- 1College of Chinese Medicine Materials, Jilin Agricultural University, Changchun, China
- 2College of Animal Science and Technology, Jilin Agricultural University, Changchun, China
- 3Laboratory of Production and Product Application of Sika Deer of Jilin Province, Jilin Agricultural University, Changchun, China
- 4Key Laboratory of Animal Production, Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, China
Background: Bovine viral diarrhea is one of the diseases that cause huge economic losses in animal husbandry. Many countries or regions have successively introduced eradication plans, but BVDV still has a high prevalence in the world. This meta-analysis aims to investigate the prevalence and risk factors of BVDV in the world in recent 10 years, and is expected to provide some reference and theoretical basis for BVDV control plans in different regions.
Method: Relevant articles published from 2010 to 2021 were mainly retrieved from NCBI, ScienceDirect, Chongqing VIP, Chinese web of knowledge (CNKI), web of science and Wanfang databases.
Results: 128 data were used to analyze the prevalence of BVDV from 2010 to 2021. BVDV antigen prevalence rate is 15.74% (95% CI: 11.35–20.68), antibody prevalence rate is 42.77% (95% CI: 37.01–48.63). In the two databases of antigen and antibody, regions, sampling time, samples, detection methods, species, health status, age, sex, breeding mode, and seasonal subgroups were discussed and analyzed, respectively. In the antigen database, the prevalence of dairy cows in the breed subgroup, ELISA in the detection method subgroup, ear tissue in the sample subgroup, and extensive breeding in the breeding mode were the lowest, with significant differences. In the antibody database, the prevalence rate of dairy cows in the breed subgroup and intensive farming was the highest, with a significant difference. The subgroups in the remaining two databases were not significantly different.
Conclusion: This meta-analysis determined the prevalence of BVDV in global cattle herds from 2010 to 2021. The prevalence of BVDV varies from region to region, and the situation is still not optimistic. In daily feeding, we should pay attention to the rigorous and comprehensive management to minimize the spread of virus. The government should enforce BVDV prevention and control, implement control or eradication policies according to local conditions, and adjust the policies in time.
1. Introduction
Bovine viral diarrhea virus (BVDV) is the main pathogen of bovine viral diarrhea BVD (1, 2), and it is the main member of flaviviridae and pestivirus genus, which consists of three species: pestivirus A (BVDV-1), pestivirus B (BVDV-2) and pestivirus H (bovine viral diarrhea virus type 3 [Hobi-like pestiviruses)] (WOAH). BVDV-1 contains at least 22 subgenotypes of 1a-1v and BVDV-2 and HoBi-like pestivirus are divided into 4 subtypes (3). Multiple species and genotypes lead to the mutation of BVDV, which brings great obstacles to its prevention and control. BVDV contains two biotypes, and BVDV can be divided into cytopathic type (CP) and non-cytopathic type (NCP) according to whether it causes pathological changes in cultured tissue cells (4). NCP BVDV can infect cows early in embryonic development and produce persistently infected (PI) calves. PI makes it more difficult to control BVDV. It is the main source of infection of BVDV, because it is immune tolerant to infected strains, does not produce antibodies, and is always infected and continuously detoxifies (5). In contrast, the risk of transient infection (TI) transmission is weaker, producing only mild clinical symptoms to the host and expelling the virus into the environment for a short period of time. However, TI damage to the immune system can exacerbate the occurrence of secondary infections, so it remains an important component of BVDV infection (6).
BVDV is widespread in the world and can cause gastrointestinal, respiratory and reproductive diseases. The induced immunosuppression can increase the probability of infection of other diseases (7). BVDV reduces the breeding and growth efficiency of livestock through various ways, increases the mortality rate of young animals and the prevalence rate of reproductive system, respiratory system and gastrointestinal diseases, and causes continuous and serious economic losses to the animal husbandry (8). BVDV can infect cattle, goats, sheep, camels, pigs and other cloven-hoofed animals (9–11). Among them, cattle are the main infection host and source of BVDV, and are most affected by diseases (12). As a major economic animal, cattle are closely related to people's life. According to the survey, the economic impact of BVD ranges from £0 to £552 per cow per year, with a mean impact of £46.50 (13). At the same time, BVDV's pollution to bovine-derived substances further endangers the accuracy of scientific research and the safety of biological products such as vaccines (14). The growing demand for beef and dairy products reminds people to focus on the health of primitive animals and avoid possible economic losses (15). Therefore, it is very important to investigate and control the prevalence of BVDV infection in cattle species. ACVIM's consensus statement clarifies the importance of BVDV control (16). Many countries have also introduced measures to control and purify BVDV. Denmark introduced the BVD eradication plan as early as 1994 (17). Northern Ireland began implementing the BVD AHWNI eradication program in 2013 and the virus positivity declined significantly by 2020 (18). Germany's 6-year mandatory plan has seen a significant decline in the number of PI by 2016, and further removal of the virus is the next challenge (19). Switzerland has had a control program since 2008 and infection rates have dropped significantly by 2020, but PI animals remain the last strong obstacle (20). In 2016–2017, the Indonesian government tried to breed beef cattle by increasing artificial insemination, hoping to reduce the vertical transmission of BVDV (21).
According to the positive rate of BVDV in different species, many articles have been meta-analyzed. Knowing the prevalence of BVDV in time can not only provide data support for the formulation of BVDV prevention and control policies, but also provide technical guidance for practical production.
This paper makes a meta-analysis on the prevalence of BVDV infection among cattle in the world in recent 10 years. Through the summary of the latest data and the thinking caused by eradication plans in different regions, the following questions are addressed: “What should we do to control BVDV? How should the control plan be carried out under different circumstances?”. We hope to observe the effectiveness of current prevention and control measures and provide reference for further prevention and control of BVDV in the future.
2. Materials and methods
2.1. Search strategy
We searched six databases of PubMed, ScienceDirect, Web of Science, CNKI, VIP, and Wanfang, and find articles published in Chinese and English from 2010 to May 20, 2021. Designed to filter prevalence data for all BVDV, the specific search process is as follows:
PubMed search strategy is as follows: According to MeSH terminology, the following keywords were used to search: “Diarrhea Viruses, Bovine Viral,” “Cattle,” and the Boolean operators “OR,” “AND” in the “Keyword/Title/Summary” field alone or in combination.
A: We search for “Cattle” based on MeSH terminology: ((((((((((((((((((((“Cattle”[Mesh]) OR (Cow)) OR (Cows)) OR (Bos indicus)) OR (Zebu)) OR (Zebus)) OR (Holstein Cow)) OR (Cow, Holstein)) OR (Dairy Cow)) OR (Cow, Dairy)) OR (Dairy Cows)) OR (Beef Cow)) OR (Beef Cows)) OR (Cow, Beef)) OR (Bos grunniens)) OR (Yak)) OR (Yaks)) OR (Bos taurus)) OR (Cow, Domestic)) OR (Domestic Cow)) OR (Domestic Cows).
B: We search for “Diarrhea Viruses, Bovine Viral” based on MeSH terminology: (((((((((((“Diarrhea Viruses, Bovine Viral”[Mesh]) OR (Bovine Viral Diarrhea Viruses)) OR (Bovine Pestivirus)) OR (Bovine Pestiviruses)) OR (Pestiviruses, Bovine)) OR (Bovine Diarrhea Virus)) OR (Bovine Diarrhea Viruses)) OR (Diarrhea Virus, Bovine)) OR (Diarrhea Viruses, Bovine)) OR (Virus, Bovine Diarrhea)) OR (Viruses, Bovine Diarrhea)) OR (Diarrhea Virus, Bovine Viral).
C: We used the Boolean operators “OR” for the entry terms and “AND” for the MeSH terms. (((((((((((((((((((((“Cattle”[Mesh]) OR (Cow)) OR (Cows)) OR (Bos indicus)) OR (Zebu)) OR (Zebus)) OR (Holstein Cow)) OR (Cow, Holstein)) OR (Dairy Cow)) OR (Cow, Dairy)) OR (Dairy Cows)) OR (Beef Cow)) OR (Beef Cows)) OR (Cow, Beef)) OR (Bos grunniens)) OR (Yak)) OR (Yaks)) OR (Bos taurus)) OR (Cow, Domestic)) OR (Domestic Cow)) OR (Domestic Cows)) AND ((((((((((((“Diarrhea Viruses, Bovine Viral”[Mesh]) OR (Bovine Viral Diarrhea Viruses)) OR (Bovine Pestivirus)) OR (Bovine Pestiviruses)) OR (Pestiviruses, Bovine)) OR (Bovine Diarrhea Virus)) OR (Bovine Diarrhea Viruses)) OR (Diarrhea Virus, Bovine)) OR (Diarrhea Viruses, Bovine)) OR (Virus, Bovine Diarrhea)) OR (Viruses, Bovine Diarrhea)) OR (Diarrhea Virus, Bovine Viral)).
Use advanced search in ScienceDirect and Web of Science databases to improve the accuracy of your results, enter subject terms “cattle,” “Diarrhea Viruses, Bovine Viral,” “prevalence” and select research articles to search. The VIP database was searched for articles by selecting the subject headings “bovine” and “bovine viral diarrheal mucosal disease” or “bovine” and “bovine viral diarrhea virus.” Wanfang and CNKI search strategies are: The theme words “bovine” and “bovine viral diarrhea mucosal disease” or: “bovine” and “bovine viral diarrhea virus” or “bovine” and “BVDV” were selected.
In order to collect comprehensive data as much as possible, Google Academic will further search the related articles of the collected articles.
2.2. Inclusion criteria and exclusion criteria
Eligible articles are screened according to the inclusion exclusion criteria below.
Inclusion criteria:
(1) Study on the prevalence of BVDV infection;
(2) Literature between 2010 and 2021.5.20;
(3) The species is cattle and the source is clear;
(4) The type of article is experimental research article;
(5) Literature published in Chinese or English.
Exclusion criteria:
(1) Repetitive articles;
(2) Articles that cannot be downloaded;
(3) Study animals were vaccinated or model animals;
(4) Research data is not clear;
(5) Sample size <30.
2.3. Data extraction
Import the search database results into the EndNote (EndNote X 9.3.3) reference manager software (Clarivate analysis, Philadelphia, Pennsylvania, USA) for screening, delete duplicate articles, and then two reviewers further screen according to the article title and abstract. Obtain key data information from all relevant studies, including the first author, sampling year, country, mainland, sample type, detection method, variety, season, health status, age, gender and breeding mode. Microsoft ® Excel ® 2019 MSO (16.0.14228.20216) 32 is used to sort and compile the data mentioned above.
2.4. Quality assessment
The level of proposal evaluation, formulation and evaluation methods determines the quality of selected literature. The scoring standard includes the following four aspects, whether it is random sampling, sampling time, whether the sampling method is detailed, whether the detection method is detailed, and whether there are more than four factors. “Yes” is 1 point, and the maximum is 5 points. Based on the above standards, the article is divided into three grades 0–1, 2–3, 4–5, respectively.
2.5. Statistical analysis
Under the guidance of PRISMA 2020, the article strictly follows its requirements and completes the systematic evaluation and meta-analysis (Supplementary material 1).
R software 4.0.0 is used to compile and calculate data. Sensitivity analyses were performed in different possible ways for all included studies, and bias tests were done by looking at funnel graphs (22). Egger's test and trim and fill analysis further illustrate whether bias occurs (23). Bias is indicated when the funnel chart is asymmetrical or when the Egger test p < 0.05. Q- test (X2 and p representation) was used to evaluate the heterogeneity among the studies, and the forest map was used for visual analysis. The degree of heterogeneity was further evaluated by I2 (24). The higher the I2, the greater the heterogeneity. The code in R for meta-analysis is in Supplementary material 2. Factors investigated in the subgroup analysis included sampling year (before 2017, after 2017), season, health status (healthy, clinically symptomatic), age (<6 months, >6 months), country, region, test method, sample origin, breed (beef cattle, dairy cows, dairy meat dual-use, breeding cattle), sex, breeding pattern (intensive, extensive).
3. Results
3.1. Flow chart and results of literature screening
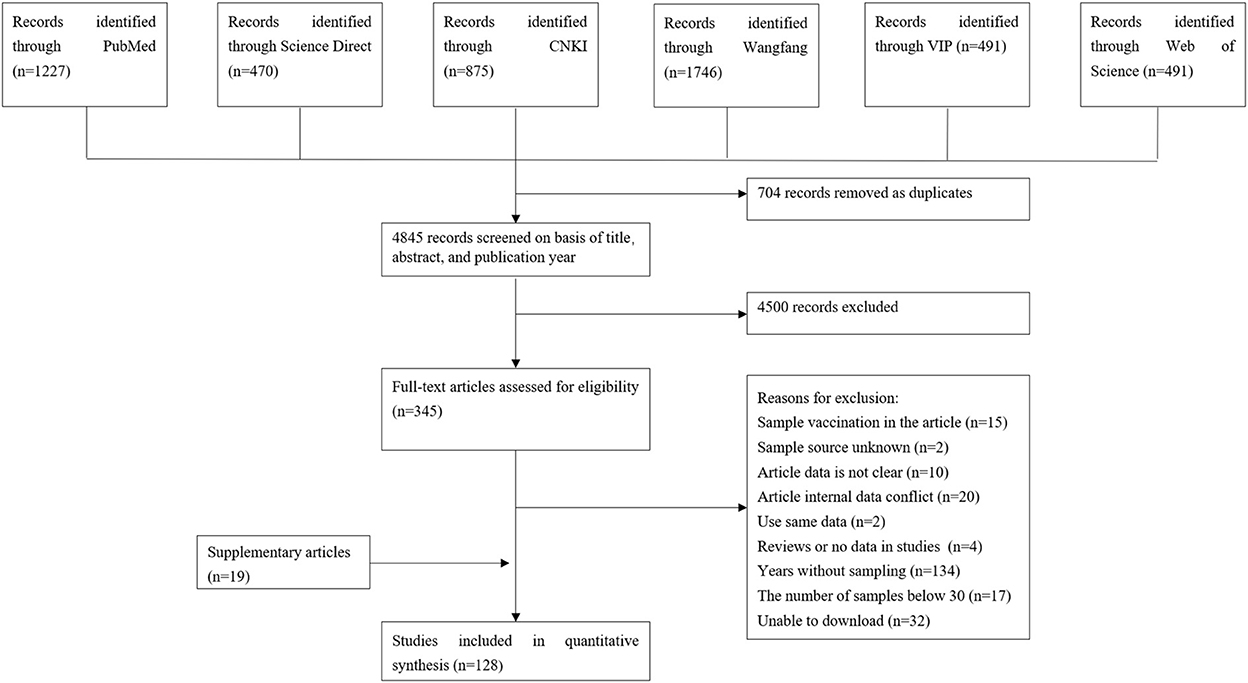
A total of 5,549 eligible articles were obtained. Seven hundred four duplicate articles were deleted, and 4,500 articles were further screened according to the title, abstract, and Year of publication. Further screening according to the inclusion and exclusion criteria, 15 articles on vaccination were deleted, 2 sample sources were unclear, 10 article data were unclear, 20 data errors were used, 2 articles were used the same data, 4 non-epidemiological investigative articles, 134 non-sampling years, 17 articles with a sample size of <30, 32 articles that could not be queried, and a total of 109 articles were included. Nineteen articles were added to Google Academic, including 128 articles in total. The specific flow chart is shown in Figure 1.
3.2. Studies included
Through literature screening, 128 studies were eligible for the meta-analysis. Among them, there were 77 articles on detecting antibodies and 51 articles on detecting antigens. Studies were identified from 19 countries worldwide, including 10 countries in Asia, two in North America, two in South America, two in Europe and three in Africa (Supplementary Figure 1).
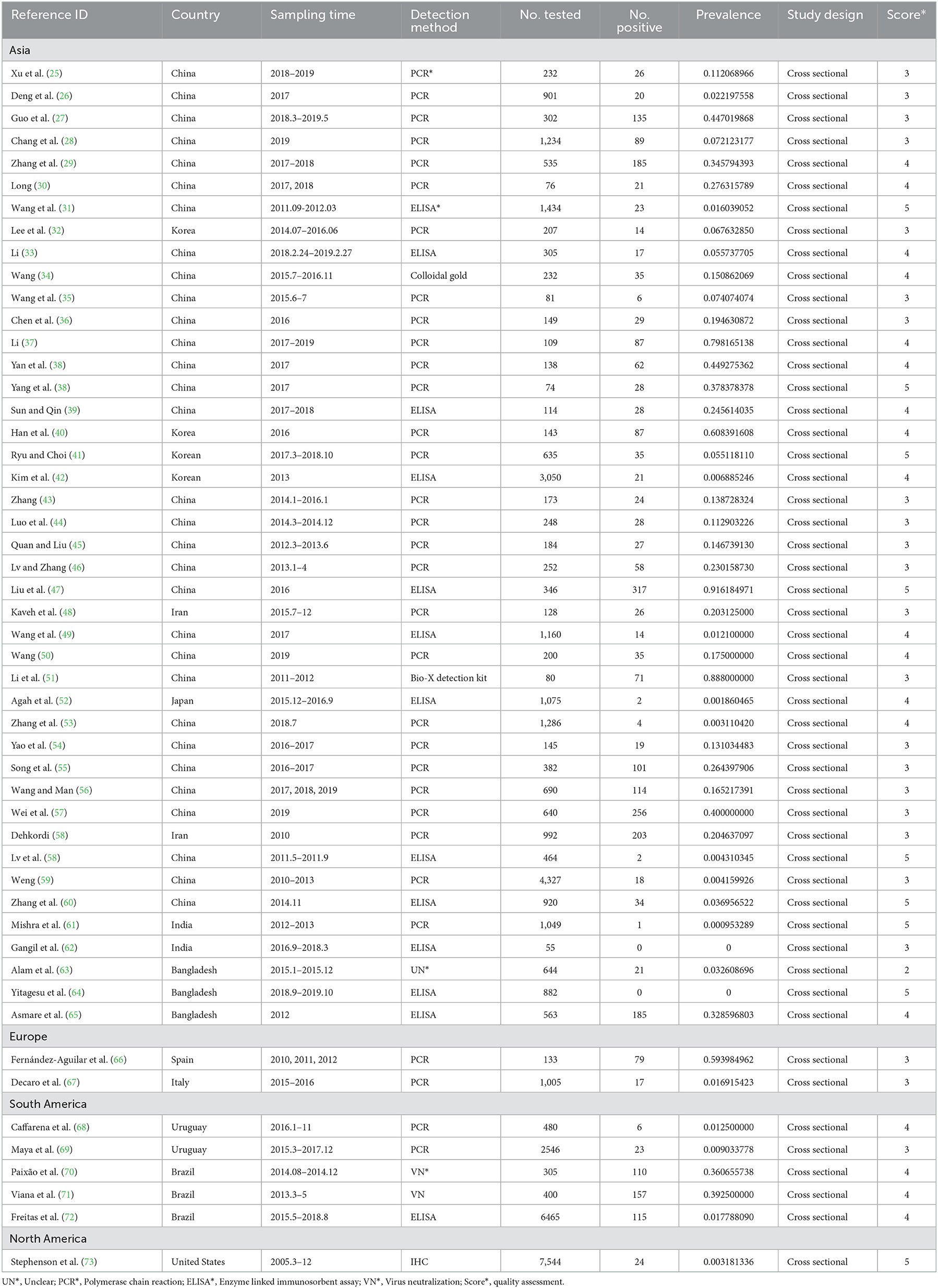
There are a total of 51 antigen detection articles, including 27 articles of 4–5 points and 24 articles of 2–3 points (Table 1). A total of 46,211 cattle were tested, and 3,488 BVDV-positive cattle were tested, with a positive infection rate of 15.74% (95% CI: 11.35–20.68 3,488/46,211, Table 2). Among the regional subgroups, Europe had the highest positive rate with a positive rate of 23.27% (95% CI: 0.00–89.41, Table 2), followed by the Asia positivity rate of 16.75% (95% CI: 11.27–23.04, Table 2), The lowest is 0.32% (95% CI: 0.20–0.46, Table 2) in North America. Spain (59.40%, 95% CI: 50.91–67.62, Supplementary Table 1) has the highest antigen-positive rate among all countries and India has the lowest positive rate. The positivity rate after 2017 was higher than before 2017. The positive rate of ear tissue in the test samples was the lowest, with a positive rate of 0.48% (95% CI: 0.05–1.20, Table 2), which was significantly different. Diary cows had the lowest positive rate of infection, with a positive rate of 11.43% (95% CI: 6.61–17.32, Table 2), which was significantly different. In the health condition subgroup, the rate of BVDV infection with clinical symptoms was higher than that of clinically healthy cattle. Summer infection rate was lowest, with a positive rate of 4.17% (95% CI: 0.12–12.59, Table 2), Spring positivity rate was highest at 21.33% (95% CI: 0.82–57.99, Table 2). ELISA had the lowest positive rate among the test methods, with a positive rate of 6.94% (95% CI: 2.12–14.16, Table 2), which was significantly different. The positive rate in adult cattle is higher than that in calves. Extensive culture mode had the lowest rate of infection, with a positive rate of 1.11% (95% CI: 0.00–5.86, Table 2), which was significantly different.
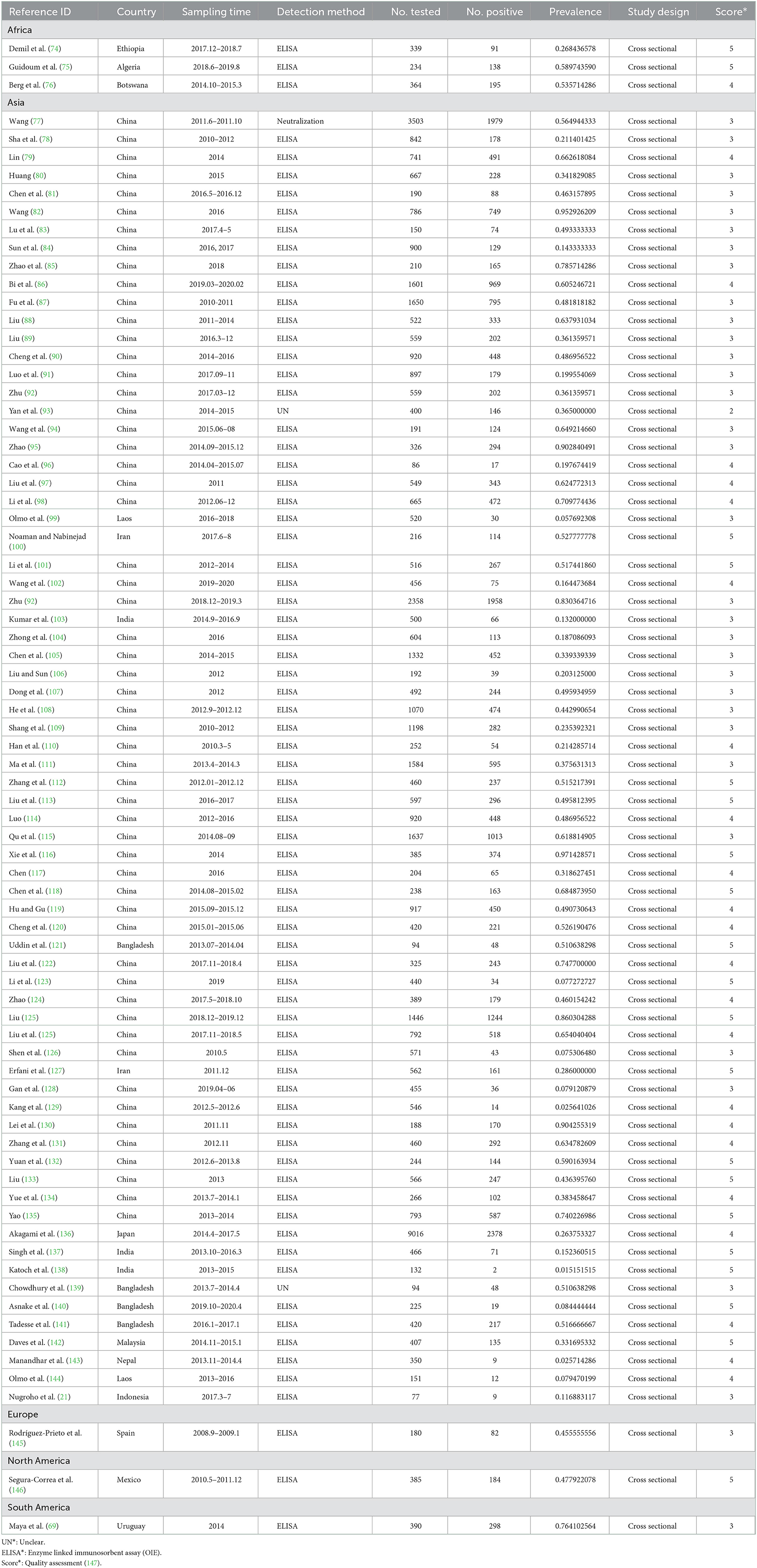
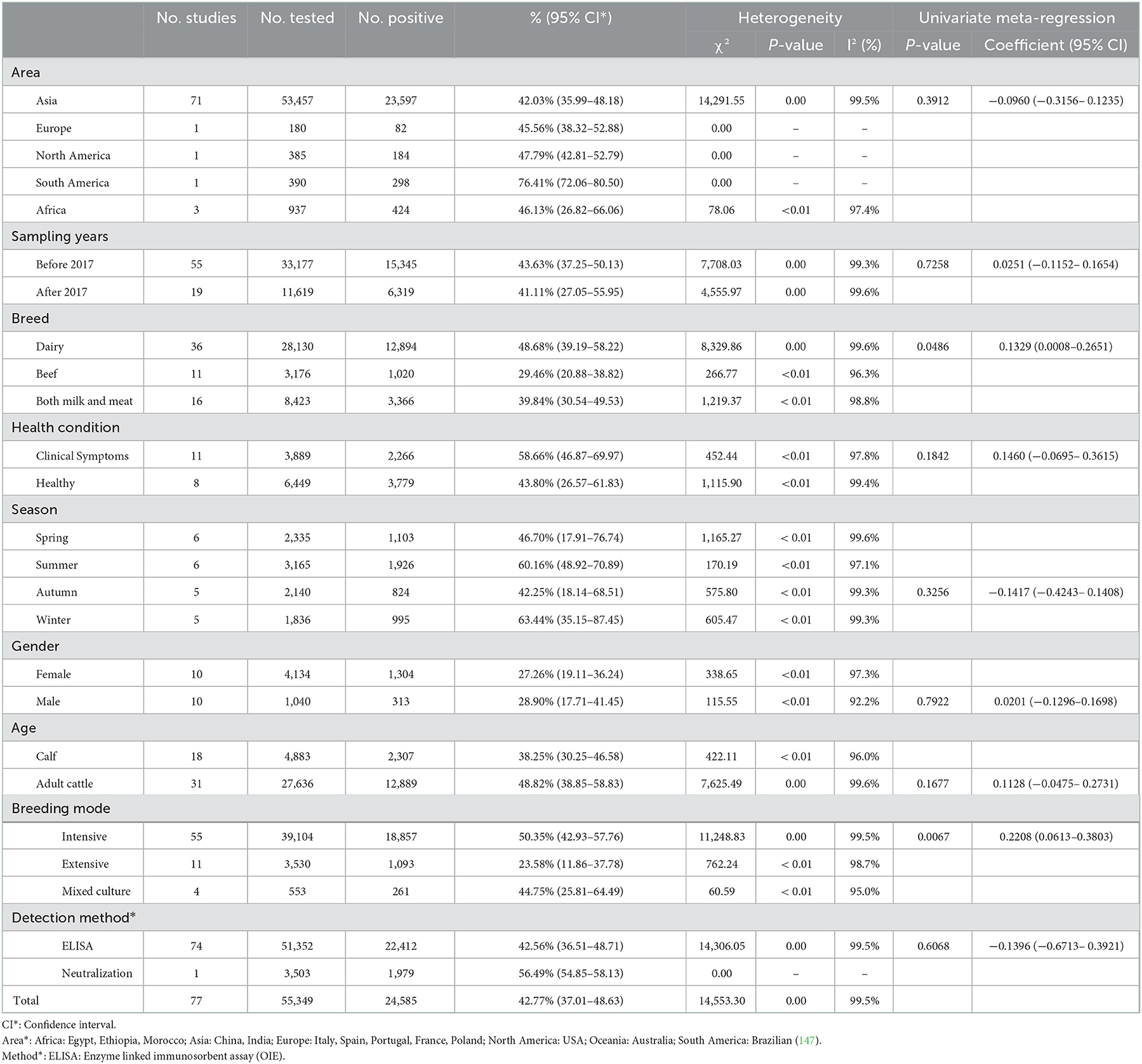
A total of 77 articles were published on the detection of BVDV antibodies, including 43 articles with 4–5 points and 34 articles with 2–3 points (Table 3). A total of 55,349 samples were tested, of which 24,585 were positive, and the positive rate was 42.77% (95% CI: 37.01–48.63, Table 4). South America had the highest prevalence in the regional subgroup, with a positivity rate of 76.4% (95% CI: 72.06–80.50, Table 4, Supplementary Table 2) followed by North America, Africa, Europe, and Asia. Infection rates have decreased after 2017 compared to before 2017. Dairy cattle had the highest prevalence rate, with a positive rate of 48.68% (95% CI: 39.19–58.22, Table 4), which was significantly different. In the health condition subgroup, the infection rate of clinically healthy cattle was relatively low, with a positive rate of 43.80% (95% CI: 26.57–61.83, Table 4). The positive rate is relatively high in summer 60.16% (95% CI: 48.92–70.89, Table 4) and winter 63.44% (95% CI: 35.15–87.45, Table 4). The positive rate for cows is lower than that of bulls, and the positive rate of calves is lower than that of adult cattle. Intensive has the highest prevalence of all culture models, with a positive rate of 50.35% (95% CI: 42.93–57.76, Table 4).
3.3. Meta-analysis based on detected antigen
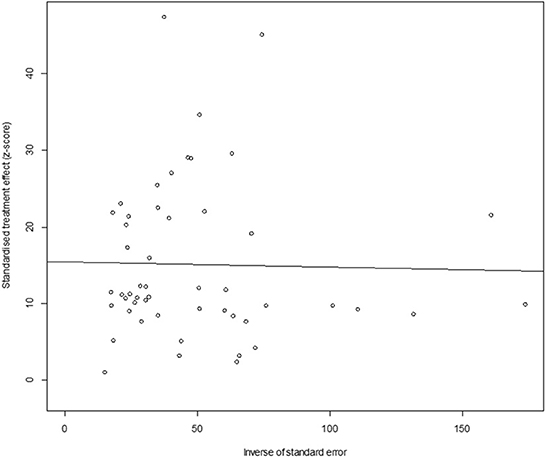
In antigen detection, a total of 46,211 cattle were tested, and 3,488 BVDV-positive cattle were tested, with a positive infection rate of 15.74% (95% CI: 11.35–20.68, Figure 2). PFT conversion rate and random effect model (χ2 = 0.0566, I2 = 99%, P = 0.00) were used (Table 5). The egger test result is t = 6.5574, p = 6.975e−08 (Supplementary Table 3, Figure 3). The funnel diagram shows that there is bias (Figure 4). The trim and fill analysis are used to correct the bias, a total of 22 articles were corrected, and the adjusted prevalence rate was 2.01% (95% CI:0.40–4.64). The results of sensitivity analysis show that the results of meta-analysis are reliable (Table 6).
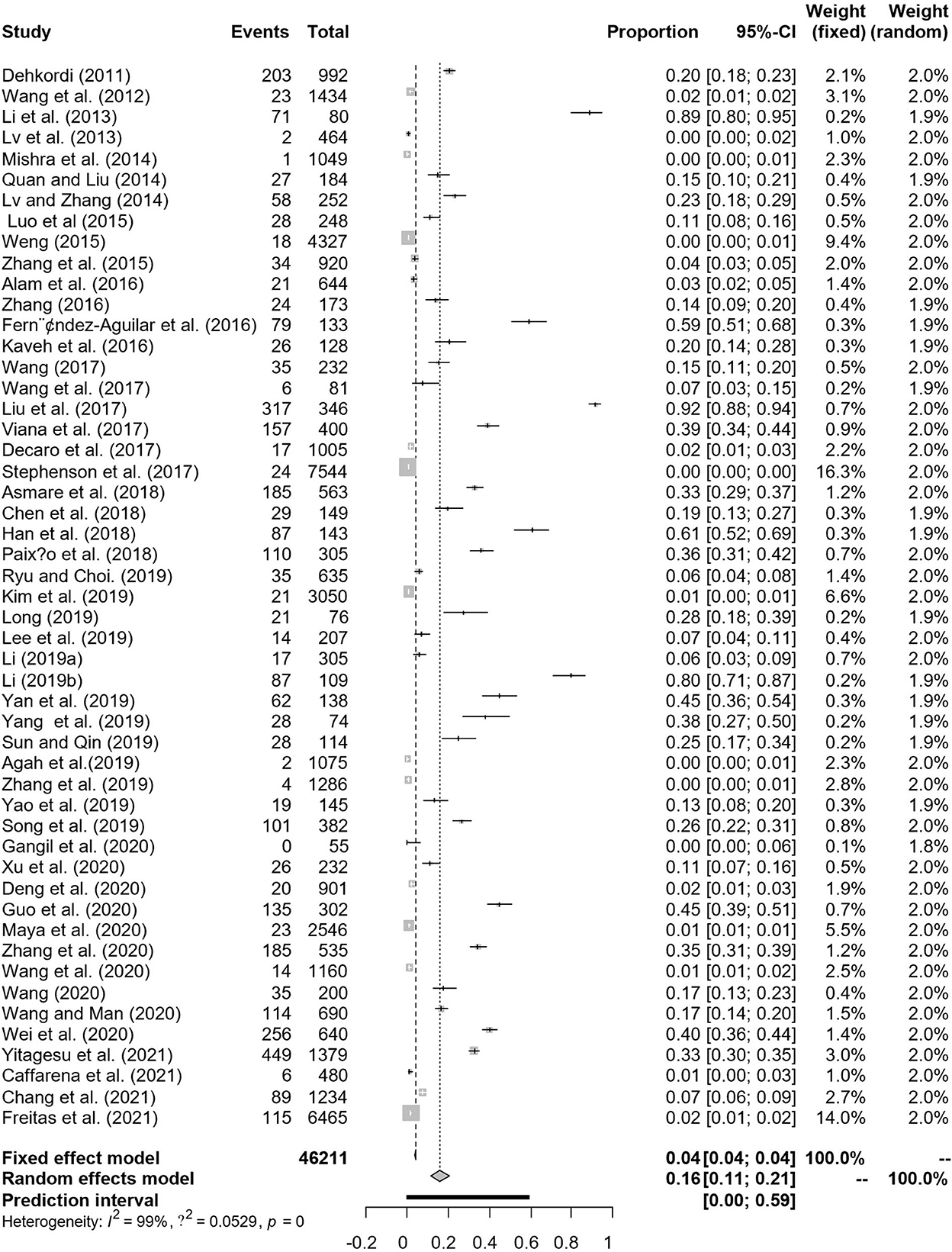
Figure 2. Forest plot of bovine viral diarrhea virus antigen prevalence in the world study conducted 2010–2021 (decetion antigen).
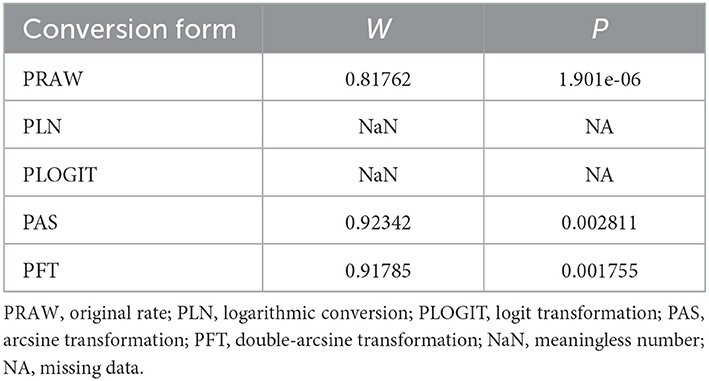
Table 5. Normal distribution test for the normal rate and the different conversion of the normal rate.
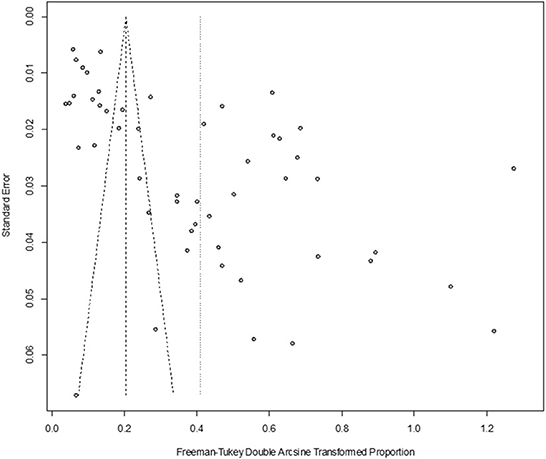
Figure 4. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias (decetion antigen).
3.4. Meta-analysis based on detected antibody
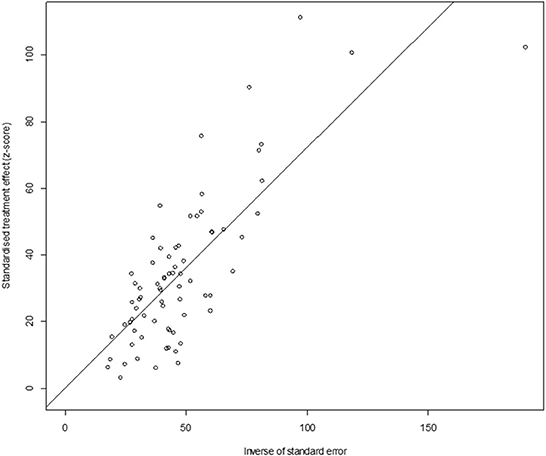
In antibody detection, a total of 55,349 samples were tested, of which 24,585 were positive, and the positive rate was 42.77% (95% CI: 37.01–48.63, Figure 5). PFT conversion rate and random effect model(χ2 = 0.0664, I2 = 100%, P = 0.00, Table 7) were used. The egger test result is t = 0.68873, p = 0.4935 (Supplementary Table 4, Figure 6). The funnel diagram shows that there is bias (Figure 7). The data from the trim and fill analysis showed that no trimming performed, and the data unchanged, meaning there may be no significant publication bias. The results of sensitivity analysis show that the results of meta-analysis are reliable (Table 8).
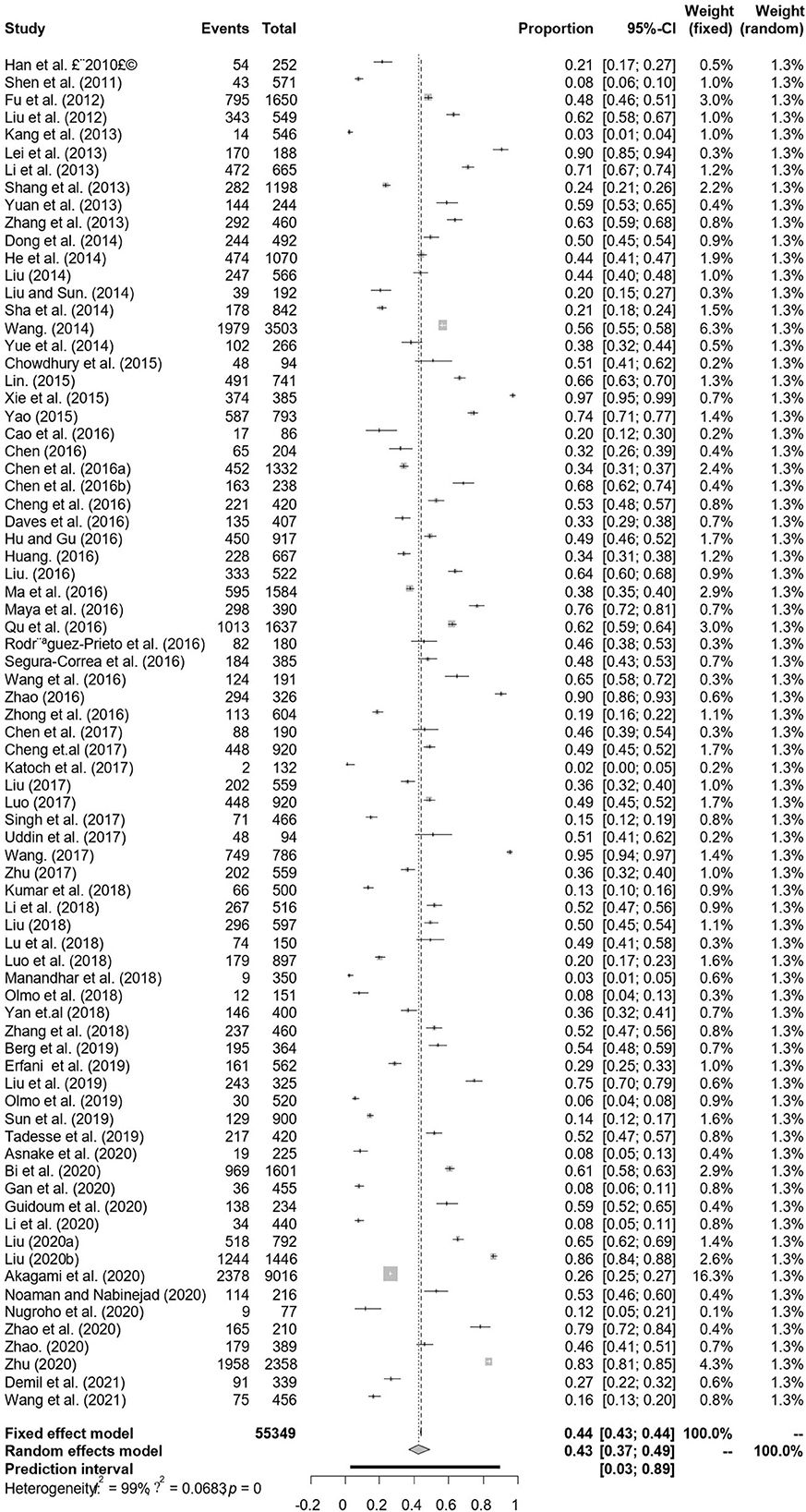
Figure 5. Forest plot of bovine viral diarrhea virus antibody prevalence in the world study conducted 2010–2021 (decetion antibody).
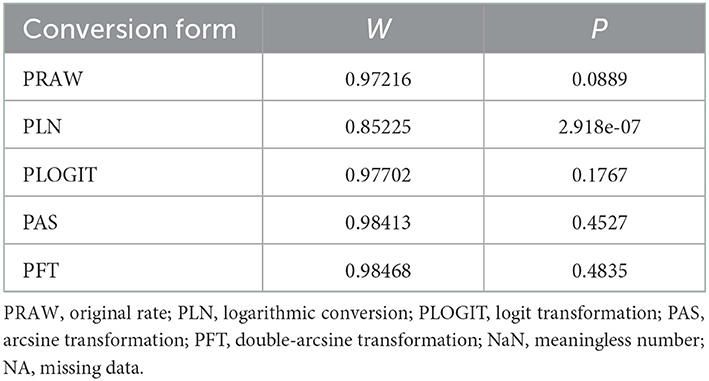
Table 7. Normal distribution test for the normal rate and the different conversion of the normal rate.
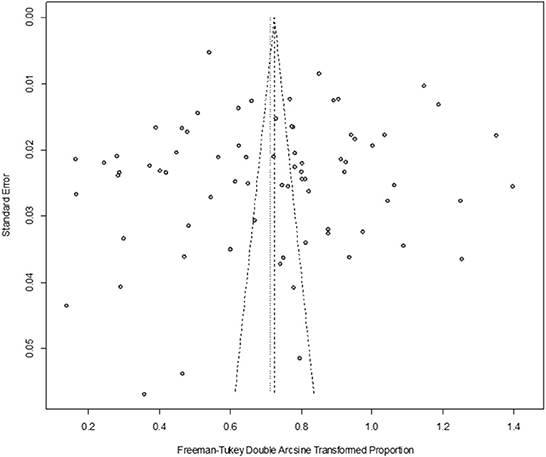
Figure 7. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias (decetion antibody).
4. Discussion
BVDV is one of the most important bovine infectious diseases with global animal health and economic impacts. BVDV infection will not only cause huge economic losses to the breeding industry, but also in animal research and medical industry related serum, vaccines and other biological not infected with BVDV but contaminated with BVDV, which has a huge economic impact (150). BVDV can be spread in many ways. BVDV is widely transmitted, not only through direct contact, but also through various excreta, contaminated materials, etc (151). However, vertical transmission plays an important role in its epidemiology and pathogenesis. PI calves produced by pregnant cows through vertical transmission are the main source of infection of the disease, and they continue to be infected and carry BVDV pathogens throughout their lives. PI cattle are the main host of the virus. A large number of viruses are excreted from urine, feces, excrement, milk and semen, causing serious obstacles to the control of the disease.
The article searched all articles on the epidemiology of bovine BVDV in 2010–2021. The meta-analysis included 128 articles. Through the analysis, it is expected to investigate the latest data on the global prevalence of BVDV and provide data support for the prevention and control of BVDV. The detection of BVDV is usually divided into the detection of antigens and the detection of antibodies. A positive antigen represents the current prevalence of animals carrying BVDV pathogens, making it clear that the virus is spreading and harming the population. Positive antibody indicates infection, vaccine immunization or transient infection. As individuals immunized with vaccine are excluded, positive antibody in the article can be considered as being infected by virus. Both have important guiding significance for the description of the BVDV epidemic.
In the regional subgroup, there were fewer test samples in Europe, possibly due to large-scale vaccinations in Europe and not included in the study; On the other hand, it may be due to the fact that many European countries have eradicated BVDV or the prevalence rate has dropped to 1.5% (152). Examples include Denmark, Norway, Sweden and Finland (153). Switzerland, Austria and Germany are in the late or final stages of eradicating BVDV, followed by plans to eradicate BVDV in the Netherlands, Ireland and Poland (19). Control measures in several countries are mainly aimed at the clearance of PI animals. As early as 1990, a non-vaccination program in the Scandinavian countries was implemented to eliminate BVDV, which was planned to detect and remove PI animals based on ear groove samples (154). The Swiss clearance program restricts action mainly on pregnant cattle and directly tests for antigenic and viral genomes (155). Ireland's clearance program focuses on monitoring ear groove samples from newborn calves (156). PI animals are immune to BVDV and are unable to develop specific antibodies against it, which increases the obstacle to virus clearance and is also the main source of infection of the disease (157). And the mutagenicity of the virus itself, as well as the infection of cp BVDV from the outside world, has developed into a fatal mucosal disease, causing serious harm to the herd (158). There is evidence that when PI animals disappear, population virus transmission is largely stopped. However, the impact of removing only PI without considering TI is still debatable. There are cases where BVDV will persist for 6 years without PI mavericks (159). The successful implementation of a BVDV control plan should consider the impact of both modes of infection. In the process of removing PI, the prevalence rate should be monitored at the same time, and TI animals should be monitored in a timely manner. While the prevalence of PI animals varies from region to region in terms of legal support, it took nearly 10 years for all countries to reach the final stages of the control plan (160, 161). The long-term implementation of the plan also suggests that in order to successfully complete the purification, strict policies, strict management, and a high degree of prevention awareness of practitioners are required.
In the regional subgroups, the low prevalence rate in North America and the high prevalence rate in South America and South America may result in a small number of articles and be unrepresentative due to the limitations of search. There is no data on antigen testing in Africa, while the prevalence of antibody testing remains high. This also reminds us that although many African countries have carried out surveillance and culling of BVDV, it will take a long time to eliminate BVDV. Asia has the largest literature and the infection rate remains high, with no significant differences in regional subgroups. The high infection rate in Asia may be due to the lack of a sound control plan and a surge in herd numbers due to the rapid development of the cattle industry. From the measures in different regions, we can find that the control plan in Poland suggests that it is very important to control the possible risk of virus transmission if the eradication plan is to be successful. From the German plan, it can be found that voluntary policies are not enough to achieve freedom from disease, and the initial implementation of voluntary policies eventually leads to mandatory plans (19). The control plan of the Netherlands points out that in areas where BVDV has been eradicated, the increase of susceptible animals makes the area more affected by BVDV, so timely detection should be carried out to reduce the possibility of transmission of BVDV (162). Monitoring plays an important role in reducing the spread of BVDV, and the comprehensiveness of the sample survey is critical to the success of the eradication plan (20). It can be concluded from the control and eradication plans implemented in different countries and regions that the identification and isolation of PI animals is the key to the eradication plan, and vaccination and appropriate safety measures are the basic methods of the control plan (163). Therefore, countries and areas that have implemented eradication plans should conduct timely and regular prevalence surveys. Other areas should implement corresponding eradication plans as soon as possible.
Due to different control measures in different regions, a subgroup analysis of time for global BVDV antigen testing and antibody testing found that the prevalence after 2017 did not decrease significantly compared with the prevalence before 2017. Previous articles have analyzed that in the global region, the prevalence of PI showed a downward trend from 1982 to 2016, while the level of antibody prevalence was relatively stable (164). Our data also shows that the prevalence of antigens and antibodies has remained relatively stable since 2017. It is suggested that we should improve the corresponding eradication policy and give certain time and patience to eliminate pathogens. BVDV still has a high infection rate, and this high spread may be due to the lack of complete prevention and control measures for BVDV, the most important reason being the failure to detect and eliminate PI animals (151). In addition, the lack of commercial vaccines and reasonable and effective prevention and control programs is one of the reasons for the high prevalence of BVDV (27). Commercial transportation, fertilization of breeding cattle, and the introduction of new herds are all indispensable factors in the spread of disease, hindering the eradication of BVDV. Therefore, it is important to improve the monitoring of BVDV and introduce relevant control measures (151, 165).
In the breed subgroup, the antibody test results were the highest among dairy cattle, with significant difference. On the one hand, it may be due to the long service period of dairy cows, which have more opportunities to contact with pathogens. Studies have also confirmed that the positive rates of tuberculosis, brucellosis and bovine leukemia virus in dairy cows are higher than those in beef cattle (165, 166). On the other hand, compared with beef cattle, there may be more contact between cows and milkers, and the cross-infection among different cows is more intensive during milking, which leads to more opportunities for virus contact and greater risk of infection (142). Among the antigen test results, the positive rate of dairy cows was the lowest, and the difference was significant. Perhaps this is because the harm of antigen-positive cows to dairy cows is more intuitive, such as decreased milk yield, stillbirth, abortion, etc (12, 167, 168), and the production performance of dairy cows needs higher health, so antigen-positive cows are eliminated in time (169–171). For different breeds of cattle, different control measures should be taken according to different economic uses and lifestyles, and strict management should be taken to reduce the prevalence of BVDV.
Age has long been considered the most common influencing factor associated with infection rates (172). The data in the article show that the prevalence of adult cattle is higher than that of calve, and there is no significant difference. Many studies have also pointed out that the prevalence of adult cattle is higher than that of calves as a factor of age (74, 149, 173). This may be due to the longer survival time of adult cattle, a higher chance of exposure to the virus, and a higher probability of infection (141). In addition, antibody prevalence in calves is much higher than antigen prevalence, possibly due to the fact that calves can obtain colostrum antibodies from the mother (174). For calves, whether they are PI cattle carrying antigens should be detected in time, and eliminated in time to prevent the spread of infection. Adult cattle should have a reasonable detection system and a sound management system to reduce the chance of contact with the virus and reduce the prevalence of the disease.
The survey data of the article shows that the prevalence rate is relatively high in winter and spring, no matter for antigen detection or antibody detection. This may be due to the breeding season in spring and winter. It has been reported that in winter and spring, both male and female animals have strong reproductive performance, which is more conducive to cattle breeding (175, 176). The spread of BVDV in the breeding process led to the birth of more PI positive calves, further promoting the increase of the positive rate. The prevalence of BVDV antibody is still high in summer. It may be that the PI animals produced continuously expel viruses to the external environment, leading to the expansion of infection. Some literature points out that there is no antiviral drug to prevent the spread of BVDV in the farm at present, and the spread of the virus can only be prevented by isolating PI animals or vaccinating (177). Therefore, BVDV detection should be done well in the breeding season to reduce the production of PI animals and control the spread of BVDV from the source.
Many articles point out that gender does not have a large influence on infection rates, and bulls are just as susceptible as cows (74). The results of this survey show that there is no significant difference, which is consistent with other research results. Investigation samples of cows are much larger than those of bulls, possibly due to the fact that bulls are mostly used as beef cattle and female cows are used for milk production and reproductive purposes. Female cattle are more affected by the disease. Since PI calves born of cow infection during the first trimester of pregnancy are the main source of infection of the disease, cow test samples are collected in antigen testing to prevent the birth of PI cattle. Therefore, the prevention and control of PI cattle can be screened for antigens from pregnant cows.
In the introduction of BVDV 2018, the diagnosis of BVDV includes nucleic acid detection of QPCR, antigen antibody detection of ELISA, IHC, VN and virus isolation. PCR method can almost meet all purposes of detection, including making group animals free from infection, individual animals free from infection before moving, promoting the implementation of eradication policy, confirmation of clinical cases and detection of infection rate. PCR detection method has the advantages of convenience, rapidity and large sample size. The ELISA method is not applicable to animals with acute infection. IHC is mainly for diagnostic investigation. VN and virus isolation are usually used in laboratory research. It can be seen in the antigen detection data that the positive rate of PCR test is higher than the positive rate of ELISA test. There have also been reports of low sensitivity and accuracy of ELISA testing compared to PCR (178). Young animals can also obtain BVDV antibodies from the milk of female animals, thereby reducing the detection rate of ELISA antigens. This is consistent with the findings of this paper. VN has the highest detection rate, but it is difficult to detect, the sample detection volume is small, and it is generally not used in epidemiological investigations (28). In epidemiological investigations, antibody testing mostly chooses the rapid and inexpensive ELISA test, while the antigen test chooses a more sensitive PCR test (179).
The results of the survey show that most of the antibody test samples are derived from serum, and the test is relatively mature. The feces positivity rate was highest among the antigen-tested samples, followed by blood samples and ear tissue sample. The ear tissue sample is significantly different from other samples, and its low prevalence rate may be due to the fact that the sample was collected from the area where BVDV purification was carried out. Feces samples and blood samples can reflect the prevalence of infection. Feces sample collection is more convenient, the harm to cattle is small, but there is a possibility of cross-infect; Stress may occur on cattle during blood sample collection; Ear tissue samples are often used for the removal of PI cattle under the purification policy of various regions. For the detection of BVDV in cattle, the most appropriate samples shall be taken according to local conditions to make the detection results comprehensive and correct (180).
Breeding mode has always been a key factor affecting BVDV infection. An article survey shows that the low prevalence of grazing and breeding is due to the low density of grazing and breeding (40). However, some data show that the prevalence of intensive farming is low, and some studies show that grazing and breeding have the opportunity to contact more pathogens. Studies have pointed out that although BVDV cannot be transmitted by flies, flies have been shown to carry the BVDV pathogen (181). In the breeding mode subgroup, the positive rate of intensive culture was higher than that of extensive culture. On the one hand, there may be errors in monitoring the infection rate due to the difficulty in sampling free range animals. On the other hand, the virus may spread widely due to the high density of intensive farming. In addition, BVDV is introduced and spread through contaminated houses, water tanks, feeds and feeding equipment (182).
When BVDV infection occurs, the clinical symptoms of acute infected animals usually include temperature rise to 40°C, diarrhea, oral erosion, etc. There are few or no clinical symptoms observed in other infections (183). Mucosal diseases induced by BVDV do not show clinical symptoms within 1 week. After 1 week, severe diarrhea, dehydration, anorexia, and lethargy will occur, and death will occur 1 week after clinical symptoms (184). Due to the low incidence of acute infection and mucosal disease, BVDV infection will not lead to obvious clinical infection, or only non-specific clinical symptoms and immunosuppression (6). The immunosuppression will be secondary to other pathogenic infections, which may cause a series of clinical symptoms and endanger the health level of livestock (185). Through the division of clinical health, the results showed that the prevalence rate of cattle with clinical symptoms was higher than that of clinical healthy cattle, indicating that regular detection of cattle health was also an important way to prevent and control infection. Therefore, whether or not having a sound management system is the key to affecting the infection rate of the intensive breeding industry. A reasonable and perfect management system can greatly reduce the spread of virus.
Through our meta-analysis, we found that the prevalence of BVDV in the world is still very high. In the areas where the eradication plan is implemented, attention should still be paid to controlling the possible transmission risk of the virus. In terms of time span, the control and elimination of BVDV requires the joint efforts of all countries and regions to develop reasonable and effective prevention and control programs to eliminate PI animals. At the same time, the elimination of BVDV requires a certain degree of patience, timely grasp the epidemic situation, and improve the prevention and control policy. Different control measures should be taken for different breeds of cattle, and strict management policies are required to reduce BVDV infection. After calves are born, they should be tested for antigen in time to reduce the birth of PI cattle. BVDV detection and elimination should be done well in winter and spring breeding seasons. For cows, it is necessary to timely detect whether there is antigen infection before pregnancy to prevent the production of PI cattle. The ear tissue samples selected for antigen monitoring are more accurate, VN detection method has a higher accuracy, while PCR detection method has a wide detection range and a large sample detection volume. Generally, ELISA is used to detect serum samples. In raising cattle, attention should be paid to the cleanliness and hygiene of the breeding environment.
To sum up, based on the epidemiological situation of BVDV in different areas, the eradication and prevention policies should be formulated and revised in time. Meanwhile, it is necessary to strengthen the awareness of herders to diseases and increase the awareness of veterinary and other related professionals to prevent and control BVDV. Our meta-analysis still has some limitations. The main reasons are as follows: 1. Due to the choice of language and database, it was not included in all studies. 2. The data cannot be downloaded or excluded from the inclusion and exclusion criteria. 3. Many countries do not have perfect testing procedures and do not test all cattle.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RD and KS: idea and concept. NS: writing and editing of the manuscript. RD: funding. H-YL, L-ML, QW, and KS: revision of the manuscript. Q-XM, T-TW, WZ, and T-LY: collection and extraction of data. TT and J-YY: database establishment. TL, N-CD, QW, and J-ML: data analysis. All the authors contributed to the editing of the manuscript and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31672577).
Acknowledgments
We thank the scientists and personnel of the College of Animal Science and Technology, Jilin Agricultural University, and the College of Chinese Medicine Materials, Jilin Agricultural University, for their collaboration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1086180/full#supplementary-material
Abbreviations
BVDV, Bovine viral diarrhea virus; PRISMA, Preferred reporting items for systematic reviews and meta-analyses.
References
1. Brownlie J. The pathogenesis of bovine viral diarrhoea virus infections. Rev Sci Tech. (1990) 9:43–59. doi: 10.20506/rst.9.1.491
2. Abdelsalam K, Rajput M, Elmowalid G, Sobraske J, Thakur N, Abdallah H, et al. The effect of bovine viral diarrhea virus (BVDV) strains and the corresponding infected-macrophages' supernatant on macrophage inflammatory function and lymphocyte apoptosis. Viruses. (2020) 12:701. doi: 10.3390/v12070701
3. Mosena A, Falkenberg SM, Ma H, Casas E, Neill, JD. Multivariate analysis as a method to evaluate antigenic relationships between BVDV vaccine and field strains. Vaccine. (2020) 38:5764–72. doi: 10.1016/j.vaccine.2020.07.010
4. Meyers G, Thiel HJ. Molecular characterization of pestiviruses. Adv Virus Res. (1996) 47:53–118. doi: 10.1016/S0065-3527(08)60734-4
5. Peterhans E, Jungi TW, Schweizer M. BVDV and innate immunity. Biologicals. (2003) 31:107–12. doi: 10.1016/S1045-1056(03)00024-1
6. Brodersen BW. Bovine viral diarrhea virus infections: manifestations of infection and recent advances in understanding pathogenesis and control. Vet Pathol. (2014) 51:453–64. doi: 10.1177/0300985813520250
7. Duan H, Ma Z, Xu L, Zhang AK, Li ZW, Xiao SQ. A novel intracellularly expressed NS5B-specific nanobody suppresses bovine viral diarrhea virus replication. Vet Microbiol. (2019) 240:108449. doi: 10.1016/j.vetmic.2019.108449
8. Arnaiz I, Cervio M, Martínez S, Fouz R, Diéguez FJ. Bovine viral diarrhea virus (BVDV) infection: effect on reproductive performance and milk yield in dairy herds. Vet J. (2021) 277:105747. doi: 10.1016/j.tvjl.2021.105747
9. Vilcek S, Nettleton PF, Paton DJ, Belák S. Molecular characterization of ovine pestiviruses. J Gen Virol. (1997) 78:725–35. doi: 10.1099/0022-1317-78-4-725
10. Vilcek S, Nettleton PF. Pestiviruses in wild animals. Vet Microbiol. (2006) 116:1–12. doi: 10.1016/j.vetmic.2006.06.003
11. de Oliveira LG, Mechler-Dreibi ML, Almeida HMS, Gatto IRH. Bovine viral diarrhea virus: recent findings about its occurrence in pigs. Viruses. (2020) 12:600. doi: 10.3390/v12060600
12. Houe H. Economic impact of BVDV infection in dairies. Biologicals. (2003) 31:137–43. doi: 10.1016/S1045-1056(03)00030-7
13. Yarnall MJ, Thrusfield MV. Engaging veterinarians and farmers in eradicating bovine viral diarrhoea: a systematic review of economic impact. Vet Rec. (2017) 181:347–347. doi: 10.1136/vr.104370
14. Al-Kubati AAG, Hussen J, Kandeel M, Al-Mubarak AIA, Hemida MG. Recent advances on the bovine viral diarrhea virus molecular pathogenesis, immune response, and vaccines development. Front Vet Sci. (2021) 8:665128. doi: 10.3389/fvets.2021.665128
15. Yue X, Steeneveld W, van der Voort M, van Schaik G, Vernooij JCM, van Duijn L, et al. The effect of bovine viral diarrhea virus introduction on milk production of Dutch dairy herds. J Dairy Sci. (2020) 104:2074–86. doi: 10.3168/jds.2020-18866
16. Walz PH, Chamorro MF, M Falkenberg S, Passler T, van der Meer F, R Woolums A. Bovine viral diarrhea virus: an updated american college of veterinary internal medicine consensus statement with focus on virus biology, hosts, immunosuppression, and vaccination. J Vet Intern Med. (2020) 43:1690–706. doi: 10.1111/jvim.15816
17. Bitsch V, Hansen KE, Rønsholt L. Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994-1998 with special reference to legislation and causes of infection. Vet Microbiol. (2000) 77:137–43. doi: 10.1016/S0378-1135(00)00270-4
18. Strain S, Verner S, Campbell E, Hodnik JJ, Santman-Berends IMGA. The Northern ireland control programmes for infectious cattle diseases not regulated by the EU. Front Vet Sci. (2021) 8:694197. doi: 10.3389/fvets.2021.694197
19. Wernike K, Gethmann J, Schirrmeier H, Schröder R, Conraths FJ, Beer M. Six Years (2011-2016) of mandatory nationwide bovine viral diarrhea control in Germany-a success story. Pathogens. (2017) 6:50. doi: 10.3390/pathogens6040050
20. Schweizer M, Stalder H, Haslebacher A, Grisiger M, Schwermer H, Di Labio E. Eradication of bovine viral diarrhoea (BVD) in cattle in Switzerland: lessons taught by the complex biology of the virus. Front Vet Sci. (2021) 8:702730. doi: 10.3389/fvets.2021.702730
21. Nugroho W, Silitonga RJP, Reichel MP, Irianingsih SH, Wicaksono MS. The epidemiology and control of bovine viral diarrhoea virus in tropical Indonesian cattle. Pathogens. (2022) 11:215. doi: 10.3390/pathogens11020215
22. Egger M, Smith GD. Bias in location and selection of studies. BMJ. (1998) 316:61–6. doi: 10.1136/bmj.316.7124.61
23. Egger M. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
24. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193. doi: 10.1037/1082-989X.11.2.193
25. Xu CQ, Hou HJ, Jiang X, Fu XB, Li LA, Ai X, et al. Isolation, characterization and molecular epidemiology of Bovine Viral Diarrhea Virus in Tianjin from 2018 to 2019. J Infect Dis. (2020) 28:24–8.
26. Deng ML, Chen N, Guidarini C, Xu ZH, Zhang JJ, Cai LJ, et al. Prevalence and genetic diversity of bovine viral diarrhea virus in dairy herds of China. Vet Microbiol. (2020) 242:108565. doi: 10.1016/j.vetmic.2019.108565
27. Guo T, Zhang J, Chen X, Wei X, Wu C, Cui Q, et al. Investigation of viral pathogens in cattle with bovine respiratory disease complex in Inner Mongolia, China. Microb Pathog. (2020) 153:104594. doi: 10.1016/j.micpath.2020.104594
28. Chang L, Qi Y, Liu D, Du Q, Zhao X, Tong D. Molecular detection and genotyping of bovine viral diarrhea virus in Western China. BMC Vet Res. (2021) 17:66. doi: 10.1186/s12917-021-02747-7
29. Zhang L, Wang JL, Sun YY, Yang HJ, Zhang YF, Jiang RX, et al. Pathogenic detection of viruses related to calf diarrhea in dairy farms in Shandong Province during 2017 to 2018. Chin J Anim Health Insp. (2020) 37:5.
30. Long MC. Epidemiological investigation of bovine viral diarrhea virus. Today Anim Husbandry Vet Med. (2019) 35:15. doi: 10.3969/j.issn.1673-4092.2019.05.011
31. Wang JT, Sang XB, Shi QW, Diao CX, Zhuang YL, et al. Serological investigation of bovine viral diarrhea antigens in large-scale dairy farms in Heilongjiang Province. Heilongjiang Anim Sci Vet Med. (2012) 91–2.
32. Lee SH, Kim HY, Choi EW, Kim D. Causative agents and epidemiology of diarrhea in Korean native calves. J Vet Sci. (2019) 20:e64. doi: 10.4142/jvs.2019.20.e64
33. Li ZY. Investigation on the Main Pathogens of Calf Diarrhea in Henan Province and Analysis of Two Pathogens. Henan Agricultural University (2019).
34. Wang HR. The investigation of major pathogens of the calf diarrhea and the analysis of biological characteristics of E. coli in 13 provinces, China. Chinese Acad Agric Sci. (2017).
35. Wang MC, Yue H, Tang C, Yang ZL. Detection and genetic evolution of diarrhea-related viruses in Chongqing beef cattle. China Anim Husbandry Vet. (2017) 44:2731–8. doi: 10.16431/j.cnki.1671-7236.2017.09.027
36. Chen XN, Xiao M, Ruan WQ, Qin SN, Yue H, Tang C, et al. Molecular epidemiological investigation and isolation of bovine viral diarrhea virus in yak in Sichuan-Tibet plateau region. Chin J Anim Vet Sci. (2018) 49:606–13.
37. Li J. Investigation and analysis of yak diarrhea in Qinghai. Today Anim Husbandry Vet Med. (2019) 35:23. doi: 10.3969/j.issn.1673-4092.2019.11.016
38. Yan ZY, Lv BL, La J, Hai CX, Li WY. Detection and analysis of five viral pathogens associated with diarrhea in yaks in Huangzhong County, Qinghai Province. Anim Husb Vet Med. (2019) 51:88–92.
39. Sun L, Qin J. Epidemiological investigation on the pathogen of calf diarrhea in Bazhou, Xinjiang. Heilongjiang Anim Sci Vet Med. (2019) 3.
40. Han DG Ryu JH, Park J, Choi KS. Identification of a new bovine viral diarrhea virus subtype in the Republic of Korea. BMC Vet Res. (2018) 14:233. doi: 10.1186/s12917-018-1555-4
41. Ryu JH, Choi KS. Genetic analysis of bovine viral diarrhea virus in pre-weaned native Korean calves. Trop Anim Health Pro. (2019) 51:2085–90. doi: 10.1007/s11250-019-01882-6
42. Kim Y, Kim Y, Lee SY, Lee KK, Lee KH, Song JC, et al. Identification of Korean native cattle persistently infected with BVDV using Ear-notch method. Korean J Vet Serv. (2019) 42:117–20. doi: 10.7853/kjvs.2019.42.2.117
43. Zhang K. Investigation of Calves Viral Diarrhea Related Pathogen of Large-Scale Dairy Farm in Northern XinJiang Region. Shihezi University (2016). Available online at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201701&filename=1016778661.nh
44. Luo YJ, Li J, Su GC, Qu YG, Cao SZ, Li Y. Investigation on infection of major viral reproductive disorders in dairy farms. China Anim Health Insp. (2015) 32:15–7.
45. Quan YC, Liu HS. Investigation on the infection of three bovine viral diarrhea pathogens in some areas of Qinghai Province. Chung-kuo Hsu Mu Shou I. (2014) 41:220–3.
46. LV JJ, Zhang LQ. Epidemiological investigation of viral diarrhea and infectious rhinotracheitis in Qinghai Yaks. Acta Ecologiae Animalis Domastici. (2014) 35:59–63.
47. Liu XP, Song K, Gao YW, Fu XZ, Wang ZS, Wu T. Serological investigation on major infectious diseases of beef cattle and crossbred beef cattle in Wujiaqu City, Xinjiang. China Cattle Sci. (2017) 43:73–5.
48. Kaveh A, Merat E, Samani S, Danandeh S, Soltan Nezhad S. Infectious causes of bovine abortion in Qazvin Province, Iran. Arch Razi Inst. (2016) 72:225–30. doi: 10.22092/ari.2017.113299
49. Wang HM, Li XH, Zhang HJ, Zhang LG, Zhang XL, Yin LL, et al. Report on detection results of bovine viral diarrhea in Weichang County, Hebei Province in 2017. Vet Orient. (2020) 2.
50. Wang TL. Investigation of BVD Infection and Isolation and Identification of Epidemic Strains in an Area of Henan. Tarim University (2020).
51. Li DL, Zhao JY, Shen JL, Shi CJ, Cao J. Risk assessment and epidemic analysis of infectious rhinotracheitis and bovine viral diarrhea in dairy farms in Beijing. China Dairy Cattle. (2013) 31–3. doi: 10.3969/j.issn.1004-4264.2013.11.009
52. Agah MA, Notsu K, El-Khaiat HM, Arikawa G, Kubo M, Mitoma S, et al. Slaughterhouse survey for detection of bovine viral diarrhea infection among beef cattle in Kyushu, Japan. J Vet Med Sci. (2019) 81:1450–4. doi: 10.1292/jvms.19-0045
53. Zhang SX, He MR, Yu HJ, He BN, Zhao SQ, Wang L, et al. Detection of Bovine viral diarrhea virus using qRT-PCR combined with double-antibody sandwich ELISA in a large-scale cattle farm in Heilongjiang Province. Heilongjiang Anim Sci Vet Med. (2019) 72–5. doi: 10.13881/j.cnki.hljxmsy.2019.01.0308
54. Yao ZL, Fu HQ, Cui PF, Zong JL. Molecular epidemiology of bovine viral diarrhea virus and identification of a BVDV−2 isolate in regional area of Jiangsu and Zhejiang provinces. J Yangzhou University. (2019) 40:40–6. doi: 10.16872/j.cnki.1671-4652.2019.01.007
55. Song WB, Ma CS, Guo JM, Ma LT, Zhang XY. Etiological investigation and analysis of yak infected with bovine viral diarrhea virus and bovine enterovirus in Haibei Prefecture, Qinghai Province from 2016 to 2017. Heilongjiang Anim Sci Vet Med. (2019) 88–90. doi: 10.13881/j.cnki.hljxmsy.2018.03.0120
56. Wang H, Man HY. Epidemiological investigation and analysis of bovine viral diarrhea virus in Liangzhou District, Wuwei City, Gansu Province. China Dairy Cattle. (2020) 26–9. doi: 10.19305/j.cnki.11-3009/s.2020.12.007
57. Wei Q, Qu YG, Chang JS, Gu SY, Wu YY, Yu HJ, et al. Molecular epidemiological investigation of bovine viral diarrhea in some areas of Xinjiang. Anim Husb Vet Med. (2020) 52:105–9.
58. Safarpoor Dehkordi F. Prevalence study of Bovine viral diarrhea virus by evaluation of antigen capture ELISA and RT-PCR assay in Bovine, Ovine, Caprine, Buffalo and Camel aborted fetuses in Iran. AMB Express. (2011) 1:32. doi: 10.1186/2191-0855-1-32
59. Weng XG. Epidemiologic Survey of Bovine Viral Diarrhea in Beijing Region, Study of IFN- α/β Response in Persistently Infected Cattle and Immunomodulatory Effects of Forsythoside A. China Agriculture University (2015).
60. Zhang X, Huang KH, Zhang KC. Serological investigation of two viral diseases in dairy cows in Shanghai. China Diary. (2015) 54–9. doi: 10.3969/j.issn.1004-4264.2015.14.015
61. Mishra N, Rajukumar K, Pateriya A, Kumar M, Dubey P, Behera SP, et al. Identification and molecular characterization of novel and divergent HoBi-like pestiviruses from naturally infected cattle in India. Vet Microbiol. (2014) 174:239–46. doi: 10.1016/j.vetmic.2014.09.017
62. Gangil R, Kaur G, Dwivedi PN. Detection of respiratory viral antigens in nasal swabs of bovine by sandwich ELISA. Indian J Anim Res. (2020) 54:354–8. doi: 10.10.18805/ijar.B-3769
63. Alam MR, Afrin K, Dash AK, Bhowmik DK, Sen AB, Nath S. Incidence and therapeutic management of viral diseases in cattle at Jaintapur, Sylhet, Bangladesh. J Adv Res. (2016) 3:13–20.
64. Yitagesu E, Jackson W, Kebede N, Smith W, Fentie T. Prevalence of bovine abortion, calf mortality, and bovine viral diarrhea virus (BVDV) persistently infected calves among pastoral, peri-urban, and mixed-crop livestock farms in central and Northwest Ethiopia. BMC Vet Res. (2021) 17:87. doi: 10.1186/s12917-021-02798-w
65. Asmare K, Sibhat B, Ayelet G, Gebremedhin EZ, Lidete KA, Skjerve E. Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg virus infections in relation to reproductive disorders in dairy cattle in Ethiopia. Acta Tropica. (2018) 178:236–41. doi: 10.1016/j.actatropica.2017.12.005
66. Fernández-Aguilar X, López-Olvera JR, Marco I, Rosell R, Colom-Cadena A, Soto-Heras S, et al. Pestivirus in alpine wild ruminants and sympatric livestock from the Cantabrian Mountains, Spain. Vet Rec. (2016) 178:586. doi: 10.1136/vr.103577
67. Decaro N, Lucente MS, Lanave G, Gargano P, Larocca V, Losurdo M, et al. Evidence for circulation of bovine viral diarrhoea virus type 2c in ruminants in Southern Italy. Transbound Emerg Dis. (2017) 64:1935–44. doi: 10.1111/tbed.12592
68. Caffarena RD, Casaux ML, Schild CO, Fraga M, Castells M, Colina R, et al. Causes of neonatal calf diarrhea and mortality in pasture-based dairy herds in Uruguay: a farm-matched case-control study. Braz J Microbiol. (2021) 52:977–88. doi: 10.1007/s42770-021-00440-3
69. Maya L, Macías-Rioseco M, Silveira C, Giannitti F, Castells M, Salvo M, et al. An extensive field study reveals the circulation of new genetic variants of subtype 1a of bovine viral diarrhea virus in Uruguay. Arch Virol. (2020) 165:145–56. doi: 10.1007/s00705-019-04446-z
70. Paixão SF, Fritzen JTT, Alfieri AF, Alfieri AA. Virus neutralization technique as a tool to evaluate the virological profile for bovine viral diarrhea virus infection in dairy water buffalo (Bubalus bubalis) herds. Trop Anim Health Prod. (2018) 50:911–4. doi: 10.1007/s11250-017-1503-5
71. Viana RB, Monteiro BM, Souza DC. Sensitivity and specificity of indirect ELISA for the detection of antibody titers against BVDV from beef cattle raised in Pará State. Semina Ciências Agrárias. (2017) 38:3049–58. doi: 10.5433/1679-0359.2017v38n5p3049
72. Freitas BB, Correa A, Valotto AA, Marcom NN, Paulino LR, Brum JS, et al. Prevalence of bovines persistently infected with bovine viral diarrhea virus (BVDV) in dairy cattle herds in Paraná State, Brazil. Pesquisa Vet Brasil. (2021) 41. doi: 10.1590/1678-5150-pvb-6622
73. Stephenson MK, Palomares RA, White BJ, Engelken TJ, Brock KV. Prevalence of bovine viral diarrhea virus (BVDV) persistently infected calves in auction markets from the southeastern United States; association between body weight and BVDV-positive diagnosis. Profess Anim Sci. (2017) 33:426–31. doi: 10.15232/pas.2017-01619
74. Demil E, Fentie T, Vidal G, Jackson W, Smith W. Prevalence of bovine viral diarrhea virus antibodies and risk factors in dairy cattle in Gondar city, Northwest Ethiopia. Chin J Prev Vet Med. (2021) 191:105363. doi: 10.1016/j.prevetmed.2021.105363
75. Guidoum KA, Benallou B, Pailler L, Espunyes J, Napp S, Cabezón O. Ruminant pestiviruses in North Africa. Prev Vet Med. (2020) 184:105156. doi: 10.1016/j.prevetmed.2020.105156
76. Berg M, Ramabu SS, Wensman JJ, Lysholm S. First-time detection of bovine viral diarrhoea virus, BVDV-1, in cattle in Botswana. Onderstepoort J Vet Res. (2019) 86:1–7.
77. Wang W. Serosurvey of major bovine resporitary viruses and identification of BVDV isolates and vaccine development. Chin Acad Agric Sci. (2014).
78. Sha JM, Ka ZC, Wang ZS. Serological investigation of three viral diarrhea diseases in cattle herds in Huangnan Prefecture, Qinghai Province. Anim Husb Vet Med. (2014) 46:94–6.
79. Lin XY. The Infection Status Investigation of Five Major Diseases in Large-scale Farms in Parts of Shandong Province. Shandong Agriculture University (2015).
80. Huang ZL. The Serosurvey of Major Diseases in Cattle Farms in Parts of Guangxi Province. Nanning: Guangxi University (2016).
81. Chen M, Liu P, Cheng ZL, Liu SD. Seroepidemiological survey of common infectious diseases in large-scale dairy farms in Shandong Province. China Cattle Sci. (2017) 43:74–7.
82. Wang QQ. Epidemiological Investigation and Analysis of Bovine Viral Diarrhea in Partial Scale Cattle Farms of the South Xinjiang. Aral: Tarim University (2017).
83. Lu CM, Yan ZG, Wang J. Serological investigation and Study on cow viral diarrhea in Jiading and Chongming areas of Shanghai. Vet Guide. (2018) 73–5.
84. Sun WM, Zhu J, Shen LH, Cao XY, Jin YC, Xu K, et al. Serological investigation of bovine viral diarrhea in Songjiang District, Shanghai. Shanghai J Anim Husbandry Vet Med. (2019) 50–2. doi: 10.14170/j.cnki.cn31-1278/s.2019.02.016
85. Zhao XL, Niu JQ, Ciren YJ, Ciren YZ, Suolang ZG, Wen DX, et al. Epidemiological Survey of Yak Diarrhea Virus BVDV, BCV and BRV. Plateau Agricult. (2020) 4:298–302. doi: 10.19707/j.cnki.jpa.2020.03.013
86. Bi YY, Song LL, Xue Y, Li JB, Li CH, Jia AQ, et al. Detection of serum antibodies against major pathogens leading to BRDC in large-scale dairy farms in four Provinces, Northern China. China Anim Quarant. (2020) 37:9–13.
87. Fu CX, Jin XJ, Zheng RF, Guo F, Han L, Li J, et al. Serological investigation of three viral diarrhea diseases in large-scale dairy farms in Beijing. Prog Vet Med. (2012) 33:85–8. doi: 10.16437/j.cnki.1007-5038.2012.05.018
88. Liu JQ. Investigation on bovine viral diarrhea in some large-scale dairy farms in Xinjiang. Modern Anim Husbandry. (2016) 6–7. doi: 10.14070/j.cnki.15-1150.2016.02.00
89. Liu Q. Serological investigation of Qinghai yak viral diarrhea virus. Contemp Livestock Poult Breed. (2017) 10–1. doi: 10.14070/j.cnki.15-1150.2017.03.008
90. Cheng SL, Wang G, Yixi CM, Luo RB, Zhou HB, Gong G, et al. Detection of serum antibody against viral diarrhea in Tibetan Yaks. Hubei J Anim Vet Sci. (2017) 38:5–6. doi: 10.16733/j.cnki.issn1007-273x.2017.05.001
91. Luo RB, Chen JC, Qu J, Cheng SL, Shen MY, Luo XL, et al. Serological investigation of viral diarrhea in Tibet yak. J Plateau Agricult. (2018) 2:261–5. doi: 10.19707/j.cnki.jpa.2018.03.007
92. Zhu GY. Epidemiological Investigation of BVDV in a Large Cattle farm in South Xinjiang. Aral: Tarim University (2020).
93. Yan XL, He YC, Li XR, Li S. Serological investigation of bovine viral diarrhea mucosal disease and infectious rhinotracheitis in Zhangye City. China Cattle Sci. (2018) 44:48–9.
94. Wang XL, Wang YM, Zhang YL, Wu YW, Li ZX, Zhang W, et al. Serological investigation of bovine viral diarrhea in some regions of Ningxia. Chin J Anim Health Insp. (2016) 33:17–9. doi: 10.3969/j.issn.1005-944x.2016.02.008
95. Zhao SY. The Isolation and Fabrication of E0 Genetic Prokaryptic Expression Vector of Bovine Viral Diarrhea Virus Isolated From Ningxia. Yinchuan: Ningxia University (2016) doi: 10.7666/d.y3109188
96. Cao ST, Guo YN, Lei YY, Bai XN, Ma Y, Xu YT, et al. Serological investigation on the causes of abortion in large-scale dairy farms in Wuzhong area of Ningxia. Prog Vet Med. (2016) 37:115–9. doi: 10.3969/j.issn.1007-5038.2016.04.025
97. Liu MY, Gao JF, Han ZQ, Zhang KR, Deng JH, Sun WD, et al. Seroprevalence of bovine viral diarrhea infection in yaks (Bos grunniens) in some counties of Qinghai- -Tibetan plateau, China. Chin Soc Zootechnics Vet Sci. (2012).
98. Li J, Li Y, Fan WX, Yuan LG, Qi YY, Pu JW, et al. Serological investigation of five epidemic diseases in some large-scale dairy farms in Xinjiang. Prog Vet Med. (2013) 24–7. doi: 10.3969/j.issn.1007-5038.2013.11.006
99. Olmo L, Reichel MP, Nampanya S, Khounsy S, Wahl LC, Clark BA, et al. Risk factors for Neospora caninum, bovine viral diarrhoea virus, and Leptospira interrogans serovar Hardjo infection in smallholder cattle and buffalo in Lao PDR. PLoS ONE. (2019) 14:e0220335. doi: 10.1371/journal.pone.0220335
100. Noaman V, Nabinejad AR. Seroprevalence and risk factors assessment of the three main infectious agents associated with abortion in dairy cattle in Isfahan province, Iran. Trop Anim Health Prod. (2020) 52:2001–9. doi: 10.1007/s11250-020-02207-8
101. Li JK, Li K, Han ZQ, Zhang H, Wang XQ, Luo HQ, et al. Serological survey of bovine viral diarrhoea virus among yaks (Bos poephagus grunniens) in Hongyuan of Sichuan, China. Pak J Zool. (2018) 50:1557–9. doi: 10.17582/journal.pjz/2018.50.4.sc7
102. Wang LP, Jin XD, Bi JL, Su YS, Yang C, Li JL, et al. Seroepidemiological investigation of bovine viral diarrhea mucosal disease in Yunnan Province. China Cattle Sci. (2021) 47:19–22.
103. Kumar SK, Palanivel KM, Sukumar K, Ronald BSM, Selvaraju G, Ponnudurai G. Herd-level risk factors for bovine viral diarrhea infection in cattle of Tamil Nadu. Trop Anim Health Prod. (2018) 50:793–9. doi: 10.1007/s11250-017-1497-z
104. Zhong YM, Zhang JF, Zhang J, Wang M, Su J, Zhang JY, et al. Monitoring and purification of breeding cattle disease in Heilongjiang Province. Chung-kuo Hsu Mu Shou I. (2016) 17–8.
105. Chen R, Fan XZ, Zhu YY, Zou XQ, Xu L, Zhang QY, et al. Prevalence study and phylogenetic analysis of bovine viral diarrhea virus in free-roaming beef cattle in Western China. Sci Agricul Sinica. (2016) 49:2634–41.
106. Liu P, Sun JW. Serological investigation on viral diarrhea and mucosal disease of dairy cows in Qian County, Shaanxi Province Farm Technology PC. Digest Magazine. (2014) 140.
107. Dong YS, Liu XQ, Li HR, Wang Y, Luo ZQ, Tang WS. Serological investigation of bovine viral diarrhea / mucosal disease in Qinghai Province. Heilongjiang Anim Sci Vet Med. (2014) 66–7. doi: 10.13881/j.cnki.hljxmsy.2014.0815
108. He ML, Zhang HR, Wang Y, Wang YX, Wang YW, Tang C. Serological investigation on three viral diarrhea diseases of Yaks in Northwest Sichuan. Chung-kuo Hsu Mu Shou I. (2014) 41:248–51.
109. Shang YP, Liu H, Zhang HL, Gao MC, Zhang WL, Wang JW. Serological investigation of bovine viral diarrhea-mucosal disease on scale dairy farms in the Northeast China. Chung-kuo Yu Fang Shou I Hsueh Pao. (2013) 35:559–61.
110. Han ZH, Quan H, He XL, Wei KF, Erdenizabu. Serological survey of viral diarrhea / mucosal disease and infectious rhinotracheitis in yaks. (2010) 18:56–9.
111. Ma JG, Cong W, Zhang FH, Feng SY, Zhou DH, Wang YM, et al. Seroprevalence and risk factors of bovine viral diarrhoea virus (BVDV) infection in yaks (Bos grunniens) in northwest China. Trop Anim Health Prod. (2016) 48:1747–50. doi: 10.1007/s11250-016-1118-2
112. Zhang XJ, Yang Y, Lin H, Zhu GQ. Epidemiological survey of bovine viral diarrhea and infectious bovine rhinotracheitis in dairy herds of Jiangsu province. Chin J Vet Sci. (2018) 38:69–76. doi: 10.16303/j.cnki.1005-4545.2018.01.10
113. Liu SQ. Serological investigation and analysis of cow viral diarrhea in some areas of Xinyang City, Henan Province. China Dairy. (2018) 54–6. doi: 10.16172/j.cnki.114768.2018.11.016
114. Luo RB. Tibet Bovine Viral Diarrhea Disease Epidemiology Investigation and Virus Isolation and Identification of Sequence Analysis. Lhasa: Tibet University (2017).
115. Qu P, Zhao BL, Hu DM, Shi H, Cao DS, Song XH. Investigation on the prevalence of bovine viral diarrhea in Western China. Heilongjiang Anim Sci Vet Med. (2016) 111–3. doi: 10.13881/j.cnki.hljxmsy.2016.0487
116. Xie CF, Yu RS, Li Z, Zhang RH, Si FS, Dong SJ. Epidemiological investigation on infectious rhinotracheitis and bovine viral diarrhea in large-scale dairy farms . China Dairy Cattle. (2015) 38–41. doi: 10.19305/j.cnki.11-3009/s.2016.04.0010
117. Chen XL. Epidemiological survey on cattle disease in Sanming city. Fujian J Anim Husbandry Vet. (2016) 38:1–3.
118. Chen FM, Cheng GM, Ma AX, Hu SL. Serological investigation of bvd-md, IBR and TB in dairy cows in Weifang City and surrounding areas. Heilongjiang Anim Sci Vet Med. (2016) 114–7. doi: 10.13881/j.cnki.hljxmsy.2016.1593
119. Hu RL, Gu JT. Epidemiological investigation of bovine viral diarrhea in large-scale dairy farms in Suzhou. Chung-kuo Hsu Mu Shou I. (2016) 32:115–6.
120. Cheng ZL, Liu P, Liu SD. Epidemiological study on three common epidemics in large-scale cattle farms in Shandong. China Cattle Sci. (2016) 42:44–8.
121. Uddin MA, Ahasan ASML, Islam K, Islam MZ, Mahmood A, Islam A, et al. Seroprevalence of bovine viral diarrhea virus in crossbred dairy cattle in Bangladesh. Vet World. (2017) 10:906–13. doi: 10.14202/vetworld.2017.906-913
122. Liu ZY, Liu ZY, Li ZJ, Guo Li, Zhang JL. Investigation of BVDV, IBRV and BRSV infection in some cattle farms of Jilin Province. Anim Husb Vet Med. (2019) 51:101–5.
123. Li BL, Tao J, Huang Z, Zhan T, Ma YL, Yu HF, et al. Serological investigation of bovine viral diarrhea in a dairy farm. Shanghai J Anim Husbandry Vet Med. (2020) 40–2. doi: 10.14170/j.cnki.cn31-1278/s.2020.04.015
124. Zhao N. Serological investigation and Study on cow viral diarrhea disease in Pingjibao area of Ningxia . Gansu Anim Vet Sci. (2020) 50:51–3. doi: 10.15979/j.cnki.cn62-1064/s.2020.01.020
125. Liu GS. Epidemiological Investigation and Prevention of IBR and BVD of Dairy Cows in Ningxia. Yinchuan: Ningxia University (2020).
126. Shen YL, Cai JS, Li J, Hu GW, Wang XR. Serological investigation of bovine viral diarrhea mucosal disease in Yushu District of Qinghai Province. Chin J Anim Health Insp. (2011) 28:52.
127. Erfani AM, Bakhshesh M, Fallah MH, Hashemi M. Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Trop Anim Health Prod. (2019) 51:313–9. doi: 10.1007/s11250-018-1687-3
128. Gan FB, Luo RB, Zhaxi CR, Baima SZ, Zhaxi WJ, Suolang SZ. Serological detection and analysis of antibody to yak viral diarrhea in bange County, Tibet . Gansu Anim Vet Sci. (2020) 50:68–70. doi: 10.15979/j.cnki.cn62-1064/s.2020.10.020
129. Kang XD, Xie XL, Wu SR, Ma C, Bai LJ. Serological investigation of viral diarrhea mucosal disease in dairy cows in Ningxia. Heilongjiang Anim Sci Vet Med. (2013) 90–1. doi: 10.13881/j.cnki.hljxmsy.2013.04.036
130. Lei CH, Guo FL, Wei L, Shu Z, Lv CH, Huang YB, et al. Serological survey of bovine viral diarrhea-mucosal disease. Chin J Vet Med. (2013) 49:18–9.
131. Zhang GW, Li Y, Hao WF, Shi XT. Epidemiological investigation of cow viral diarrhea in Taiyuan area. China Dairy. (2013) 39–42.
132. Yuan XJ, Zhang Y, Li NZ, Li CM, Yang BF, Wang ZL, et al. Serological investigation of six major bovine infectious diseases in some areas of Southwest China [C] / / National Conference on bovine disease control and industrial development Huazhong Agricultural University; Laboratory of disease control function of national modern agriculture (beef / yak) industrial technology system (2013).
133. Liu MY. Seroprevalence of Bovine Viral Diarrhea Infection in Yaks and BVDV Vaccine, Swine Fever Vaccine Immune Effects on Yaks. Wuhan: Huazhong Agricultural University. (2014).
134. Yue RC, Cheng ZL, Li N, Liu SD. Serological survey and analysis of bovine common infectiou ssome cattle farms in Shandong province. China Anim Health Insp. (2014) 31:58–61.
135. Yao W. Serologic study on bovine viral diarrhea in diary cattle from scale dairy farms in Liaoning. Modern J Anim Husbandry Vet Med. (2015) 36–40. doi: 10.3969/j.issn.1672-9692.2015.04.007
136. Akagami M, Seki S, Kashima Y, Yamashita K, Oya S, Fujii Y, et al. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Res Vet Sci. (2020) 129:187–92. doi: 10.1016/j.rvsc.2020.02.001
137. Singh V, Mishra N, Kalaiyarasu S, Khetan RK, Hemadri D, Singh RK, et al. First report on serological evidence of bovine viral diarrhea virus (BVDV) infection in farmed and free ranging mithuns (Bos frontalis). Trop Anim Health Prod. (2017) 49:1149–56. doi: 10.1007/s11250-017-1310-z
138. Katoch S, Dohru S, Sharma M, Vashist V, Chahota R, Dhar P, et al. Seroprevalence of viral and bacterial diseases among the bovines in Himachal Pradesh, India. Vet World. (2017) 10:1421–26. doi: 10.14202/vetworld.2017.1421-1426
139. Chowdhury MMR, Afrin F, Saha SS, Jhontu S, Asgar MA. Prevalence and haematological parameters for bovine viral diarrhoea (BVD) in South Bengal areas in Bangladesh. Bangladesh Vet. (2015) 32:48–54. doi: 10.3329/bvet.v32i2.30610
140. Asnake P, Lemma A, Tesfaye A., Gizaw D, Guta S, Dima C, et al. Seroprevalence of Bovine Viral Diarrhea Virus (BVDV) and its associated risk factors in dairy cattle in and Around Assela Town, South East Ethiopia. Research Article. (2020) doi: 10.21203/rs.3.rs-128860/v1
141. Tadesse T, Deneke Y, Deresa B. Seroprevalence of bovine viral diarrhea virus and its potential risk factors in dairy cattle of Jimma town, southwestern Ethiopia. JDVAR. (2019) 8:11–7. doi: 10.15406/jdvar.2019.08.00235
142. Daves L, Yimer N, Arshad SS, Sarsaifi K, Omar MA, Yusoff R, et al. Seroprevalence of Bovine Viral Diarrhea Virus infection and associated risk factors in cattle in Selangor, Malaysia. Univ Putra Malaysia Institutional. (2016) 1:22–8. doi: 10.17140/VMOJ-1-105
143. Manandhar S, Yadav GP, Singh DK. Epidemiological survey of bovine viral diarrhea in dairy cattle in Nepal. OIE Bullet Newsfeed. (2018) doi: 10.20506/bull.2018.NF.2860
144. Olmo L, Dye MT, Reichel MP, Youngd JR, Nampanyaac S, Khounsyc S, et al. Investigation of infectious reproductive pathogens of large ruminants: are neosporosis, brucellosis, leptospirosis and BVDV of relevance in Lao PDR? Acta Tropica. (2018) 177:118–26. doi: 10.1016/j.actatropica.2017.10.007
145. Rodríguez-Prieto V, Kukielka D, Rivera-Arroyo B, Martínez-López B, de las Heras AI, et al. Evidence of shared bovine viral diarrhea infections between red deer and extensively raised cattle in south-central Spain. BMC Vet Res. (2016) 12:11. doi: 10.1186/s12917-015-0630-3
146. Segura-Correa JC, Zapata-Campos CC, Jasso-Obregón JO, Martinez-Burnes J, López-Zavala R. Seroprevalence and risk factors associated with bovine herpesvirus 1 and bovine viral diarrhea virus in North-Eastern Mexico. Open Vet J. (2016) 6:143–9. doi: 10.4314/ovj.v6i2.12
147. Gong QL, Ge GY, Wang Q, Tian T, Liu F, Diao NC, et al. Meta-analysis of the prevalence of Echinococcus in dogs in China from 2010 to 2019. PLoS Negl Trop Dis. (2021) 2;15(4):e0009268. doi: 10.1371/journal.pntd.0009268
148. Lv JJ, Gao LY, Shang HZ. Serological investigation of bovine viral diarrhea mucosal disease in Haixi District of Qinghai Province. Chin Qinghai J Anim Vet Sci. (2013) 43:35.
149. Handel IG, Kim W, Fiona L, Bronwyn K, Morgan KL, Tanya VN, et al. Seroepidemiology of bovine viral diarrhoea virus (BVDV) in the adamawa region of cameroon and use of the SPOT test to identify herds with PI calves. PLoS ONE. (2011) 6:e21620. doi: 10.1371/journal.pone.0021620
150. Antos A, Rola J, Bednarski M, Krzysiak MK, Larska M. Is contamination of bovine-sourced material with bovine viral diarrhea virus still a problem in countries with ongoing eradication campaigns? Ann Anim Sci. (2020) 21. doi: 10.2478/aoas-2020-0056
151. Lotuffo ZN, Bracamonte Pérez MB, Hidalgo Díaz MA. Seroprevalence of bovine viral diarrhea in milk producing herds at Barinas State, Venezuela. Rev Soc Ven Microbiol. (2013) 33:162–8. Available online at: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1315-25562013000200014&lng=en.
152. Sthl K, Alenius S. BVDV control and eradication in Europe - an update. Jpn J Vet Res. (2012) 60(Suppl):S31–9. doi: 10.14943/jjvr.60.suppl.s31
153. Nielsen LR, Houe H, Nielsen SS. Narrative review comparing principles and instruments used in three active surveillance and control programmes for Non-EU-regulated diseases in the Danish cattle population. Front Vet Sci. (2021) 8:685857. doi: 10.3389/fvets.2021.685857
154. Lindberg AL, Alenius S. Principles for eradication of bovine viral diarrhoea virus (BVDV) infections in cattle populations. Vet Microbiol. (1999) 64:197–222. doi: 10.1016/S0378-1135(98)00270-3
155. Presi P, Struchen R, Knight-Jones T, Scholl S, Heim D. Bovine viral diarrhea (BVD) eradication in Switzerland-experiences of the first two years. Chin J Prev Vet Med. (2011) 99:112–21. doi: 10.1016/j.prevetmed.2011.01.012
156. Graham DA, Lynch M, Coughlan S, Doherty ML, O'Neill R, Sammin D, et al. Development and review of the voluntary phase of a national BVD eradication programme in Ireland. Vet Rec. (2014) 174:67–67. doi: 10.1136/vr.101814
157. Ezanno P, Fourichon C, Seegers H. Influence of herd structure and type of virus introduction on the spread of bovine viral diarrhoea virus (BVDV) on the spread of bovine viral diarrhoea virus (BVDV) within a dairy herd. Vet Rec. (2008) 39:39. doi: 10.1051/vetres:2008016
158. Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhoea: pathogenesis and diagnosis. Vet J. (2013) 199:201–9. doi: 10.1016/j.tvjl.2013.07.024
159. Moen A, Sol J, Sampimon O. Indication of transmission of BVDV in the absence of persistently infected (PI) animals. Chin J Prev Vet Med. (2005) 72:93–8. doi: 10.1016/j.prevetmed.2005.08.014
160. Hult L, Lindberg A. Experiences from BVDV control in Sweden. Chin J Prev Vet Med. (2005) 72:143–8. doi: 10.1016/j.prevetmed.2005.04.005
161. Rikula U, Nuotio L, Aaltonen T, Ruoho O. Bovine viral diarrhoea virus control in Finland 1998-2004. Chin J Prev Vet Med. (2005) 72:139–42. doi: 10.1016/j.prevetmed.2005.08.010
162. Van Duijn L, Santman-Berends I, Biesheuvel M, Mars J, Waldeck F, van Schaik G. Why test purchased cattle in BVDV control programs? Front Vet Sci. (2021) 8:686257. doi: 10.3389/fvets.2021.686257
163. Toker EB, Aytogu G, Kadiroglu B, Ates O, Yesilbag K. Failure in dry period vaccination strategy for bovine viral diarrhea virus. Vet Microbiol. (2020) 247:108797. doi: 10.1016/j.vetmic.2020.108797
164. Scharnböck B, Roch FF, Richter V, Funke C, Firth CL, Obritzhauser W, et al. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci Rep. (2018) 8:14420. doi: 10.1038/s41598-018-32831-2
165. Ma CB, Liu XP, Chen WW, Cui L, Wang ZS, Zhang ZR. “Tow diseases” situation retrospective analysis of monitoring data in different cattle breeding patterns. China Cattle Sci. (2015) 41:53–5. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=BULL201505014&DbName=CJFQ2015
166. Yang Y. Molecular Epidemiological Investigation of Bovine Leukemia Virus and Its Pathogenicity [D]. Yangzhou University (2018).
167. Nagayama K, Oguma K, Sentsui H. Survey on vertical infection of bovine viral diarrhea virus from fetal bovine sera in the field. J Vet Med Sci. (2015) 77:1531–4. doi: 10.1292/jvms.14-0556
168. Wang ZY. Establishment of Multiplex PCR Detection Methods for Cow BVDV, Pm and Kp[D]. Baoding, HJ: Hebei Agricultural University (2019).
169. Cwr C, Mcdougall S, Heuer C. Bovine viral diarrhoea virus in dairy cattle in New Zealand - studies on its prevalence, biologic and economic impact. Proc NZ Soc Anim Prod. (2006) 66:162–7.
170. Wang JJ, Liu S, Peng R, Chen X, Cao ZJ. Calves and heifers management on China large-scale dairy farms: descriptive characteristics of disease and culling of dairy replacement. China Dairy Cattle. (2022) 61–6. doi: 10.19305/j.cnki.11-3009/s.2022.02.014
171. Ramon A, Lorenzo F. Descriptive study for culling and mortality in five high-producing Spanish dairy cattle farms (2006–2016). Acta Vet Scand. (2018) 60:45. doi: 10.1186/s13028-018-0399-z
172. Vásquez NFR, Argaiz DV, Silva J, Pino LJ, Ngel MEO. Seropreval ence and risk factors of several bovine viral diseases in dairy farms of San Pedro de los Milagros, Antioquia, Colombia. Revista Ces Medicina Veterinaria Y Zootecnia. (2016) 11:15–25. doi: 10.21615/cesmvz.11.1.2
173. Kassaye A, Berhanu S, Gelagay A, Eystein S, Gebremedhin EZ, Kassahun A. Seroprevalence and factors associated with bovine viral diarrhea virus (BVDV) infection in dairy cattle in three milksheds in Ethiopia. Trop Anim Health Prod. (2018) 50:1821–7. doi: 10.1007/s11250-018-1624-5
174. McDougall S. Effect of calf age on bovine viral diarrhea virus tests. J Vet Diagn Invest. (2021) 33:528–37. doi: 10.1177/1040638721998821
175. Perumal P, Savino N, Sangma CTR, Chang S, Sangtam TZT, Khan MH, et al. Effect of season and age on scrotal circumference, testicular parameters and endocrinological profiles in mithun bulls. Theriogenology. (2017) 98:23–9. doi: 10.1016/j.theriogenology.2017.04.049
176. Báez F, López Darriulat R, Rodríguez-Osorio N, Viñoles C. Effect of season on germinal vesicle stage, quality, and subsequent in vitro developmental competence in bovine cumulus-oocyte complexes. J Therm Biol. (2022) 103:103171. doi: 10.1016/j.jtherbio.2021.103171
177. Ibba R, Riu F, Delogu I, Lupinu I, Carboni G, Loddo R, et al. Benzimidazole-2-Phenyl-carboxamides as dual-target inhibitors of BVDV entry and replication. Viruses. (2022) 14:1300. doi: 10.3390/v14061300
178. Izzo MM, Kirkland PD, Gu X, Lele Y, Gunn AA, House JK. Comparison of three diagnostic techniques for detection of rotavirus and coronavirus in calf faeces in Australia. Aust Vet J. (2012) 90:122–9. doi: 10.1111/j.1751-0813.2011.00891.x
179. Bitsch V, Rønsholt L. Control of bovine viral diarrhea virus infection without vaccines. Vet Clin North Am Food A. (1995) 11:627–40. doi: 10.1016/S0749-0720(15)30471-0
180. Wang JL. Epidemiological Investigation of Bovine Viral Diarrhea in Henan Province and Identification of Local Isolates[D]. Zhengzhou, HY: Henan Agricultural University (2013).
181. Chamorro MF, Passler T, Givens MD, Edmondson MA, Wolfe DF, Walz PH. Evaluation of transmission of bovine viral diarrhea virus (BVDV) between persistently infected and naive cattle by the horn fly (Haematobia irritans). Vet Res Commun. (2011) 35:123–9. doi: 10.1007/s11259-010-9453-7
182. Negrón M, Raizman EA, Pogranichniy R, Hilton WM, Lévy M. Survey on management practices related to the prevention and control of bovine viral diarrhea virus on dairy farms in Indiana, United States. Prev Vet Med. (2011) 99:130–5. doi: 10.1016/j.prevetmed.2010.12.008
183. OLAFSON P, Maccallum AD, Fox FH. An apparently new transmissible disease of cattle. Cornell Vet. (1946) 36:205–13.
184. Lunardi M, Headley SA, Lisbôa JA, Amude AM, Alfieri AA. Outbreak of acute bovine viral diarrhea in Brazilian beef cattle: clinicopathological findings and molecular characterization of a wild-type BVDV strain subtype 1b. Res Vet Sci. (2008) 85:599–604. doi: 10.1016/j.rvsc.2008.01.002
Keywords: bovine viral diarrhea virus, cattle, meta-analysis, antigen prevalence, antibody prevalence, risk factors
Citation: Su N, Wang Q, Liu H-Y, Li L-M, Tian T, Yin J-Y, Zheng W, Ma Q-X, Wang T-T, Li T, Yang T-L, Li J-M, Diao N-C, Shi K and Du R (2023) Prevalence of bovine viral diarrhea virus in cattle between 2010 and 2021: A global systematic review and meta-analysis. Front. Vet. Sci. 9:1086180. doi: 10.3389/fvets.2022.1086180
Received: 03 November 2022; Accepted: 22 December 2022;
Published: 17 January 2023.
Edited by:
Yasser Mahmmod, Higher Colleges of Technology, United Arab EmiratesReviewed by:
Ahmed N. F. Neamat-Allah, Zagazig University, EgyptAndrea Verna, Instituto de Innovación para la Producción Agropecuaria y el Desarrollo Sostenible (IPADS), Argentina
Vahid Rahmanian, Jahrom University of Medical Sciences, Iran
Copyright © 2023 Su, Wang, Liu, Li, Tian, Yin, Zheng, Ma, Wang, Li, Yang, Li, Diao, Shi and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Shi,  c2sxOTgxNTIxQDEyNi5jb20=; Rui Du,
c2sxOTgxNTIxQDEyNi5jb20=; Rui Du,  ZHVydWkxOTcxMDFAc2luYS5jb20=
ZHVydWkxOTcxMDFAc2luYS5jb20=
†These authors have contributed equally to this work
 Nuo Su
Nuo Su Qi Wang
Qi Wang Hong-Ying Liu1
Hong-Ying Liu1 Lian-Min Li
Lian-Min Li Ting Li
Ting Li Jian-Ming Li
Jian-Ming Li Nai-Chao Diao
Nai-Chao Diao Kun Shi
Kun Shi Rui Du
Rui Du