- College of Biological Engineering, Henan University of Technology, Zhengzhou, Henan, China
This study was conducted to determine the subclinical symptom of broilers exposure to mycotxoins from corn naturally contaminated, and the preventive effect with peroxisome proliferator activated receptor-α (PPARα) agonist (Wy-14643) supplementation. A total of 360 one-day -old male Arbor Acres broilers were randomly distributed into 4 treatments with 9 replicates of 10 birds. Dietary treatments included: treatment 1, normal corn diets group, treatment 2, normal corn + Wy-14643 diets group, treatment 3, mycotoxin-contaminated corn diets group, treatment 4, mycotoxin-contaminated corn + Wy-14643 diets group. The supplementation of Wy-14643 was added at the expense of 1 and 2 mg/kg in starter and grower diets, respectively. Birds fed mycotoxin diets had lower (P < 0.05) final body weight (BW), Body weight gain (BWG), feed intake (FI), and had higher (P < 0.05) feed conversion ratio (FCR). Feeding mycotoxin diets reduced (P < 0.05) the levels of serum superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), catalase (CAT), total antioxidative capacity (T-AOC) and high-density lipoprotein cholesterol (HDL-C), but higher malondialdehyde (MDA), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and fatty acid synthetase (FAS). The supplementation of Wy-14643 increased (P < 0.05) the level of serum T-AOC, but reduced (P < 0.05) TG and LDL-C. Interactive effect was not observed (P > 0.05) in growth performance and blood profiles. The relative expression of PPARα mRNA and 3-Hydroxy-3-MethylGlutaryl-CO enzyme A (HMGCoA) mRNA was higher (P < 0.05) in treatment 3 and treatment 4 than treatment 1 and treatment 2, and there was significant difference (P <0.05) between treatment 3 and treatment 4. There was significant difference (P < 0.05) between groups of the relative expression of recombinant carnitine palmitoyl transferase 1 (CPT1) mRNA. The relative expression of acyl CoA oxidase (ACO) mRNA was higher (P < 0.05) in treatment 1 and treatment 4 than treatment 2 and treatment 3, and there was significant difference (P < 0.05) between treatment 1 and treatment 4. The relative expression of apolipoprotein A (APO-A) mRNA was higher (P < 0.05) in treatment 1 and treatment 4 than treatment 2 and treatment 3. The relative expression of sterol regulatory element binding protein (SREBP) mRNA was lower (P < 0.05) in treatment 2, treatment 3 and treatment 4 than treatment 1, and there was significant difference (P < 0.05) between treatment 3 and treatment 4. Overall, feeding naturally contaminated mycotoxin diets caused negative effects on growth performance and blood profiles, while diet supplementation with Wy-14643 alleviate the detrimental effects on gene and expression related to liver fat metabolism in broilers.
Introduction
Poultry are widely exposed to mycotoxin contaminants, which are a group of secondary metabolites or molds generated by naturally occurring metabolic processes in fungi on various cereals and feedstuffs (1). Among them, aflatoxin B1 (AFB1), ochratoxin (OA), deoxynivalenol (DON), zearalenone (ZEA), T2 toxin and fumonisin (FM) have the most toxicity to poultry health (2). It has been well-documented that various mycotoxins could result in detrimental effects on growth performance, intestinal health, immunity, hepatic function as well as biochemical and hematological parameters in broilers (1, 3). The naturally contaminated diets were more toxic than those diets with purified mycotoxins (4). In China, the corn as the major energy source accounted for over 50% of broilers feed and was extremely susceptible to mycotoxins. However, there were few literatures about the effects of corn naturally contaminated with mycotoxins of low concentrations on broilers. Previous studies demonstrated that low levels of mycotoxins may also be harmful to broilers and could alter normal metabolic functions in various organs, especially the liver (5–7).
PPARα is one of members of the peroxisome proliferator activated receptors (PPARs), which expressed in many metabolically active tissues, especially high in the liver (8). PPARα can not only differentiate adipocytes and participate in lipid and energy metabolism, but also inhibit inflammation (9).
Wy-14643 is a fibrate analog and potent agonist of PPARα. Previous studies found that the hypolipidemic effects of Wy-14643 were liver dependent and feed intake was mediated directly by PPARα activation within hepatocytes in mice (10). This may be due to the similar crosstalk between hepatic PPARα and the central nervous system in feeding behavior by fatty acid and insulin (8, 11). Wy-14643 may beneficially regulate LPS-induced inflammation in synovial fibroblasts via NF-kB pathway (12) and decrease liver injury via the deacetylase enzyme sirtuin1 and endoplasmic reticulum stress (13). Furthermore, Wy-14643 exert its antioxidant and anti-inflammatory effects as PPARα agonist by reducing neutrophil infiltration and proinflammatory cytokine expression and thus decrease the formation of reactive oxygen species (14–16).
The feeding of diets contaminated with mycotoxins decreases performance, alters the metabolism and induces the organs injury. It has been found that mycotoxins can be detoxified using physical, chemical, and biological methods (17, 18). Is there any other way to solve this problem? And What is the mechanism of action? PPARα might play a key role in regulating nutrient metabolism and energy homeostasis in liver. Therefore, the current experiment was conducted to determine the impact of PPARα agonist on growth performance, blood profiles, gene and expression related to liver fat metabolism in broilers fed diets containing corn naturally contaminated with mycotoxins, and to evaluate whether activating PPARα can reduce the hazard of mycotoxins to broilers.
Materials and methods
Experimental design and broiler husbandry
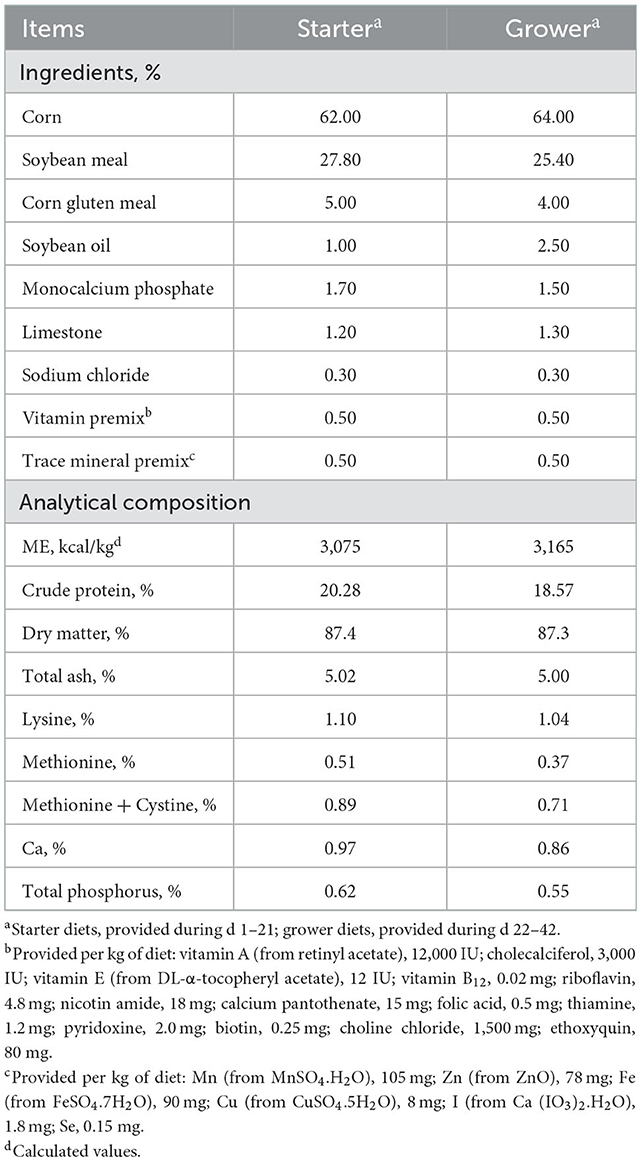
The Animal Welfare Committee of Henan University of Technology approved the animal care protocol used for these experiments (HUT20210417).
A total of 360 one-day-old male Arbor Acres broilers with an average initial body weight (BW) of 41.4 ± 0.2 g were divided into 1 of 4 dietary treatments with 9 replicates, each replicate consisting of 10 birds. The treatments were in a 2 × 2 factorial arrangement, with 2 levels of corn naturally contaminated with mycotoxins (0 and 100%) and 2 levels of Wy-14643 (1 mg/kg in the starter phase and 2 mg/kg in the growing phase). The starter phase was from d 1 to 21 and the growing phase was from d 22 to 42. All birds were housed in an environmentally controlled facility with stainless steel battery brooders. Each cage was provided with a stainless steel feeder and one nipple waterer, which allowed ad libitum access to feed and water throughout the experiment. All diets were fed in pellet form and formulated to meet or exceed the NRC (19) requirements for broilers (Table 1). The four treatments were fed with normal corn diets, normal corn + Wy-14643 diets, mycotoxin-contaminated corn diets and mycotoxin-contaminated corn + Wy-14643 diets, respectively. The PPARα agonist was added at the expense of corn. The temperature was kept at 33°C from 1 to 3 d of age and then it was reduced gradually to ~25°C until 14 d of age and was kept at approximately 16 to 22°C thereafter. The feed samples were analyzed for dry matter, crude protein, total ash, calcium, phosphorus and amino acids according to the standard procedures of the AOAC (20).
Sampling and measurements
The content of mycotoxins in the diets was measured by ELISA method (kits, Neogen Company, MN, US; Microplate Reader, Model 680, Bio-Rad, Hercules, CA, US) according to the manufacturer's instructions.
At the end of the experiment, final BW and feed consumption each cage was determined. BWG, FI, and FCR of broilers corrected by mortality were calculated based on each cage. FCR was calculated as the ratio of feed intake to weight gain.
On d 42, two birds from each replicate were randomly selected and blood samples were collected from the jugular vein into a sterile syringe. Blood samples were then centrifuged at 3,500 x g for 15 min and serum was separated. The concentrations of SOD, T-AOC, CAT, GSH-PX, MDA, TC, HDL-C, LDL-C, and TG in the serum were analyzed using assay kits from Nanjing Jiancheng Biotechnology Institute according to the manufacturer's instructions. The activities of ALT, AST, and FAS in the serum were measured with an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan) using the assay kits (Jiancheng Biotechnology Institute, Nanjing, China).
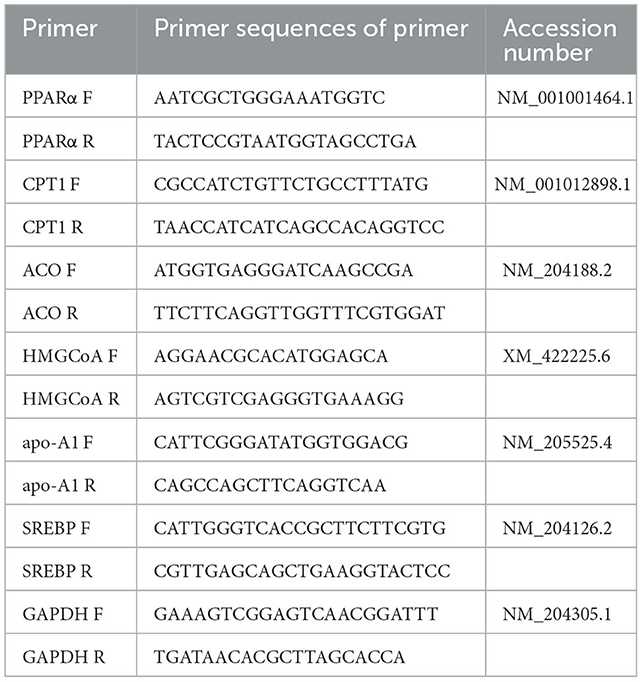
After blood collection, the same birds were electrically stunned and then sacrificed immediately by decapitation and eviscerated manually. The liver was removed manually by the same trained person. The liver samples were immediately frozen in liquid nitrogen, then stored at −80°C until gene expression analysis. The gene and expression related to liver fat metabolism were then measured. Briefly, total RNA of tissues was extracted with RNAiso Reagent (TaKaRa, Japan) using Trizol method and reverse-transcribed with RT Reagents (TaKaRa, Japan) according to manufacturer's instructions. Quantitative real-time PCR was performed using 96-well iCycler iQTM Real-Time PCR Detection System (BIO-RAD, USA). The gene-specific primers used were listed in Table 2 and purchased from TaKaRa (Japan). The PCR system consisted of 12.5 μL SYBR Green PCR Master Mix (TaKaRa, Japan), 2.0 μL of cDNA, 8.5 μL of PCR-grade water and 2.0 μL of primer pairs (100 mM forward and 100 mM reverse) for a total volume of 25 μL. The house-keeping gene β-actin was used as internal control to normalize the expression of target genes. All samples were assayed in triplicate. Cycling conditions were as follows: 95°C for 10 s, and 40 cycles involving a combination of 95°C for 5 s, 58°C for 30 s and 72°C for 30 s. The relative quantification of gene amplification by RT-PCR was performed using the value of the threshold cycle (Ct). Relative expressions of target genes were determined by the 2-ΔΔCt method.
Statistical analysis
All data were analyzed using the GLM procedure of SAS (38). The data were analyzed as a completely randomized design with treatments arranged in a 2 × 2 factorial, the main effects of mycotoxin-contaminated corn (0 and 100%), PPAR-α agonist (1 mg/kg in starter, 2 mg/kg in grower), and the interactions among these factors were analyzed with the least squares means using the Bonferroni test. The probability level of P < 0.05 was considered to be statistically significant.
Results
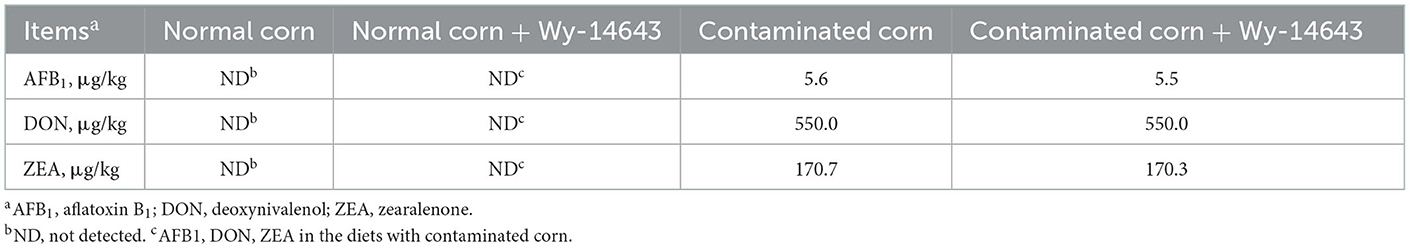
Mycotoxin content of the diets
The concentrations of AFB1, DON and ZEA were not detected in normal corn and normal corn + Wy-14643 diets (Table 3). The concentrations of AFB1, DON and ZEA were 5.6, 550.0 μg/kg, 170.7 μg/kg in contaminated corn diets, and 5.5, 550.0 μg/kg, 170.3 μg/kg in contaminated corn + Wy-14643 diets, respectively. Mycotoxins other than these were below the limits of detection.
Growth performance
FCR did not differ among dietary groups (P > 0.05). Feeding mycotoxin diets decreased (P < 0.05) final BW, BWG and FI (Table 4), there were no differences between contaminated corn diets and contaminated corn + Wy-14643 diets. Interactive effect was not observed (P > 0.05) in final BW, BWG, FI and FCR.
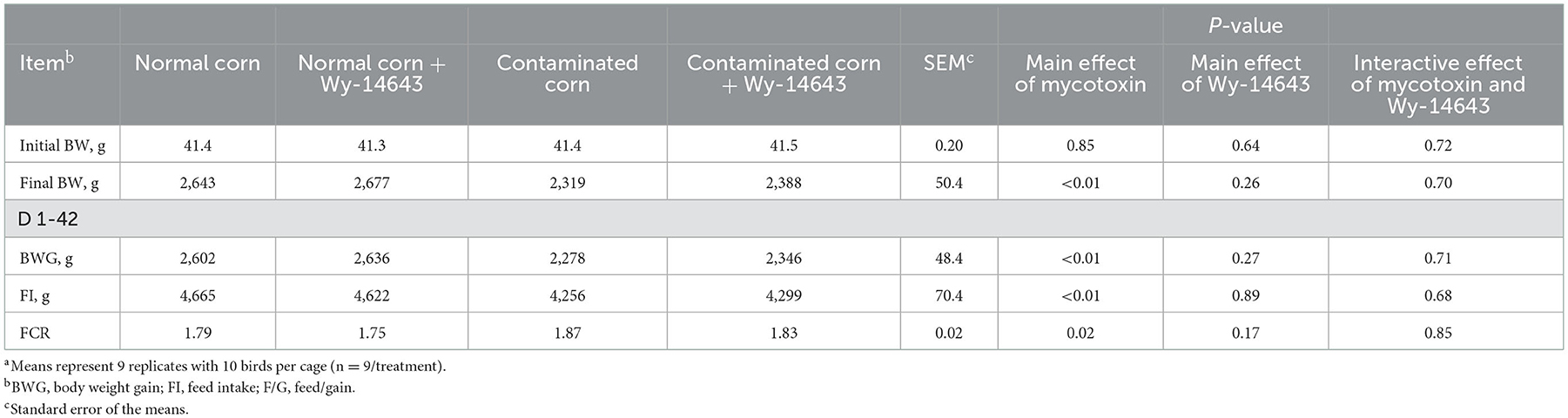
Table 4. Effects of PPAR-α agonist on growth performance in broilers fed mycotoxin contaminated corna.
Antioxidant activity
Birds fed with mycotoxin-contaminated diets had lower (P < 0.05) levels of serum SOD, GSH-Px, CAT and T-AOC, but higher (P < 0.05) MDA than the birds fed with normal diets (Table 5). The supplementation of Wy-14643 increased (P < 0.05) the level of serum T-AOC than that of mycotoxin-contaminated corn group, and dietary Wy-14643 did not influence (P > 0.05) serum SOD, GSH-Px, CAT or MDA. Interactions between mycotoxin and Wy-14643 were not significant (P > 0.05).
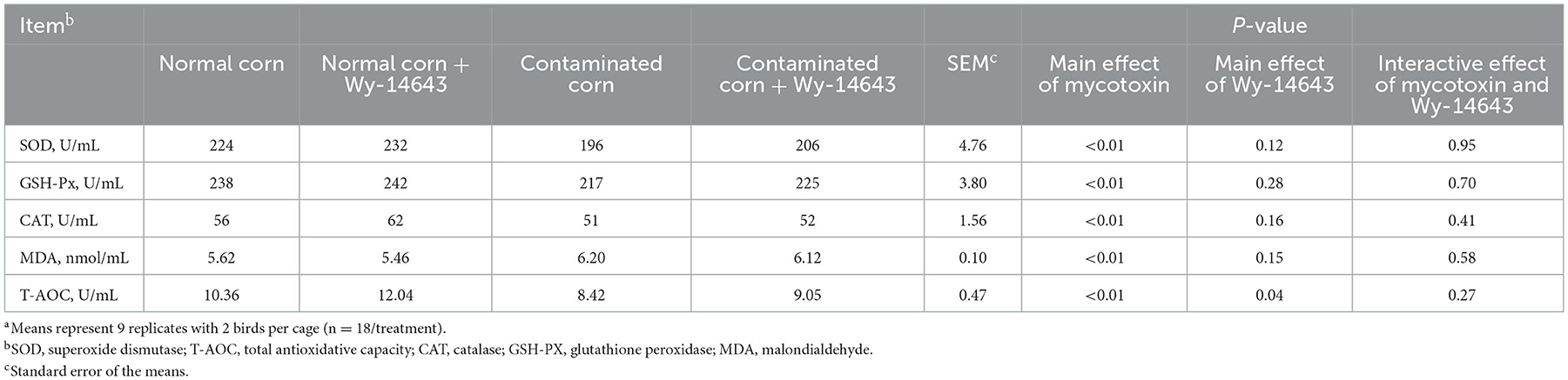
Table 5. Effects of PPAR-α agonist on antioxidant activity in broilers fed mycotoxin-contaminated corna.
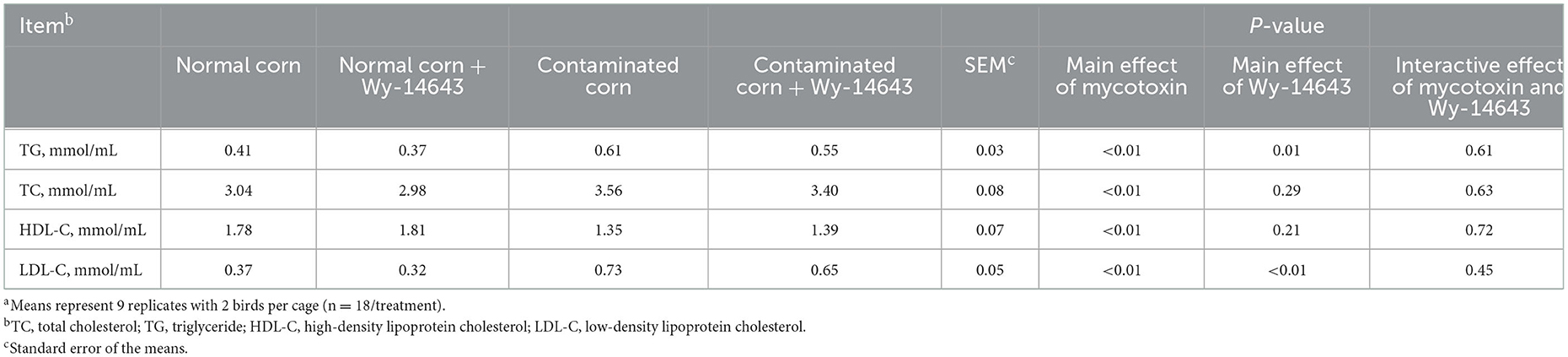
Serum lipid
Birds fed with mycotoxin-contaminated diets had lower (P < 0.05) level of serum HDL-C, but higher (P < 0.05) TG, TC and LDL-C than the birds fed with normal diets (Table 6). The supplementation of Wy-14643 decreased (P < 0.05) TG and LDL-C in the serum than that of mycotoxin-contaminated corn group, and dietary Wy-14643 did not influence (P > 0.05) serum HDL-C or TC. No interactions were observed (P > 0.05) in serum lipids between mycotoxin and Wy-14643.
Lipid metabolism-related enzyme
Birds fed with mycotoxin-contaminated diets had higher (P < 0.05) serum AST, ALT and FAS than the birds fed with normal diets (Table 7). Dietary Wy-14643 did not influence (P > 0.05) serum AST, ALT or FAS. Interactions between mycotoxin and Wy-14643 were not significant (P > 0.05).
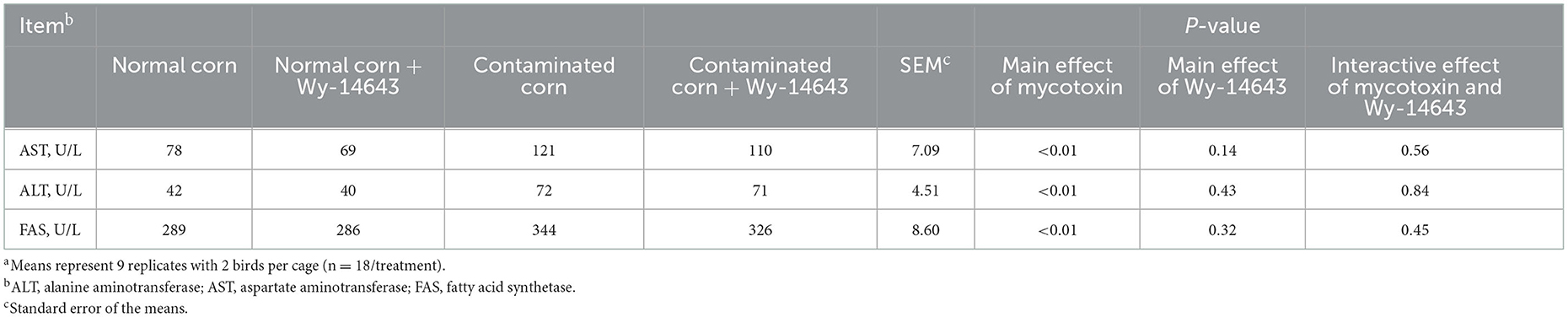
Table 7. Effects of PPAR-α agonist on enzyme concerned with lipid metabolism in broilers fed mycotoxin contaminated corna.
Gene and expression related to liver fat metabolism
Feeding mycotoxin-contaminated diets (treatment 1 and treatment 2) increased (P < 0.05) the relative expression of PPARα mRNA and HMGCoA mRNA than the birds of treatment 3 and treatment 4 (Figure 1). The relative expression of CPT1 mRNA in treatment 1 was greater (P < 0.05) in broilers compared with that of treatment 2, treatment 3 and treatment 4. Birds of treatment 2 and treatment 3 had lower (P < 0.05) relative expression of ACO mRNA, while birds of treatment 4 had higher (P < 0.05) relative expression of ACO mRNA compared with the birds of treatment 1. The relative expression of APO-A mRNA was lower (P < 0.05) in broilers of treatment 2 and treatment 3 diets compared with the birds of treatment 1. Birds of treatment 2, treatment 3 and treatment 4 had lower (P < 0.05) relative expression of SREBP mRNA than that of treatment 1.
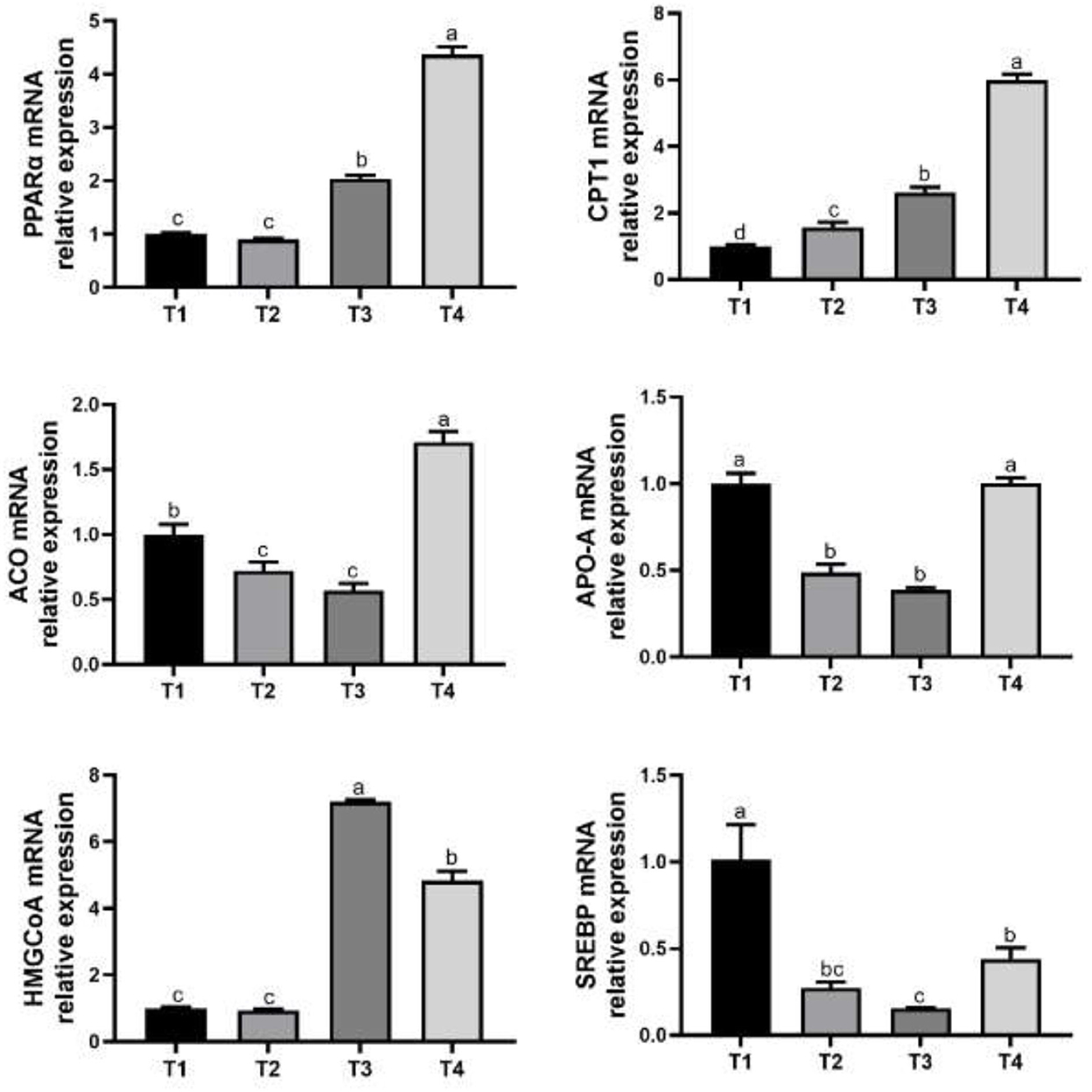
Figure 1. Effects of PPAR-α agonist on genes expression related to liver fat metabolism in broilers. T1, normal corn diets group; T2, normal corn + Wy-14643 diets group; T3, mycotoxin-contaminated corn diets group; T4, mycotoxin-contaminated corn + Wy-14643 diets group. The a, b, c, and d different letters indicate the significant differences.
Discussion
The feeding of grain naturally contaminated with mycotoxins has been previously shown to reduce growth performance. Studies demonstrated that mycotoxins decreased FI by 9–12%, BWG by 13–14% and FCR by 7% in broilers (1, 21). Moreover, The DON (1,845 μg/kg) and ZEA (534 μg/kg) decreased BWG by 18% and increased FCR by 19% in broilers without any effect on FI (22). Others also observed the similar results in broilers fed diets containing 1,680 μg/kg DON and 145 μg/kg ZEA (23). In agreement with previous studies, feeding diets containing corn naturally contaminated low concentrations of mycotoxins decreased BWG and final BW by 12% in broilers in the current study.
Some studies have reported a modest enhancement in the body weight of chickens exposed to aflatoxins in their diet due to hormesis, which is a dose-response phenomenon characterized by low-dose stimulation (24). In the current studies, the diets were naturally contaminated with low concentrations of mycotoxins, however, no similar results were observed in growth performance, which could be explained by the fact that feeding of grains contaminated naturally with several mycotoxins sometimes resulted in toxicological synergy (25).
It is expected that the PPARα agonist (Wy-14643) may exert its lipid-lowering, antioxidant and anti-inflammatory effects on target liver, and then have the potential to improve the health status and growth performance like conjugated linoleic acid as the PPARα agonist (8). Unfortunately, no effects of the supplementation of Wy-14643 were observed on the final BW, BWG, FI or FCR. Similarly, the addition of PPARα agonist (0.5% clofibrate) also did not affect BWG, FI or FCR in broilers (26).
The co-occurrence of mycotoxins caused detrimental effects on serum antioxidant parameters in broilers in the present study, which were in agreement with previous studies. Dietary mycotoxins may result in oxidative stress, which can be characterized by increased serum MDA and decreased antioxidant enzymes activities, such as serum SOD, GSH-Px, CAT and T-AOC (27). Moreover, mycotoxins increased serum AST and ALT in broilers (3), which were consistent with our results. Similarly, the serum TC was increased in broilers fed diets containing aflatoxin B1, zearalenone, deoxynivalenol, and fumonisin (28), which may be attributed to the hepatotoxic effects of AFL, ZEN, FUM, and DON or their synergetic effect that characterized by impairment of transport and lipid metabolism of liver (29).
As expected, the supplementation of Wy-14643 improved the level of T-AOC in broilers fed mycotoxins diets. The data supported our inference that the increased antioxidant activity may be involved in the regulation of liver injury by Wy-14643 through PPARα in broilers fed mycotoxins diets. This was in agreement with previous studies (14–16), which indicated that Wy-14643 might prevented the formation of reactive oxygen species. A significant reduction in MDA formation in the liver of rats pretreated with Wy-14643 was previously reported (13).
PPARα deficiency led to accumulation of hepatic TG (30). PPARα reduced circulating triacylglycerol levels via transcriptional modulation of genes related to lipolysis, cellular uptake, β-oxidation of fatty acids, and decreased synthesis of fatty acids and TG (31). Dietary Wy-14643 exactly decreased serum TG and LDL-C in broilers fed mycotoxins diets. Previous studies demonstrated that the major physiological effects of Wy-14643 agonist acted primarily through hepatic PPARα, then activated lipid catabolism and lowered consistently TG (8, 32, 33). This study confirmed the lipid-lowering effect of Wy-14643 in broilers. Li et al. (8) proposed that PPARα activation principally facilitates lipid-lowering effects that PPARα activation is necessary for the hypolipidemic actions of PPARα agonists.
Previous studies have demonstrated that the liver gene expression involved in the immune system, antioxidant function and biotransformation can be influenced by mycotoxins in broilers (4, 22, 34). In our study, we particularly evaluated the liver gene expression on the PPARα signal pathway. PPARα is the main regulatory factor of body and liver metabolism, which can regulate liver energy consumption by up-regulating fatty acid oxidation capacity and controlling fatty acid intake (35). The mRNA expression of PPARα was increased expectedly by the mycotoxins treatments, which may reflect the liver damage characterized by the adverse effects on the serum parameters about antioxidant capacity, liver function and lipid metabolism in the present study. Our study showed that feeding mycotoxins increased the relative mRNA expression of CPT1 and HMGCoA, while decreased the relative mRNA expression of APO-A and SERBP, which may mirror the liver injury and increase in serum TC, TG, and FAS. Similarly, previous study used the perfluorooctanoic acid to create the liver injury model and observed that the level of PPARα mRNA was increased, while the ACO and APO-A mRNA expression was decreased in pregnant female rats (36).
PPARα has been previously shown to upregulate many genes involved in fatty acid utilization, including several genes for hepatic clearance of LDL-C, fatty acid uptake, fatty acid activation and transport into the mitochondria, peroxisomal and mitochondrial β-oxidation, and some enzymes for mitochondrial respiration (37). The agonist of PPARα (Wy-14643) has been proven to increase the mRNA relative expression of PPARα, CPT1, ACO and HMGCoA, while decrease relative expression of SREBP mRNA in broilers fed mycotoxins diets. This indicated that the agonist of PPARα (Wy-14643) exactly activated PPARα and interfered the lipid metabolism.
However, to the best of our knowledge, this is the first study to evaluate the PPARα agonist (Wy-14643) in broilers fed diets containing corn naturally contaminated with mycotoxins. More studies are needed to determine the effects of Wy-14643 on broilers.
Conclusion
This study indicates that diets contaminated with mycotoxins could reduce growth performance and antioxidant capacity. Although the supplementation of Wy-14643 in mycotoxin-contaminated diets failed to mitigate the adverse effects on growth performance, the addition of Wy-14643 could partially enhance the antioxidant capacity and lipid-lowering effects in broilers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Welfare Committee of Henan University of Technology approved the animal care protocol used for these experiments (HUT20210417). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
YZ: writing-reviewing and editing and funding acquisition. MW and HD: methodology. MW: writing—original draft HD: data curation. HD and TY: formal analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFD1300300), Henan Science and Technology Research Project (222102110446), and Natural Science Foundation of Henan (22230042042).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Andretta I, Kipper M, Lehnen CR, Hauschild L, Vale MM, Lovatto PA, et al. Meta-analytical study of productive and nutritional interactions of mycotoxins in broilers. Poult Sci. (2011) 90:1934–40. doi: 10.3382/ps.2011-01470
2. Binder EM. Managing the risk of mycotoxins in modern feed production. Anim Feed Sci Technol. (2007) 133:149–66. doi: 10.1016/j.anifeedsci.2006.08.008
3. Andretta I, Kipper M, Lehnen CR, Lovatto PA. Meta-analysis of the relationship of mycotoxins with biochemical and hematological parameters in broilers. Poult Sci. (2012) 91:376–82. doi: 10.3382/ps.2011-01813
4. Liu JB, Yan HL, Cao SC, Hu YD, Zhang HF. Effects of absorbents on growth performance, blood profiles and liver gene expression in broilers fed diets naturally contaminated with aflatoxin. Asian Austral J Anim Sci. (2020) 33:294–304. doi: 10.5713/ajas.18.0870
5. Kolawole O, Graham A, Donaldson C, Owens B, Abia W, Meneely J, et al. Low doses of mycotoxin mixtures below EU regulatory limits can negatively affect the performance of broiler chickens: a longitudinal study. Toxins. (2020) 12:433–48. doi: 10.3390/toxins12070433
6. Miazzo R, Rosa CA, Carvalho ED, Magnoli C, Chiacchiera SM, Palacio G, et al. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks. Poult. Sci. (2000) 79:1–6. doi: 10.1093/ps/79.1.1
7. Santos RR, Eerden EV. Impaired performance of broiler chickens fed diets naturally contaminated with moderate levels of deoxynivalenol. Toxins. (2021) 22:170–87. doi: 10.3390/toxins13020170
8. Li GL, Brocker CN, Xie C, Yan TT, Noguchi A, Krausz KW, et al. Hepatic peroxisome proliferator-activated receptor alpha mediates the major metabolic effects of Wy-14643. J Gastroenterol Hepatol. (2018) 33:1138–45. doi: 10.1111/jgh.14046
9. Hashizume S, Akaike M, Azuma H, Ishikawa K, Yoshida S, Sumitomoueda Y, et al. Activation of peroxisome proliferator-activated receptor α in megakaryocytes reduces platelet-derived growth factor-bb in platelets. J Atheroscler Thromb. (2011) 18:138–47. doi: 10.5551/jat.5868
10. Fu J, Gaetani S, Oveisi F, Verme JL, Serrano A, Rodríguez F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nat. (2003) 425:90–3. doi: 10.1038/nature01921
11. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nat. (2006) 443:289–95. doi: 10.1038/nature05026
12. Huang H, McIntosh AL, Martin GG, Petrescu AD, Landrock KK, Landrock D, et al. Inhibitors of fatty acid synthesis induce PPAR? -regulated fatty acid?-oxidative genes: Synergistic roles of L-FABP and glucose. PPAR Res. (2013) 2013:865604. doi: 10.1155/2013/865604
13. Pantazi E, Folch-Puy E, Bejaoui M, Panisello A, Varela AT, Rolo AP, et al. PPARα agonist wy-14643 induces sirt1 activity in rat fatty liver ischemia-reperfusion injury. Biomed Res Int (2015. (2015) 894579–86. doi: 10.1155/2015/894679
14. Collino M, Aragno M, Mastrocola R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radical Bio Med. (2006) 41:579–89. doi: 10.1016/j.freeradbiomed.2006.04.030
15. Patel NSA, Paola RDi, Mazzon E, Britti D, Thiemermann C, Cuzzocrea S, et al. Peroxisome proliferator-activated receptor-α contributes to the resolution of inflammation after renal ischemia/reperfusion injury. J Pharmacol Exp Ther. (2009) 328:635–43. doi: 10.1124/jpet.108.146191
16. Gao Z, Li YH. Antioxidant stress and anti-inflammation of PPARα on warm hepatic ischemia-reperfusion injury. PPAR Res (2012. (2012) 738785–93. doi: 10.1155/2012/738785
17. Zhu J. The latest research progress and development trend of vomit toxin. Animal Husbandry Abroad. (2013) 33:102–4.
18. Zhang H, Wang X, Zhi A, Zhang M, Wang Z. Vomitotoxin pollution status, toxic effects, harmful effects and detoxification utilization. J Henan Univ Sci Technol. (2018) 46:40–5.
19. National Research Council. Nutrient Requirements of Poultry. 9th revised. Washington, DC: National Academy Press (1994).
20. International AOAC. Official Methods of Analysis of AOAC International.19th ed. Gaithersburg, MD: AOAC Int (2012).
21. Weaver AC, Weaver DM, Yiannikouris A, Adams N. Meta-analysis of the effects of mycotoxins and yeast cell wall extract supplementation on the performance, livability, and environmental sustainability of broiler production. Poult Sci. (2022) 101:102043–56. doi: 10.1016/j.psj.2022.102043
22. Chen XP, Ishfaq M, Wang J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune responses and Salmonella Pullorum infection resistance of broilers challenged with Aflatoxin B1. Poult Sci. (2022) 101:101651–62. doi: 10.1016/j.psj.2021.101651
23. Yunus AW, Blajet-Kosicka A, Kosicki R, Khan MZ, Rehman H, Böhm J, et al. Deoxynivalenol as a contaminant of broiler feed: intestinal development, absorptive functionality, and metabolism of the mycotoxin. Poult Sci. (2012) 91:852–61. doi: 10.3382/ps.2011-01903
24. Kouda K, Iki M. Beneficial effects of mild stress (hormetic effects): dietary restriction and health. J Physiol Anthropol (29. (2010) 127–32. doi: 10.2114/jpa2.29.127
25. Smith TK, Chowdhury SR, Swamy HVN. Comparative aspects of fusarium mycotoxicosis in broiler chickens, laying hens and turkeys and the efficacy of a polymeric glucomannan mycotoxin adsorbent: Mycosorb. Lyons TP, Jacques KA (Eds). In: Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech's 20th Annual Symposium. Nottingham University Press. (2004), pp. 103–109.
26. Zhang BY, He DC, Wang F, Zhang HG, Zhang YQ, Shang GMJ, et al. The effect of dietary CB on growth performance, slaughter performance, meat quality and immune function of broilers. J Shanxi Agr Sci. (2014) 42:1121–4.
27. Fouad A, Ruan D, El-Senousey H, Chen W, Jiang S, Zheng C, et al. Harmful effects and control strategies of Aflatoxin B1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry: review. Toxins. (2019) 11:176–96. doi: 10.3390/toxins11030176
28. Shang QH, Yang ZB, Yang WR, Li Z, Zhang GG, Jiang SZ, et al. Toxicity of mycotoxins from contaminated corn with or withoutyeast cell wall adsorbent on broiler chickens. Asian-Australas J Anim Sci. (2016) 29:674–80. doi: 10.5713/ajas.15.0165
29. Rosa CAR, Miazzo R, Magnoli C, Salvano M, Chiacchiera SM, Ferrero S, et al. Evaluation of the efficacy of bentonite from the south of Argentina to ameliorate the toxic effects of aflatoxin in broilers. Poult Sci. (2001) 80:139–44. doi: 10.1093/ps/80.2.139
30. Sugden MC, Bulmer K, Gibbons GF, Knight BL, Holness MJ. Peroxisome-proliferator-activated receptor-alpha (pparalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J. (2002) 364:361–8. doi: 10.1042/bj20011699
31. Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. (1996) 1302:93–109. doi: 10.1016/0005-2760(96)00066-5
32. Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW, et al. Peroxisome proliferator-activated receptor (PPAR)-a activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-c activation. Diabetes. (2001) 50:411–7. doi: 10.2337/diabetes.50.2.411
33. Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res. (2010) 20:124–37. doi: 10.1038/cr.2010.13
34. Chen X, Horn N, Applegate TJ. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult Sci. (2014) 93:2037–47. doi: 10.3382/ps.2014-03984
35. Misra P, Reddy JK. Peroxisome proliferator-activated receptor-alpha activation and excess energy burning in hepatocarcinogenesis. Biochimie. (2014) 98:63–74. doi: 10.1016/j.biochi.2013.11.011
36. Li DY. Amelioration of Perfluorooctanoic Acid in Utero Exposure Induced Hepatotoxicity in Female Offspring Mice by Lycium Barbarum Polysaccharide (Master Thesis). Hebei Agricultural University, Baoding, China. (2019).
Keywords: broilers, liver fat metabolism, mycotoxin, performance, peroxisome proliferator activated receptor-α agonist
Citation: Zhang Y, Wang M, Dong H and Yang T (2023) Effects of peroxisome proliferator activated receptor-α agonist on growth performance, blood profiles, gene expression related to liver fat metabolism in broilers fed diets containing corn naturally contaminated with mycotoxins. Front. Vet. Sci. 9:1103185. doi: 10.3389/fvets.2022.1103185
Received: 20 November 2022; Accepted: 09 December 2022;
Published: 04 January 2023.
Edited by:
Yongpeng Guo, Henan Agricultural University, ChinaReviewed by:
Rohollah Ebrahimi, Khuzestan University of Agricultural Sciences and Natural Resources, IranSabreen Ezzat Fadl, Matrouh University, Egypt
Copyright © 2023 Zhang, Wang, Dong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang,  eW9uZ3poYW5nMjA4QDE2My5jb20=
eW9uZ3poYW5nMjA4QDE2My5jb20=
 Yong Zhang
Yong Zhang Mengchen Wang
Mengchen Wang