- 1Instituto de Ciencias Clínicas, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile
- 2Instituto de Patología Animal, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile
- 3Instituto de Farmacología y Morfofisiología, Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile
Mesenchymal stem/stromal cells (MSCs) therapy has been a cornerstone of regenerative medicine in humans and animals since their identification in 1968. MSCs can interact and modulate the activity of practically all cellular components of the immune response, either through cell-cell contact or paracrine secretion of soluble mediators, which makes them an attractive alternative to conventional therapies for the treatment of chronic inflammatory and immune-mediated diseases. Many of the mechanisms described as necessary for MSCs to modulate the immune/inflammatory response appear to be dependent on the animal species and source. Although there is evidence demonstrating an in vitro immunomodulatory effect of MSCs, there are disparate results between the beneficial effect of MSCs in preclinical models and their actual use in clinical diseases. This discordance might be due to cells' limited survival or impaired function in the inflammatory environment after transplantation. This limited efficacy may be due to several factors, including the small amount of MSCs inoculated, MSC administration late in the course of the disease, low MSC survival rates in vivo, cryopreservation and thawing effects, and impaired MSC potency/biological activity. Multiple physical and chemical pre-conditioning strategies can enhance the survival rate and potency of MSCs; this paper focuses on hypoxic conditions, with inflammatory cytokines, or with different pattern recognition receptor ligands. These different pre-conditioning strategies can modify MSCs metabolism, gene expression, proliferation, and survivability after transplantation.
Introduction
Mesenchymal stromal/stem cells (MSCs) have been studied extensively for more than 40 years, with a large body of evidence supporting that MSCs can resolve inflammation and promote tissue repair in various inflammatory conditions, since they can interact with and modulate both innate and adaptive immune responses (1). Many of the mechanisms involved in these processes have been elucidated, but their relevance depending on the cell species and source of the cells remains to be studied, especially in large animals.
The activation of local resident sensor cells of the immune response, responsible for the non-specific detection of microorganisms or tissue damage, leads to the secretion of first-order cytokines that promote the arrival of cells of the adaptive immune response; depending on the inciting cause, local resident sensor cells (epithelial, mast cells, innate lymphoid, and effector memory cells) trigger cellular and humoral effector responses (2). In this sense, the immune and inflammatory response can be modulated at different levels, and MSCs can actively and passively influence both innate and adaptive components of the immune response (3). Recent works in animals deal in-depth with the molecules and pathways that confer MSCs their ability to inhibit lymphocyte proliferation and activation, modify macrophage and lymphocyte polarization, promote cell survival, and therefore modulate inflammation (4–7). However, these mechanisms can be enhanced through different physical and chemical alternatives, which could in turn augment the therapeutic effects of MSCs. The objective of this review is to describe the use of low oxygen tension, pro-inflammatory cytokines or Pattern Recognition Receptors (PRR) as MSCs pre-conditioning mechanisms which could potentially increase their effectiveness and translational potential for inflammatory conditions in livestock animals.
Improving MSCs Immunomodulatory Function
Low oxygen (O2) tension, high concentrations of inflammatory cytokines, and even presence of microorganisms, are all hallmarks of an inflamed tissue which play a key role in the metabolism of the cells present at the site of injury, and can modulate their functions. It is in these environments where transplanted MSCs will have to perform, by modulating the inflammatory/immune response and promoting the regenerative process. This has led to exploration of ways to pre-condition MSCs in order to “jumpstart” their physiology and strengthen their therapeutic mechanisms, looking to increase the amount of cells that arrive to the site of inflammation and their survival, while also increasing their anti-inflammatory and regenerative effects (8–10). Thus far, pre-conditioning strategies for MSC oriented therapies in veterinary medicine have focused especially on small animals (11–14). In livestock species, studies on MSCs have mostly been published in the last decade (Table 1), with some of their immunomodulatory mechanisms described (Table 2). Much remains to be determined regarding pre-conditioning strategies for MSCs in livestock.

Table 1. Effects of mesenchymal stromal/stem cells (MSCs) on inflammatory conditions in livestock animals.
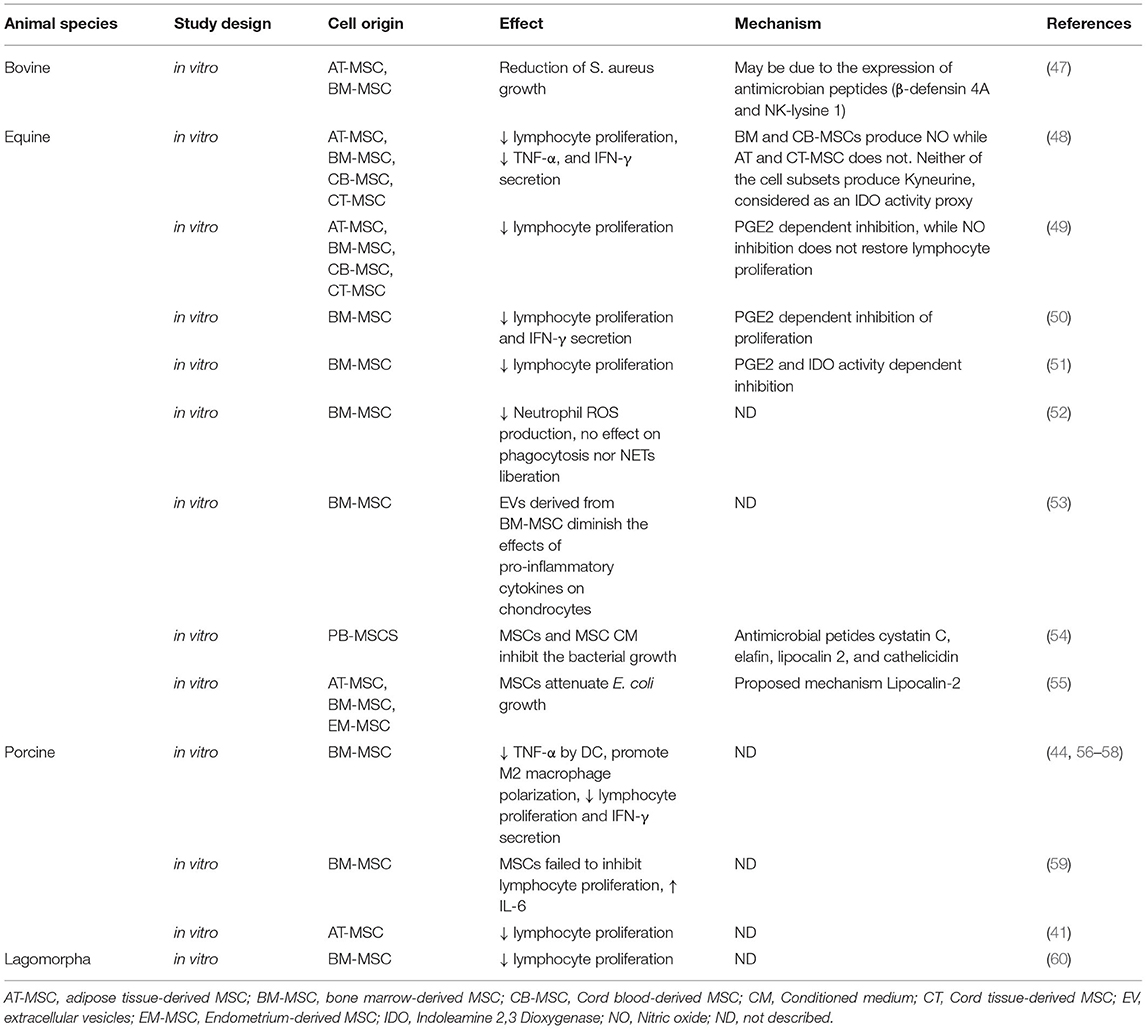
Table 2. In vitro studies that explore antiinflammatory/immunomodulatory mechanisms and effects of MSCs in livestock.
Hypoxia
The issue of optimal culture conditions for MSCs has been under investigation for many years, and the optimum oxygen tension in which to culture cells is an important consideration. Physiological oxygen tension varies greatly between different tissues and can range from 12% in blood to values below 1% (61). MSCs may experience a variety of oxygen tensions, for instance 1–7% in bone marrow or 10–15% in adipose tissue: higher levels of oxygen may lead to early senescence, oxidative stress, DNA damage and lower proliferation (62–64). Regardless of the value, physiological oxygen tension in tissues is significantly lower than the atmospheric tension of the gas generally used in MSCs cultures.
Hypoxic pre-conditioning greatly modifies MSCs physiology, increasing colony formation unit (CFU) number and survival while limiting apoptosis and senescence, mainly but not exclusively through the Hypoxia-Inducible Factor (HIF)-1α mediated pathway (65–68). Some authors have pointed out that these effects could entail an impairment in differentiation capacity (69, 70), but there are mixed opinions on this topic, since other authors describe that low O2 tension culture conditions preserve and even increase MSCs differentiation ability (71–73). Additionally, hypoxia has been shown to increase MSC migration both in vitro and in vivo, modulating the expression of chemokine receptors and integrins (72, 74–77), and also promoting their vasculogenic potential by increasing expression of VEGF and Angiopoietin (Ang-1) (78–80), both important factors that increase MSCs therapeutic potential. Furthermore, in different pre-clinical models, hypoxic pre-conditioning favors the therapeutic effect of MSCs, enhancing their arrival and engraftment into injured tissue where they promote the survival of tissue resident cells, all of which could be beneficial in clinical settings (76, 81–86). Hypoxic pre-conditioning also enhances MSCs regulation of the inflammatory response by increasing expression of key molecules that modulate the immune response. Hypoxia elevates expression of Indoleamine-pyrrole 2,3-dioxygenase (IDO), HLA-G, Prostaglandin-endoperoxide synthase 2 (PTGS-2), and interleukin (IL)-10 (87–89) and decreases pro-inflammatory molecules such as tumor necrosis factor (TNF)-α, IL-1β and IL-6, and nitric oxide (NO) (87) in different contexts both in vitro and in vivo, all of which modify cellular fate and condition the inflammatory response by decreasing inflammation and promoting a pro-resolutive context through different pathways (90, 91). Authors have also described that hypoxic pre-conditioning modifies MSCs metabolism and may cause metabolic disruption by increasing glucose uptake and usage, leading to lactate accumulation which attenuates T cell division (92). Interestingly, the MSCs expression of the soluble form of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) increases under hypoxic conditions, which could affect the lymphocyte activation process by interacting with co-stimulatory molecules of antigen presenting cells (93). Hypoxic priming of MSCs also modifies T cell polarization, by stimulating regulatory T cell proliferation via paracrine mechanisms (89). It is also important to note that hypoxic culture conditions do not impair MSCs ability to respond to inflammatory stimuli nor their immunosuppressive capacities (94). In fact, HIF-1α expression by MSCs induced by hypoxic culture conditions increases their modulatory capacities (95): experimental overexpression of HIF-1α, mimicking hypoxia, has been shown to enhance MSC immunomodulatory properties, while its silencing decreases their immunosuppresive potential (95–98).
There is little information regarding the effect of low O2 tension culture conditions upon MSCs physiology in livestock animals, and mixed results have been reported. In bovines, a recent study shows that culture with low oxygen tension may increase BM-MSCs survival and proliferation with limited effect on gene expression, mainly upregulating the expression of genes related to cell stress, growth, and metabolism (99). Similar results were described in buffalo AT-MSCs, in which hypoxic culture conditions (5% O2) enhanced proliferation, colony formation and differentiation potential, increasing expression of HIF-1α and secretion of basic fibroblast growth factor and vascular endothelial growth factor (VEGF) (100). In sheep, hypoxic culture resulted in faster bone marrow (BM)-MSC population doublings per day, and cell colony formation and viability were not significantly affected (101, 102). Although hypoxia enhanced in vitro BM-MSC chondrogenesis (102), this did not translate into increased cartilaginous repair tissue formation following cell transplantation into cartilage defects in vivo (103). While some authors report that hypoxic pre-conditioning of porcine MSCs had no effect on proliferation or cell migration (104, 105), others do describe an increase in cell proliferation and impaired osteogenic differentiation in both BM and adipose tissue (AT)-MSCs under hypoxic conditions (106). These conflicting results could be due to differences between culture protocols; for example, Antebi et al. (79) describe that MSCs proliferate significantly faster during 48 h of culture than during 10 days of culture, in both cases under 1% O2 hypoxic conditions. Additionally, authors mention that porcine MSCs cultured under hypoxia had upregulated expression of VEGF and the anti-inflammatory cytokines IL-1 receptor antagonist (RA) and granulocyte-macrophage colony-stimulating factor (GM-CSF), with concomitant downregulation of the pro-inflammatory cytokine IL-8 (79).
In horses, MSCs therapy is of special interest in musculoskeletal diseases such as osteoarthritis, tendon and ligament injuries, bone repair, among others (Table 1). Low oxygen tension culture conditions (5%) attenuate the proliferative capacity of equine AT-MSCs but not BM-MSCs; however, in normoxic (21% O2) conditions a greater proportion of cells were in S phase of cellular cycle, indicating that both cell populations were more active (69). Hypoxic culture seems to keep cells more undifferentiated than normoxic culture, and this is supported by a tendency of hypoxic MSCs to increase expression of embryonic markers (69). This is also described by Griffon et al. (70) who found that hypoxic (5%) culture together with chitosan affected cell yield but improved the stemness of UC-MSCs, with increased expression of embryonic markers such as NANOG, OCT4, and SOX2. In an in vitro fracture hematoma model in horses, hypoxic conditions (1% O2) favored survival of MSCs and an increase in osteogenesis, and MSCs survival was correlated with a decrease in live lymphocytes (107).
Hypoxic conditions (1% O2) produce an increase in tenogeneic gene expression in rabbit BM-MSC, which correlates with their increased capacity in promoting patellar tendon repair in vivo determined either by both tissue reparation and biomechanical analysis (108). Similar beneficial results are described in a study using hypoxic (1% O2) pre-conditioning in rabbit BM-MSCs used in combination with hyaluronic acid for the treatment of osteoarthritis. Those results show that the addition of hypoxic pre-conditioned BM-MSCs reduce cartilage loss and surface abrasion, with an improvement in histological features compared with hyaluronic acid alone (109). Hypoxic pre-conditioning (1% O2) also enhances the therapeutic effects of rabbit BM-MSC on a disc degeneration model, greatly improving MSC ability to reduce damage and improving extracellular matrix deposition (110).
Pro-inflammatory Cytokines
MSCs -and even their culture supernatant- can modulate phagocyte functions in vitro and in vivo without requiring activation (52, 111). However, activation of MSCs with pro-inflammatory cytokines sets in motion several pathways involved in the arrival of MSCs to the injured tissue and is also relevant to development of their full immunomodulatory potential (61). This notion about the need of pro-inflammatory stimuli to activate the immunomodulatory capacity of MSCs is particularly interesting because it could allow identification of the mechanisms required for suppression of the immune response (112). This evidence indicates the potential of MSCs for the treatment of diverse inflammatory conditions in large animals (Table 1).
TNF-α and interferon (IFN)-γ are the most common pro-inflammatory cytokines used for pre-conditioning MSCs, either alone or in combination. IFN-γ is a key modulator of the IDO-Kyneurine pathway which has been shown to be a key component of the immunomodulatory arsenal of MSCs and is involved in several of the reported effects on immune cells (3). In this regard, it is logical to find that pre-conditioning MSCs with IFN-γ results in increased expression of IDO, and of other key immunomodulatory molecules such as prostaglandin (PG)E-2, transforming growth factor (TGF)-β, NO, IL-10, tumor necrosis factor-inducible gene 6 protein (TSG-6) and Programmed death-ligand 1 (PD-L1). These molecules can suppress the cytotoxic activity of natural killer (NK) cells, lymphocyte proliferation and cytokine secretion (113–116), and macrophage polarization (116, 117). Additionally, priming MSCs with IFN-γ has been shown to impair a type 3 immune response characterized by cells that produce IL-17 (118). Regarding function of B cells, pre-treatment with IFN-γ increases MSCs' capacity to reduce B-cell proliferation and immunoglobulin production (119). Some of these effects of IFN-γ are increased with the addition of TNF-α, since the combination of both cytokines increases the expression of immunomodulatory molecules such as IDO, PTGS-2 and inducible nitric oxide synthase (iNOS) (51). This improvement of immunomodulation by MSCs pre-conditioned in vitro with IFN-γ and/or TNF-α has also been observed in vivo when the cells are transplanted into animals in different preclinical models of inflammatory conditions, such as colitis, diabetes, or graft-vs.-host disease (GVHD) (117, 118, 120–123).
One potential complication of pre-conditioning with IFN-γ is that along with the rise of MSCs immunomodulatory ability there is also an increase in immunogenicity: in horses and rabbits, IFN-γ pre-conditioning has been shown to upregulate MHC class I and II (60, 124), which can be avoided by pretreating cells with TGF-β2 (125). Pre-conditioning with IL-17 could be an alternative, since it not only confers MSCs greater immunomodulatory capacities such as suppression of T cell proliferation and activation, inhibition of type 1 response, and increased induction of Treg cell, but also unlike IFN-γ, it does not induce immunogenicity (126).
In swine, stimulation of MSCs with TNF-α or IL-1 increased their migratory capacity although this effect seems to be dependent on the tissue source (127). Additionally, treatment of porcine AT-MSCs with a IFN-γ, TNF-α and IL-6 cytokine cocktail increased the expression of immunomodulatory molecules such as GBP4, IL1-RA, and IDO while impairing pro-inflammatory cytokines IL-6, IL-17, and TNF-α in vitro (128). In horses, MSCs incubation with TNF-α and IFN-γ produces a great increase in the main immunomodulatory paracrine molecules secreted by MSCs such as PTGS-2, IDO, iNOS, and IL-6, while downregulating the expression of other molecules like IL-10, TGF-β1, TSG-6, which are relevant in other species but have no proven role in large animal MSCs immunomodulatory mechanisms (129).
Pattern Recognition Receptors Ligands
The innate immune system is the first line of defense against microorganisms and is constantly surveying the body for the presence of pathogen-associated molecular patterns (PAMPs), which are detected by highly conserved receptors known as PRR. One of the main PRR families are Toll-like receptors (TLR), and MSCs preferentially express TLR 1-6 (9, 130). Another important PRR family are the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR), whose activation of NLR proteins results in inflammatory responses mediated either by NF-κB, MAPK or Caspase-1 activation, accompanied by subsequent secretion of pro-inflammatory cytokines (131). Ligands for both types of PRR have been considered for pre-conditioning of MSCs, since they not only influence their immunomodulatory functions but also promote antibacterial activity.
The effects on MSCs immune modulation will vary depending on which TLR ligand is used. For instance, Fuenzalida et al. (132) compared the stimulation of TLR-3 and TLR-4 by using polyinosinic-polycytidylic acid [poly(I:C)] and lipopolysaccharide (LPS), respectively. They determined that TLR3 ligands produced a stronger immunosuppressive phenotype compared with MSCs preconditioned with TLR4 ligands, since poly(I:C) induces greater IDO expression which correlates with inhibition of lymphocyte proliferation in vitro and improved DSS-induced colitis in vivo. Similar results were described by another study in which pre-conditioning with poly(I:C) improved human MSCs immune modulatory properties, decreasing pro-inflammatory cytokines and increasing systemic IL-10 levels in colonic tissues. This was associated with the inhibition of type 1 and 3 immune responses and promotion of Treg differentiation (133).
In horses, there are a few studies about MSCs pre-conditioning using TLR ligands. Cassano et al. (134) showed that TLR-3 or 4 stimulation in MSCs enhances their ability to suppress mitogen stimulated T cells proliferation, with MHC class II positive MSCs having a stronger immunosuppressive activity than MHC class II negative MSCs. Interestingly, MSCs have also been shown to have antimicrobial activity, both by constitutively secreted factors such as defensins, hepcidin and lipocalins, and indirectly by activation of innate immune effector cells (135). Similarly, equine MSCs constitutively express the antimicrobial lipocalin-2 whose expression is augmented by stimulation with LPS, and which could mediate the limitation of the growth of Escherichia coli by MSCs-conditioned media (55). Furthermore, MSCs stimulation with poly(I:C) and [γ-d-Glu-mDAP (IE-DAP)], an NLR agonist, stimulates antimicrobial peptide production and increases bactericidal activity, also suppressing biofilm formation and enhancing neutrophil bactericidal functions (136).
Conclusion
Pre-conditioning with hypoxia, pro-inflammatory cytokines, or Pattern Recognition Receptors Ligands seem to enhance MSC survival, arrival, engraftment, proliferation, immunomodulatory or pro-regenerative functions, and may increase their therapeutic efficacy. Despite limitations in their use in veterinary medicine, especially due to cost and high variability between species, culture and cryopreservation protocols, and tissue sources, MSCs seem to be a promising alternative therapy for inflammatory and immune-mediated conditions. It is critical to further our understanding of known and novel mechanisms by which MSCs modulate inflammatory processes in livestock animals.
Author Contributions
BU: manuscript writing and edition. AP and CH: manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by FONDECYT Grant No. 1210839, Chilean Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
CH wants to acknowledge the members of the Instituto de Farmacología y Morfofisiología (UACh) for their constant support, scientific enthusiasm, and creative feedback.
References
1. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. (2011) 6:457–78. doi: 10.1146/annurev-pathol-011110-130230
2. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. (2015) 135:626–35. doi: 10.1016/j.jaci.2014.11.001
3. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
4. Dias IE, Pinto PO, Barros LC, Viegas CA, Dias IR, Carvalho PP. Mesenchymal stem cells therapy in companion animals: useful for immune-mediated diseases? BMC Vet Res. (2019) 15:358. doi: 10.1186/s12917-019-2087-2
5. Macdonald ES, Barrett JG. The potential of mesenchymal stem cells to treat systemic inflammation in horses. Front Vet Sci. (2019) 6:507. doi: 10.3389/fvets.2019.00507
6. Voga M, Adamic N, Vengust M, Majdic G. Stem cells in veterinary medicine-current state and treatment options. Front Vet Sci. (2020) 7:278. doi: 10.3389/fvets.2020.00278
7. Bukowska J, Szostek-Mioduchowska AZ, Kopcewicz M, Walendzik K, Machcinska S, Gawronska-Kozak B. Adipose-derived stromal/stem cells from large animal models: from basic to applied science. Stem Cell Rev Rep. (2021) 17:719–38. doi: 10.1007/s12015-020-10049-y
8. Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WC. Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int. (2016) 2016:3924858. doi: 10.1155/2016/3924858
9. Najar M, Krayem M, Merimi M, Burny A, Meuleman N, Bron D, et al. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm Res. (2018) 67:467–77. doi: 10.1007/s00011-018-1131-1
10. Lee BC, Kang KS. Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther. (2020) 11:397. doi: 10.1186/s13287-020-01920-3
11. Yu J, Liu XL, Cheng QG, Lu SS, Xu XQ, Zu QQ, et al. G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Exp Ther Med. (2016) 12:1822–8. doi: 10.3892/etm.2016.3535
12. Kim SM, Li Q, An JH, Chae HK, Yang JI, Ryu MO, et al. Enhanced angiogenic activity of dimethyloxalylglycine-treated canine adipose tissue-derived mesenchymal stem cells. J Vet Med Sci. (2019) 81:1663–70. doi: 10.1292/jvms.19-0337
13. Park SM, Li Q, Ryu MO, Nam A, An JH, Yang JI, et al. Preconditioning of canine adipose tissue-derived mesenchymal stem cells with deferoxamine potentiates anti-inflammatory effects by directing/reprogramming M2 macrophage polarization. Vet Immunol Immunopathol. (2020) 219:109973. doi: 10.1016/j.vetimm.2019.109973
14. Park SM, An JH, Lee JH, Kim KB, Chae HK, Oh YI, et al. Extracellular vesicles derived from DFO-preconditioned canine AT-MSCs reprogram macrophages into M2 phase. PLoS ONE. (2021) 16:e0254657. doi: 10.1371/journal.pone.0254657
15. Peralta OA, Carrasco C, Vieytes C, Tamayo MJ, Munoz I, Sepulveda S, et al. Safety and efficacy of a mesenchymal stem cell intramammary therapy in dairy cows with experimentally induced Staphylococcus aureus clinical mastitis. Sci Rep. (2020) 10:2843. doi: 10.1038/s41598-020-59724-7
16. Lange-Consiglio A, Gusmara C, Manfredi E, Idda A, Soggiu A, Greco V, et al. Antimicrobial effects of conditioned medium from amniotic progenitor cells in vitro and in vivo: toward tissue regenerative therapies for bovine mastitis. Front Vet Sci. (2019) 6:443. doi: 10.3389/fvets.2019.00443
17. Miranda MS, Nascimento HS, Costa MP, Costa NN, Brito KN, Lopes CT, et al. Increasing of blastocyst rate and gene expression in co-culture of bovine embryos with adult adipose tissue-derived mesenchymal stem cells. J Assist Reprod Genet. (2016) 33:1395–403. doi: 10.1007/s10815-016-0779-0
18. Sadeghian Chaleshtori S, Mokhber Dezfouli MR, Abbasi J, Dehghan MM, Jabbari Fakhr M, Yadollahi S, et al. Prevention of LPS-induced acute respiratory distress syndrome in sheep by bone marrow-derived mesenchymal stem/stromal cells. Life Sci. (2020) 263:118600. doi: 10.21203/rs.3.rs-41981/v1
19. Kocyildirim E, Cardenes N, Ting A, Caceres E, Bermudez C, Rojas M. The use of GMP-produced bone marrow-derived stem cells in combination with extracorporeal membrane oxygenation in ARDS: an animal model. ASAIO J. (2017) 63:324–32. doi: 10.1097/MAT.0000000000000566
20. Aridas JD, Mcdonald CA, Paton MC, Yawno T, Sutherland AE, Nitsos I, et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb. J Physiol. (2016) 594:1421–35. doi: 10.1113/JP271104
21. Li J, Yawno T, Sutherland AE, Gurung S, Paton M, Mcdonald C, et al. Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia. Exp Neurol. (2018) 308:120–31. doi: 10.1016/j.expneurol.2018.07.006
22. Paton MCB, Allison BJ, Fahey MC, Li J, Sutherland AE, Pham Y, et al. Umbilical cord blood versus mesenchymal stem cells for inflammation-induced preterm brain injury in fetal sheep. Pediatr Res. (2019) 86:165–73. doi: 10.1038/s41390-019-0366-z
23. Martinello T, Gomiero C, Perazzi A, Iacopetti I, Gemignani F, Debenedictis GM, et al. Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Vet Res. (2018) 14:202. doi: 10.1186/s12917-018-1527-8
24. Khan MR, Dudhia J, David FH, De Godoy R, Mehra V, Hughes G, et al. Bone marrow mesenchymal stem cells do not enhance intra-synovial tendon healing despite engraftment and homing to niches within the synovium. Stem Cell Res Ther. (2018) 9:169. doi: 10.1186/s13287-018-0900-7
25. Dooley LM, Abdalmula A, Washington EA, Kaufman C, Tudor EM, Ghosh P, et al. Effect of mesenchymal precursor cells on the systemic inflammatory response and endothelial dysfunction in an ovine model of collagen-induced arthritis. PLoS ONE. (2015) 10:e0124144. doi: 10.1371/journal.pone.0124144
26. Abdalmula A, Dooley LM, Kaufman C, Washington EA, House JV, Blacklaws BA, et al. Immunoselected STRO-3(+) mesenchymal precursor cells reduce inflammation and improve clinical outcomes in a large animal model of monoarthritis. Stem Cell Res Ther. (2017) 8:22. doi: 10.1186/s13287-016-0460-7
27. Costa CRM, Feitosa MLT, Rocha AR, Bezerra DO, Leite YKC, Argolo Neto NM, et al. Adipose stem cells in reparative goat mastitis mammary gland. PLoS ONE. (2019) 14:e0223751. doi: 10.1371/journal.pone.0223751
28. Carvalho Ade M, Badial PR, Alvarez LE, Yamada AL, Borges AS, Deffune E, et al. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. Stem Cell Res Ther. (2013) 4:85. doi: 10.1186/scrt236
29. Mund SJK, Macphee DJ, Campbell J, Honaramooz A, Wobeser B, Barber SM. Macroscopic, histologic, and immunomodulatory response of limb wounds following intravenous allogeneic cord blood-derived multipotent mesenchymal stromal cell therapy in horses. Cells. (2021) 10:2972. doi: 10.3390/cells10112972
30. Iacono E, Merlo B, Pirrone A, Antonelli C, Brunori L, Romagnoli N, et al. Effects of mesenchymal stem cells isolated from amniotic fluid and platelet-rich plasma gel on severe decubitus ulcers in a septic neonatal foal. Res Vet Sci. (2012) 93:1439–40. doi: 10.1016/j.rvsc.2012.04.008
31. Marx C, Gardner S, Harman RM, Wagner B, Van De Walle GR. Mesenchymal stromal cell-secreted CCL2 promotes antibacterial defense mechanisms through increased antimicrobial peptide expression in keratinocytes. Stem Cells Transl Med. (2021) 10:1666–79. doi: 10.1002/sctm.21-0058
32. Angelone M, Conti V, Biacca C, Battaglia B, Pecorari L, Piana F, et al. The contribution of adipose tissue-derived mesenchymal stem cells and platelet-rich plasma to the treatment of chronic equine laminitis: a proof of concept. Int J Mol Sci. (2017) 18:2122. doi: 10.3390/ijms18102122
33. Magri C, Schramme M, Febre M, Cauvin E, Labadie F, Saulnier N, et al. Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: a clinical pilot study. PLoS ONE. (2019) 14:e0221317. doi: 10.1371/journal.pone.0221317
34. Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. (2013) 95:1535–41. doi: 10.1097/TP.0b013e318291a2da
35. Vega A, Martin-Ferrero MA, Del Canto F, Alberca M, Garcia V, Munar A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. (2015) 99:1681–90. doi: 10.1097/TP.0000000000000678
36. Saldinger LK, Nelson SG, Bellone RR, Lassaline M, Mack M, Walker NJ, et al. Horses with equine recurrent uveitis have an activated CD4+ T-cell phenotype that can be modulated by mesenchymal stem cells in vitro. Vet Ophthalmol. (2020) 23:160–70. doi: 10.1111/vop.12704
37. Falomo ME, Ferroni L, Tocco I, Gardin C, Zavan B. Immunomodulatory role of adipose-derived stem cells on equine endometriosis. Biomed Res Int. (2015) 2015:141485. doi: 10.1155/2015/141485
38. Ferris RA, Frisbie DD, Mccue PM. Use of mesenchymal stem cells or autologous conditioned serum to modulate the inflammatory response to spermatozoa in mares. Theriogenology. (2014) 82:36–42. doi: 10.1016/j.theriogenology.2014.02.015
39. Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. (2005) 102:11474–9. doi: 10.1073/pnas.0504388102
40. Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. (2006) 48:2116–24. doi: 10.1016/j.jacc.2006.06.073
41. Noort WA, Oerlemans MI, Rozemuller H, Feyen D, Jaksani S, Stecher D, et al. Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J Cell Mol Med. (2012) 16:1827–39. doi: 10.1111/j.1582-4934.2011.01455.x
42. Ebrahimi B, Eirin A, Li Z, Zhu XY, Zhang X, Lerman A, et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS ONE. (2013) 8:e67474. doi: 10.1371/journal.pone.0067474
43. Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. (2013) 31:117–25. doi: 10.1002/stem.1263
44. Eirin A, Zhang X, Zhu XY, Tang H, Jordan KL, Grande JP, et al. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant. (2014) 29:274–82. doi: 10.1093/ndt/gft305
45. Eirin A, Zhu XY, Puranik AS, Tang H, Mcgurren KA, Van Wijnen AJ, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. (2017) 92:114–24. doi: 10.1016/j.kint.2016.12.023
46. Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, et al. Mesenchymal stem/stromal cells and their extracellular vesicle progeny decrease injury in poststenotic swine kidney through different mechanisms. Stem Cells Dev. (2020) 29:1190–200. doi: 10.1089/scd.2020.0030
47. Cahuascanco B, Bahamonde J, Huaman O, Jervis M, Cortez J, Palomino J, et al. Bovine fetal mesenchymal stem cells exert antiproliferative effect against mastitis causing pathogen Staphylococcus aureus. Vet Res. (2019) 50:25. doi: 10.1186/s13567-019-0643-1
48. Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells(). Cell Med. (2012) 4:1–11. doi: 10.3727/215517912X647217
49. Carrade Holt DD, Wood JA, Granick JL, Walker NJ, Clark KC, Borjesson DL. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. (2014) 23:1258–65. doi: 10.1089/scd.2013.0537
50. Colbath AC, Dow SW, Phillips JN, Mcilwraith CW, Goodrich LR. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties in vitro. Stem Cells Dev. (2017) 26:503–11. doi: 10.1089/scd.2016.0266
51. Caffi V, Espinosa G, Gajardo G, Morales N, Duran MC, Uberti B, et al. Pre-conditioning of equine bone marrow-derived mesenchymal stromal cells increases their immunomodulatory capacity. Front Vet Sci. (2020) 7:318. doi: 10.3389/fvets.2020.00318
52. Espinosa G, Plaza A, Schenffeldt A, Alarcon P, Gajardo G, Uberti B, et al. Equine bone marrow-derived mesenchymal stromal cells inhibit reactive oxygen species production by neutrophils. Vet Immunol Immunopathol. (2020) 221:109975. doi: 10.1016/j.vetimm.2019.109975
53. Hotham WE, Thompson C, Szu-Ting L, Henson FMD. The anti-inflammatory effects of equine bone marrow stem cell-derived extracellular vesicles on autologous chondrocytes. Vet Rec Open. (2021) 8:e22. doi: 10.1002/vro2.22
54. Harman RM, Patel RS, Fan JC, Park JE, Rosenberg BR, Van De Walle GR. Single-cell RNA sequencing of equine mesenchymal stromal cells from primary donor-matched tissue sources reveals functional heterogeneity in immune modulation and cell motility. Stem Cell Res Ther. (2020) 11:524. doi: 10.1186/s13287-020-02043-5
55. Cortes-Araya Y, Amilon K, Rink BE, Black G, Lisowski Z, Donadeu FX, et al. Comparison of antibacterial and immunological properties of mesenchymal stem/stromal cells from equine bone marrow, endometrium, and adipose tissue. Stem Cells Dev. (2018) 27:1518–25. doi: 10.1089/scd.2017.0241
56. Wang L, Lu XF, Lu YR, Liu J, Gao K, Zeng YZ, et al. Immunogenicity and immune modulation of osteogenic differentiated mesenchymal stem cells from Banna minipig inbred line. Transplant Proc. (2006) 38:2267–9. doi: 10.1016/j.transproceed.2006.06.048
57. Kumar G, Hara H, Long C, Shaikh H, Ayares D, Cooper DK, et al. Adipose-derived mesenchymal stromal cells from genetically modified pigs: immunogenicity and immune modulatory properties. Cytotherapy. (2012) 14:494–504. doi: 10.3109/14653249.2011.651529
58. Khatri M, O'brien TD, Chattha KS, Saif LJ. Porcine lung mesenchymal stromal cells possess differentiation and immunoregulatory properties. Stem Cell Res Ther. (2015) 6:222. doi: 10.1186/s13287-015-0220-0
59. Brunswig-Spickenheier B, Boche J, Westenfelder C, Peimann F, Gruber AD, Jaquet K, et al. Limited immune-modulating activity of porcine mesenchymal stromal cells abolishes their protective efficacy in acute kidney injury. Stem Cells Dev. (2010) 19:719–29. doi: 10.1089/scd.2009.0494
60. Yuan T, Li K, Guo L, Fan H, Zhang X. Modulation of immunological properties of allogeneic mesenchymal stem cells by collagen scaffolds in cartilage tissue engineering. J Biomed Mater Res A. (2011) 98:332–41. doi: 10.1002/jbm.a.33121
61. Noronha NC, Mizukami A, Caliari-Oliveira C, Cominal JG, Rocha JLM, Covas DT, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. (2019) 10:131. doi: 10.1186/s13287-019-1224-y
62. Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. (2005) 1049:1–8. doi: 10.1196/annals.1334.001
63. Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. (2007) 6:745–57. doi: 10.1111/j.1474-9726.2007.00336.x
64. Betous R, Renoud ML, Hoede C, Gonzalez I, Jones N, Longy M, et al. Human adipose-derived stem cells expanded under ambient oxygen concentration accumulate oxidative DNA lesions and experience procarcinogenic dna replication stress. Stem Cells Transl Med. (2017) 6:68–76. doi: 10.5966/sctm.2015-0401
65. Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, et al. Hypoxic preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. PLoS ONE. (2015) 10:e0138477. doi: 10.1371/journal.pone.0138477
66. Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. (2015) 33:1818–28. doi: 10.1002/stem.1976
67. Zhang J, Feng Z, Wei J, Yu Y, Luo J, Zhou J, et al. Repair of critical-sized mandible defects in aged rat using hypoxia preconditioned BMSCs with up-regulation of Hif-1alpha. Int J Biol Sci. (2018) 14:449–60. doi: 10.7150/ijbs.24158
68. Liu J, He J, Ge L, Xiao H, Huang Y, Zeng L, et al. Hypoxic preconditioning rejuvenates mesenchymal stem cells and enhances neuroprotection following intracerebral hemorrhage via the miR-326-mediated autophagy. Stem Cell Res Ther. (2021) 12:413. doi: 10.1186/s13287-021-02480-w
69. Ranera B, Remacha AR, Alvarez-Arguedas S, Romero A, Vazquez FJ, Zaragoza P, et al. Effect of hypoxia on equine mesenchymal stem cells derived from bone marrow and adipose tissue. BMC Vet Res. (2012) 8:142. doi: 10.1186/1746-6148-8-142
70. Griffon DJ, Cho J, Wagner JR, Charavaryamath C, Wei J, Wagoner Johnson A. Effects of hypoxia and chitosan on equine umbilical cord-derived mesenchymal stem cells. Stem Cells Int. (2016) 2016:2987140. doi: 10.1155/2016/2987140
71. Volkmer E, Kallukalam BC, Maertz J, Otto S, Drosse I, Polzer H, et al. Hypoxic preconditioning of human mesenchymal stem cells overcomes hypoxia-induced inhibition of osteogenic differentiation. Tissue Eng Part A. (2010) 16:153–64. doi: 10.1089/ten.tea.2009.0021
72. Feng Y, Zhu M, Dangelmajer S, Lee YM, Wijesekera O, Castellanos CX, et al. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. (2015) 6:e1797. doi: 10.1038/cddis.2015.176
73. Lambertini E, Penolazzi L, Angelozzi M, Bergamin LS, Manferdini C, Vieceli Dalla Sega F, et al. Hypoxia preconditioning of human MSCs: a direct evidence of HIF-1alpha and collagen type XV correlation. Cell Physiol Biochem. (2018) 51:2237–49. doi: 10.1159/000495869
74. Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE. (2007) 2:e416. doi: 10.1371/journal.pone.0000416
75. Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. (2008) 26:2173–82. doi: 10.1634/stemcells.2007-1104
76. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. (2012) 7:e34608. doi: 10.1371/journal.pone.0034608
77. Saller MM, Prall WC, Docheva D, Schonitzer V, Popov T, Anz D, et al. Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression. Biochem Biophys Res Commun. (2012) 423:379–85. doi: 10.1016/j.bbrc.2012.05.134
78. Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. (2010) 18:1545–52. doi: 10.1038/mt.2010.108
79. Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, et al. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:265. doi: 10.1186/s13287-018-1007-x
80. Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. (2019) 109:59–68. doi: 10.1016/j.biocel.2019.01.017
81. Jaussaud J, Biais M, Calderon J, Chevaleyre J, Duchez P, Ivanovic Z, et al. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur J Cardiothorac Surg. (2013) 43:1050–7. doi: 10.1093/ejcts/ezs549
82. Liu YY, Chiang CH, Hung SC, Chian CF, Tsai CL, Chen WC, et al. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS ONE. (2017) 12:e0187637. doi: 10.1371/journal.pone.0187637
83. Schive SW, Mirlashari MR, Hasvold G, Wang M, Josefsen D, Gullestad HP, et al. Human adipose-derived mesenchymal stem cells respond to short-term hypoxia by secreting factors beneficial for human islets in vitro and potentiate antidiabetic effect in vivo. Cell Med. (2017) 9:103–16. doi: 10.3727/215517917X693401
84. Wang JW, Qiu YR, Fu Y, Liu J, He ZJ, Huang ZT. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. J Neurosci Res. (2017) 95:2059–70. doi: 10.1002/jnr.24025
85. Wang W, Wang Y, Deng G, Ma J, Huang X, Yu J, et al. Transplantation of hypoxic-preconditioned bone mesenchymal stem cells retards intervertebral disc degeneration via enhancing implanted cell survival and migration in rats. Stem Cells Int. (2018) 2018:7564159. doi: 10.1155/2018/7564159
86. Hu Y, Chen W, Wu L, Jiang L, Qin H, Tang N. Hypoxic preconditioning improves the survival and neural effects of transplanted mesenchymal stem cells via CXCL12/CXCR4 signalling in a rat model of cerebral infarction. Cell Biochem Funct. (2019) 37:504–15. doi: 10.1002/cbf.3423
87. Jiang CM, Liu J, Zhao JY, Xiao L, An S, Gou YC, et al. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. (2015) 94:69–77. doi: 10.1177/0022034514557671
88. Du WJ, Reppel L, Leger L, Schenowitz C, Huselstein C, Bensoussan D, et al. Mesenchymal stem cells derived from human bone marrow and adipose tissue maintain their immunosuppressive properties after chondrogenic differentiation: role of HLA-G. Stem Cells Dev. (2016) 25:1454–69. doi: 10.1089/scd.2016.0022
89. Kadle RL, Abdou SA, Villarreal-Ponce AP, Soares MA, Sultan DL, David JA, et al. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PLoS ONE. (2018) 13:e0193178. doi: 10.1371/journal.pone.0193178
90. Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. (2018) 32:2672–84. doi: 10.1038/s41375-018-0151-8
91. Yu H, Xu Z, Qu G, Wang H, Lin L, Li X, et al. Hypoxic Preconditioning enhances the efficacy of mesenchymal stem cells-derived conditioned medium in switching microglia toward anti-inflammatory polarization in ischemia/reperfusion. Cell Mol Neurobiol. (2021) 41:505–24. doi: 10.1007/s10571-020-00868-5
92. Wobma HM, Kanai M, Ma SP, Shih Y, Li HW, Duran-Struuck R, et al. Dual IFN-gamma/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J Immunol Regen Med. (2018) 1:45–56. doi: 10.1016/j.regen.2018.01.001
93. Gaber T, Schonbeck K, Hoff H, Tran CL, Strehl C, Lang A, et al. CTLA-4 mediates inhibitory function of mesenchymal stem/stromal cells. Int J Mol Sci. (2018) 19:2312. doi: 10.3390/ijms19082312
94. Roemeling-Van Rhijn M, Mensah FK, Korevaar SS, Leijs MJ, Van Osch GJ, Ijzermans JN, et al. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol. (2013) 4:203. doi: 10.3389/fimmu.2013.00203
95. Liu J, Qiu P, Qin J, Wu X, Wang X, Yang X, et al. Allogeneic adipose-derived stem cells promote ischemic muscle repair by inducing M2 macrophage polarization via the HIF-1alpha/IL-10 pathway. Stem Cells. (2020) 38:1307–20. doi: 10.1002/stem.3250
96. Martinez VG, Ontoria-Oviedo I, Ricardo CP, Harding SE, Sacedon R, Varas A, et al. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. (2017) 8:208. doi: 10.1186/s13287-017-0659-2
97. Contreras-Lopez R, Elizondo-Vega R, Paredes MJ, Luque-Campos N, Torres MJ, Tejedor G, et al. HIF1alpha-dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. FASEB J. (2020) 34:8250–64. doi: 10.1096/fj.201902232R
98. Zielniok K, Burdzinska A, Kaleta B, Zagozdzon R, Paczek L. Vadadustat, a HIF prolyl hydroxylase inhibitor, improves immunomodulatory properties of human mesenchymal stromal cells. Cells. (2020) 9:2396. doi: 10.3390/cells9112396
99. Peck SH, Bendigo JR, Tobias JW, Dodge GR, Malhotra NR, Mauck RL, et al. Hypoxic preconditioning enhances bone marrow-derived mesenchymal stem cell survival in a low oxygen and nutrient-limited 3D microenvironment. Cartilage. (2021) 12:512–25. doi: 10.1177/1947603519841675
100. Deng Y, Huang G, Chen F, Testroet ED, Li H, Li H, et al. Hypoxia enhances buffalo adipose-derived mesenchymal stem cells proliferation, stemness, and reprogramming into induced pluripotent stem cells. J Cell Physiol. (2019) 234:17254–68. doi: 10.1002/jcp.28342
101. Deschepper M, Oudina K, David B, Myrtil V, Collet C, Bensidhoum M, et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J Cell Mol Med. (2011) 15:1505–14. doi: 10.1111/j.1582-4934.2010.01138.x
102. Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. (2015) 6:84. doi: 10.1186/s13287-015-0075-4
103. Bornes TD, Adesida AB, Jomha NM. Articular cartilage repair with mesenchymal stem cells after chondrogenic priming: a pilot study. Tissue Eng Part A. (2018) 24:761–74. doi: 10.1089/ten.tea.2017.0235
104. Bukowska J, Slowinska M, Cierniak P, Kopcewicz M, Walendzik K, Frazier T, et al. The effect of hypoxia on the proteomic signature of pig adipose-derived stromal/stem cells (pASCs). Sci Rep. (2020) 10:20035. doi: 10.1038/s41598-020-76796-7
105. Archacka K, Grabowska I, Mierzejewski B, Graffstein J, Gorzynska A, Krawczyk M, et al. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res Ther. (2021) 12:448. doi: 10.1186/s13287-021-02530-3
106. Burian E, Probst F, Palla B, Riedel C, Saller MM, Cornelsen M, et al. Effect of hypoxia on the proliferation of porcine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells in 2- and 3-dimensional culture. J Craniomaxillofac Surg. (2017) 45:414–9. doi: 10.1016/j.jcms.2016.12.014
107. Pfeiffenberger M, Bartsch J, Hoff P, Ponomarev I, Barnewitz D, Thone-Reineke C, et al. Hypoxia and mesenchymal stromal cells as key drivers of initial fracture healing in an equine in vitro fracture hematoma model. PLoS One. (2019) 14:e0214276. doi: 10.1371/journal.pone.0214276
108. Chen G, Zhang W, Zhang K, Wang S, Gao Y, Gu J, et al. Hypoxia-induced mesenchymal stem cells exhibit stronger tenogenic differentiation capacities and promote patellar tendon repair in rabbits. Stem Cells Int. (2020) 2020:8822609. doi: 10.1155/2020/8822609
109. Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS ONE. (2016) 11:e0149835. doi: 10.1371/journal.pone.0149835
110. Chiang ER, Ma HL, Wang JP, Chang MC, Liu CL, Chen TH, et al. Use of allogeneic hypoxic mesenchymal stem cells for treating disc degeneration in rabbits. J Orthop Res. (2019) 37:1440–50. doi: 10.1002/jor.24342
111. Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. (2019) 10:1191. doi: 10.3389/fimmu.2019.01191
112. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. (2008) 2:141–50. doi: 10.1016/j.stem.2007.11.014
113. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. (2013) 13:392–402. doi: 10.1016/j.stem.2013.09.006
114. Noone C, Kihm A, English K, O'dea S, Mahon BP. IFN-gamma stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. (2013) 22:3003–14. doi: 10.1089/scd.2013.0028
115. Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, et al. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-gamma produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front Immunol. (2020) 11:54. doi: 10.3389/fimmu.2020.00054
116. Park SG, An JH, Li Q, Chae HK, Park SM, Lee JH, et al. Feline adipose tissue-derived mesenchymal stem cells pretreated with IFN-gamma enhance immunomodulatory effects through the PGE(2) pathway. J Vet Sci. (2021) 22:e16. doi: 10.4142/jvs.2021.22.e16
117. Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, et al. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. (2019) 13:1792–804. doi: 10.1002/term.2930
118. Yang R, Huang H, Cui S, Zhou Y, Zhang T, Zhou Y. IFN-gamma promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. (2020) 11:603. doi: 10.1038/s41419-020-02788-0
119. Luk F, Carreras-Planella L, Korevaar SS, De Witte SFH, Borras FE, Betjes MGH, et al. Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Front Immunol. (2017) 8:1042. doi: 10.3389/fimmu.2017.01042
120. Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, Van Zuylen VL, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. (2011) 29:1549–58. doi: 10.1002/stem.698
121. Boland L, Burand AJ, Brown AJ, Boyt D, Lira VA, Ankrum JA. IFN-gamma and TNF-alpha pre-licensing protects mesenchymal stromal cells from the pro-inflammatory effects of palmitate. Mol Ther. (2018) 26:860–73. doi: 10.1016/j.ymthe.2017.12.013
122. Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-gamma. EBioMedicine. (2018) 28:261–73. doi: 10.1016/j.ebiom.2018.01.002
123. Lim JY, Kim BS, Ryu DB, Kim TW, Park G, Min CK. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-gamma and poly(I:C) priming through promoting the expression of indoleamine 2,3-dioxygenase. Stem Cell Res Ther. (2021) 12:37. doi: 10.1186/s13287-020-02087-7
124. Cassano JM, Schnabel LV, Goodale MB, Fortier LA. Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res Ther. (2018) 9:82. doi: 10.1186/s13287-018-0840-2
125. Berglund AK, Fisher MB, Cameron KA, Poole EJ, Schnabel LV. Transforming growth factor-beta2 downregulates major histocompatibility complex (MHC) I and MHC II surface expression on equine bone marrow-derived mesenchymal stem cells without altering other phenotypic cell surface markers. Front Vet Sci. (2017) 4:84. doi: 10.3389/fvets.2017.00084
126. Sivanathan KN, Rojas-Canales DM, Hope CM, Krishnan R, Carroll RP, Gronthos S, et al. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells. (2015) 33:2850–63. doi: 10.1002/stem.2075
127. Calle A, Barrajon-Masa C, Gomez-Fidalgo E, Martin-Lluch M, Cruz-Vigo P, Sanchez-Sanchez R, et al. Iberian pig mesenchymal stem/stromal cells from dermal skin, abdominal and subcutaneous adipose tissues, and peripheral blood: in vitro characterization and migratory properties in inflammation. Stem Cell Res Ther. (2018) 9:178. doi: 10.1186/s13287-018-0933-y
128. Venkataiah VS, Handa K, Njuguna MM, Hasegawa T, Maruyama K, Nemoto E, et al. Periodontal regeneration by allogeneic transplantation of adipose tissue derived multi-lineage progenitor stem cells in vivo. Sci Rep. (2019) 9:921. doi: 10.1038/s41598-018-37528-0
129. Barrachina L, Remacha AR, Romero A, Vazquez FJ, Albareda J, Prades M, et al. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet Immunol Immunopathol. (2016) 171:57–65. doi: 10.1016/j.vetimm.2016.02.007
130. Van Den Akker F, De Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. (2013) 2013:181020. doi: 10.1155/2013/181020
131. Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. (2008) 83:13–30. doi: 10.1189/jlb.0607402
132. Fuenzalida P., Kurte M., Fernandez-O'ryan C., Ibanez C., Gauthier-Abeliuk M., Vega-Letter A. M., et al. (2016). Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy 18, 630–641. doi: 10.1016/j.jcyt.2016.02.002
133. Qiu Y, Guo J, Mao R, Chao K, Chen BL, He Y, et al. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. (2017) 10:727–42. doi: 10.1038/mi.2016.78
134. Cassano JM, Schnabel LV, Goodale MB, Fortier LA. The immunomodulatory function of equine MSCs is enhanced by priming through an inflammatory microenvironment or TLR3 ligand. Vet Immunol Immunopathol. (2018) 195:33–9. doi: 10.1016/j.vetimm.2017.10.003
135. Chow L, Johnson V, Impastato R, Coy J, Strumpf A, Dow S. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Transl Med. (2020) 9:235–49. doi: 10.1002/sctm.19-0092
Keywords: mesenchymal stem cells, mesenchymal stromal cells, livestock animals, pre-conditioning, inflammation, hypoxia
Citation: Uberti B, Plaza A and Henríquez C (2022) Pre-conditioning Strategies for Mesenchymal Stromal/Stem Cells in Inflammatory Conditions of Livestock Species. Front. Vet. Sci. 9:806069. doi: 10.3389/fvets.2022.806069
Received: 31 October 2021; Accepted: 16 February 2022;
Published: 16 March 2022.
Edited by:
Tereza Cristina Cardoso, Universidade Estadual de São Paulo, BrazilReviewed by:
Valerie Johnson, Michigan State University, United StatesIris Maria Gerner, University of Veterinary Medicine Vienna, Austria
Copyright © 2022 Uberti, Plaza and Henríquez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Henríquez, Y2xhdWRpby5oZW5yaXF1ZXpAdWFjaC5jbA==
 Benjamin Uberti
Benjamin Uberti Anita Plaza2
Anita Plaza2 Claudio Henríquez
Claudio Henríquez