
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 24 March 2022
Sec. Animal Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.802051
This article is part of the Research Topic The Interaction between Digestive Tract Microbes and Hosts in Poultry View all 25 articles
The objective of this experiment was to study the effects of catalase (CAT) on growth performance, antioxidant capacity, intestinal morphology, and microbial composition of yellow broilers. Male Lingnan yellow broilers (360), aged 1 day, were randomly divided into control group (CON) (fed with a basic diet), R1 group (fed with basic diet + 150 U/kg catalase), and R2 group (fed with basic diet + 200 U/kg catalase). Each group had 8 replicates and 15 chickens in each replicate. The test is divided into the early stage (1–30 days) and the later stage (31–60 days). The results showed that compared with the control group, groups R1 and R2 significantly (p < 0.05) increased the weight gain and reduced (p < 0.05) the ratio of feed to gain in the early and the whole stages; prominently increased (p < 0.05) the concentration of total antioxidant capacity (T-AOC), the activities of CAT, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) in livers, the activities of CAT and GSH-Px in serum, and CAT in the jejunum in the early and the later stages; markedly increased (p < 0.05) the villus height and the ratio of villus height to crypt depth of the duodenum in the early and the later stages, the villus height and the villus height:crypt depth ratio of the jejunum and ileum in the early stage, and significantly lowered (p < 0.05) the crypt depth of the duodenum (in the early and the later stages), jejunum, and ileum (in early stage); memorably (p < 0.05) increased the number of total bacteria and Bacteroidetes in ceca, as well as the number of Lactobacillus in the jejunum (p < 0.05) on the 30th; significantly (p < 0.05) increased the mRNA expression of junction adhesion molecule 2 (JAM2), mucin 2 (MCU2), and occlusal protein (occludin) in the duodenum in the early stage, and increased (p < 0.05) the mRNA expression of JAM2 in the jejunum in the later stage. Collectively, adding catalase (CAT) to the diet of yellow broilers can improve the growth performance and the antioxidant capacity, promoting the integrity of intestinal morphology, optimizing the composition of intestinal microorganisms, and upregulating the mRNA expression of tight junction protein.
In the daily life activities of animals, free radicals (1) are constantly produced and cleared in the body. Under normal circumstances, the two processes maintain a dynamic balance (2, 3). However, when animals are affected by heat stress, disease, immunity, oxidized oil, mycotoxins, excessive metal ions, or other adverse factors (4–9), the balance between the production and clearance of free radicals is broken. The production of free radicals exceeds the ability and speed of body clearance, and the redox balance in the body is destroyed (10), causing the oxidative stress response of the body (11–15). Excess free radicals would attack biological macromolecules including DNA and protein, resulting in the peroxidation and dysfunction of biomacromolecules, thus, reducing the transcription level of genes and affecting the normal growth and development of the body. Growth performance, immune function, antioxidant function, intestinal microflora composition, intestinal morphology, and health status of animals are often affected and damaged (15–27).
The antioxidant system of animal is composed of the antioxidant enzyme system and non-antioxidant system (including various antioxidants), which can remove free radicals produced in the body (28–37). The antioxidant enzyme system is composed of CAT, SOD, GSH-Px, etc. CAT is a key enzyme in the antioxidant enzyme system, which has anti-inflammatory and antioxidant effects and widely exists in microorganisms, animals, and plants. It can catalyze the decomposition of hydrogen peroxide (H2O2), preventing iron chelates from using H2O2 and oxygen (O2) to generate more toxic hydroxyl radicals, preventing lipid oxidation of cell membrane, reducing oxidative damage.
The biological antioxidant system of yellow broilers is fragile due to rapid growth. Ambient high temperature, oxidized grease, high lipid, and protein diet (38), and high-density raising can cause oxidative stress easily (39). The scavenging capacity of free oxygen radicals and peroxides in the body decreases sharply, resulting in a large amount of accumulation (40–42), which affects the growth performance of the intestinal structure and body health of broilers. Therefore, this experiment was conducted to evaluate the effect of catalase (CAT) on growth performance, antioxidant capacity, intestinal morphology, microbial composition, and tight junction protein expression using 1-day-old male Lingnan yellow broilers as our research objective.
Catalase (CAT) was purchased from Shandong Longkete Enzyme Preparation Co., Ltd. (Shandong, China). The CAT is a food-grade enzyme preparation, and its enzyme activity is 5,000 U/g. Moreover, the definition of enzyme activity is at pH 7.0 and 30°C, decomposing 1 μmol H2O2 per minute; the amount of enzyme required is defined as 1 activity unit, expressed in U/g. The 1-day-old male Lingnan yellow broilers were purchased from the market chick seeding seller.
The basic diet was divided into the early stage (1–30 days) and the later stage (31–60 days). The diet was prepared in accordance with the standard National Research Council (43) and the industrial standard NY/T33-2004 of the People's Republic of China. The feed shape is powder, and the composition and nutritional level of the basic diet are listed in Table 1.
A total of 360 healthy 1-day-old male Lingnan yellow broilers with similar weight were randomly divided into control group (CON) (fed basic diet), R1 group (fed basic diet + 150 U/kg CAT), and R2 group (fed basic diet + 200 U/kg CAT). There were 8 replicates in each group and 15 chickens in each replicate. The test period lasted for 60 days. The chickens were raised in three-layer cages and were immunized normally according to the feeding and management requirements of yellow broilers. Artificial light, free feeding, and clean drinking water were applied during the experiment period, and the daily behavior and health status of the chickens were observed in the experiment.
On the 30th and 60th days of the experiment, one chicken per replicate was selected for blood sample collection. The collected blood was centrifuged after standing, and the obtained serum was stored in the refrigerator at −20°C for subsequent testing.
After venous bloodletting to death, the bodies were immediately dissected, and the intestines were separated. The chyme of the jejunum and cecum was taken out in a sterile environment and stored at −80°C for total DNA extraction.
About 1 cm of duodenum, ileum, and jejunum were extracted, respectively, and gently washed away the contents with normal saline and fixed in 10% formalin solution. After dehydration, transparent treatment with xylene and paraffin embedding, the fixed intestinal segment was made into sections, which were stained and sealed for microscopic observation.
Intestinal segments of duodenum, jejunum, and ileum were cut out and washed with sterile phosphate buffer, then cut into pieces and stored at −80°C for RNA extraction.
Liver and jejunum tissues were washed off chyme with normal saline and placed in 2-ml cryopreservation tubes, respectively, and put in a −20°C refrigerator for analysis.
On the morning of the 1st, 30th, and 60th days of the experiment, the test chickens were weighed on an empty stomach (fasted for 12 h, only for drinking water). In addition, the daily feed intake and the number and weight of dead chickens were recorded to adjust the feed conversion ratio. Then the total feed intake and body weight on the 30th and 60th days of each repetition were counted, and the body weight gain, feed intake, and feed:gain ratio of each chicken at each stage were calculated as well.
Total antioxidant capacity (T-AOC), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) activity, and malondialdehyde (MDA) content were detected in livers, serum, and jejunum using commercial assay kits, which were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The thawed liver and jejunum samples were washed with precooled normal saline, dried with filter paper, and weighed. The weighed liver and jejunum samples were homogenized in the ice bath with precooled normal saline in a ratio of 1:9 by mass volume, centrifuged at 4°C for 20 min (rotating speed 10,000 r/min), and the supernatant was taken to determine the corresponding indices according to the xanthine oxidase method, colorimetry, spectrophotometry, and thiobarbital method.
The prepared sections of the duodenum, ileum, and jejunum were photographed after selecting the typical field of vision with a fluorescence microscope, observed, and analyzed with Lecia Qwin image analysis system, and measured the villus height and crypt depth, respectively. Four visual field areas were taken from each section, and their average value was taken, and then the villus height:crypt depth was calculated (44).
On the 30th day, 0.3 g each of thawed chyme of the jejunum and cecum were weighed, respectively, and the total DNA of the intestinal chyme was extracted by CTAB (cetyltrimethylammonium bromide), bead beating, and phenol–chloroform methods referring to the method of Zoetendal et al. (45). Simultaneously, using the kit (QIAamp Fast DNA Stool Mini Kit, Qiagen, Hilden, Germany), the total DNA from intestinal mucosa was extracted. With utilizing specific primers (Table 2), quantitative analysis of the total number of bacteria, Firmicutes, Bacteroidetes, Clostridium cluster IV, Clostridium cluster XIVα, Lactobacillus, and Escherichia coli were detected.
The 100 mg of duodenum, jejunum, and ileum tissues were weighed, homogenized by liquid nitrogen grinding method, and RNA was extracted by EASYspin Plus tissue/cell RNA rapid extraction kit (Beijing Adlai Biotechnology Co., Ltd. Beijing China).
The RNA concentration was determined by NanoDrop microspectrophotometer (nd-1000uv0vis, Thermo Fisher Scientific Inc.). Samples (cDNA>100 ng/μl) with OD260:OD280 values of 2.0–2.2 were stored at −20°C for reverse transcription. cDNA was synthesized by reverse transcription Kit (Prime Script TM RT reagent kit and gDNA Eraser kit, RR047a, Takara, Japan).
Gene quantitative PCR (qPCR) took cDNA as a template, and the primer sequence is shown in Table 3. The reaction system was: 10 μl f SYBR® Premix Ex Taq (TaKaRa Biotechnology, Dalian, China), 2 μl f DNA template, 0.3 μl each of upstream and downstream primers, and 8.4 μl of water. Quantification was performed using the CFX96 PCR System (Bio-Rad, USA). Quantitative results used glyceraldehyde-3-phosphate dehydrogenase (GADPH) as the internal reference gene. Each sample was repeated three times, and the gene expression was analyzed by the 2−ΔΔCt method.
After the test data were processed by Excel 10, SPSS 19.0 statistical software was adopted for analysis of variance. When the difference was significant, Duncan's method was used for multiple comparisons. The test results were expressed as mean ± standard deviation, and p < 0.05 was significant.
As Table 4 shows, there was a significant difference in body weight gain and feed:gain ratio among the three test groups from 1 to 30 days (p < 0.05), and compared with the control group and R2 group, the R1 group increased feed intake significantly (p < 0.05). From 31 to 60 days, the body weight gain of the three experimental groups showed an upward trend, but there was no remarkable difference (p > 0.05); however, groups R1 and R2 decreased the ratio of feed to gain significantly (p < 0.05) compared with the control group in this stage. In the whole course (from 1 to 60 days), body weight gain and feed:gain ratio of R1 and R2 groups were significantly improved (p < 0.05) than those of the control group. Simultaneously, there was a significant difference in feed intake between the group R1 and the control group (p < 0.05).
The data on antioxidant indices are summarized in Table 5. On the 30th day, the activities of CAT in jejunums showed a significant increase (p < 0.05) among the three test groups; compared with the control group, adding 150 and 200 U CAT to the diet significantly increased (p < 0.05) the concentration of T-AOC, the activities of CAT, SOD, and GSH-Px in the liver, and the activities of CAT and GSH-Px in serum. In the meantime, dietary supplementation with 200 U CAT displayed a notable decrease (p < 0.05) in the content of MDA in the serum and jejunum. On the 60th day, groups R1 (150 U CAT) and R2 (200 U CAT) increased (p < 0.05) the concentration of T-AOC, the activities of CAT, SOD, and GSH-Px in the liver, the concentration of T-AOC and the activity of GSH-Px in the serum, and the activity of CAT in jejunums. CAT activity in the serum increased prominently among the three groups (p < 0.05).
Data in Table 6 shows that on the 30th day, compared with the control group, the addition of 150 and 200 U CAT in the diet could markedly (p < 0.05) increase the villus height and villus height:crypt depth ratio of the duodenum, jejunum, and ileum, and significantly reduce (p < 0.05) crypt depth. On the 60th day, in comparison with the control group, the villus height and the ratio of villus height to crypt depth of the duodenum and jejunum were improved notably (p < 0.05) after adding 150 and 200 U CAT (p < 0.05). Dietary supplementation with 200 U of CAT notably lowered (p < 0.05) crypt depth of the ileum and significantly increased (p < 0.05) the ratio of villus height to crypt depth. At the same time, there were conspicuous differences in the crypt depth of the duodenum among the three groups (p < 0.05).
According to the data in Table 7 and Figure 1, on the 30th day, the diet supplemented with 200 U of CAT prominently increased (p < 0.05) the jejunal Firmicutes and Bacteroidetes counts and significantly decreased the number of Escherichia coli in the ceca (p < 0.05). Adding 150 and 200 U of CAT could remarkably increase (<0.05) the number of Bacteroidetes and total bacteria in the ceca; diet with the 150 U of CAT could effectively increase (p < 0.05) the amount of Clostridium cluster IV, Clostridium cluster XIVα, and Lactobacillus counts in the ceca. Also, the quantity of Lactobacillus in the jejunum increased markedly among the three groups (p < 0.05).
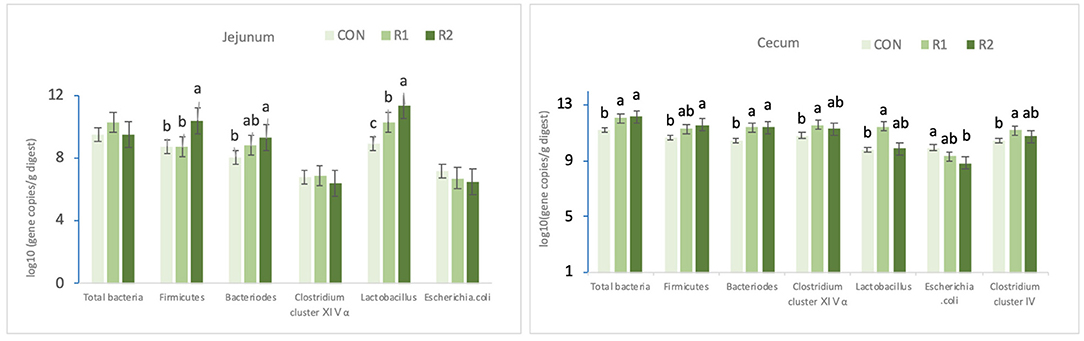
Figure 1. Effects of dietary catalase supplementation on intestinal microbial composition of 30-day-old yellow broilers. Data are the mean ± SEM (n = 8). CON-broilers fed the basal diet, R1-broilers fed the basal diet supplemented with 150U/kg CAT, R2-broilers fed the basal diet supplemented with 200U/kg CATa,b,c within the same intestinal segment and the same strain, means with different superscript letters differ (P < 0.05).
The results obtained from the mRNA expression of tight junction protein are presented in Figure 2. On the 30th day, compared with the control group, group R1 (150 U CAT) and R2 (200 U CAT) increased (p < 0.05) the expression levels of JAM2, MUR2, and occludin in the duodenum. Additionally, group R2 (200 U CAT) significantly upregulated (p < 0.05) jejunal, occludin, ileal JAM2, and occludin mRNA expression. On the 60th day, compared with the control group, group R2 (200 U CAT) increased notably (p < 0.05) the expressions of duodenal MUR2, jejunal MUR2, occludin, and closed small cyclic protein-1 (ZO-1), ileal JAM2, MUR2, and Occludin. In the meantime, groups R1 and R2 dramatically heightened (p < 0.05) the expression level of jejunal JAM2.
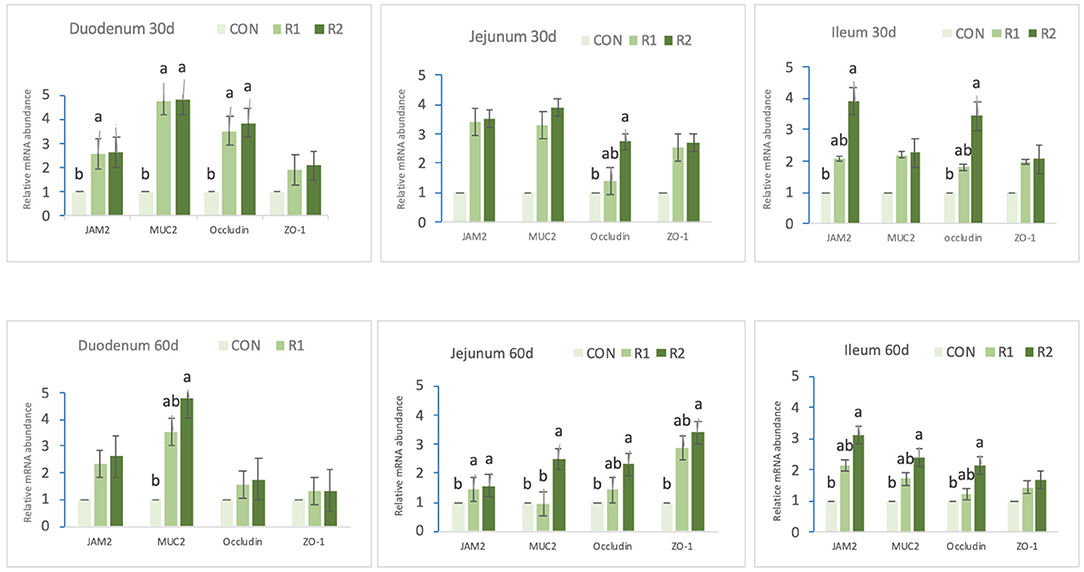
Figure 2. Effects of dietary catalase supply on tight junction protein mRNA expression in the intestinal mucosa of yellow broilers. Data are the mean ± SEM (n = 8). CON-broilers fed the basal diet, R1-broilers fed the basal diet supplemented with 150 U/kg CAT, R2-broilers fed the basal diet supplemented with 200 U/kg CATa,b,c within the same intestinal segment and the same protein, means with different superscript letters differ (p < 0.05).
Rapid growth, high lipid and protein feed, and high-density cage feeding are high-intensity stresses for broilers. These stresses (53) can lead to the production of a large number of free radicals, break the steady-state balance of free radical production and clearance in the body, and then induce oxidative stress in the digestive tract, damage the intestinal mucosa (54–56), and affect the enzyme activity. As a result, the digestion and absorption capacity of the digestive tract to feed decreased (57, 58), showing that the growth of broilers slowed down, and the feed:gain ratio increased (59, 60). CAT, as a very important enzyme in the antioxidant enzyme system, can quickly decompose H2O2 and eliminate the harm caused by H2O2. The active unpaired electrons in the outer orbit of free radicals can be transferred between different atoms or ions, so the free radicals can be transformed with each other in a chain reaction (61). At the same time, CAT, SOD, and GSH-Px have synergistic effects on free radical scavenging. Therefore, CAT can remove free radicals in the intestine by continuously decomposing H2O2, attenuate or eliminate intestinal oxidative stress, ameliorate the structure of intestinal mucosa, improve the digestion and absorption capacity of nutrients (62–66), promote the growth of broilers, and abate the F:G ratio. The results showed that the addition of 150 and 200 U of CAT in the diet could significantly enhance the body weight gain of yellow broilers in the early and the whole stages, and dramatically reduce the ratio of feed:gain in the early, the later, and the whole stages.
In the life process of animals, free radicals widely exist in the body. Low-dose free radicals can act as signal transduction molecules (67, 68), mediate biological defense (69, 70), regulate the expression of antioxidant enzymes in the body, and maintain the internal balance of redox (67, 71). However, a high concentration of free radicals will reduce the activity of antioxidant enzymes, do harm to the body, and threaten the growth, development, and health of animals (72). CAT, SOD, and GSH-Px together constitute the antioxidant enzyme system of the body, and they work together to clean up free radicals (73, 74) and maintain the balance of free radical production and elimination. The addition of CAT to the diet cannot only remove free radicals, reduce the concentration of free radicals, decrease the production of MDA in the body, and save the consumption of other antioxidant enzymes but also induce the expression of CAT, SOD, and GSH-Px in the body (67, 75), and improve the antioxidant capacity of the body. As expected, the results displayed that adding 150 and 200 U of CAT to the diet could significantly increase the T-AOC concentration, SOD, CAT, and GSH-Px activities in the liver, CAT and GSH-Px activities in serum and jejunal CAT activities in the early stage, and remarkably heighten the T-AOC concentration, SOD, CAT, and GSH-Px activities in the liver, T-AOC concentration, CAT and GSH-Px activities in the serum, and jejunal CAT activities in the later stage. Furthermore, the addition of 200 U of CAT significantly decreased the content of MDA in the serum and jejunum in the early stage.
Endogenous or exogenous stress (53) causes the production of excess free radicals in the intestine (76), which triggers oxidative stress in the digestive tract, leads to the apoptosis and abscission of intestinal mucosal cells (77), and the differentiation and maturation of crypt intestinal stem cells are destroyed. Therefore, after oxidative damage, intestinal mucosal villi atrophy and fall off, villus height decreases, and crypt depth increases (58). Brollegier et al. found that heat stress markedly reduced the height, volume, and surface area of jejunal villi in young hens. The addition of CAT in the diet can remove excessive free radicals in the intestine, dispel or weaken intestinal oxidative stress, return to the normal differentiation and maturation of intestinal stem cells, and repair the injured intestinal mucosa, improving the height of villi and debasing the depth of the recess.
The study results indicated that diet supplemented with 150 and 200 U of CAT can significantly increase the villus height and villus height:crypt depth ratio of the duodenum (in the early and the later stages), jejunum (in the early stage), and ilea (in the early stage), as well as significantly reduce (p < 0.05) crypt depth. The addition of 200 U of CAT prominently lowered crypt depth and dramatically raised the ratio of villus height to crypt depth of the ileum in the later stage.
The intestinal microorganisms are divided into mucosal microorganisms and intestinal microorganisms (78). The mucosal microorganisms mainly colonize the mucus layer of intestinal epithelial cells. Meanwhile, the intestinal microorganisms exist in the intestinal cavity. The intestinal microorganisms continuously plant in the intestinal epithelium, and the mucosal microorganisms continuously inoculate into the intestinal cavity. Under healthy conditions, this state maintains a dynamic balance (79, 80). When the intestinal tract is subjected to oxidative stress of free radicals, the balance is broken and changes the composition of intestinal microorganisms (81, 82). The influence of intestinal oxidative stress on intestinal microorganisms is mainly caused by the following reasons: ① Oxidative stress leads to intestinal mucosal damage, intestinal epithelial cell apoptosis, destroys the colonization basis of mucosal microorganisms, and affects the proliferation of microorganisms. ② After the intestinal mucosa is damaged, the permeability of the intestinal mucosa is improved, the barrier function of the intestinal mucosa is reduced, and the migration of microorganisms inside and outside the intestinal mucosa becomes easier, thus, affecting the composition of microorganisms. ③ After the intestinal mucosa is damaged, the osmotic pressure and permeability are increased (83), mucus release and electrolyte secretion occur (57), which changes the pH in the intestine and breaks the microenvironment for the growth of intestinal microorganisms. ④ The free radicals produced by oxidative stress directly attack the biofilm of intestinal microorganisms (84), causing harm to the normal life activities of microorganisms. There are great differences in the antioxidant enzyme systems of different intestinal microorganisms, so their tolerance to oxidative stress is different. After intestinal oxidative stress, the number of different microorganisms changes greatly. A number of studies have shown that dietary supplementation with essential oil, probiotics, acidifier, and iron oxide nanozyme can inhibit the growth of harmful bacteria, reduce their infection ability, increase the number of beneficial bacteria and consolidate their dominant position, and alleviate the effects of stress on intestinal microorganisms. The addition of CAT can eliminate free radicals in the intestine, alleviate oxidative stress, reduce the damage of intestinal mucosa, and regulate the composition of intestinal microorganisms (85–87).
In the current study, adding 150 U of CAT could significantly increase the cecal Clostridium cluster IV, Clostridium cluster XIVα, and Lactobacillus amounts. The addition of 200 U of CAT could prominently heighten the number of Firmicutes, Bacteroidetes in the jejunum and Firmicutes in the ceca, and markedly reduce the number of Escherichia coli in the ceca. On the other hand, dietary supplementation with 150 and 200 U of CAT could significantly enhance the quantity of Lactobacillus in the jejunum and total bacteria, Bacteroidetes in the ceca.
The tight junction is the main connection mode between intestinal epithelial cells (88), which is composed of the transmembrane protein and plaque protein, regulates the permeability of epithelial cells, and mediates material exchange and energy metabolism (89). Transmembrane proteins consist of occludin, claudin, and adhesion molecule (JAM), and plaque proteins are mainly formed of zonula (ZO) family proteins (90). Occludin forms the occludin–ZO complex on the outside of the cell membrane and plays its physiological role. Occludin and ZO-1 are the two most important tight junction proteins (91). Previous studies have shown that the decreased expression of claudin and ZO-1 in the intestinal tissue will increase intestinal permeability, and the intestinal barrier function could be compromised (92, 93). JAM2, as an important adhesion molecule, binds to tight junction proteins such as ZO-1 through a special domain (PDZ domain). Therefore, regulating the expression of JAM2 can promote and enlarge the generated quantity of tight junction proteins (94, 95) and enhance the barrier function of the intestinal mucosa. Mucus covering the surface of the intestinal epithelial cells is the macromolecular glycoprotein secreted by goblet cells, forming a mucus barrier and protecting intestinal mucosa (96, 97). MUR2 is an important component of mucin and a marker gene to judge the integrity of the intestinal mucus barrier (98). The debased expression of MUR2 affects and destroys the barrier function of mucus (99). Free radicals produced by oxidative and disease stress attack intestinal epithelial cells, resulting in DNA, protein, and lipid peroxidation (100–105), lessen the expression of occludin, ZO-1, JAM2, and MUR2 (106, 107), improve the permeability of intestinal epithelial cells, and weaken the barrier function (92, 93, 108, 109).
Assimakopoulos et al. (110) found that obstructive jaundice markedly downregulated the expression of intestinal occludin. Yang et al. (111) confirmed that obstructive jaundice significantly reduced the expression of occludin and ZO. Forder et al. (112) reported that the mRNA expression of MUR2 in the jejunum decreased remarkably after Eimeria and Clostridium perfringens double infection in broilers. When CAT was added to the diet to rapidly scavenge free radicals and weaken intestinal oxidative stress injury, the expression of a series of proteins such as occludin, ZO-1, JAM2, and MUR2 increased.
Results of this study indicated that the addition of 150 and 200 U of CAT to the diet could significantly upregulate the mRNA expression of JAM2, MUR2, and occludin in the duodenum of yellow broilers in the early stage and the mRNA expression of JAM2 in the jejunum in the later stage. Adding 200 U of CAT to the diet prominently increased the mRNA expression of occludin in the jejunum, and JAM2 and occludin in the ilea in the early stage, and significantly raised the expression of MUR2 in the duodenum, MUR2, occludin, ZO-1 in the jejunum and JAM2, MUR2, and occludin in the ilea in the later stage. In this experiment, we verified the influence of catalase on the gene transcription level of tight junction protein and mucin. As for the influence on their protein expression level, our team will continue to conduct in-depth research.
The addition of CAT in the diet can increase the body weight gain and reduce the feed:gain ratio of yellow broilers, improve the antioxidant capacity of the body and the activity of antioxidant enzymes in the body, increase the villus height and the ratio of villus height-to-crypt depth of the intestinal tract and reduce the crypt depth, enlarge the number of beneficial microorganisms in the intestine, and upregulate the mRNA expression levels of tight junction protein and mucin.
The results of this study and previous studies by our team showed that adding 200 U/kg of CAT to the diet of yellow broilers has the best effect.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Animal Welfare Committee of Hunan Agricultural University.
MT conceived and designed the study, performed the experiments, and wrote the original paper. MT, JX, and KY analyzed the sequencing data and experimental results. YL corrected the manuscript. RF obtained the project funding, guided the experiment, and revised the manuscript. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
We greatly appreciate the funding support of the National Key R&D Program of China (2018YFD0501403).
YL was employed by the company Shanghai Menon Biotechnology Co., LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (homocuprein). J Biol Chemistr. (1969) 244:6049–55. doi: 10.1016/S0021-9258(18)63504-5
2. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. (1997) 82:291–5. doi: 10.1113/expphysiol.1997.sp004024
3. Packer L, Carol C. The Antioxidant Miracle: Put Lipoic Acid, Pycnogenol, and Vitamins E and C to work for you. New York, NY: John Wiley and sons, Inc.(1999).
4. Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa inflammatory bowel disease. Dig Dis Sci. (1996) 41:2078–86.
5. Assimakopoulos SF, Scopa CD, Zervoudakis G, Mylonas PG, Georgiou C, Nikolopoulou V, et al. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann Surg. (2005) 241:159–67. doi: 10.1097/01.sla.0000149306.35717.8b
6. Quinteiro-Filho WM, Ribeiro A. Ferraz-de-Paula V, Pinheiro ML, Sakai M, Sa L. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Sci. (2010) 89:1905–14. doi: 10.3382/ps.2010-00812
7. Osselaere A, Santos R, Hautekiet V, De Backer P, Chiers K, Ducatelle R, et al. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE. (2013) 8:e69014. doi: 10.1371/journal.pone.0069014
8. Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, et al. Dietary nickel chloride induces oxidative intestinal damage in broiler. Int J Environ Res Public Health. (2013) 10:2109–19. doi: 10.3390/ijerph10062109
9. Li Y, Ma QG, Zhao LH, Guo YQ, Duan GX, Zhang JY, et al. Protective efficacy of alpha-lipoic acid against aflatoxinB1-induced oxidative damage in the liver. Asian-australas J Anim Sci. (2014) 27:907–15. doi: 10.5713/ajas.2013.13588
10. Sies H. Oxidative stress: from basic research to clinical application. Am J Med. (1991) 91:s31–8. doi: 10.1016/0002-9343(91)90281-2
11. Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. (2009) 89:27–71. doi: 10.1152/physrev.00014.2008
12. Fang YZ, Yang S, Wu GY. Free radicals, antioxidants, and nutrition. Nutrition. (2002) 18:872–79. doi: 10.1016/S0899-9007(02)00916-4
13. Wang LL, Chen L, Madsen J. Tackling oxidative stress in pigs. Anim Sci Abroad (pigs and poultry). (2014) 12:19–21. CNKI:SUN:GWXM.0.2014-12-007.
14. Sohal RS, Allen RG. Oxidative stress as a causal factor in differentiation and aging: A unifying hypothesis. Exp Gerontol. (1990) 25:499–522. doi: 10.1016/0531-5565(90)90017-V
15. Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochimica et Biophysica Acta: Molecular Cell Research. (2006) 1763:1755–66. doi: 10.1016/j.bbamcr.2006.09.006
16. Halliwell B. biochemistry of oxidative stress. Biochem Soc Trans. (2007) 35:1147–50. doi: 10.1042/BST0351147
17. Kovacic P, Jacintho JD. Mechanisms of carcinogenesis: Focus on oxidative stress and electron transfer. Curr Med Chem. (2001) 8:773–96. doi: 10.2174/0929867013373084
18. Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci U S A. (2005) 102:13147–52. doi: 10.1073/pnas.0502979102
19. De La Fuente M. Effects of antioxidants on immune system aging. Eur J Clin Nutr. (2002) 56:s5–8. doi: 10.1038/sj.ejcn.1601476
20. Gou Z, Jiang S, Zheng C, Tian Z, Lin X. Equol inhibits LPS-induced oxidative stress and enhances the immune response in chicken HDmacrophages. Cell Physiol Biochemistr. (2015) 36:611–21. doi: 10.1159/000430124
21. Xu H, Shao X, Zhang Z, Zou Y, Wu X, Yang L. Oxidative stress and immune related gene expression following exposure to di-n-butyl phthalate and diethyl phthalate in zebrafish embryos. Ecotoxicol Environ Saf. (2013) 93:39–44. doi: 10.1016/j.ecoenv.2013.03.038
22. Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. (1995) 19:227–50. doi: 10.1016/0891-5849(95)00017-R
23. Bhatnagar A. Biochemical mechanism of irreversible cell injury caused by free radical-initiated reactions. Mol Cell Biochem. (1994) 137:9–16. doi: 10.1007/BF00926034
24. Liu JF, Huang CJ. Dietary oxidized frying oil enhances tissue alpha-tocopherol depletion and radioisotope tracer excretion in vitamin E-deficient rats. J Nutri. (1996) 126:2227–35. doi: 10.1093/jn/126.9.2227
25. Kruidenier L, Verspaget HW. Review article oxidative stress as a pathogenic factor in inflammatory bowel disease-radicals or ridiculous? Aliment Pharmacol Ther. (2002) 16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x
26. Fubini B, Hubbard A. Reactive oxygen species(ROS) and reactive nitrogen species(RNS) generation by silica in inflammation and fibrosis. Free Rad Biol Med. (2003) 34:1507–16. doi: 10.1016/S0891-5849(03)00149-7
27. Poersch AB, Trombetta F, Braga AC, Boeira SP, Oliveira MS, Dilkin P, et al. Involvement of oxidative stress in subacute toxicity induced by fumonisin B1 in broiler chicks. Vet Microbiol. (2014) 174:180–5. doi: 10.1016/j.vetmic.2014.08.020
28. Deng Q, Xu J, Yu B, He J, Zhang K, Ding X, et al. Effect of dietary tea polyphenols on growth performance and cell-mediated immune response of post-weaning piglets under oxidative stress. Arch Anim Nutr. (2010) 64:12–21. doi: 10.1080/17450390903169138
29. Bai XM, Ma QG, Zhao LH Xi L, Ji C. Effects of alpha-lipoic acid supplementation on antioxidative ability and performance of sows and nursing piglets. J Anim Physiol Anim Nutr. (2012) 96:955–61. doi: 10.1111/j.1439-0396.2011.01205.x
30. Brenneisen P, Steinbrenner H, Sies H. Selenium, oxidative stress, and health aspects. Mol Aspects Med. (2005) 26:256–67. doi: 10.1016/j.mam.2005.07.004
31. Sarma AD, Sreelakshmi Y, Sharma R. Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry. (1997) 45:671–4. doi: 10.1016/S0031-9422(97)00057-5
32. Mates JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. (2000) 153:83–104. doi: 10.1016/S0300-483X(00)00306-1
33. Eid YZ, Ohtsuka A, Hayashi K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br Poult Sci. (2003) 44:127–32. doi: 10.1080/0007166031000085427
34. Stephensen CB, Marquis GS, Douglas SD, Wilson CM. Immune activation and oxidative damage in HIV-positive and HIV-negative adolescents. JAIDS J Acquir Immune Deficiency Syndromes. (2005) 38:180–90. doi: 10.1097/00126334-200502010-00009
35. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J Nutr. (2003) 133:3275–84. doi: 10.1093/jn/133.10.3275S
36. Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J Nutri Biochemistr. (2005) 16:577–86. doi: 10.1016/j.jnutbio.2005.05.013
37. Chen P, Ma QG Ji C, Zhang JY, Zhao LH, Zhang Y, et al. Dietary lipoic acid influences antioxidant capability and oxidative status of broilers. Int J Mol Sci. (2011) 12:8476–88. doi: 10.3390/ijms12128476
38. Machilin LJ, Bandito A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. Offic. Publicat. Federat. Am. Soc. Experiment. Biol. (1987) 1:441–5. doi: 10.1096/fasebj.1.6.3315807
39. Wu G, Flynn NE, Flynn SP, Jolly CA, Davis PK. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr. (1999) 129:1347–54. doi: 10.1093/jn/129.7.1347
40. Zhang HJ, Guo YM, Tian YD, Yuan JM. Dietary conjugated linoleic acid improves antioxidant capacity in broiler chicks. Br Poult Sci. (2008) 49:213–21. doi: 10.1080/00071660801989836
41. Ghosh B, Hanevold CD, Dobashi K, Orak JK, Singh I. Tissue differences in antioxidant enzyme gene expression in response to endotoxin. Free Rad Biol Med. (1996) 21:533–40. doi: 10.1016/0891-5849(96)00048-2
42. Abd-Allah AR, Helal GK, Al-Yahya AA, Aleisa AM, Al-Rejaie SS, Al-Bakheet SA. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxid Med Cell Longev. (2009) 2:73–81. doi: 10.4161/oxim.2.2.8177
43. National Research Council. Nutrient Requirements of Poultry: Ninth Revised Edition. Washington, DC: National Academies Press (1994). p. 16–78.
44. Gu Y, Song Y, Yin H, Lin S, Zhang X, Che L, et al. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal FGF19expression, and intestinal acetate fermentation. J Anim Sci. (2017) 95:226–38. doi: 10.2527/jas.2016.0911
45. Zoetendal EG, Akkermans ADL, de Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. (1998) 64:3854–9. doi: 10.1128/AEM.64.10.3854-3859.1998
46. Suzuki MT, Taylor LT. deLong EF. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl Environ Microbiol. (2000) 66:4605–14. doi: 10.1128/AEM.66.11.4605-4614.2000
47. Guo X, Xia X, Tang R, Wang K. Real-time PCR quantification of the predominant bacterial divisions in the distal gut of meishan and landrace pigs. Anacerobe. (2008) 14:224–8. doi: 10.1016/j.anaerobe.2008.04.001
48. Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16s rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. (2004) 70:7220–8. doi: 10.1128/AEM.70.12.7220-7228.2004
49. Khafipour E, Li S, Plaizier JC, Krause DO. Rumen microbiome composition determine using two nutritional models of subacute ruminal acidosis. Appl Environ Microbiol. (2009) 7:7115–24. doi: 10.1128/AEM.00739-09
50. Huijsdens XW, Linskens RK, Mak M, Meuwissen SG, Vandenbroucke-Grauls CM, Savelkoul PH. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. J Clin Microbiol. (2002) 40:4423–7. doi: 10.1128/JCM.40.12.4423-4427.2002
51. Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Prot. (2017) 19:397–405. doi: 10.1007/s12602-017-9275-9
52. De Boever S, Vangestel C, De Backer P, Croubels S, Sys SU. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chichens. Vet Immunol Immunopathol. (2008) 122:312–7. doi: 10.1016/j.vetimm.2007.12.002
53. Zhu LH, Zhao KL, Chen XL, Xu JX. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. (2012) 90:2581–9. doi: 10.2527/jas.2011-4444
54. Bagchi M, Williams CB, Milnes M, Balmoori J, Ye X, Bagchi D, et al. Acute and chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract. Nutri Res. (1999) 19:1189–99. doi: 10.1016/S0271-5317(99)00080-9
55. Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res. (2000) 33:819–30. doi: 10.1080/10715760000301341
56. Nieto N, Torres MI, Fernandez MI, Giron MD, Rios A, Suarez MD, et al. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. (2000) 45:1820–7. doi: 10.1023/A:1005565708038
57. Boros M, Takaichi S, Hatanaka K. Ischemic time-dependent microvascular changes and reperfusion injury in the rat small intestine. J Surg Res. (1995) 59:311–20. doi: 10.1006/jsre.1995.1170
58. Hu XF, Guo YM. Corticosterone administration alters small intestinal morphology and function of broiler chickens. Asian-australas J Anim Sci. (2008) 21:1773–8. CNKI:SUN:DWYX.0.2008-04-011. doi: 10.5713/ajas.2008.80167
59. Ansaldo M, Najle R, Luquet CM. Oxidative stress generated by diesel seawater contamination in the digestive gland of the antarctic limpet nacella concinna. Mar Environ Res. (2005) 59:381–90. doi: 10.1016/j.marenvres.2004.06.003
60. Lukkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. The Veterinary J. (2007) 173:502–11. doi: 10.1016/j.tvjl.2006.06.005
61. Kaufmann JA, Bickford PC, Taglialatela G. Free radical-dependent changes in constitutive Nuclear factor kappa B in the aged hippocampus. Neuroreport. (2002) 13:1917–28. doi: 10.1097/00001756-200210280-00017
62. Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. (1992) 55:299–308. doi: 10.1093/ajcn/55.1.299s
63. Ferraris RP, Diamond JM. Regulation of intestinal sugar transport. Physiol Rev. (1997) 77:257–302. doi: 10.1152/physrev.1997.77.1.257
64. BrÖer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. (2008) 88:249–86. doi: 10.1152/physrev.00018.2006
65. Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. (2002) 82:245–89. doi: 10.1152/physrev.00026.2001
66. Langhout DJ, Schutte JB, Van Leeuwen P, Wiebenga J, Tamminga S. Effect of dietary high-and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br Poult Sci. (1999) 40:340–7. doi: 10.1080/00071669987421
67. Bedard K, Kranze KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. (2007) 87:245. doi: 10.1152/physrev.00044.2005
68. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, et al. NF-kappa B inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK and necrotic cell death. The EMBO J. (2003) 22:3898–909. doi: 10.1093/emboj/cdg379
69. Benzie IFF. Evolution of antioxidant defense mechanisms. Eur J Nutr. (2000) 39:55–61. doi: 10.1007/s003940070030
70. Halliwell B. Antioxidant defense mechanisms: from the beginning to the end (of the beginning). Free Rad Res. (1999) 31:261–72. doi: 10.1080/10715769900300841
71. Finkel T, Hlobrook NJ. Oxidants, oxidative stress and the biology of aging. Nature. (2000) 408:239–47. doi: 10.1038/35041687
72. Lin H, Decuypere E, Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comparative Biochemistr Physiol Part A. (2006) 144:11–7. doi: 10.1016/j.cbpa.2006.01.032
73. Harris ED. Regulation of antioxidant enzymes. The FASEB J. (1992) 6:2675–83. doi: 10.1096/fasebj.6.9.1612291
74. MatÉs JÉM, Pérez-Gómez C, De Catro IN. Antioxidant enzymes and human diseases. Clin Biochem. (1999) 32:595–603. doi: 10.1016/S0009-9120(99)00075-2
75. Mikhailov VE, Mazurik VK, Burlakova EB. Signal function of the reactive oxygen species in regulatory networks of the cell reaction to damaging effects: contribution of radiosensitivity and genome instability. Radiatsionnaia Biologiia, Radioecologiia/Rossiiskaia Akadermiia Nauk. (2003) 43:5–18.
76. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clinic Obstetr Gynaecol. (2011) 25:287–99. doi: 10.1016/j.bpobgyn.2010.10.016
77. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. (2000) 5:415–8. doi: 10.1023/A:1009616228304
78. Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. (2002) 68:3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002
79. Ahmed S, Macfarlane GT, Fite A, McBain AJ, Gilbert P, Macfarlane S. Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl Environ Microbiol. (2007) 73:7435–42. doi: 10.1128/AEM.01143-07
80. Lepage P, Seksik P, Sutren M, de la Cochetiere MF, Jian R, Marteau P, et al. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD inflammatory. Bowel Dis. (2005) 11:473–80. doi: 10.1097/01.MIB.0000159662.62651.06
81. Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. (2007) 87:545–64. doi: 10.1152/physrev.00012.2006
82. Podolsky DKV. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol Gastrointestinal Liver Physiol. (1999) 277:G495–9. doi: 10.1152/ajpgi.1999.277.3.G495
83. Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. (2006) 55:1512–20. doi: 10.1136/gut.2005.085373
84. Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. (1979) 59:527–605. doi: 10.1152/physrev.1979.59.3.527
85. Zhang S, Shen YR, Wu S, Xiao YQ, He Q. Shi SR. The dietary combination of essential oils and organic acids reduces salmonella enteritidis in challenged chick. Poultry Sci. (2019) 98:6349–55. doi: 10.3382/ps/pez457
86. Zhang S, Zhong G, Shao D, Wang Q, Hu Y, Wu T, et al. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. (2021) 100:100935. doi: 10.1016/j.psj.2020.12.032
87. Shi S, Wu S, Shen Y, Zhang S, Xiao Y, He X, et al. Iron oxide nanozyme suppresses intracelluar salmonella enteritidis growth and alleviates infection in vivo. Theranostics. (2018) 8:6149–62. doi: 10.7150/thno.29303
88. Reynolds JV, O'farrelly C, Feighery C, Murchan P, Leonard N, Fulton G, et al. Impaired gut barrier function in malnourished patients. Br J Surg. (1996) 83:1288–91. doi: 10.1046/j.1365-2168.1996.02330.x
89. Anderson JM, Van Itallie CM. Tight junctions and molecular basis for regulation of paracellular permeability. Am J Physiol. (1995) 269:467–75. doi: 10.1152/ajpgi.1995.269.4.G467
90. Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. (2002) 51:507–13. doi: 10.1136/gut.51.4.507
91. Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. (1994) 127:1617–26. doi: 10.1083/jcb.127.6.1617
92. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. (1998) 273:29745–53. doi: 10.1074/jbc.273.45.29745
93. Anderson JM. Molecular structure of tight junctions and their role in epithelial transport. News in Physiol Sci. (2001) 16:126–30. doi: 10.1152/physiologyonline.2001.16.3.126
94. Zhao H, Yu H, Martin TA, Zhang Y, Chen G, Jiang WG. Effect of junction adhesion molecule-2 expression on cell growth, invasion and migration in human colorectal cancer. Int J Oncol. (2016) 48:929–36. doi: 10.3892/ijo.2016.3340
95. Soendergaard C, Young JA, Kopchick JJ. Growth hormone resistance-special focus on inflammatory bowel disease. Int J Mol Sci. (2017) 18:1110–9. doi: 10.3390/ijms18051019
96. Cornick S, Kumar M, Moreau F, Gaisano H, Chadee K. VAMP8-mediated MUR2 mucin exocytosis from colonic goblet cells maintains innate intestinal homeostasis. Nat Commun. (2019) 10:e982426–330. doi: 10.1038/s41467-019-11811-8
97. Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. (2002) 5:685–94. doi: 10.1097/00075197-200211000-00012
98. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two MUR2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. (2008) 105:15064–9. doi: 10.1073/pnas.0803124105
99. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. MUR2-deficient mice spontaneously develop colitis, indicating that MUR2 is critical for colonic protection. Gastroenterology. (2006) 131:117–29. doi: 10.1053/j.gastro.2006.04.020
100. Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Nat Acad Sci. (1997) 94:514–9. doi: 10.1073/pnas.94.2.514
101. Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Internat Microbiol. (2010) 3:3–8. doi: 10.0000/PMID10963327
102. Çakatay U, Telci A, Kayali R, Tekeli F, Akçay T, Sivas A. Relation of aging with oxidative protein damage parameters in the rat skeletal muscle. Clin Biochem. (2003) 36:51–6. doi: 10.1016/S0009-9120(02)00407-1
103. Jana CK, Das N, Sohal RS. Specificity of age-related carbonylation of plasma proteins in the mouse and rat. Arch Biochem Biophys. (2002) 397:433–9. doi: 10.1006/abbi.2001.2690
104. Chatgilialoglu C, O'Neill P. Free radicals associated with DNA damage. Experimental Gerontology. (2001) 36:1459–1471 doi: 10.1016/S0531-5565(01)00132-2
105. Wickens AP. Aging and the free radical theory. Respir Physiol. (2001) 128:379–91. doi: 10.1016/S0034-5687(01)00313-9
106. Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. (2005) 393:69–77. doi: 10.1042/BJ20050959
107. Brownlee IA, Knight J, Dettmar PW, Pearson JP. Action of reactive oxygen species on colonic mucus secretions. Free Rad Biol Med. (2007) 43:800–88. doi: 10.1016/j.freeradbiomed.2007.05.023
108. Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circulat Physiol. (2001) 280:H509–21. doi: 10.1152/ajpheart.2001.280.2.H509
109. Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. (2010) 6:392. doi: 10.1038/msb.2010.46
110. Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, et al. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. (2004) 198:748–57. doi: 10.1016/j.jamcollsurg.2003.12.017
111. Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, et al. Bile modulates intestinal epithelial barrier function via an extracellular related kinase 1/2 dependent mechanism. Intensive Care Med. (2005) 31:709–17. doi: 10.1007/s00134-005-2601-9
Keywords: catalase, yellow broilers, growth performance, intestinal morphology, antioxidant capacity, microbial composition, junction protein
Citation: Tang M, Fang R, Xue J, Yang K and Lu Y (2022) Effects of Catalase on Growth Performance, Antioxidant Capacity, Intestinal Morphology, and Microbial Composition in Yellow Broilers. Front. Vet. Sci. 9:802051. doi: 10.3389/fvets.2022.802051
Received: 26 October 2021; Accepted: 04 January 2022;
Published: 24 March 2022.
Edited by:
Shourong Shi, Poultry Institute (CAAS), ChinaReviewed by:
Kun Li, Nanjing Agricultural University, ChinaCopyright © 2022 Tang, Fang, Xue, Yang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rejun Fang, ZmFuZ3JqNjNAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.