Melotti
Luca Melotti Anna Carolo
Anna Carolo Noha Elshazly
Noha Elshazly Filippo Boesso3
Filippo Boesso3 Laura Da Dalt
Laura Da Dalt Gianfranco Gabai
Gianfranco Gabai Anna Perazzi
Anna Perazzi Ilaria Iacopetti
Ilaria Iacopetti Marco Patruno
Marco Patruno