
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 11 January 2023
Sec. Veterinary Neurology and Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1107315
This article is part of the Research Topic Managing Dog and Owner Health in Canine Epilepsy View all 5 articles
 Maud F. N. Hamers1
Maud F. N. Hamers1 Marta Plonek2
Marta Plonek2 Sofie F. M. Bhatti3
Sofie F. M. Bhatti3 Niklas Bergknut4
Niklas Bergknut4 M. Montserrat Diaz Espineira1
M. Montserrat Diaz Espineira1 Koen M. Santifort2
Koen M. Santifort2 Paul J. J. Mandigers1,2*
Paul J. J. Mandigers1,2*Epilepsy in dogs is a common chronic and serious disorder and may have an impact on the quality of life of the owners as well as the dogs themselves. The aim of this pilot study was to investigate the QoL score of dogs suffering from idiopathic epilepsy and their owners and if possible, investigate whether a breed specific difference exists. Owners, either Dutch or Belgium, were asked to participate in a web based SurveyMonkey questionnaire. A total of 402 questionnaires representing 402 dogs with epilepsy were suitable for further analysis. Of the 402 dogs, 253 were males and 149 were females. Ninety-nine different breeds were represented. Fourteen breeds (177 dogs in total) were used to calculate breed specific scores; Australian Shepherd (n = 8), Beagle (n = 7), Belgian Tervuren dog (n = 9), Belgian Groenendaeler dog (n = 8), Border Collie (n = 38), Chihuahua (n = 9), Dachshund (n = 13), Drentsche Patrijshond (a Dutch partridge dog) (n = 14), French Bulldog (n = 12), Golden Retriever (n = 17), Labrador Retriever (n = 18), and Rottweiler (n = 12). For the Border Collie, there was a statistically significant correlation between “epilepsy related death,” the severity of the seizures (p < 0.001) and cluster seizures (p < 0.001). The quality of life of the Border Collie was scored lower compared to all other dogs (p = 0.02). There were three breeds that had a minimal decrease in the overall quality of life score compared to all other dogs: the Chihuahua (p = 0.03), Dachshund (p = 0.001), and Golden retriever (p = 0.01). The score for “caring for my epileptic dog decreases my own QoL” was high for the Border Collie, Boxer, French Bulldog, and Rottweiler, but was only found to be statistically significantly higher in the Border Collie (p = 0.01). Scores for the Golden Retriever (p = 0.04) and Labrador (p = 0.006) were lower. In conclusion, this study reports breed specific quality of life scores of dogs with epilepsy and their owners, and underlines that breed by itself, is also an important factor when managing epilepsy in dogs.
Epilepsy is the most common chronic canine neurological syndrome and has serious implication on the quality of life (QoL) of both the dogs and owners (1–4). Determining the etiology of canine epilepsy is important and has been the focus of the International Veterinary Epilepsy Taskforce (IVETF), leading to the creation of manuscripts addressing its classification, terminology, and diagnostics (5, 6).
The prevalence of canine epilepsy is estimated to range from 0.6 to 0.75 percent (7, 8), the majority of which is idiopathic, genetic or presumed genetic (9). There is a clear breed predisposition in hunting dogs, shepherds and molosser dogs (9, 10), and several recent studies focussed on the genetics of epilepsy (11–16). But there is also growing interest into the impact of epilepsy and the use of anti-seizure medication (ASM) on owners and their dogs. The goal of ASM is to reduce the number and severity of the seizures. However, the efficacy of various registered ASM's varies and ranges from 70 to 80% with phenobarbital (17, 18), 60% with bromide (17), and just 50% with imepitoin (19–21). Hence, up to 20–30% of all epileptic dogs do not respond favorably to registered ASM's and require multi-drug treatment with unlicensed ASM's such levetiracetam (22, 23), gabapentin (24) or zonisamide (25). These combined therapies introduce considerable side-effects (18, 26).
Hence, several studies have been published investigating the effect of both epilepsy and ASM treatment on the QoL of dogs and their owner (1, 4, 26, 27). Dogs may show behavioral and cognitive changes such as fear/anxiety, aggression, abnormal perception, inattention, and excitability (3, 28, 29). Not only will this have a negative effect on the QoL of the dog but also of the owner (3, 28).
But not all breeds respond equally to ASM. For instance, the outcome in BCs differs clearly from the general population (30). Up to 50% of all young male BCs are euthanized before the age of two as they do not respond to the ASM's (30, 31). Similar observations have been noted for Australian Shepherds (32), Italian Spinone (33), and Rottweiler (34). When there is a breed specific response to ASM's, there is usually also a breed specific QoL score for both dog and owner.
The aim of this pilot study was to investigate the QoL score of dogs suffering from idiopathic epilepsy and their owners and if possible, investigate whether a breed specific difference exists.
Owners, either from the Netherlands or Belgium, were asked to participate in a web based SurveyMonkey questionnaire. Owners were approached using social media, direct e-mailing, adverts on canine epilepsy fora and indirectly by spreading the questionnaire with the help of veterinarians in the Netherlands and of Belgium.
The questionnaire consisted of forty questions, based on earlier questionnaires used to evaluate owner perception of dogs with epilepsy in Ohio and Glasgow (27, 35) and the proposed Quality of Life assessment of Wessmann et al. (2). The questions focussed on general aspects of the dog—including breed, age and gender, the number and type of seizures (focal, generalized or other), effect of therapeutic modalities, side effects, the QoL of the dog, and the QoL of the owner. All questions were accompanied with an explanation of the used medical terms, a website page was available with an explanation in Dutch and examples of a tonic-clonic seizure and focal seizures were made available on YouTube. An example of the questionnaire (translated from Dutch into English) can be found in Appendix 1. The questionnaire contained both open and closed questions and some questions allowed multiple answers. The questions addressing the QoL were scored on a scale of 1 to 10. Two groups of questions were formulated. One group addressed the QoL of the dog, the second group the QoL of the owner.
Questions addressing the QoL of the dog:
• Score the severity of the tonic-clonic (large) seizures on the QoL of your dog (1 = not, 10 = severe)
• Score the severity of the focal (small) seizures on the QoL of your dog (1 = not, 10 is severe)
• Score whether the side effects of ASM are acceptable (1 = no side effects, 10 = severe side-effects)
• Score the decrease in QoL now compared to the moment the dog was still free of seizures (0 = 100% decrease, 100 = no decrease)
• Score the quality of life of your dog (1 = zero QoL, 10 = optimal QoL)
Questions addressing the QoL of the owner:
• The severity of seizures is acceptable to me (1 = not acceptable, 10 = no problem at all)
• I dare to leave my dog alone at home for a while (disagree = 1, strongly agree = 10)
• Caring for a dog with epilepsy is worth the effort (1 = it is not worth the effort, 10 = worth)
• I find it difficult to administer ASM to my dog (1 = no, 10 = yes, very much)
• The costs of the treatment are acceptable (1 = no, 10 = yes, very much)
• Regular visits to the consulting vet/specialist make life difficult (1 = not at all, 10 = yes, extremely)
• In the past 3 months I worried about the frequency of seizures in my dog (1 = not at all, 10 = yes, extremely
• Caring for a dog with epilepsy limits my daily activities and causes a decrease of my own quality of life (1 = no not at all, 10 = yes, extremely)
All completed questionnaires were collected with the online survey development software SurveyMonkey within a timeframe of 2 months. Questionnaires were only analyzed if the dog had at least more than two, idiopathic epileptic seizures as described earlier (5) and the questionnaire had been completed. Owners were advised to contact us if there were questions and, on a voluntary base, could sent us the medical record of their dog. Results were analyzed with version 28 of the IBM SPSS Software and included descriptive statistics, chi-squared tests, t-tests and Pearson's correlation tests. Means ± standard deviations were calculated for all dogs and compared with the results of specific breeds. Each breed was compared with the entire group of dogs minus the breed to be tested. Breed scores were only calculated if at least six dogs were of the same breed. Results were considered statistically significant if the p-value was < 0.05.
A total of 622 owners (partly or completely) filled in the questionnaire. Based on the selection criteria, 402 questionnaires were suitable for further analysis.
Of the 402 dogs, 253 were males and 149 were females. Males were overrepresented with statistical significance (p = 0.001). Owners of 99 different breeds participated. Fourteen breeds (177 dogs in total) could be used to calculate breed specific scores; Australian Shepherd (n = 8), Beagle (n = 7), Belgian Tervuren (n = 9), Belgian Groenendaeler dog (n = 8), Border Collie (n = 38), Chihuahua (n = 9), Dachshund (n = 13), Drentsche Patrijshond (a Dutch partridge dog) (n = 14), French Bulldog (n = 12), Golden Retriever (n = 17), Labrador Retriever (n = 18), and Rottweiler (n = 12). The remaining 225 dogs were either listed as mixed breeds (n = 42) or belonged to various other breeds (183 dogs). A complete list of breeds is available in Appendix 2.
Based on the owners' submissions, 305 of 402 dogs showed mainly generalized tonic-clonic seizures and 97 showed only focal seizures. No association was found between seizure type and breed (p = 0.867). On average, for the complete group of 402 dogs, the age of onset was 38 ± 30 months. Three breeds, the Border collie (p = 0.003), French Bulldog (p = 0.018), and Boxer (p = 0.04), showed a statistically significant lower age of seizure onset compared to all other dogs (Table 1 and Figure 1). Compared to all other dogs, the Drentsche Patrijshond showed a statistically significantly higher age of seizure onset (p = 0.04). The number of seizures for all dogs was 24 ± 163 over a period of 12 months. Only the Drentsche Patrijshond showed a statistically significant higher number of clusters during a period of 12 months compared to all other dogs (p = 0.002) (Table 1). There was no statistically significant difference for the number of status epilepticus events, visits made to the veterinarian or specialist, or number of needed nightly visits, except for the Labrador retriever and Golden retriever. Owners of these two breeds reported statistically significant fewer nightly visits compared to all other owners (p < 0.001) (Table 1).
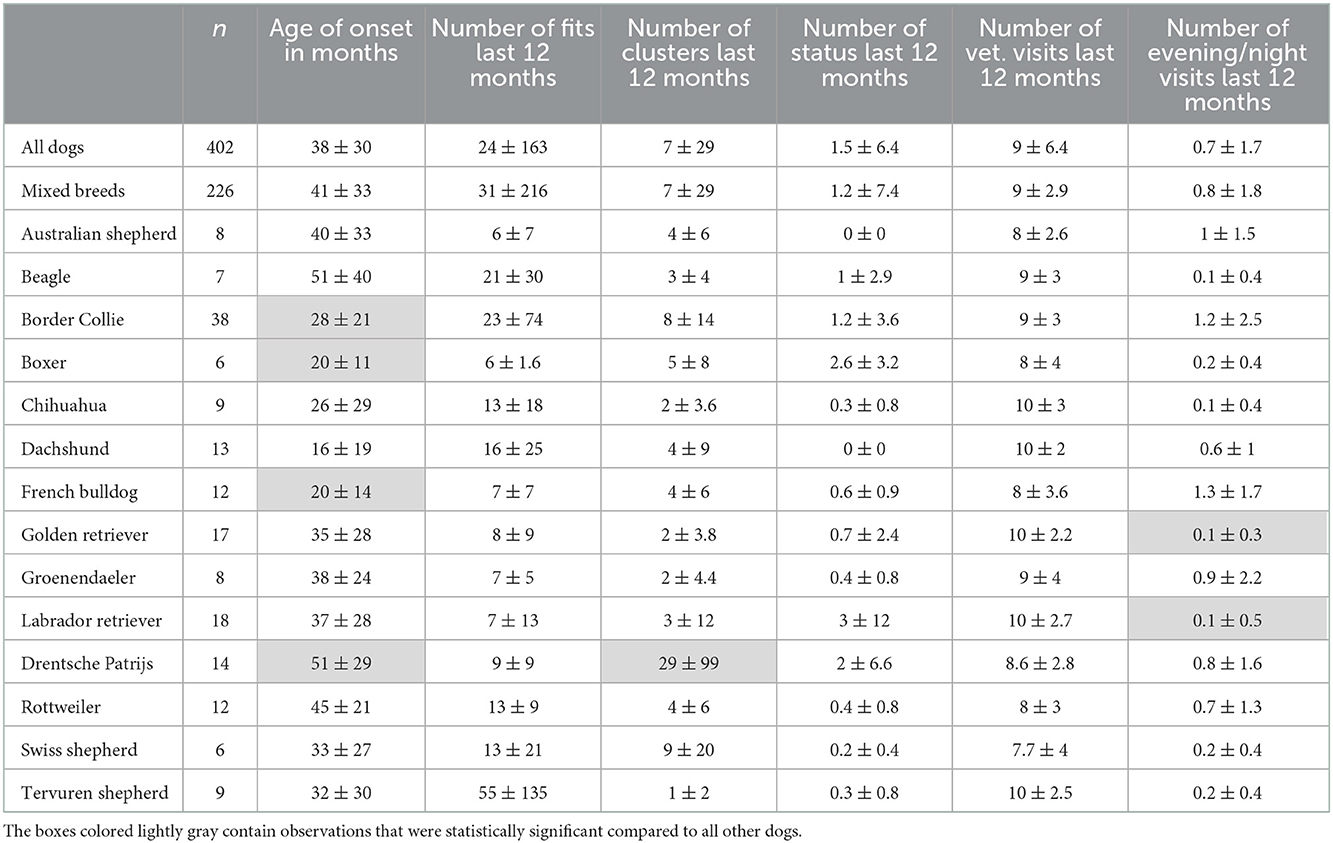
Table 1. Descriptive statistics (number, mean ± standard deviation) for all dogs and the 14 selected breeds.
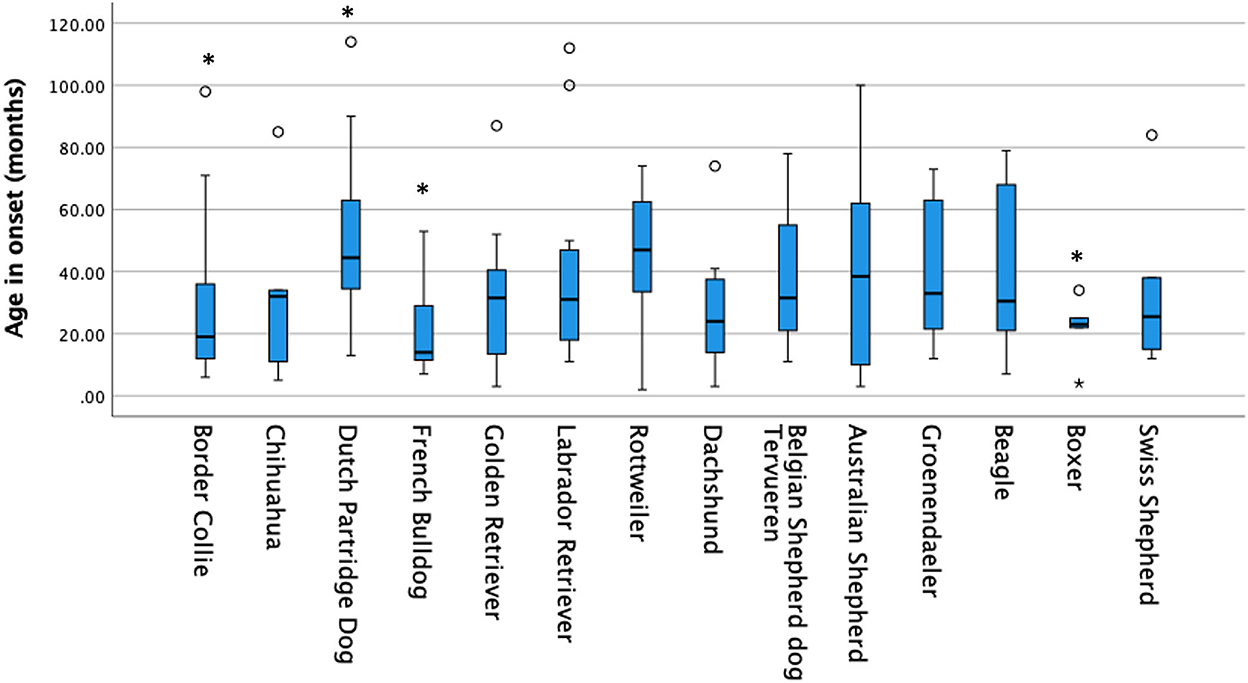
Figure 1. Age in onset for all breeds. The age of onset was for the Border collie, French Bulldog and Boxer significantly lower compared to all other breeds and for the Dutch Partridge Dog higher.
At the time of the survey, 308 dogs were alive, and 94 dogs were dead. The mean age ± SD of the dogs that were alive (308 dogs) was 6.6 ± 3.2 years. The mean age ± SD at death (94 dogs) was 5.6 ± 4.0 years. Epilepsy-related issues were the cause of death in 71% of the dogs, hence in 29% of the dogs it was not. In the group of Border collies, there was a clear statistically significant association of seizures and death compared to the other breeds (p = 0.047; Figure 2). Only in the Border Collie was there a clear, statistically significant correlation of “death,” the severity of the seizures (p < 0.001) and clustering (p < 0.001). For all other breeds, no statistically significant differences were found.
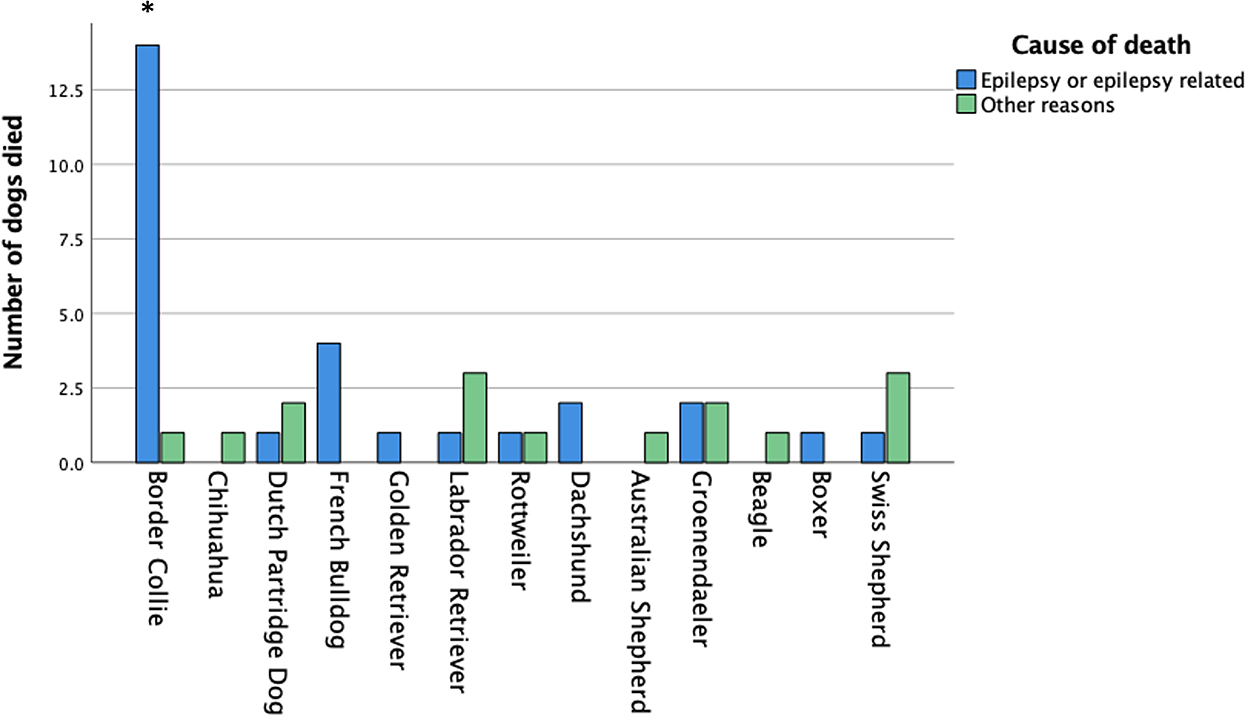
Figure 2. Cause of death for the dogs that had died. Only for the Border collie the ‘epilepsy related death' was statistically significant higher compared to all other dogs.
Several ASM strategies were used, either as monotherapy or combination-therapy. Two hundred and eighteen of the 402 dogs were treated with phenobarbital: either in monotherapy (n = 92) or in combination with other ASM (n = 126). Imepitoin was administered to 85 of the 402 dogs: either in monotherapy (n = 39) or in combination with other ASM (n = 46). Potassium bromide was used in 135 of 402 dogs: either in monotherapy (n = 22) or in combination with other ASM (n = 113). Levetiracetam (n = 22), gabapentin (n = 5) and slow-release phenytoin (n = 5) were used less frequently and only in a combination-therapy. Diazepam applied rectally during a seizure event, was used in 78 of 402 dogs. Some owners only used diazepam to stop the seizure and did not give other ASM (n = 12). ASMs were not used at all in 60 dogs.
The most used combination-therapies were phenobarbital and potassium bromide (n = 77), phenobarbital and imepitoin (n = 22), imepitoin and bromide (n = 9) and phenobarbital, imepitoin and bromide (n = 11). Alternative treatment options were employed in 64 out of 402 cases; 12 dogs were fed special diets; 22 dogs were administered cannabidiol (CBD) oil and 8 CBD/ tetrahydrocannabinol (THC) oil. Other alternative treatments were phytotherapy (n = 3) and music therapy (n = 1).
Up to 90% of all Border collies (BC) received at least one ASM and up to 47% received at least two different ASM's. Forty-five percent of all Labrador Retrievers and 50% of all Belgian Groenendaeler dogs did not use any ASM. The majority of the Drentsche Patrijshond, the Dachshund and Belgian Tervuren dogs used only one ASM. Bromide was most frequently used in the BC and Australian Shepherd. Although minimal, this difference was not statistically significant (p = 0.054).
The most reported side effects were lethargy (n = 123), sleepiness (n = 117), an increased appetite (n = 140) and polydipsia (n = 125). The use of monotherapy potassium bromide in dogs had the most side effects. Breed specific scores are discussed in the Questions addressing the QoL of the dog.
Five questions addressed the QoL of the dog. Interestingly, there was no statistically significant difference in the severity of the TC seizures for any of the 14 breeds compared to all other dogs minus the tested breed (Table 2). There was a statistically significant lower focal seizure severity in the Belgian Groenendaeler dog compared to the other breeds (p = 0.04). The owners of the Dachshund (p = 0.0003) and Golden retriever (p = 0.001) assessed the ASM side effects as very mild compared to all other dogs. ASM side effects were considered more severe compared to all other dogs in the Australian shepherd (p = 0.01) and Rottweiler (p = 0.007).
Owners were also asked to score the decrease in QoL compared to the moment the dog was still seizure free and to score the overall QoL. All dogs had a decrease in QoL after seizure onset, but the Dachshund (p = 0.001), Golden retriever (0.06) and Labrador retriever (p = 0.006), had the lowest decrease in QoL (Table 2). The Border Collie and Swiss Shepherd had the highest decrease in QoL (average of 40%) compared to all other dogs (average of 34%) but this was not found to be statistically significant. The overall QoL score for all dogs was 7.2 ±2.7. Although the Border Collie, Australian Shepherd and Belgian Groenendaeler dog had an average QoL score of < 6.5, only in the Border Collie was it statistically significantly lower compared to all other dogs (p = 0.02). There were three breeds that had a minimal decrease in the overall QoL score compared to all other dogs: the Chihuahua (p = 0.03), Dachshund (p = 0.001) and Golden retriever (p = 0.01) (Table 2).
Eight questions addressed the impact of the dog's epilepsy on the owner. Overall scores were calculated for all dogs (Table 3). Up to half of all BC owners, compared to all other owners, found it difficult to leave their dog alone at home for a brief period (p = 0.028), while Dachshund owners were very confident to leave their dog at home (p = 0.005, Table 3). Australian Shepherd owners scored lower compared to other owners on the question asking whether taking care of their dog was worth the effort (p = 0.009). Interestingly, Dachshund (p < 0.001), Chihuahua (p < 0.001), and Rottweiler owners (p = 0.007) scored very high: they found that taking care of their dog was very much worth the effort. The overall score for that question, for all owners, was 8.9 ± 2.2 (Table 3). The owners of Dachshunds found the costs of treatment significantly less burdening compared to all other owners (p = 0.049). The overall score was 6.4 ± 3. There was no breed specific statistically significant difference concerning the question addressing whether visits to the veterinarian/specialist or ASM administration were troublesome. Veterinary visits were less of an issue for Belgian Groenendaeler dog owners, compared with the other owners (p = 0.004). The owners of the Dachshund (p = 0.013) and Golden Retriever (p = 0.03) reported a lower severity of the seizures compared to all other owners. There was no breed specific difference for “worried during the last 3 months.”
The score for “caring for my epileptic dog decreases my own QoL” was high for the Border Collie, Boxer, French Bulldog and Rottweiler was found to be statistically significantly lower compared to all other breeds only in the Border Collie (p = 0.01). A positive difference was noted for the Golden Retriever (p = 0.04) and Labrador (p = 0.006) (Table 3).
The assessment of QoL in epileptic dogs and their owners has been previously studied in various countries (1, 3, 4, 26–28, 36) but limited information is available for breed specific QoL scores. To date, two breed specific studies addressing QoL score have been published. One was performed in 2016 in the Italian Spinone (33) and a second one recently in the Border Collie (30). Furthermore, breed comparisons for QoL of dogs and owners are, to the authors knowledge, limited. The aim of this pilot study was to investigate breed specific QoL scores for dogs suffering from idiopathic epilepsy and their owners. During a period of 2 months, 402 questionnaires were obtained from the owners of several dog breeds. In total, 14 breeds were overrepresented, which enabled a comparison of breed specific QoL scores of both owners and their epileptic dogs. Several interesting breed specific observations were noted. Compared with all breeds/dogs in this pilot study, the breed with the poorest statistically significant outcome appeared to be the Border Collie. This breed suffers not only from early onset seizuring (Figure 1), a low survival rate due to the severity of the seizures and the development of cluster seizures (Figures 2, 3), and up to 90% of all dogs required ASM and no < 47% was on a combination-therapy of ASMs. Sadly enough, this breed also had the lowest, statistically significant, QoL for both dog (Table 2) and owner (Table 3). Similar observations were noted in 2010 by Hulsmeyer et al. and just recently confirmed by our group (31). Wessmann et al. (1) reported that QoL was predominantly influenced by seizure frequency rather than the severity or clustering. Although not statistically significant, this was also the observation in this study. The Belgian Tervuren dog had the highest number of seizures (Table 1) compared to all other breeds/dogs, although this was not coupled with a high owner severity score (Table 3). In this study, the Border collie owners scored the severity of the seizures as high (Table 2).
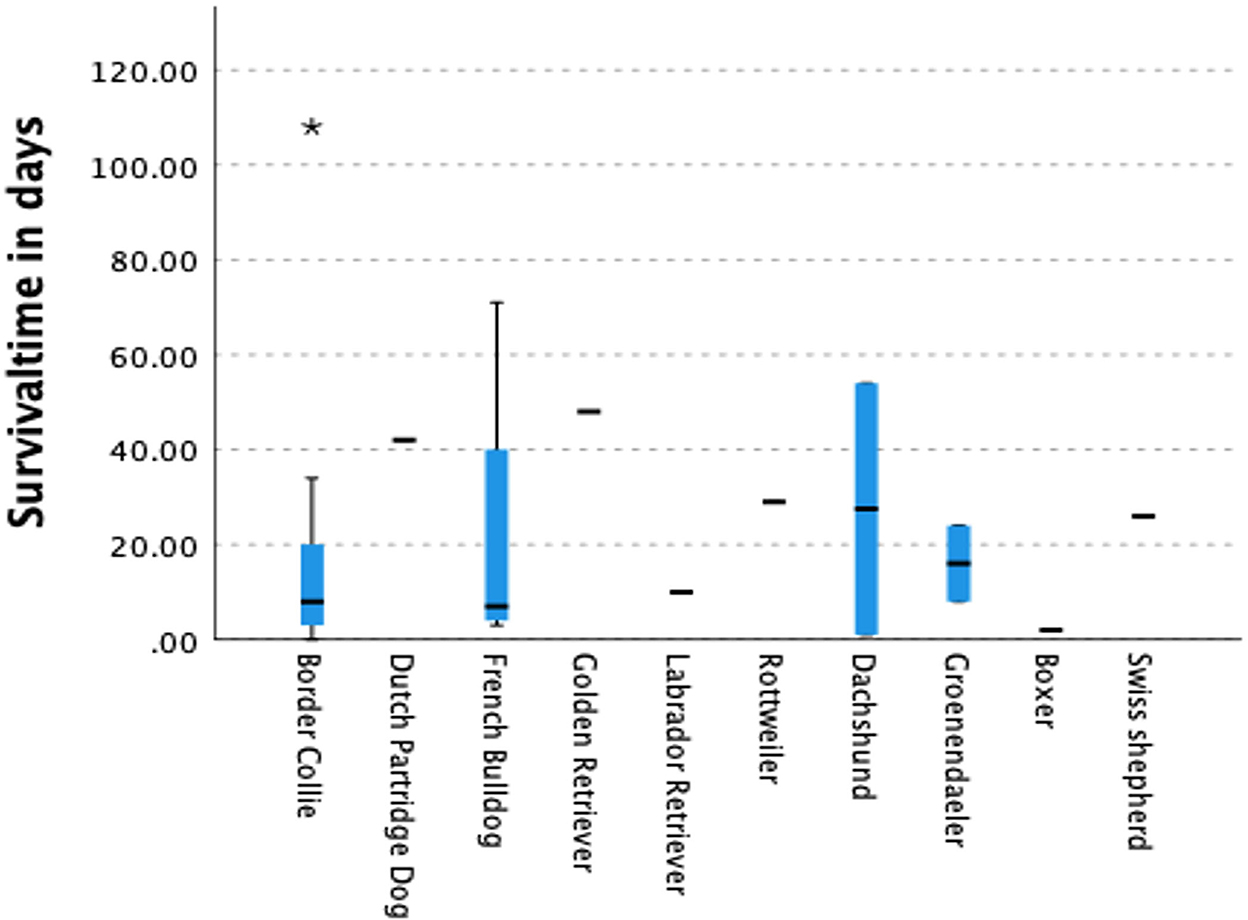
Figure 3. Survival time in days after the onset of the seizures. Only for the Border collie the survival time was statistically significant lower compared to all other dogs. It was also low for the French Bulldog and Boxer although not statistically significant.
An analogous poor outcome, like that of the Border Collie, was expected for the Australian Shepherd. An earlier study describing the characteristics of idiopathic epilepsy in this breed (32) was comparable with a similar study in the Border Collie (31). Although the owners of the Australian Shepherds score their dogs' and their own QoL low, it was not statistically significant compared with the other breeds. Surprisingly, the survival outcome in the Australian Shepherd was clearly different to that of the Border Collie (Figure 2). The only clear difference between these two breeds was, in this study, the age of onset which was in the Border collie much lower. But this was in two other breeds also statistically significant: the French Bulldog and the Boxer. The survival time in both breeds was also low, although this difference was not statistically significant. As far as the Border Collie is concerned this observation has just recently been confirmed in another study (30).
The only breed, in this study, with a similar, but not statistically significant, low survival rate was the French Bulldog (Figures 1, 2). Surprisingly, the French Bulldog, Boxer and Border Collie all had a statistically significant early age of seizure onset (Table 1). The number of seizures was low in the French Bulldog and Boxer, but the severity of seizures was scored as high, although this difference was not statistically significant for the French Bulldog. In contrast to the earlier study of Wessmann et al. (1), we found that there is most likely a combination of factors that influence the QoL in these breeds rather than just the number of seizures. Next to the seizure numbers, early age of seizure onset and the severity of the seizures were also predictors of outcome in some breeds. The number of clusters was, except for the Drentsche Patrijshond, apparently not important. The Drentsche Patrijshond, a Dutch hunting dog, had the highest age of onset and the highest number of cluster seizures. However, the owners scored their dog's and their own QoL comparable to that of other dog owners/dogs. Hence, clustering appears to not be a predominant factor for QoL.
The QoL was scored statistically significantly higher for the Chihuahua, Dachshund and Golden retriever than the other breeds. The QoL score of the owners of Golden retrievers and Labrador retrievers was least affected by the epilepsy (Table 3). Owners of the Chihuahua, Dachshund and the Drentsche Patrijshond also reported an overall low impact of their dog's seizures on their QoL. Surprisingly, the number of seizures was high in the Chihuahua and Dachshund compared to the Labrador retriever, Golden retriever and Drentsche Patrijshond (Table 2). However, the number of seizures was not the predominant factor influencing the QoL score.
The effects of ASM side effects on QoL were also assessed. The acceptability of ASM side effects was statistically significantly higher in the Chihuahua and Golden retriever (Table 2). In other breeds, the ASM side effects appear to have no major influence on the QoL score of the dog. The same could be concluded for the number of visits to the consulting veterinarian/specialist (Table 1). There were statistically significantly fewer evening or night emergency visits in the Golden retriever and Labrador retriever than in other breeds, while the most emergency visits were reported in the Border Collie and French Bulldog (Table 2).
In the Border Collie, French Bulldog, and Boxer, costs of care were scored as least acceptable, although this was not statistically significant (Table 3). These costs were not due to the number of needed (evening/nightly) veterinary visits, as these were comparable to all other breeds/dogs (Table 1) but the owners scored them as higher compared to the other breeds/dogs (Table 3).
An important factor that influences the QoL scores of an owner is the ability to lead a normal life. The owners of Border Collies scored being able to leave the dog alone statistically significantly lower compared to other breeds, and this question also scored low in breeds such as the Boxer and French Bulldog. Owners of these breeds scored their own QoL low. This question also scored low for the Chihuahua owners, although this breed had the highest QoL score (Table 2).
This seems to suggest that there is not only a breed specific, but also an owner-specific impact on QoL. Owners choose their dogs for various reasons. A Border Collie owner may plan to use their dog for other tasks than a Chihuahua owner. Expectations, or disappointments, will have a clear influence on the outcome. Owners usually choose their dogs based on breed-specific traits, which may be strongly impacted by epilepsy-related factors. It is interesting to note that owners of some breeds accept disease-related changes more easily than others. This warrants further research.
This study has limitations. A significant limitation of any on-line questionnaire is that the population studied is self-selected. There is a possibility that owners with good QoL and good seizure control are underrepresented in the study. As there is a clear breed difference we expect that this bias is minimal. And although we were able to collect 402 questionnaires, the number of questionnaires obtained were low for five of the 14 breeds. Obtaining more filled-in questionnaires may have allowed a broader breed-specific analysis. However, seven breeds were clearly overrepresented to provide first insights into breed specific QoL scores for both dogs and owners.
This is the first study reporting breed-specific differences in the perceived QoL of dogs with idiopathic epilepsy and their owners. In this study, Border Collies and their owners had statistically significantly lower QoL scores than other dog breeds. What influences the QoL score differs for each breed, although the age of seizure onset, the number of seizures and their severity have a negative influence on the perceived QoL. Owners of Labrador retrievers and Golden retrievers reported a minimal impact of the seizures on their pet's QoL, with Chihuahua and Dachshund owners also reporting an overall good QoL of their dogs. Costs of medical care, veterinary visits, and the inability to leave the dog alone may be of negative influence in breeds that already have a negative outcome.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because it is a questionnaire web-based study. Written informed consent was obtained from the owners for the participation of their animals in this study.
PM was responsible for the study conception and data collection in co-operation with co-authors. Statistical analysis, data analysis, and manuscript writing were performed by MH and PM. PM supervised data analysis and manuscript editing, in co-operation with MP, MD, SB, and KS. All authors contributed to the article and approved the submitted version.
The publication of this manuscript was financially supported by the Utrecht University Open Access fund.
Our gratitude goes out to all owners and veterinarians who offered to help with this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1107315/full#supplementary-material
IE, idiopathic epilepsy; IVETF, International Veterinary Epilepsy Task Force; CS, cluster seizures; ES, epileptic seizures; SE, status epilepticus; ASM, anti-seizure medication; DOB, date of birth.
1. Wessmann A, Volk HA, Packer RM, Ortega M, Anderson TJ. Quality-of-life aspects in idiopathic epilepsy in dogs. Vet Rec. (2016) 179:229. doi: 10.1136/vr.103355
2. Wessmann A, Volk HA, Parkin T, Ortega M, Anderson TJ. Evaluation of quality of life in dogs with idiopathic epilepsy. J Vet Intern Med. (2014) 28:510–4. doi: 10.1111/jvim.12328
3. Packer RM, Volk HA. Epilepsy beyond seizures: a review of the impact of epilepsy and its comorbidities on health-related quality of life in dogs. Vet Rec. (2015) 177:306–15. doi: 10.1136/vr.103360
4. Nettifee JA, Munana KR, Griffith EH. Evaluation of the impacts of epilepsy in dogs on their caregivers. J Am Anim Hosp Assoc. (2017) 53:143–9. doi: 10.5326/JAAHA-MS-6537
5. Berendt M, Farquhar RG, Mandigers PJ, Pakozdy A, Bhatti SF, De Risio L, et al. International Veterinary Epilepsy Task Force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. (2015) 11:182. doi: 10.1186/s12917-015-0461-2
6. Bhatti SF, De Risio L, Munana K, Penderis J, Stein VM, Tipold A, et al. International Veterinary Epilepsy Task Force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet Res. (2015) 11:176. doi: 10.1186/s12917-015-0464-z
7. Kearsley-Fleet L, O'Neill DG, Volk HA, Church DB, Brodbelt DC. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec. (2013) 172:338. doi: 10.1136/vr.101133
8. Heske L, Nodtvedt A, Jaderlund KH, Berendt M, Egenvall A. A cohort study of epilepsy among 6,65,000 insured dogs: incidence, mortality and survival after diagnosis. Vet J. (2014) 202:471–6. doi: 10.1016/j.tvjl.2014.09.023
9. Hulsmeyer VI, Fischer A, Mandigers PJ, DeRisio L, Berendt M, Rusbridge C, et al. International Veterinary Epilepsy Task Force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. (2015) 11:175. doi: 10.1186/s12917-015-0463-0
10. Jaggy A, Bernardini M. Idiopathic epilepsy in 125 dogs: a long-term study. Clinical and electroencephalographic findings. J Small Anim Pract. (1998) 39:23–9. doi: 10.1111/j.1748-5827.1998.tb03665.x
11. Jokinen TS, Metsahonkala L, Bergamasco L, Viitmaa R, Syrja P, Lohi H, et al. Benign familial juvenile epilepsy in Lagotto Romagnolo dogs. J Vet Intern Med. (2007) 21:464–71. doi: 10.1111/j.1939-1676.2007.tb02991.x
12. Wielaender F, Sarviaho R, James F, Hytonen MK, Cortez MA, Kluger G, et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc Natl Acad Sci USA. (2017) 114:2669–74. doi: 10.1073/pnas.1614478114
13. Koskinen LL, Seppala EH, Belanger JM, Arumilli M, Hakosalo O, Jokinen P, et al. Identification of a common risk haplotype for canine idiopathic epilepsy in the ADAM23 gene. BMC Genomics. (2015) 16:465. doi: 10.1186/s12864-015-1651-9
14. Koskinen LL, Seppala EH, Weissl J, Jokinen TS, Viitmaa R, Hanninen RL, et al. ADAM23 is a common risk gene for canine idiopathic epilepsy. BMC Genet. (2017) 18:8. doi: 10.1186/s12863-017-0478-6
15. Belanger JM, Heinonen T, Famula TR, Mandigers PJJ, Leegwater PA, Hytonen MK, et al. Validation of a chromosome 14 risk haplotype for idiopathic epilepsy in the Belgian shepherd dog found to be associated with an insertion in the RAPGEF5 gene. Genes. (2022) 13:1–7. doi: 10.3390/genes13071124
16. Jenkins CA, Risio LD, Heinonen T, Johnson J, Kennedy LJ, Short AD, et al. Idiopathic epilepsy in the Border Collie: using genome-wide association study and whole genome sequencing approaches to identify genetic risk factors. In: International Conference on Canine and Feline Genetics and Genomics Conference. Huntsville, AL (2022).
17. Boothe DM, Dewey C, Carpenter DM. Comparison of phenobarbital with bromide as a first-choice antiepileptic drug for treatment of epilepsy in dogs. J Am Vet Med Assoc. (2012) 240:1073–83. doi: 10.2460/javma.240.9.1073
18. Charalambous M, Pakozdy A, Bhatti SFM, Volk HA. Systematic review of antiepileptic drugs' safety and effectiveness in feline epilepsy. BMC Vet Res. (2018) 14:64. doi: 10.1186/s12917-018-1386-3
19. Tipold A, Keefe TJ, Loscher W, Rundfeldt C, de Vries F. Clinical efficacy and safety of imepitoin in comparison with phenobarbital for the control of idiopathic epilepsy in dogs. J Vet Pharmacol Ther. (2015) 38:160–8. doi: 10.1111/jvp.12151
20. Stabile F, van Dijk J, Barnett CR, De Risio L. Epileptic seizure frequency and semiology in dogs with idiopathic epilepsy after initiation of imepitoin or phenobarbital monotherapy. Vet J. (2019) 249:53–7. doi: 10.1016/j.tvjl.2019.05.007
21. Gallucci A, Gagliardo T, Menchetti M, Bianchi E, Bucci D, Gandini G. Long-term efficacy of imepitoin in the treatment of naive dogs affected by idiopathic epilepsy. Vet Rec. (2017) 181:144. doi: 10.1136/vr.104187
22. Munana KR, Thomas WB, Inzana KD, Nettifee-Osborne JA, McLucas KJ, Olby NJ, et al. Evaluation of levetiracetam as adjunctive treatment for refractory canine epilepsy: a randomized, placebo-controlled, crossover trial. J Vet Intern Med. (2012) 26:341–8. doi: 10.1111/j.1939-1676.2011.00866.x
23. Fredso N, Sabers A, Toft N, Moller A, Berendt M. A single-blinded phenobarbital-controlled trial of levetiracetam as mono-therapy in dogs with newly diagnosed epilepsy. Vet J. (2016) 208:44–9. doi: 10.1016/j.tvjl.2015.10.018
24. Platt SR, Adams V, Garosi LS, Abramson CJ, Penderis J, De Stefani A, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. (2006) 159:881–4. doi: 10.1136/vr.159.26.881
25. Dewey CW, Guiliano R, Boothe DM, Berg JM, Kortz GD, Joseph RJ, et al. Zonisamide therapy for refractory idiopathic epilepsy in dogs. J Am Anim Hosp Assoc. (2004) 40:285–91. doi: 10.5326/0400285
26. Shihab N, Bowen J, Volk HA. Behavioral changes in dogs associated with the development of idiopathic epilepsy. Epilepsy Behav. (2011) 21:160–7. doi: 10.1016/j.yebeh.2011.03.018
27. Chang Y, Mellor DJ, Anderson TJ. Idiopathic epilepsy in dogs: owners' perspectives on management with phenobarbitone and/or potassium bromide. J Small Anim Pract. (2006) 47:574–81. doi: 10.1111/j.1748-5827.2006.00203.x
28. Packer RMA, Volk HA, Fowkes RC. Physiological reactivity to spontaneously occurring seizure activity in dogs with epilepsy and their carers. Physiol Behav. (2017) 177:27–33. doi: 10.1016/j.physbeh.2017.04.008
29. Jokinen TS, Tiira K, Metsahonkala L, Seppala EH, Hielm-Bjorkman A, Lohi H, et al. Behavioral abnormalities in lagotto romagnolo dogs with a history of benign familial juvenile epilepsy: a long-term follow-up study. J Vet Intern Med. (2015) 29, 1081–1087. doi: 10.1111/jvim.12611
30. Santifort KM, Bertijn E, Bhatti SFM, Leegwater P, Fischer A, Mandigers PJJ. Phenotypic characterization of idiopathic epilepsy in Border Collies. Front Vet Sci. (2022) 9:880318. doi: 10.3389/fvets.2022.880318
31. Hulsmeyer V, Zimmermann R, Brauer C, Sauter-Louis C, Fischer A. Epilepsy in Border Collies: clinical manifestation, outcome, and mode of inheritance. J Vet Intern Med. (2010) 24:171–8. doi: 10.1111/j.1939-1676.2009.0438.x
32. Weissl J, Hulsmeyer V, Brauer C, Tipold A, Koskinen LL, Kyostila K, et al. Disease progression and treatment response of idiopathic epilepsy in Australian shepherd dogs. J Vet Intern Med. (2012) 26:116–25. doi: 10.1111/j.1939-1676.2011.00853.x
33. De Risio L, Freeman J, Shea A. Evaluation of quality of life of carers of Italian spinoni with idiopathic epilepsy. Vet Rec Open. (2016) 3:e000174. doi: 10.1136/vetreco-2016-000174
34. Heske L, Korberg IB, Nodtvedt A, Jaderlund KH. Clinical characteristics of epilepsy of unknown origin in the Rottweiler breed. Acta Vet Scand. (2015) 57:75. doi: 10.1186/s13028-015-0168-1
35. Lord LK, Podell M. Owner perception of the care of long-term phenobarbital-treated epileptic dogs. J Small Anim Pract. (1999) 40:11–5. doi: 10.1111/j.1748-5827.1999.tb03246.x
Keywords: idiopathic epilepsy, dog, breed, impact, quality of life, owner, treatment
Citation: Hamers MFN, Plonek M, Bhatti SFM, Bergknut N, Diaz Espineira MM, Santifort KM and Mandigers PJJ (2023) Quality of life in dogs with idiopathic epilepsy and their owners with an emphasis on breed—A pilot study. Front. Vet. Sci. 9:1107315. doi: 10.3389/fvets.2022.1107315
Received: 24 November 2022; Accepted: 28 December 2022;
Published: 11 January 2023.
Edited by:
Catherine Elizabeth Stalin, University of Glasgow, United KingdomReviewed by:
Tarja Susanna Jokinen, University of Helsinki, FinlandCopyright © 2023 Hamers, Plonek, Bhatti, Bergknut, Diaz Espineira, Santifort and Mandigers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul J. J. Mandigers,  cC5qLmoubWFuZGlnZXJzQHV1Lm5s
cC5qLmoubWFuZGlnZXJzQHV1Lm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.