
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 14 March 2022
Sec. Comparative and Clinical Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.813524
Objective: To assess the prognostic value of neutrophil-to-lymphocyte ratio (NLR) for cardiac death in cats with hypertrophic cardiomyopathy.
Study Design: Prospective observation study.
Animals: Ninety-six client-owned cats.
Methods: Complete blood count samples were collected from 38 healthy and 58 cats with hypertrophic cardiomyopathy (HCM), and the NLR ratios were analyzed. All cats had echocardiographic measurements performed on the same day as blood collection. Spearman rank correlation was used to assess the relationship between echocardiographic measurements and NLR. Long-term outcome data were obtained, and time to cardiac death and variables associated with cardiac death were analyzed using Kaplan–Meier survival curves and Cox proportional hazards models, respectively.
Results: The NLR was significantly higher in cats with confirmed congestive heart failure. When evaluating HCM patients, cats in the third NLR tertile had a significantly higher risk of cardiac death with a hazard ratio of 10.26 (95% CI: 1.84–57.14; p = 0.0001) when compared with that of patients in the first tertile. NLR was significantly associated with echocardiographic measures of left atrial size, left auricular function, the presence of left atrial spontaneous echo contrast (SEC), and thrombus formation.
Conclusions and Clinical Relevance: Increased NLR is a negative prognostic indicator in cats with HCM.
Hypertrophic cardiomyopathy (HCM) is the most common acquired cardiac disease in cats, affecting ~16–34% of apparently healthy cats (1, 2).The prognosis for cats with HCM is highly variable, with reported median survival times between 2 and 4, 418 days (3–6). Risk stratification and prognosis in feline HCM is based largely on echocardiographic indices of left atrial size and left ventricular function and wall thickness (7). While echocardiography is excellent at evaluating heart size and function, it requires advanced technical expertise and is not widely available to most general practitioners. Several biomarkers previously identified in humans have been successfully translated into veterinary species, including N-terminal probrain natriuretic peptide (NT-proBNP), cardiac troponin I (cTnI), and cardiac troponin T (cTnT) (8–13). These biomarkers have been found to be useful to identify patients who should undergo further cardiac evaluation, and severely elevated cTnI is a predictor of adverse cardiac outcomes in cats with HCM (10). Another study evaluated the association of NT-proBNP in cats with heart failure and found that cats with a larger percentage decrease in NT-proBNP during hospitalization had longer survival times (14). Unfortunately, absolute NT-proBNP concentrations were not associated with survival, and three precise time points were required to monitor the changes in NT-proBNP, resulting in onerous guidelines for veterinarians and extensive costs for owners. In exploring new approaches to determe prognosis in cats with HCM, we evaluated a widely available hematological test, the neutrophil-to-lymphocyte ratio (NLR).
The NLR is an easily accessible and widely available hematological marker of inflammation and stress (15–20).It also serves as a good prognostic marker and has been studied in veterinary patients with oncological and systemic inflammatory disorders. In humans, previous research has revealed a predictive value of NLR in peripheral artery disease, calcific aortic stenosis, coronary artery disease, heart failure, pulmonary hypertension, and recently HCM (21–23). A higher NLR is associated with poor prognosis in most studies. To date, the NLR has not been evaluated in cats with HCM.
The aim of this study was to determine the prognostic value of NLR in cats with HCM. We hypothesized that an elevated NLR would be significantly associated with reduced time to cardiac death.
Ninety-six client-owned cats were prospectively recruited and enrolled over a 5-year period from April 2016 to May 2021. Tests performed included physical examination, indirect blood pressure by Doppler method, CBC, biochemical analysis including total thyroxine concentration, urinalysis, transthoracic echocardiogram, and thoracic radiographs. For inclusion, cats had to have all diagnostic tests performed and blood samples collected within 24-h of presentation to the hospital. Exclusion criteria included cats with congenital or non-HCM cardiac disease, systemic hypertension (systolic blood pressure > 160 mmHg), pulmonary hypertension estimated by Doppler echocardiography (systolic pulmonary artery pressure > 55 mmHg), corticosteroid use in the previous 3 months, hyperthyroidism, or any systemic illness that may limit survival significantly (i.e., neoplasia). All patients with heart failure had to be receiving furosemide orally upon discharge from the hospital.
Cats were considered healthy if all diagnostic tests were within normal limits. A diagnosis of HCM was determined by a diastolic left ventricular free-wall or interventricular septal thickness ≥6 mm on echocardiography (24). Heart failure was determined by thoracic radiographs showing cardiomegaly, vertebral heart score >8.1 on the lateral projection, as well as evidence of either pulmonary edema or pleural effusion with concurrent clinical signs such as tachypnea (respiratory rate >50/min in the examination room or resting respiratory rate >40/min at home, or both) and dyspnea that must resolve with diuretic treatment. Cats were staged as healthy, subclinical (Stage B), or heart failure (Stage C) according to the ACVIM consensus statement guidelines for classification, diagnosis, and management of cardiomyopathy in cats (25). None of the healthy cats or Stage B cats with HCM received medications other than topical heartworm and flea prevention at the time of enrollment.
All cats had a complete transthoracic echocardiographic examination performed by a board-certified cardiologist or cardiology resident under direct supervision, by use of recommended standardized transthoracic imaging planes (26–28). A diagnosis of HCM was determined by a diastolic left ventricular free-wall or interventricular septal thickness ≥6 mm on echocardiography (24). Echocardiographic measurements obtained for each cat included, left ventricular (LV) size and function performed using standard right parasternal short-axis and long-axis views and left apical parasternal long-axis views. Two-dimensional variables measured included LV internal dimensions at end-diastole (LVIDd) and end-systole (LVIDs), LV free-wall thickness at end-diastole (LVFWd) and end-systole (LVFWs), and interventricular septal thickness at end-diastole (IVSd) and end-systole (IVSs). The LV fractional shortening was calculated using the following formula: LV FS = [LVIDd – LVIDs]/LVIDd × 100%. Assessment of left atrial (LA) size was performed from standard right parasternal long-axis and short-axis views. Variables measured included LA diameter (LASAX) and aortic diameter (Ao) measured from a right parasternal short-axis view in early diastole timed to the earliest frame in which the closed aortic value cusps could be visualized. The ratio between LASAX and Ao (LA:Ao) were calculated. Additionally, the LA septal-to-free wall dimension maximum (LADMax) and minimum (LADMin) were measured from the right parasternal long-axis four-chamber view. The LADMax and LADMin were measured mid-chamber approximately parallel to the mitral annuls at end LV systole immediately prior to mitral valve opening (LADMax) and end LV diastole immediately after mitral valve closure (LADMin). The LA fractional shortening was calculated using the following formula: LA FS = [LADMax – LADMin]/LADMax × 100%. Left atrial appendage emptying velocities (LAA) were recorded using pulsed-wave Doppler from the left cranial short-axis view, and the presence or absence of spontaneous echo contrast (SEC) or thrombus was noted. Transmitral velocities were recorded using pulsed-wave Doppler from a left apical parasternal long-axis view with the sampling gate place in line with color Doppler flow at the level of the open mitral valve tips. Variables measured included peak velocity of early diastolic transmitral flow (E), deceleration time of early diastolic transmitral flow (DTE), and peak velocity of late diastolic transmitral flow (A). Ratio between peak E to peak A (E:A) was calculated. Tissue Doppler imaging (TDI) was performed with the highest available transducer frequency to record the velocity of lateral mitral annular motion from the left apical parasternal long-axis view with the sampling gate placed on the lateral mitral annulus. The following variables were measured: peak early diastolic velocity (E′), peak late diastolic velocity (A′), and peak systolic velocity (S′).
Whole blood was drawn from a peripheral vein within 24-h of presentation, and a complete blood count, biochemistry analysis, and total thyroid (T4) were assessed. The complete blood count was performed using an automated analyzer (Abbott Cell-Dyn 3700) and manually verified by a board-certified veterinary clinical pathologist.
Thoracic radiography was performed at presentation for all cats with suspected heart failure or an enlarged left atrium on echocardiography using right lateral and ventrodorsal or dorsoventral projections. The presence and severity of pulmonary edema (none, mild interstitial density, moderate interstitial density, alveolar pattern, and severe consolidation) were assessed as well as the presence and severity of pleural effusion (none, mild, moderate, severe).
The outcome of the cats was determined by follow-up examinations and telephone interviews with the owner. The primary endpoint was sudden cardiac death or euthanasia for HF. When a cat died spontaneously or was euthanized, the investigator determined whether the cause of death was cardiac or noncardiac. In cases in which the cause of death was considered noncardiac, the reason for death or euthanasia was noted. Survival time was defined as the time from enrollment into the trial to the endpoint.
Statistical analyses were performed using commercially available software (GaphPad Prism, version 8, GraphPad Software, Inc, San Diego, CA, USA; MedCal for Windows, version 19.7, Ostend, Belgium). Normality was assessed using the Shapiro–Wilk test and continuous variables were expressed as mean ± standard deviation (SD) when normally distributed, or median and range when the distribution was nonnormal. Based on the results of the normality, comparisons between groups were assessed using one-way ANOVA or a Kruskal–Wallis test, and Spearman rank correlation was used to assess the relationship between echocardiographic measurements and NLR. Survival analysis was performed in cats that reached the primary endpoint (cardiac death) and all cats alive or dying of noncardiac disease were right-censored. The continuous variables, LAmax and NLR subclassified by tertile, were analyzed using Kaplan–Meier curves. To investigate independent prognostic factors, we used Cox proportional hazard analyses on all continuous variables. After the univariate analysis, only variables with p-value <0.1 were used in the multivariate analysis. The results were described as hazard ratios (HR) and 95% CI. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic accuracy of NLR to predict cardiac death. A diagnostic cutoff was chosen on the basis of the highest of various combinations of sensitivity and specificity using Youden's index (Y = sensitivity + specificity-1). p-value <0.05 was defined as statistically significant.
One-hundred and forty-four cats were eligible for inclusion after initial screening. Forty-eight were excluded because of concurrent non-HCM cardiac disease (mitral valve dysplasia 10, transient myocardial thickening 7, ventricular septal defect 4, atrial septal defect 2), hyperthyroidism (9), incomplete diagnostics (8), and systemic hypertension (8), leaving a total of 96 cats that met all inclusion criteria. Demographic, physical examination, echocardiographic, and hematologic data are summarized in Table 1.
A total of 43/96 (44.8%) cats died by the end of the study period. One healthy cat was euthanized because of septic peritonitis. Four Stage B cats with HCM were euthanized because of advanced kidney disease (3) and a soft tissue sarcoma (1). All Stage C cats were euthanized because of refractory pulmonary edema (17), refractory pleural effusion (5), arterial thromboembolism (4), or died suddenly (3). None of the cats were lost to follow-up.
Stage C cats had significantly high NLR compared with clinically healthy and Stage B cats (Figure 1). The NLR was significantly correlated with LAA (ρs = −0.85), LA FS (ρs = −0.74), LAmax (ρs = 0.64), LA:Ao (ρs = 0.61), LVFWd (ρs = 0.37), and IVSd (ρs = 0.26). The degree of multicollinearity assessed by the variance inflation factor for each of these significant correlations was <10 (1.74–7.80).
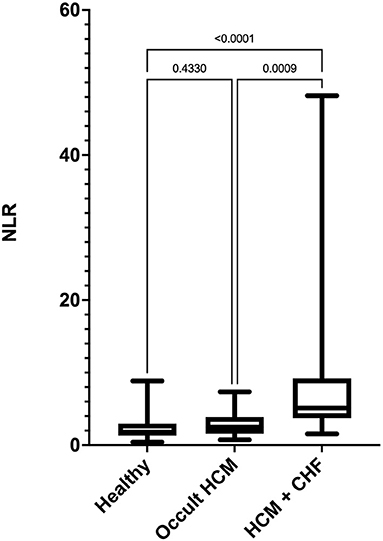
Figure 1. Box-and-whiskers plot of (NLR) in 96 cats. For each plot, the box represents the interquartile range, the horizontal line in each box represents the median, and the whiskers denote the range. The Stage C HCM (n = 29) group had significantly high NLR than Stage B HCM (n = 29) and healthy groups (n = 38). NLR, neutrophil-to-lymphocyte ratio; HCM, hypertrophic cardiomyopathy; CHF, congestive heart failure.
There were significant interactions among echocardiographic variables that were considered when performing multivariable Cox proportional hazard analysis. Significant correlation was present between LAmax and LAA (ρs = −0.62), LA FS (ρs = −0.81), LA:Ao (ρs = 0.80), IVSd (ρs = 0.40), LVFWd (ρs = 0.56), and LV FS (ρs = −0.36). Additionally, LVFWd was correlated with LAA (ρs = −0.42), LA FS (ρs = −0.54), LA:Ao (ρs = 0.52), and IVSd (ρs = 0.34) and LA FS with LV FS (ρs = 0.42). Significantly higher NLR (P = <0.0001) was present in cats with left atrial SEC or thrombus (8.62, CI 4.17−48.18) compared to cats with HCM that did not have SEC or thrombus (2.1, CI 0.79 – 6.83).
The median follow-up time for the whole population was 344 days (19–1, 500 days). Given a lower than 50% death rate in the whole population, survival analysis was evaluated in only the HCM affected cats. In this cohort, the median survival time (MST) was 265 days (19–1, 043 days). Stage C cats had significantly shorter MST 188 days (19–546) compared with Stage B cats 456 days (67–1, 043 days) p = 0.004. Both LAmax and NLR had a significant impact on MST (Figure 2). Cats with LAmax ≥ 18 mm had a significantly shorter MST (199 days, 95% CI 115–277 vs. 897 days, 95% CI 546–908 days, p = <0.0001), HR 9.5, 95% CI 4.59–19.67. There was a significant reduction in MST in the top 2 NLR tertiles (p = <0.001). The MST for cats in tertile 1 (NLR <5) was 456 days, 95% CI 322–908 days; tertile 2 was 275 days, 95% CI 114–621 days, HR 2.18, 95% CI.0.96–4.94; and tertile 3 was 56 days, 95% CI 19–100 days, HR 10.26, 95% CI 1.84–57.14.
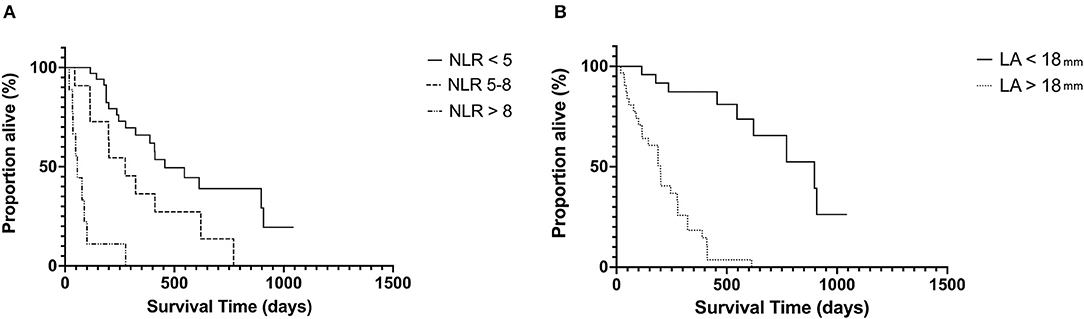
Figure 2. Kaplan–Meier curves showing the effect of NLR (A) and LA size (B) on survival in cats with hypertrophic cardiomyopathy at the univariable level. NLR, neutrophil-to-lymphocyte ratio; LA, left atrium.
For all cats with HCM, the follow variables were significant in the univariate Cox proportional hazards model: NLR (HR 1.23, 95% CI 1.11–1.37, p = <0.001), LAmax (HR 1.78, 95% CI 1.49–2.11, p = 0.001), IVSd (HR 2.14, 95% CI 1.38 – 3.32, p = <0.001), LVFWd (HR 1.97, 95% CI 1.30 – 2.98, p = 0.003), and LV FS (HR 0.89, 95% CI 0.83–0.96, p = 0.001). In the multivariable Cox proportional hazards model only LAmax (HR 1.54, 95% CI 1.25–1.92, p = 0.001) and NLR (HR 1.11, 95% CI 1.03–1.21, p = 0.006) remained significant. Indicating that LA size remains a strong predictor of outcome in cats with HCM and NLR may also provide prognostic information. Results of receiver operating characteristic indicated that NLR (AUC = 0.86, 95% CI = 0.77–0.96) provided very good diagnostic accuracy to predict cardiac death in cats with HCM. Using a cutoff for NLR of >3.43 (Youden's Index = 67) provided a sensitivity of 82% and specificity of 86%, and a cutoff of >4.46 (Youden's Index = 53) provided a sensitivity of 58% and specificity of 95% at predicting cardiac death.
When evaluating the Stage C cats alone, the significant variables in univariate Cox proportional hazards model were LAmax (HR 1.39, 95% CI 1.09–1.77, p = 0.01) and NLR (HR 1.12, 95% CI 1.05–1.21, p = 0.001), and in the multivariable analysis only NLR (HR 1.11, 95% CI 1.03–1.19, p = 0.002) remained significant, indicating that in this population of heart failure cats, NLR was an independent predictor of cardiac death.
We demonstrated that NLR is a significant prognostic indicator in cats with HCM. Moreover, an increased NLR was associated with reduced MST in both Stage B and Stage C cats suggesting that higher NLR is associated with reduced time to cardiac-related death. Based on Cox proportional models, for each unit increase in NLR, there was an 11% increased risk of cardiac death in cats with Stage B and Stage C HCM. A NLR >4.46 was associated with a 95% specificity in predicting cardiac death.
The NLR is the number of neutrophils divided by the number of lymphocytes. In cats, increases in NLR have been documented in inflammatory and neoplastic disorders (17–20). However, endogenous cortisol and catecholamines will increase under physiologic stress and have the effect of increasing the number of neutrophils, while decreasing the number of lymphocytes. Thus, NLR is not solely an indication of neoplasia or inflammation, and in humans any cause of physiologic stress may increase the NLR (29).
While it is unclear if the elevated NLR in this population of cats was the result of physiologic stress, inflammation, or both, systemic and myocardial inflammation has been documented in feline HCM. Cats with congestive heart failure caused by cardiomyopathies including HCM have demonstrated increased plasma concentrations of tumor necrosis factor alpha (TNF-α) and serum amyloid A concentrations (30, 31). There is evidence to support that TNF-α may be a driving force in peripheral lymphocyte apoptosis further contributing to lymphopenia (32). Inflammatory cell infiltrates, specifically lymphocytes and neutrophils, have been identified in the myocardium of cats with mild preclinical HCM (33). Recently, it was confirmed that systemic diseases with a generalized inflammatory response activate the transcription of inflammatory cytokines and remodeling enzymes in the myocardium (34). While inflammation may be present, it is unclear if this ultimately leads to myocyte injury and death. A specific marker of myocyte injury and death, cTnI, has been shown to predict cardiac mortality in cats and perhaps the combination of cTnI and NLR will provide superior prognostic information than either alone (10). In humans, accumulating evidence has suggested the existence of low-grade systemic and local inflammation in HCM. Mild chronic inflammatory cell infiltration was observed in the myocardium of patients with HCM (35–37). Several studies also reported increased circulating inflammatory markers in HCM such as TNF-α and interleukin-6 (38–40). While associations are continuing to mount, the exact mechanism for the inflammatory cell infiltration of the myocardium in cats and humans with HCM is unclear. Possible etiologies including altered mechanical stress inducing inflammatory cytokine expression (41), hypoxia. and ischemia induced TNF-α, IL-8, and monocyte chemoattractant peptide (42) and triggered inflammatory response during wound repair (43).
One complication of HCM is arterial thromboembolism (ATE) (3, 5, 44). Affected cats often present with left atrial enlargement, impaired atrial function, and reduced blood flow velocities (45, 46). However, not all cats with left atrial enlargement develop an ATE, suggesting that additional factors might contribute to ATE formation and hypercoagulability is suggested in some cases (47, 48). One study demonstrated that the feline myocardium constitutively transcribes proinflammatory and remodeling markers, generally at higher levels in atria than in the ventricular myocardium (49), and specific marker activation in the atria could therefore contribute to an environment that supports thrombus formation. In this population of cats, increased NLR was significantly associated with the presence of SEC or thrombus within the left atrium further supporting an association between ATE and inflammation.
This study has several limitations. This was an observational study and there may have been residual or unmeasured confounding factors as a possible alternative explanation of our observational results. We did not measure variables, including NLR, overtime that could have changed the results. Serially monitoring may improve the prognostic ability of NLR or could identify those patients at greatest risk for progression. This study did not evaluate circulating inflammatory cytokines or antemortem assessment of myocardial inflammation via cardiac magnetic resonance imaging, which could greatly improve the understanding of the role of inflammation in HCM. Finally, we did not analyze all-cause mortality and focused solely on cardiac death. It is therefore possible that some causes of death may have been misclassified and given the option for euthanasia; MST may not be interchangeable with different populations and owners.
Increased NLR is a negative prognostic indicator in cats with HCM and is associated with an increased likelihood of spontaneous echo contrast or thrombus formation in the left atrium and a shorter median time to cardiac death.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee at the University of Illinois at Urban-Champaign. Written informed consent was obtained from the owners for the participation of their animals in this study.
RF: study design, data acquisition and interpretation, and preparation of manuscript SK: study design, data acquisition and interpretation, and manuscript revision. JS, LK, and GW: study design, data acquisition, manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by Winn Feline Foundation Grants (MTW17-009 and W18-031).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge Kathryn McConnell and Candice Simpson for their technical expertise.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.813524/full#supplementary-material
1. Paige CF, Abbott JA, Elvinger F, Pyle RL. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc. (2009) 234:1398–403. doi: 10.2460/javma.234.11.1398
2. Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. (2010) 51:540–7. doi: 10.1111/j.1748-5827.2010.00989.x
3. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990-1999). J Am Vet Med Assoc. (2002) 220:202–7. doi: 10.2460/javma.2002.220.202
4. Ferasin L, DeFrancesco T. Management of acute heart failure in cats. J Vet Cardiol Off J Eur Soc Vet Cardiol. (2015) 17:S173–189. doi: 10.1016/j.jvc.2015.09.007
5. Atkins CE, Gallo AM, Kurzman ID, Cowen P. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985-1989). J Am Vet Med Assoc. (1992) 201:613–8.
6. Fox PR, Maron BJ, Basso C, Liu SK, Thiene G. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: A new animal model similar to the human disease. Circulation. (2000) 102:1863–70. doi: 10.1161/01.CIR.102.15.1863
7. Payne JR, Borgeat K, Connolly DJ, Boswood A, Dennis S, Wagner T, et al. Prognostic indicators in cats with hypertrophic cardiomyopathy. J Vet Intern Med. (2013) 27:1427–36. doi: 10.1111/jvim.12215
8. Fox PR, Oyama MA, Reynolds C, Rush JE, DeFrancesco TC, Keene BW, et al. Utility of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) to distinguish between congestive heart failure and non-cardiac causes of acute dyspnea in cats. J Vet Cardiol Off J Eur Soc Vet Cardiol. (2009) 11:S51–61. doi: 10.1016/j.jvc.2008.12.001
9. Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg. (2003) 5:209–16. doi: 10.1016/S1098-612X(03)00007-X
10. Borgeat K, Sherwood K, Payne JR, Luis Fuentes V, Connolly DJ. Plasma cardiac troponin I concentration and cardiac death in cats with hypertrophic cardiomyopathy. J Vet Intern Med. (2014) 28:1731–7. doi: 10.1111/jvim.12459
11. Langhorn R, Tarnow I, Willesen JL, Kjelgaard-Hansen M, Skovgaard IM, Koch J. Cardiac Troponin I and T as Prognostic Markers in Cats with Hypertrophic Cardiomyopathy. J Vet Intern Med. (2014) 28:1485–91. doi: 10.1111/jvim.12407
12. Hori Y, Iguchi M, Heishima Y, Yamashita Y, Nakamura K, Hirakawa A, et al. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J Vet Intern Med. (2018) 32:922–9. doi: 10.1111/jvim.15131
13. Hertzsch S, Roos A, Wess G. Evaluation of a sensitive cardiac troponin I assay as a screening test for the diagnosis of hypertrophic cardiomyopathy in cats. J Vet Intern Med. (2019) 33:1242–50. doi: 10.1111/jvim.15498
14. Pierce KV, Rush JE, Freeman LM, Cunningham SM, Yang VK. Association between Survival Time and Changes in NT-proBNP in Cats Treated for Congestive Heart Failure. J Vet Intern Med. (2017) 31:678–84. doi: 10.1111/jvim.14690
15. Benites-Zapata VA, Hernandez AV, Nagarajan V, Cauthen CA, Starling RC, Wilson Tang WH. Usefulness of Neutrophil-to-Lymphocyte Ratio in Risk Stratification of Patients With Advanced Heart Failure. Am J Cardiol. (2015) 115:57–61. doi: 10.1016/j.amjcard.2014.10.008
16. Gori E, Pierini A, Lippi I, Lubas G, Marchetti V. Leukocytes Ratios in Feline Systemic Inflammatory Response Syndrome and Sepsis: A Retrospective Analysis of 209 Cases. Anim Open Access J MDPI. (2021) 11:1644. doi: 10.3390/ani11061644
17. Naito E, Yuki M, Hirano T, Kainuma D, Aoyama R. Prognostic utility of preoperative neutrophil-lymphocyte ratio in cats with malignant mammary tumors. Res Vet Sci. (2021) 135:349–54. doi: 10.1016/j.rvsc.2020.10.015
18. Chiti LE, Martano M, Ferrari R, Boracchi P, Giordano A, Grieco V, et al. Evaluation of leukocyte counts and neutrophil-to-lymphocyte ratio as predictors of local recurrence of feline injection site sarcoma after curative intent surgery. Vet Comp Oncol. (2020) 18:105–16. doi: 10.1111/vco.12534
19. Neumann S. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in dogs and cats with acute pancreatitis. Vet Clin Pathol. (2021) 50:45–51. doi: 10.1111/vcp.12979
20. Petrucci GN, Lobo L, Queiroga F, Martins J, Prada J, Pires I, et al. Neutrophil-to-lymphocyte ratio is an independent prognostic marker for feline mammary carcinomas. Vet Comp Oncol. (2021) 19:482–91 doi: 10.1111/vco.12686
21. Ozyilmaz S, Akgul O, Uyarel H, Pusuroglu H, Gul M, Satilmisoglu MH, et al. The importance of the neutrophil-to-lymphocyte ratio in patients with hypertrophic cardiomyopathy. Rev Port Cardiol. (2017) 36:239–46. doi: 10.1016/j.repc.2016.09.014
22. González-Fajardo JA, Brizuela-Sanz JA, Aguirre-Gervás B, Merino-Díaz B, Del Río-Solá L, Martín-Pedrosa M, et al. Prognostic significance of an elevated neutrophil–lymphocyte ratio in the amputation-free survival of patients with chronic critical limb ischemia. Ann Vasc Surg. (2014) 28:999–1004. doi: 10.1016/j.avsg.2013.06.037
23. Shah N, Parikh V, Patel N, Patel N, Badheka A, Deshmukh A, et al. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: Insights from the National Health and Nutrition Examination Survey-III. Int J Cardiol. (2014) 171:390–7. doi: 10.1016/j.ijcard.2013.12.019
24. Häggström J, Luis Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. (2015) 17:S134–149. doi: 10.1016/j.jvc.2015.07.003
25. Luis Fuentes V, Abbott J, Chetboul V, Côté E, Fox PR, Häggström J, et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J Vet Intern Med. (2020) 34:1062–77. doi: 10.1111/jvim.15745
26. Schober KE, Maerz I. Doppler echocardiographic assessment of left atrial appendage flow velocities in normal cats. J Vet Cardiol. (2005) 7:15–25. doi: 10.1016/j.jvc.2004.11.001
27. Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, et al. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. (1993) 7:247–52. doi: 10.1111/j.1939-1676.1993.tb01015.x
28. Abbott JA, MacLean HN. Two-dimensional echocardiographic assessment of the feline left atrium. J Vet Intern Med. (2006) 20:111–9. doi: 10.1111/j.1939-1676.2006.tb02830.x
29. Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11:55–9. doi: 10.1586/erc.12.159
30. van Hoek I, Hodgkiss-Geere H, Bode EF, Hamilton-Elliott J, Mõtsküla P, Palermo V, et al. Associations among echocardiography, cardiac biomarkers, insulin metabolism, morphology, and inflammation in cats with asymptomatic hypertrophic cardiomyopathy. J Vet Intern Med. (2020) 34:591–9. doi: 10.1111/jvim.15730
31. Meurs KM, Fox PR, Miller MW, Kapadia S, Mann DL. Plasma concentrations of tumor necrosis factor-alpha in cats with congestive heart failure. Am J Vet Res. (2002) 63:640–2. doi: 10.2460/ajvr.2002.63.640
32. Takano T, Hohdatsu T, Hashida Y, Kaneko Y, Tanabe M, Koyama H, et al. “possible” involvement of TNF-alpha in apoptosis induction in peripheral blood lymphocytes of cats with feline infectious peritonitis. Vet Microbiol. (2007) 119:121–31. doi: 10.1016/j.vetmic.2006.08.033
33. Khor KH, Campbell FE, Owen H, Shiels IA, Mills PC. Myocardial collagen deposition and inflammatory cell infiltration in cats with pre-clinical hypertrophic cardiomyopathy. Vet J Lond Engl 1997. (2015) 203:161–8. doi: 10.1016/j.tvjl.2014.11.018
34. Fonfara S, Kitz S, Monteith G, Hahn S, Kipar A. Myocardial transcription of inflammatory and remodeling markers in cats with hypertrophic cardiomyopathy and systemic diseases associated with an inflammatory phenotype. Res Vet Sci. (2021) 136:484–94. doi: 10.1016/j.rvsc.2021.03.027
35. Lamke GT, Allen RD, Edwards WD, Tazelaar HD, Danielson GK. Surgical pathology of subaortic septal myectomy associated with hypertrophic cardiomyopathy. a study of 204 cases (1996-2000). Cardiovasc Pathol. (2003) 12:149–58. doi: 10.1016/S1054-8807(03)00036-X
36. Baandrup U, Olsen EG. Critical analysis of endomyocardial biopsies from patients suspected of having cardiomyopathy. I: Morphological and morphometric aspects. Br Heart J. (1981) 45:475–86. doi: 10.1136/hrt.45.5.475
37. Phadke RS, Vaideeswar P, Mittal B, Deshpande J. Hypertrophic cardiomyopathy: an autopsy analysis of 14 cases. J Postgrad Med. (2001) 47:165–70.
38. Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. (1994) 72:561–6. doi: 10.1136/hrt.72.6.561
39. Zen K, Irie H, Doue T, Takamiya M, Yamano T, Sawada T, et al. Analysis of circulating apoptosis mediators and proinflammatory cytokines in patients with idiopathic hypertrophic cardiomyopathy: comparison between nonobstructive and dilated-phase hypertrophic cardiomyopathy. Int Heart J. (2005) 46:231–44. doi: 10.1536/ihj.46.231
40. Högye M, Mándi Y, Csanády M, Sepp R, Buzás K. Comparison of circulating levels of interleukin-6 and tumor necrosis factor-alpha in hypertrophic cardiomyopathy and in idiopathic dilated cardiomyopathy. Am J Cardiol. (2004) 94:249–51. doi: 10.1016/j.amjcard.2004.03.078
41. Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, et al. Increased expression of interleukin-1β and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res. (1997) 81:664–71. doi: 10.1161/01.RES.81.5.664
42. Aukrust P, Gullestad L, Ueland T, Damås JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: Potential therapeutic implications. Ann Med. (2005) 37:74–85. doi: 10.1080/07853890510007232
43. Fang L, Ellims AH, Beale AL, Taylor AJ, Murphy A, Dart AM. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am J Transl Res. (2017) 9:5063–73. doi: 10.1016/j.hlc.2017.06.155
44. Payne JR, Borgeat K, Brodbelt DC, Connolly DJ, Luis Fuentes V. Risk factors associated with sudden death vs. congestive heart failure or arterial thromboembolism in cats with hypertrophic cardiomyopathy. J Vet Cardiol. (2015) 17:S318–28. doi: 10.1016/j.jvc.2015.09.008
45. Hogan DF, Brainard BM. Cardiogenic embolism in the cat. J Vet Cardiol. (2015) 17:S202–14. doi: 10.1016/j.jvc.2015.10.006
46. Linney CJ, Dukes-Mcewan J, Stephenson HM, López-Alvarez J, Fonfara S. Left atrial size, atrial function and left ventricular diastolic function in cats with hypertrophic cardiomyopathy. J Small Anim Pract. (2014) 55:198–206. doi: 10.1111/jsap.12186
47. Bédard C, Lanevschi-Pietersma A, Dunn M. Evaluation of coagulation markers in the plasma of healthy cats and cats with asymptomatic hypertrophic cardiomyopathy. Vet Clin Pathol. (2007) 36:167–72. doi: 10.1111/j.1939-165X.2007.tb00203.x
48. Tablin F, Schumacher T, Pombo M, Marion CT, Huang K, Norris JW, et al. Platelet activation in cats with hypertrophic cardiomyopathy. J Vet Intern Med. (2014) 28:411–8. doi: 10.1111/jvim.12325
Keywords: mortality, inflammation, neutrophil-to-lymphocyte ratio, prognosis, hypertrophic
Citation: Fries RC, Kadotani S, Stack JP, Kruckman L and Wallace G (2022) Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Cats With Hypertrophic Cardiomyopathy. Front. Vet. Sci. 9:813524. doi: 10.3389/fvets.2022.813524
Received: 19 November 2021; Accepted: 09 February 2022;
Published: 14 March 2022.
Edited by:
Arianna Miglio, University of Perugia, ItalyReviewed by:
Carlo Guglielmini, University Hospital of Padua, ItalyCopyright © 2022 Fries, Kadotani, Stack, Kruckman and Wallace. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan C. Fries, cmZyaWVzQGlsbGlub2lzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.