- 1Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, College of Animal Science, Guizhou University, Guiyang, China
- 2Testing Center for Livestock and Poultry Germplasm, Guizhou Agricultural and Rural Affairs Office, Guiyang, China
- 3Institute of Animal Nutrition and Feed Science, Guizhou University, Guiyang, China
The objective of this study was to investigate the effects of anthocyanin-rich purple corn extract (PCE) on performance, antioxidant potential, egg quality, egg amino acid and fatty acid profiles of laying hens during the late laying period. A total of 360 88-wk-old laying hens were randomly divided into 4 groups, and fed a basal diet (CON) or a basal diet supplemented with 120 (LP), 240 (MP), and 360 mg/kg (HP) PCE, respectively. No significant difference (P > 0.05) was observed in the ADFI or average egg weight among the groups. However, the mean feed to egg ratio was quadratically decreased (P < 0.05) in the LP and HP treatments. The mean TAC was linearly and quadratically increased (P < 0.05) in all PCE supplemented treatments. The mean SOD was linearly and quadratically increased (P < 0.05) in the HP treatment compared with CON and MP groups. The GPX was linearly and quadratically lower in the HP treatment compared to the CON and LP groups. Differently, the MDA was linearly and quadratically lower (P < 0.05) in the PCE treatments compared with the CON. The eggshell thickness value in MP and HP treatments were linearly and quadratically higher (P < 0.05) than that of the CON and LP groups. Hens fed PCE was linearly and quadratically increased (P < 0.05) most individual amino acids, essential amino acid and umami amino acid profiles in egg. The PCE treatments showed linearly and quadratic (P < 0.05) effect on the myristoleate, heptadecenoic acid, elaidic acid, eicosenoic acid, heneicosanoic acid, and eicosatrienoic acid concentrations. Moreover, dietary supplementation of PCE was quadratically increased egg stearic acid, oleic acid, arachidic acid, linolenic acid methyl ester, arachidonic acid, diphenylamine, docosahexaenoic acid, monounsaturated fatty acid, and polyunsaturated fatty acid compared to the CON. Therefore, dietary anthocyanin-rich PCE can enhance plasma antioxidant potential, is beneficial to egg production, and improves amino acids and fatty acids in hen eggs during the late laying period.
1. Introduction
Currently, the residues of antibiotics and drug resistance are becoming increasingly concerning, and green and safe poultry products have been increasingly welcomed by consumers (1). In recent years, many countries including China prohibited the use of antibiotics as feed additives. Thus, finding a safe substitute has been imperative. The plant extract is an excellent natural substitution for antibiotics because its addition to the diet could improve the growth, gut health, and improve performance and egg quality of chickens (2, 3). These reports have indicated that the secondary metabolites of plants can promote the growth of animals, enhance immunity, resist bacteria and disease, and improve the quality of animal products (4). Therefore, plant extracts are promising green feed additives that can replace antibiotics.
Anthocyanins are plant secondary metabolites that have excellent antioxidative capacity and antibacterial properties, which as natural antioxidants, have potential benefits for livestock and poultry production (5). Anthocyanin has strong antioxidant, immune regulation and anti-inflammation effects, which may improve the immune function and antioxidant effects of the animal, alleviating the breeding process of a variety of stress responses (6). This is because anthocyanins can provide hydrogen atoms for phenols and trap free radicals, thereby stabilizing compounds through various resonant forms, and showing high antioxidant activity (7). Hence, anthocyanins have strong antioxidative and radical scavenging activities and are a source of an attractive natural antioxidant (8). Kaya et al. (9) found that the addition of anthocyanin-rich grape seed extract could improve egg haugh units of laying hens. Sabet et al. (10) found that anthocyanin-rich sour tea (Hibiscus Sabdariffa) plant extract did not affect functional or immune parameters but did improve the plasma antioxidant status in laying hens.
Currently, eggs are an important component of human food, and thus can be enriched with antioxidants through manipulation of poultry feed (11). Various studies have showed that dietary supplementation with anthocyanin plant extract can improve the quality of egg production in poultry (12, 13). However, in the late laying period, laying hens are prone to convert excess nutrients into fat, causing imbalance of lipid oxidation and reducing antioxidant function, and leading to the decrease of performance (14). Additionally, purple corn is rich in anthocyanin, which may be an important source of anthocyanin extraction and has wide development and application prospects (15). However, reports about the role and mechanism of anthocyanins in laying hens during the late laying period are relatively rare. We hypothesized that the feeding of purple corn extract (PCE) can increase antioxidant activities and improve egg production, amino acids and fatty acids in hen eggs. Accordingly, the current study observed the effects of anthocyanins from PCE on the performance, plasma antioxidant activity, egg production, amino acids and fatty acids in egg of Chishui black-bone laying hens during the late laying period.
2. Materials and methods
2.1. Animals, diets, and experimental design
Chishui black-bone hen is one of the famous local poultry breeds in the Guizhou Province of China (16). Moreover, the feeding trial was carried out at the Guizhou Zhuxiang Chicken Breeding Co. Ltd., Chishui, China (28.590337 N, 105.697472 E). Amer et al. (17) demonstrated that the inclusion of 200 and 400 mg/kg anthocyanin-rich roselle, Hibiscus sabdariffa L. extract could improve antioxidant activity in chicken. In this study, 360 healthy and similar body weight (BW 1,728 ± 147 g, mean±standard deviation). Chishui black bone hens from 88 weeks during the late laying period were used. All hens were randomly allocated into four groups consisting of a basal diet (CON) or the basal diet supplemented with 120 (LP), 240 (MP), and 360 mg/kg (HP) anthocyanin-rich PCE. Each group had six replicates, and each replicate had 15 laying hens. The hen housed was kept at a cage, and each cage (0.076 m3) was kept at 3 hens. All experimental hens were under the same conditions with 16 h/d light, 21°C temperature and 45~65% humidity.
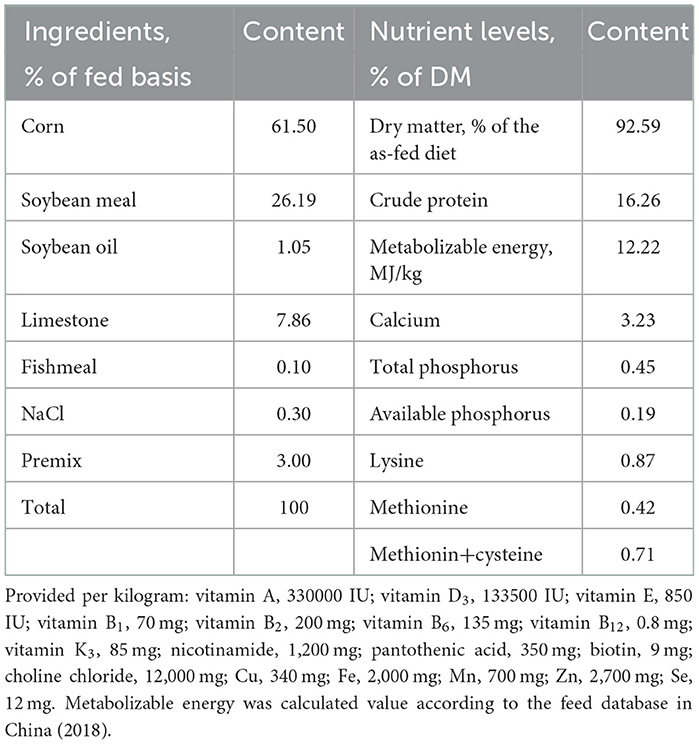
The PCE (Nanjing Herd Source Biotechnology Co., Ltd., Nanjing, China), a commercial extract, had a total anthocyanin concentration of 2,619 μg/g according to our previous study (18). The PCE was first mixed with chopped concentrate, then mixed with roughage to prepare basal diet. There was 14 d a preparation period prior to a 60 d formal experimental period of the feeding trial. Feed and water were provided ad libitum during the whole period. The nutritional requirements of laying hens were determined according to the farm requirements and the Chinese Standard for the Feeding Standard of chickens (NY/T33-2004) (19). The chemical compositions for dry matter (930.15), crude protein (988.05), calcium (927.02), and phosphorus (964.06) of feed were analyzed as per the method of the AOAC (20). The chemical composition and nutrition composition of basal diet as shown in Table 1.
2.2. Performance
During the entire experimental period, the daily feed amount and surplus were collected to calculate average daily feed intake (ADFI). The daily egg yield and egg weight were recorded to calculate average egg weight (g) = total daily weight/total egg weight; moreover, laying rate per hen-day (HD, %) = (laying number/layer number) × 100, and feed to egg ratio = total feed intake (g)/total egg weight (g).
2.3. Plasma antioxidant activity parameters
Two hens from each replicate for a total of twelve hens per group at 20, 40, and 60 d before feeding were randomly selected, blood (about 5 mL) was obtained from the inferior wing vein by a negative pressure vacuum tube with 12.5 IU/mL heparin sodium (Nanchang Ganda Medical Instrument Co., Ltd., Nanchang, China). Blood samples were centrifuged (KJH80-2, Jiangsu KangJianhua Medical Supplies Co., Ltd., Jiangsu, China) at 4,000 × g for 15 min, and the plasma was collected and kept at a −80°C fridge for further analysis. Total antioxidant capacity (TAC, A015-1), superoxide dismutase (SOD, A001-3), glutathione peroxidase (GPX, A005), catalase (CAT, A007-1), and malondialdehyde (MDA, A003-1) were determined, and all kids purchased from Nanjing Jiangcheng Bioengineering Institute (Nanjing, China).
2.4. Egg quality
Two hens from each replicate for a total of twelve hens per group at 20 d, 40 d and 60 d were randomly selected, and egg weight, albumen height, yolk weight, yolk color, and haugh unit were analyzed by an egg quality meter (EA-01, ORKA, Israel). Moreover, eggshell strength was analyzed by an eggshell strength tester (KQ-1A, Nannong Animal Husbandry Technology Co., Ltd., Beijing, China). Moreover, the egg shape index and the thickness of the eggshell were determined using cursor calipers (3 V, MASTERPROOF professional TOOL, Germany).
2.5. Egg amino acids and fatty acids
Two eggs from each replicate and a total of twelve eggs per group at the end of experimental period were randomly selected, and transferred into a vacuum freeze dryer (LYOQUEST-85 Plus, Bole Technology, Spain). The condenser was set at −80°C, and the vacuum pump was set to 0.000 m Bar. Next, the egg samples were ground by a high-speed grinder after 72 freeze-drying cycles. Amino acids and fatty acids were detected according to our previous study (16). Briefly, amino acid was analyzed using an automatic amino acid analyser (Biochrom 30, Biochrom Ltd., Cambridge, the United Kingdom). The amino acid analyser conditions were as follows: the chromatographic column was a sulfonic acid cation resin; detection wavelengths were 570 and 440 nm, respectively; individual amino acid was determined using peak area according to external standard method. For fatty acid, approximately 1 g of sample was weighed for hydrolysis; then, the fat extract was obtained, fat saponification and fatty acid esterification were processed, and the upper solution was collected and kept in a sample bottle. The individual fatty acids were detected by an Agilent 6,890 gas chromatograph (Agilent Technologies Co. Ltd., Palo Alto, USA). The gas chromatograph conditions were as follows: the injection volume was 1.0 μL; capillary column was a polar stationary phase of 100 m × 0.25 mm × 0.2 μm polydicyanide siloxane; 270°C of injector temperature; 280°C of detector temperature. The individual fatty acids were calculated using the peak area normalization method.
2.6. Statistical analysis
All raw data were recorded with Microsoft Excel 2010. All observations were analyzed by the general linear models procedure using in Statistical Product and Service Solutions 20.0 software (SPSS Inc., Chicago, Illinois, USA) with the least square mean noted by Tukey's test. All variables were tested for linear and quadratic effects, and the level of significance was assessed at P < 0.05.
3. Results
3.1. Performance
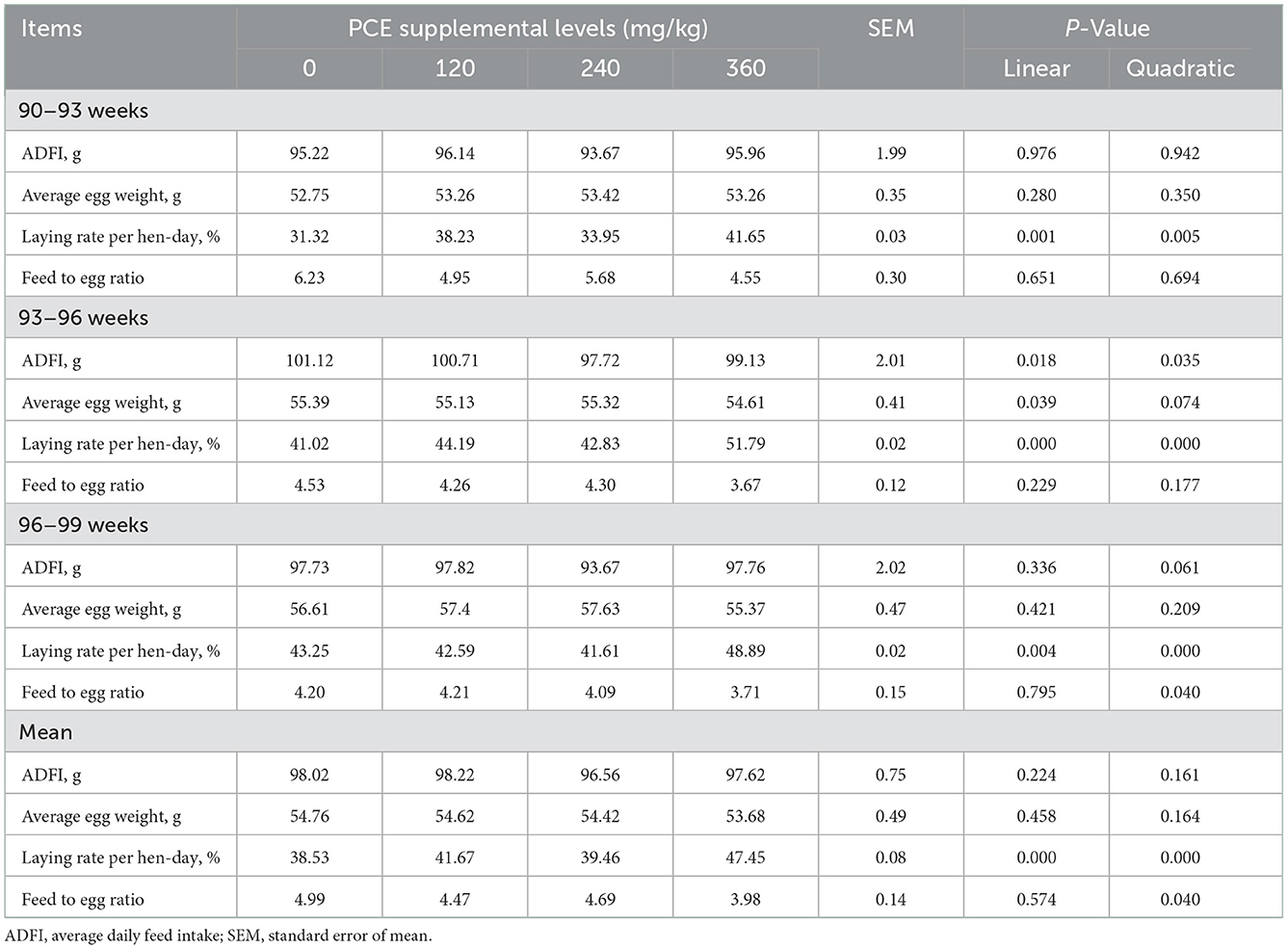
The mortality of hens did not differ (P > 0.05) throughout the feeding experimental period (data not shown; Table 2). No significant difference (P > 0.05) was observed in the ADFI or average egg weight among the groups during the entire experimental period. Compared to the CON, dietary supplementation of PCE exhibited linear and quadratic effect (P < 0.05) on the HD, with the highest values noted at HP treatment. Moreover, the feed to egg ratio was not significantly (P > 0.05) different in all groups from d 21 to 40 and d 21 to 40 except for the d 41 to 60, which was quadratically decreased relative to the CON group. In addition, the mean feed to egg ratio was quadratically decreased in the LP and HP treatments.
3.2. Antioxidant activity parameters
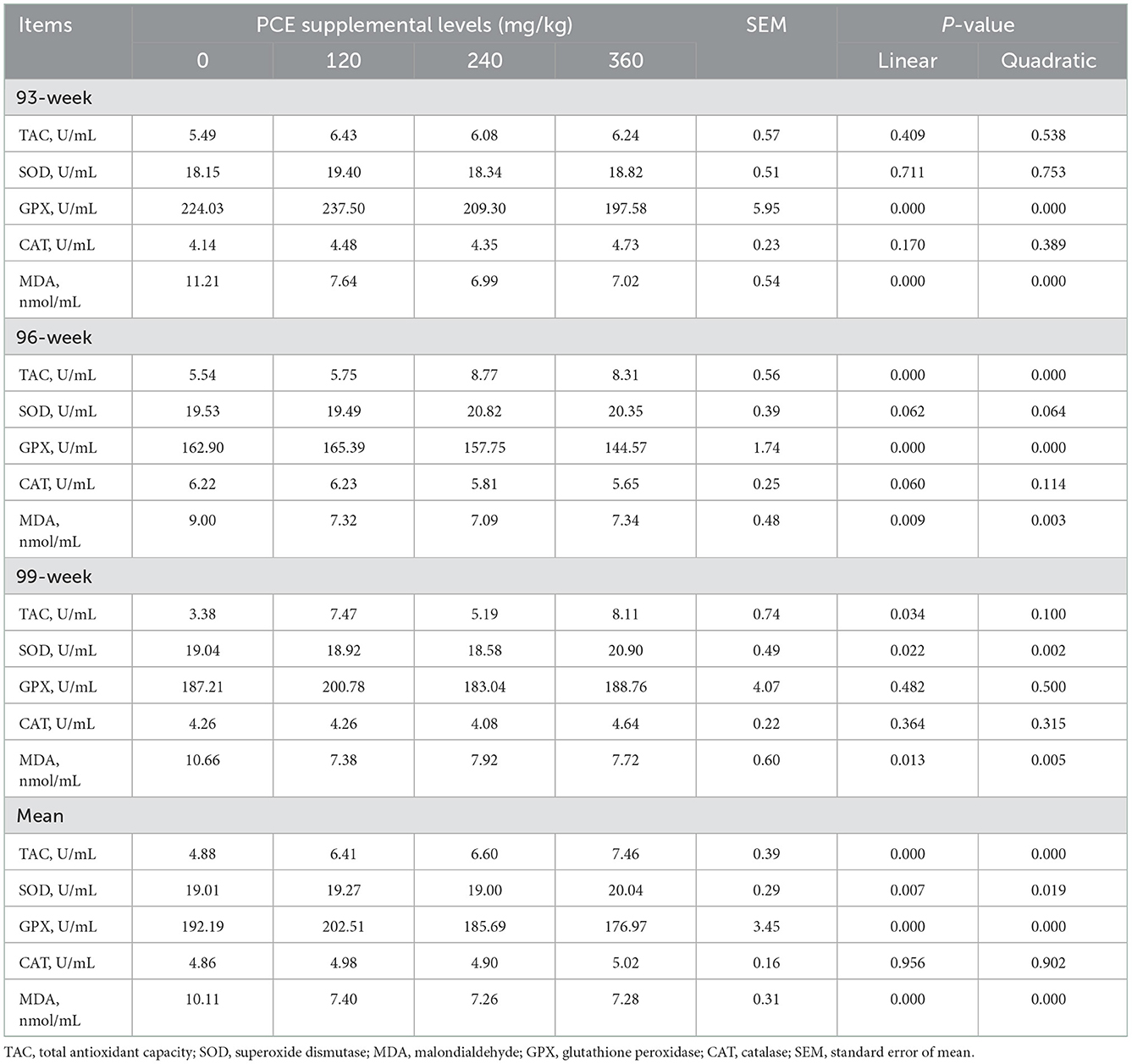
For day 20, there was no differences (P > 0.05) among groups in terms of TAC, SOD, and CAT concentrations (Table 3). The GPX was linearly and quadratically decreased (P < 0.05) in the HP treatment. For day 40, the TAC was linearly and quadratically increased (P < 0.05) in MP and HP treatments. The SOD was not significantly (P > 0.05) different between all groups. The GPX was linearly and quadratically decreased (P < 0.05) in the HP treatment. For day 60, the TAC and SOD were linearly and quadratically increased (P < 0.05) in the HP treatment. The mean TAC was linearly and quadratically increased (P < 0.05) in all PCE supplemented treatments. The mean SOD was linearly and quadratically increased (P < 0.05) in the HP treatment compared with CON and MP groups. The mean GPX was linearly and quadratically lower in the HP compared to the CON and LP groups. During the whole experimental period, no significant (P > 0.05) differences were found in the plasma CAT among the four groups; whereas the MDA was linearly and quadratically lower (P < 0.05) in the PCE treatments compared with the CON group.
3.3. Egg quality
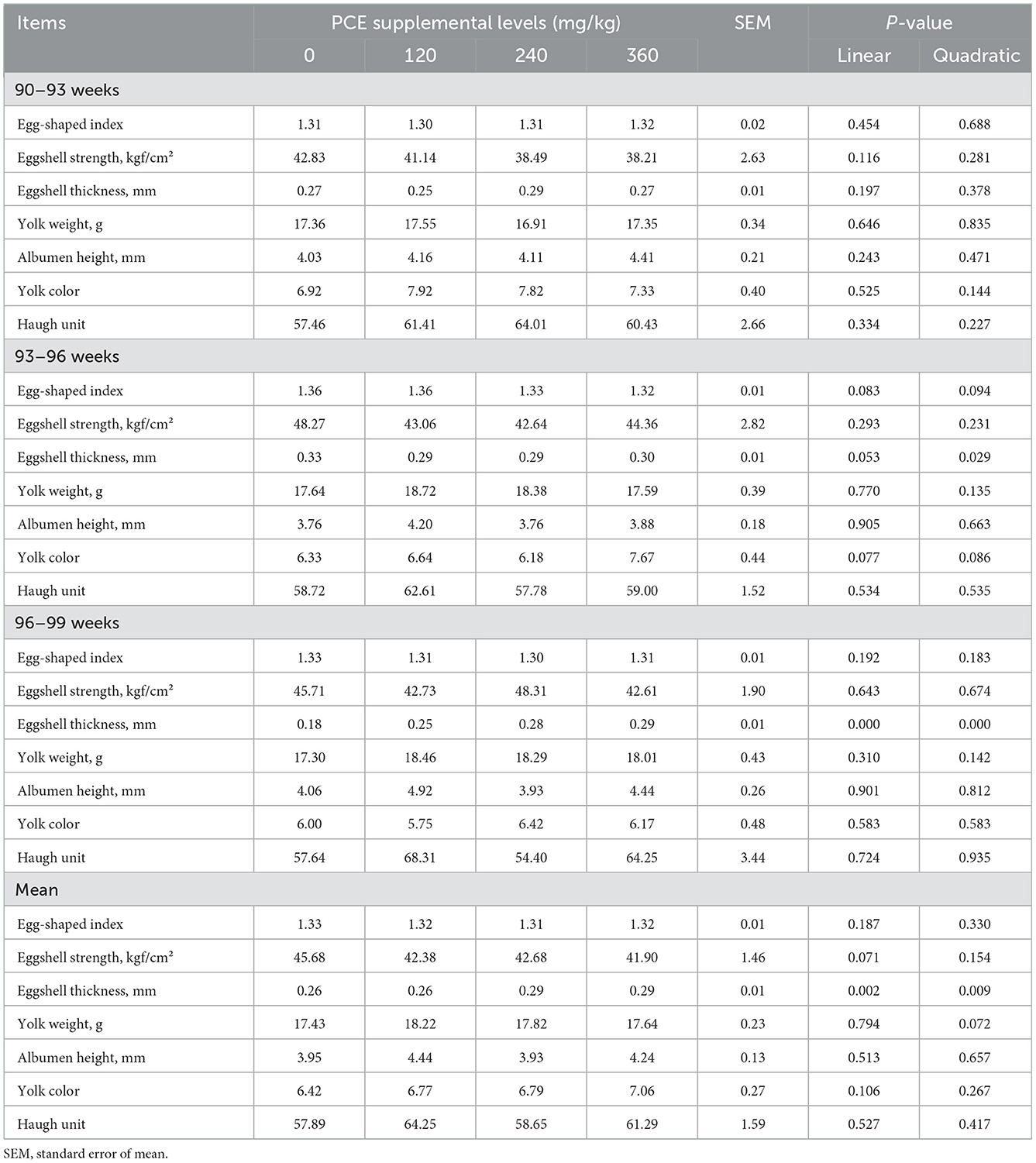
No significant difference (P > 0.05) was detected in egg shape index, eggshell strength, yolk weight, albumen height, yolk color, and haugh unit among the four groups during the entire experimental period (Table 4). The eggshell thickness was quadratically decreased (P < 0.05) in the LP and MP treatments at day 40. The eggshell thickness was linearly and quadratically increased (P < 0.05) in all PCE supplemented treatments at day 60. Moreover, the eggshell thickness value in MP and HP treatments were linearly and quadratically higher (P < 0.05) than that of the CON and LP groups.
3.4. Egg amino acids
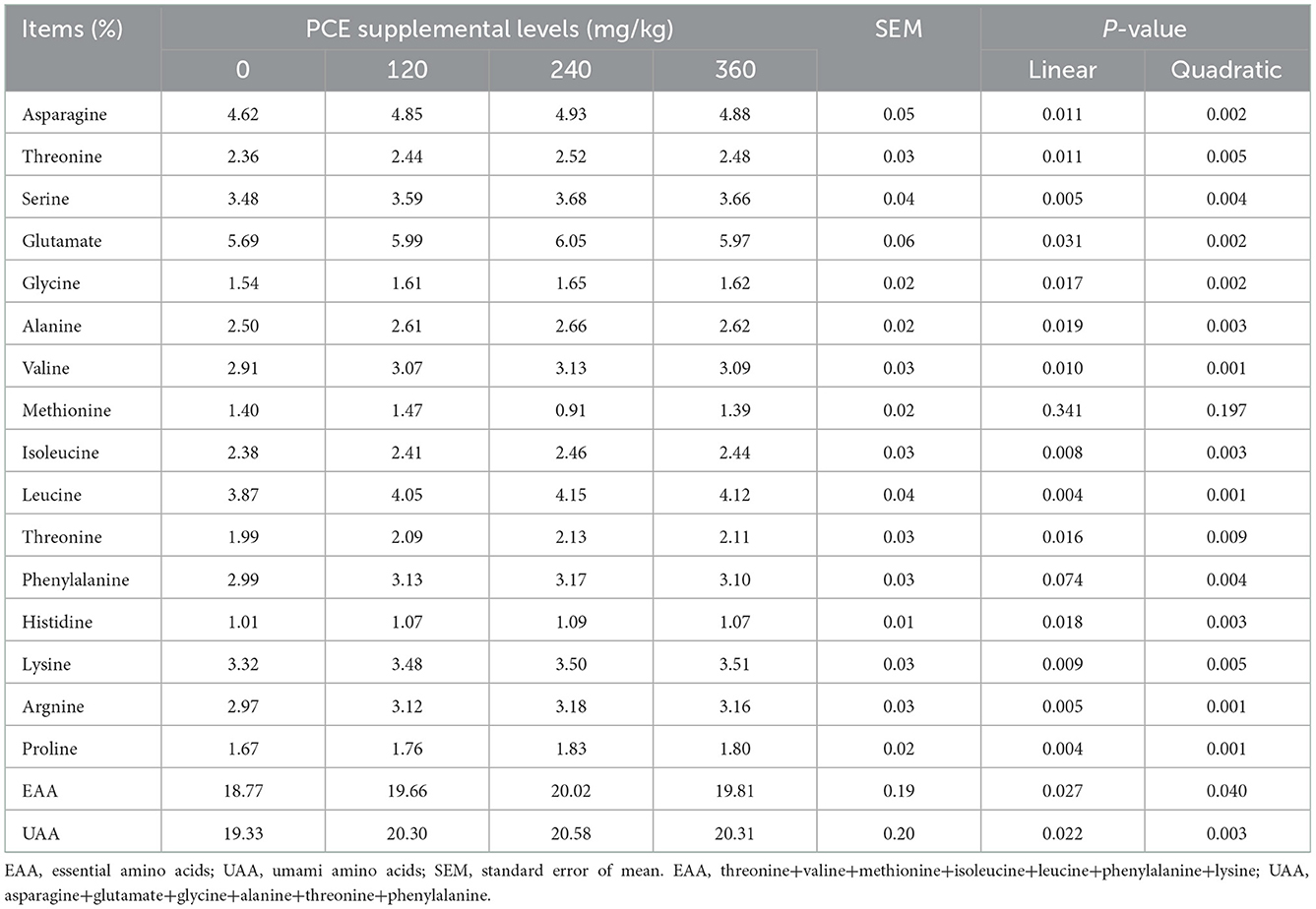
The inclusion of PCE was linearly and quadratically increased (P < 0.05) most individual amino acid (including aspartate, threonine, serine, glutamate, glycine, alanine, valine, isoleucine, leucine, threonine, histidine, lysine (Lys), arginine, and proline) concentrations in eggs compared to the without PCE group (Table 5). Dietary PCE addition had no significant effect (P > 0.05) on the egg methionine content among four groups. The phenylalanine was quadratically increased (P < 0.05) in the PCE treatments relative to the CON group. In addition, hens fed PCE was linearly and quadratically increased in essential amino acid (EAA) and umami amino acid (UAA) profiles in egg.
3.5. Egg fatty acids
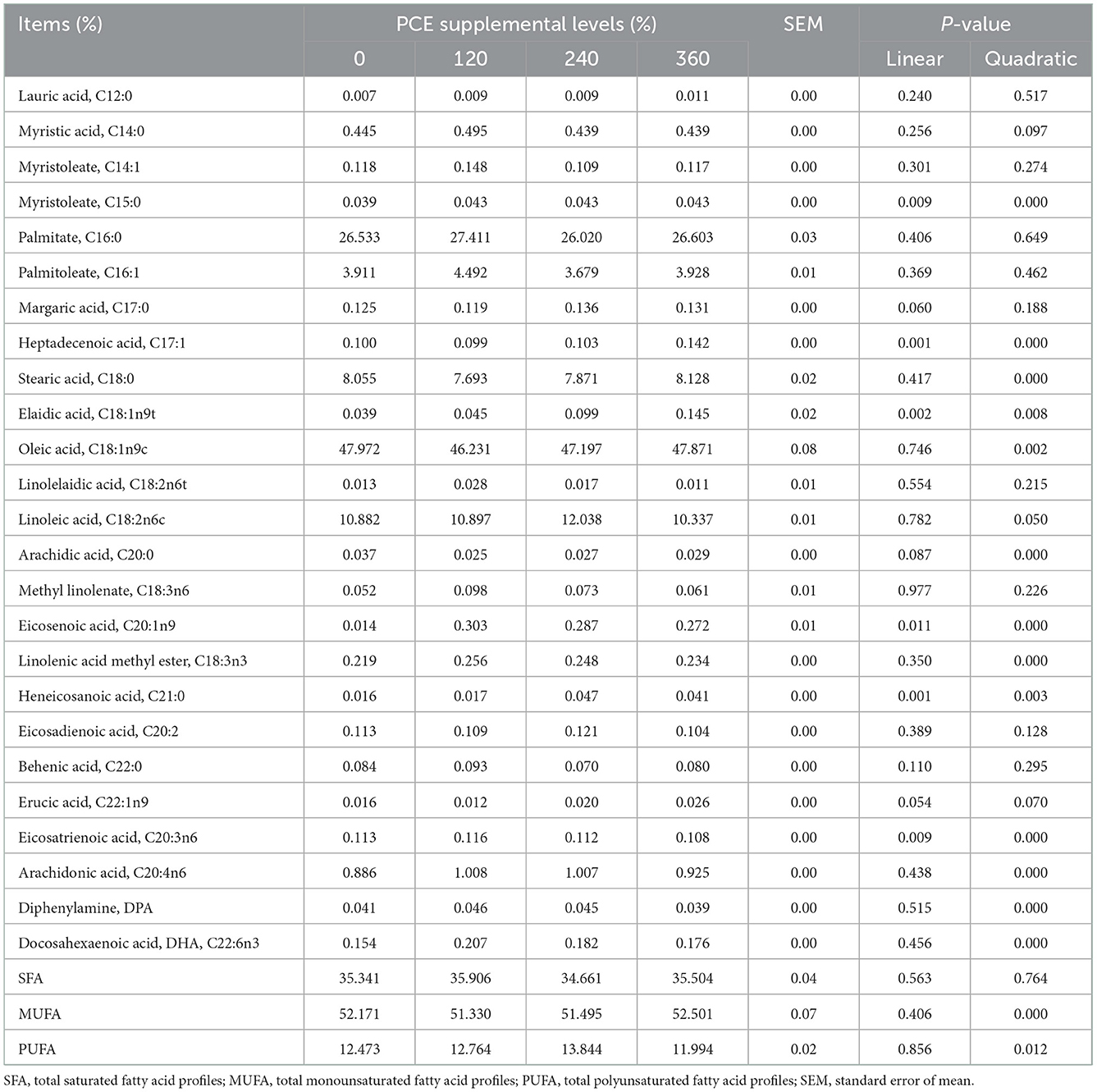
There were no significant differences (P > 0.05) for lauric acid (C12:0), myristic acid (C14:0), myristoleate (C14:1), palmitate (C16:0), palmitoleate (C16:1), margaric acid (C17:0), linolelaidic acid (C18:2n6t), linoleic acid (C18:2n6c), methyl linolenate (C18:3n6), eicosadienoic acid (C20:2), behenic acid (C22:0), erucic acid (C22:1n9), and SFA in egg among all the groups (Table 6). The PCE treatments showed linearly and quadratic (P < 0.05) effect on the myristoleate (C15:0), heptadecenoic acid (C17:1), elaidic acid (C18:1n9t), eicosenoic acid (C20:1n9), heneicosanoic acid (C21:0), eicosatrienoic acid (C20:3n6) concentrations. Moreover, dietary supplementation of PCE was quadratically increased egg stearic acid (C18:0), oleic acid (18:1n9c), arachidic acid (C20:0), linolenic acid methyl ester (C18:3n3), arachidonic acid (C20:4n6), diphenylamine (DPA), docosahexaenoic acid (DHA), monounsaturated fatty acid, and polyunsaturated fatty acid (PUFA) compared to the CON group.
4. Discussion
The free radicals in poultry are usually in homeostasis and have important biological functions under normal circumstances (21). However, free radicals accumulated and resulted in animals entering the oxidative status, reducing the resistance and production performance of poultry (22). Plant flavonoids could improve the reproductive capacity of animals by releasing hormones, such as growth hormone, in livestock and poultry (23). Additionally, animals receiving anthocyanin extract can enhance digestive enzyme activity, thus improving feed digestibility and affecting nutrient absorption (17). Hence, we found that PCE could improve the HD values, which might be related to anthocyanin improve reproductive capacity and nutrient absorption. Consistent with our results, Tufarelli et al. (24) found that the feeding of anthocyanin-rich dried grape pomace in a laying hen diet did not affect the ADFI, whereas it could improve egg production. Duenas et al. (25) suggested that broiler chickens receiving anthocyanin-rich cranberry extract had an improved feed conversion ratio relative to the control group.
Radical reactions in the body must be in dynamic balance. Oxidative injury induced by excess free radicals is an etiology of several diseases. As a high-yielding animal species, laying hens have a more vigorous metabolism, producing higher levels of reactive oxygen free radicals. Generally, the activity of antioxidant enzymes decreases, and free radicals accumulate, and thus negatively affecting health and production of laying hens (26). Anthocyanin-rich plants can improve antioxidant potential by decreasing thio-barbituric acid-reactive substance values in breast meat in broilers (27). In addition, plant flavonoids can decrease the production of proliferation, T-cell activation, and proinflammatory cytokines, and thus regulating the immune system in animals (28). Saleh et al. (29) concluded that dietary supplementation with anthocyanin-rich sorghum can modulate the abundances of the mRNAs of genes related to antioxidative properties, such as GPX and SOD, which were increased in chickens. Moreover, anthocyanin plants can relieve the inflammatory reaction by the expression of genes related to interleukin-1β and interleukin-6 in chickens, thus enhancing antioxidants in broiler chickens (30). Hence, we found that the inclusion of PCE in hen diets can increase the concentration of TAC, SOD, and GPX, as well as decrease the content of MDA in blood. This might be because anthocyanins can provide excess phenolic hydrogen atoms to free radicals and neutralize free radicals in the animal body (31); and anthocyanins can participate in the metabolism of phospholipids, arachidonic acid and protein phosphorylation to protect lipids from oxidative damage (32). Consistent with our findings, Wang et al. (33) showed that the addition of 100 mg/kg anthocyanin-rich bilberry extract could increase SOD and GPX, but it could decrease MDA of yellow-feathered chickens. Additionally, Reis (34) demonstrated that laying hens receiving anthocyanin-rich grape pomace flour could increase TAC, SOD and GPX in the serum compared to the control.
Generally, with the increase in the daily age of laying layers, the eggshell quality of eggs produced decreases (35). Free radicals accumulate in the body due to their long oxidative metabolism, which may also damage the formation of egg protein and reduce the protein haugh unit of the laying hens at the later laying period (36). Moreover, the decline in eggshell quality were decreases of estrogen levels, calcium absorption and metabolic capacity in late egg laying (37). Notably, the inclusion of phenolic-rich plant extract may regulate the intestinal microflora profile in chickens, and improving health condition (38). Of interest, anthocyanins and their metabolites can reduce pathogenic bacteria and improve beneficial bacteria (Lactobacillus and Enterococcus), improving animal health and increasing production performance in poultry (39). Thus, we found that laying hens fed a diet containing anthocyanin-rich PCE showed higher value of eggshell thickness in eggs, perhaps due to anthocyanin can improve the intestinal environment and liver SOD concentration in laying hens (40, 41). Furthermore, Kara et al. (42) demonstrated that supplementing laying hen diets with 4% anthocyanin-rich grape pomace has the potential to increase egg weight, improving the egg quality of 80-week-old 96 Bovans laying hens. In short, anthocyanin-rich PCE improved the nutritional value of eggs, which has a higher market value and is more conducive to future application in production.
The amino acid content of eggs is balanced, which is easily absorbed and utilized by consumers, and it is the most ideal source of high-quality protein in natural food (43). Dietary supplementation with natural antioxidants in animal diets could improve UAA concentrations because it can increase antioxidant potential and regulate related umami gene expression in the body (44). In the current research, we found that the feeding of anthocyanins from PCE could improve UAA concentrations in eggs compared to the CON group. This possibly due to purple corn anthocyanins might take part in the UAA signaling pathway in laying hens, resulting in the higher EAA and UAA content in eggs in Chishui black bone hens. The exact reasons remain unclear, and further observations are needed. Our observations are in agreement with Omar et al. (45), who showed that phenolic-rich onion extract could improve the amino acid ileal digestibility of amino acids in broiler chicken. Mishra et al. (46), who found that anthocyanins could enhance higher levels of antioxidant activity, and improve the concentrations of Lys and tryptophan in eggs in white leghorn layer chickens.
The PUFAs are important to the human body, and the intake of a certain amount of unsaturated fatty acids has a good regulatory effect on blood fat, vision and immune function. However, PUFA molecules could transform into hydroperoxides, conjugated dienes, and peroxy radicals (47). Anthocyanins have antioxidant properties, which can prevent unsaturated fatty acids on the cell membrane from being oxidized. Crescenti et al. (48) showed that anthocyanin-rich grape seed can promote related fatty acid absorption and beta-oxidation genes overexpression, which can subsequently ameliorate obesity and metabolic disorders. Thus, MP and HP treatments increased UFA concentration in eggs, perhaps because anthocyanins not only exhibit a higher oxygen radical absorbance capacity; but also can provide hydrogen donors to the lipid free radicals, and thus resulting in the inhibition of lipid peroxidation (49–51). Consistent with our observations, Untea et al. (52) found that anthocyanin-rich bilberry leaves could improve egg UFA profiles (C18:2n6, C18:3n6, C20:3n6 and total n-6 fatty acids) in laying hens.
5. Conclusions
The results of the current research suggest that dietary supplementation with PCE can enhance plasma antioxidation ability, increase egg production, and improve egg amino acid and fatty acid profiles during the late laying period of laying hens.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and all experimental animal care procedures were approved by the Rules of Animal Welfare and Experimental Animal Ethics of Guizhou University (EAE-GZU-2021-P017), Guizhou, China.
Author contributions
JL conducted writing–original draft, data curation, methodology, and software. DZ and HL conducted resources, investigation, and supervision. QLuo, XW, JQ, and YX conducted resources and validation. QLu contributed by investigation. XT contributed by resources, writing–review and editing, software, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This study has gained the support of the Joint Research Project of Local Poultry Industry of Guizhou Province (Qiannongcai (2020)175), the National Natural Science Foundation of China (32260849), the Science and Technology Project of Guizhou Province (Qiankehe foundation-ZK [2021] General 164), the Youth Science and Technology Talent Development Project of Guizhou Province (Qianjiaohe KY [2022] 150), and the Cultivating Project of Guizhou University (2019-33).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu H, Ye X, Chen S, Sun A, Zhang Y. Chitosan as additive affects the bacterial community accelerates the removals of antibiotics and related resistance genes during chicken manure composting. Sci Total Environ. (2021) 792:148381. doi: 10.1016/j.scitotenv.2021.148381
2. Shirzadi H, Shariatmadari F, Torshizi MAK, Rahimi S, Masoudi AA, Zaboli G, et al. Plant extract supplementation as a strategy for substituting dietary antibiotics in broiler chickens exposed to low ambient temperature. Arch Anim Nutr. (2020) 74:206–21. doi: 10.1080/1745039X.2019.1693860
3. Choe HS, Song TH, Han OK, Park TI, Ryu KS. Effect of barley containing different levels of anthocyanin on the performance and egg quality of laying hens. J Life Sci. (2013) 23:237–41. doi: 10.5352/JLS.2013.23.2.237
4. Lee JH, Cho S, Paik HD, Choi CW, Nam KT, Hwang SG, et al. Investigation on antibacterial and antioxidant activities phenolic and flavonoid contents of some Thai edible plants as an alternative for antibiotics. Asian Austral J Anim Sci. (2014) 27:1461–8. doi: 10.5713/ajas.2013.13629
5. Khurana S, Venkataraman K, Hollingsworth A, Piche M. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. (2013) 5:3779–827. doi: 10.3390/nu5103779
6. Jian H, Monica GM. Anthocyanins: natural colorants with health promoting properties. Annu Rev Food Sci T. (2010) 1:163–87. doi: 10.1146/annurev.food.080708.100754
7. Brewer MS. Natural antioxidants: sources compounds mechanisms of action and potential applications. Compr Rev Food Sci F. (2011) 10:221–47. doi: 10.1111/j.1541-4337.2011.00156.x
8. Copetti CLK, Diefenthaeler F, Hansen F, Vieira FGK, Di Pietro PF. Fruit-derived anthocyanins: effects on cycling-induced responses and cycling performance. Antioxidants. (2022) 11:387. doi: 10.3390/antiox11020387
9. Kaya A, Yildirim BA, Kaya H, Gül M, Çelebi S. The effects of diets supplemented with crushed and extracted grape seed on performance egg quality parameters yolk peroxidation and serum traits in laying hens. Eur Poultry Sci. (2014) 78:1–10. doi: 10.1399/eps.2014.59
10. Sabet SS, Hosseini SM, Farhangfar SH. The effect of sour tea (Hibiscus sabdariffa) plant extract on immune system plasma antioxidant status oxidant balance and blood biochemical and functional parameters of old laying hens. Iran J Anim Sci Res. (2020) 12:87–101. Available online at: https://www.sid.ir/paper/383631/en
11. Chamila N, Wu J. Hen egg as an antioxidant food commodity: A review. Nutrients. (2015) 7:8274–93. doi: 10.3390/nu7105394
12. Yuan ZH, Zhang KY, Ding XM, Luo YH, Bai SP, Zeng QF, et al. Effect of tea polyphenols on production performance egg quality and hepatic antioxidant status of laying hens in vanadium-containing diets. Poultry Sci. (2016) 95:1709–17. doi: 10.3382/ps/pew097
13. Lv ZP, Yan SJ, Li G, Liu D, Guo YM. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poultry Sci. (2019) 98:7022–9. doi: 10.3382/ps/pez367
14. Chen F, Zhang H, Du E, Jin F, Zheng C, Fan Q, et al. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poultry Sci. (2021) 100:835–43. doi: 10.1016/j.psj.2020.10.047
15. Abdel-Moneim AM, Shehata AM, Alzahrani SO, Shafi ME, Mesalam NM, Taha AE, et al. The role of polyphenols in poultry nutrition. J Anim Physiol Anim Nutr. (2020) 104:1851–66. doi: 10.1111/jpn.13455
16. Luo Q, Li J, Li H, Zhou D, Wang X, Tian Y, et al. The effects of purple corn pigment on growth performance blood biochemical indices meat quality muscle amino acids and fatty acids of growing chickens. Foods. (2022) 11:1870. doi: 10.3390/foods11131870
17. Amer SA, Al-Khalaifah HS, Gouda A, Osman A, Goda NIA, Mohammed HA, et al. Potential effects of anthocyanin-rich roselle (Hibiscus sabdariffa L)extract on the growth intestinal histomorphology blood biochemical parameters and the immune status of broiler chickens. Antioxidants. (2022) 11:544. doi: 10.3390/antiox11030544
18. Tian XZ, Li JX, Luo Q, Wang X, Wang T, Zhou D, et al. Effects of purple corn anthocyanin on growth performance meat quality muscle antioxidant status and fatty acid profiles in goats. Foods. (2022) 11:1255. doi: 10.3390/foods11091255
19. Chinese Standard NY/T 33-2004; Feeding Standard of Chicken. The Standard Press of PR China: Beijing China. (2004). (In Chinese).
20. Association of Official Analytical Chemists. Association of Official Analytical Chemists Official Methods of Analysis. 18th ed. Washington DC USA: AOAC. (2005).
21. Liu J, Liu D, Wu X, Pan C, Wang S, Ma L, et al. quantitative proteomics analysis reveals the effects of transport stress on iron metabolism in the liver of chicken. Animals. (2022) 12:52. doi: 10.3390/ani12010052
22. Huang S, Wang L, Liu L, Hou Y, Li L. Nanotechnology in agriculture livestock and aquaculture in China. A review. Agron Sustain Dev. (2014) 35:369–400. doi: 10.1007/s13593-014-0274-x
23. Liu Y, Li Y, Liu HN, Suo YL, Hu LL, Feng XA, et al. Effect of quercetin on performance and egg quality during the late laying period of hens. Brit Poultry Sci. (2013) 54:510–4. doi: 10.1080/00071668.2013.799758
24. Tufarelli V, Baghban-Kanani P, Azimi-Youvalari S, Hosseintabar-Ghasemabad B, Laudadio V. Effect of dietary flaxseed meal supplemented with dried tomato and grape pomace on performance traits and antioxidant status of laying hens. Anim Biotechnol. (2021) 4:1–8. doi: 10.1080/10495398.2021.1914070
25. Duenas M, Munoz-Gonzalez I, Cueva C, Jimenez-Giron A, Sanchez-Patan F, Santos-Buelga C, et al. survey of modulation of gut microbiota by dietary polyphenols. BioMed Res Int. (2015) 2015:850902. doi: 10.1155/2015/850902
26. Lei K, Li YL, Yu DY, Rajput IR, Li WF. Influence of dietary inclusion of Bacillus licheniformis on laying performance egg quality antioxidant enzyme activities and intestinal barrier function of laying hens. Poultry Sci. (2013) 9:2389–95. doi: 10.3382/ps.2012-02686
27. Aditya S, Ohh S, Ahammed M, Lohakare J. Supplementation of grape pomace (Vitis vinifera)in broiler diets and its effect on growth performance apparent total tract digestibility of nutrients blood profile and meat quality. Anim Nutr. (2018) 4:210–4. doi: 10.1016/j.aninu.2018.01.004
28. Hasted TL, Sharif S, Boerlin P, Diarra MS. Immunostimulatory potential of fruits and their extracts in poultry. Front Immunol. (2021) 12:641696. doi: 10.3389/fimmu.2021.641696
29. Saleh AA, Abudabos AM, Ali MH, Ebeid TA. The effects of replacing corn with low-tannin sorghum in broiler's diet on growth performance nutrient digestibilities lipid peroxidation and gene expressions related to growth and antioxidative properties. J Appl Anim Res. (2019) 47:532–9. doi: 10.1080/09712119.2019.1680377
30. Csernus B, Biró S, Babinszky L, Komlósi I, Jávor A, Stündl L, et al. Effect of carotenoids oligosaccharides and anthocyanins on growth performance immunological parameters and intestinal morphology in broiler chickens challenged with Escherichia coli lipopolysaccharide. Animals. (2020) 10:347. doi: 10.3390/ani10020347
31. Tian XZ, Wang X, Ban C, Luo QY, Li JX, Lu Q. Effect of purple corn anthocyanin on antioxidant activity volatile compound and sensory property in milk during storage and light prevention. Front Nutr. (2022) 9:862689. doi: 10.3389/fnut.2022.862689
32. Cevallos-Casals BA, Cisneros-Zevallos L. Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red -fleshed sweetpotato. J Agric Food Chem. (2003) 51:3313–9. doi: 10.1021/jf034109c
33. Wang Y, Ma X, Ye J, Zhang S, Jiang S. Effects of dietary supplementation with bilberry extract on growth performance immune function antioxidant capacity and meat quality of yellow-feathered chickens. Animals. (2021) 11:1989. doi: 10.3390/ani11071989
34. Reis JH, Gebert RR, Barreta M, Boiago MM, Souza CF, Baldissera MD, et al. Addition of grape pomace flour in the diet on laying hens in heat stress: impacts on health and performance as well as the fatty acid profile and total antioxidant capacity in the egg. J Therm Biol. (2019) 80:141–9. doi: 10.1016/j.jtherbio.2019.01.003
35. Marques RH, Gravena RA, Della J, Silva TD, Roccon J, Picarelli J, et al. Effect of supplementation of diets for quails with vitamins A D and E on performance of the birds and quality and enrichment of eggs. R Bras Zootec. (2011) 40:1222–32. doi: 10.1590/S1516-35982011000600010
36. Guo Y, Zhao Z, Pan Z, An L, Balasubramanian B, Liu W. New insights into the role of dietary marine-derived polysaccharides on productive performance egg quality antioxidant capacity and jejunal morphology in late-phase laying hens. Poult Sci. (2020) 99:2100–7. doi: 10.1016/j.psj.2019.12.032
37. Jiang MJ, Zhao JP, Jiao HC, Wang XJ, Zhang Q, Lin H. Dietary supplementation with sodium bicarbonate improves calcium absorption and eggshell quality of laying hens during peak production. Brit Poultry Sci. (2015) 56:740–7. doi: 10.1080/00071668.2015.1113499
38. Abdel-Moneim AME, Shehata AM, Khidr RE, Paswan VK, Ibrahim NS, El-Ghoul AA, et al. Nutritional manipulation to combat heat stress in poultry-A comprehensive review. J Therm Biol. (2021) 98:102915. doi: 10.1016/j.jtherbio.2021.102915
39. Iqbal Y, Cottrell JJ, Suleria HAR, Dunshea FR. Gut microbiota-polyphenol interactions in chicken: a review. Animals. (2020) 10:1391. doi: 10.3390/ani10081391
40. Refaie AM, El-Maged MH, El-Halim HHA, Alghonimy HRH, Shaban SAM. Performance physiological parameters and egg quality traits of laying hens as affected by dietary supplementation of date palm pollen. J Anim Health Prod. (2022) 10:21–8. doi: 10.17582/journal.jahp/2022/10.1.21.28
41. Liu H, Liu Y, Hu L, Suo Y, Zhang L, Jin F, et al. Effects of dietary supplementation of quercetin on performance egg quality cecal microflora populations and antioxidant status in laying hens. Poult Sci. (2014) 93:347–53. doi: 10.3382/ps.2013-03225
42. Kara K, Güçlü BK, Baytok E, Sentürk M. Effects of grape pomace supplementation to laying hen diet on performance egg quality egg lipid peroxidation and some biochemical parameters. J Appl Anim Res. (2016) 44:303–10. doi: 10.1080/09712119.2015.1031785
43. Bonekamp R, Lemme A, Wijtten P, Sparla J. Effects of amino acids on egg number and egg mass of brown (heavy breed) and white (light breed) laying hens. Poult Sci. (2010) 89:522–9. doi: 10.3382/ps.2009-00342
44. Tian XZ, Li JX, Luo QY, Wang X, Xiao MM, Zhou D, et al. Effect of supplementation with selenium-yeast on muscle antioxidant activity meat quality fatty acids and amino acids in goats. Front Vet Sci. (2022) 8:813672. doi: 10.3389/fvets.2021.813672
45. Omar AE, Al-Khalaifah HS, Mohamed WAM, Gharib HSA, Osman A, Al-Gabri NA, et al. Effects of phenolic-rich onion (Allium cepa L)extract on the growth performance behavior intestinal histology amino acid digestibility antioxidant activity and the immune status of broiler chickens. Front Vet Sci. (2020) 7:582612. doi: 10.3389/fvets.2020.582612
46. Mishra R, Patel AB, Bhagora NJ, Dhruv JJ, Savaliya FP. Influence of feeding different maize varieties on production performance and egg quality of white leghorn birds in India. Indian J Vet Sci Biotechnol. (2022) 18:49–53. doi: 10.21887/ijvsbt.18.1.10
47. Schneider C. An update on products and mechanisms of lipid peroxidation. Mol Nutr Food Res. (2009) 53:315–21. doi: 10.1002/mnfr.200800131
48. Crescenti A, del Bas JM, Arola-Arnal A, Oms-Oliu G, Arola L, Caimari A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J Nutr Biochem. (2015) 26:912–20. doi: 10.1016/j.jnutbio.2015.03.003
49. Tian X, Lu Q. Anthocyanins in dairy cow nutrition: a review. Agriculture. (2022) 12:1806. doi: 10.3390/agriculture12111806
50. Lachowicz S, Oszmiański J, Wilczyńska M, Zagua G, Saletnik B, Puchalski C. Impact mineralization of chokeberry and cranberry fruit juices using a new functional additive on the protection of bioactive compounds and antioxidative properties. Molecules. (2020) 25:659. doi: 10.3390/molecules25030659
51. Rossetto M, Vanzani P, Lunelli M, Scarpa M, Mattivi F, Rigo A. Peroxyl radical trapping activity of anthocyanins and generation of free radical intermediates. Free Radical Res. (2007) 41:854–9. doi: 10.1080/10715760701261533
Keywords: anthocyanin, antioxidant activity, egg quality, amino acid, fatty acid, laying hen
Citation: Li J, Zhou D, Li H, Luo Q, Wang X, Qin J, Xu Y, Lu Q and Tian X (2023) Effect of purple corn extract on performance, antioxidant activity, egg quality, egg amino acid, and fatty acid profiles of laying hen. Front. Vet. Sci. 9:1083842. doi: 10.3389/fvets.2022.1083842
Received: 29 October 2022; Accepted: 12 December 2022;
Published: 06 January 2023.
Edited by:
Abdel-Moneim Eid Abdel-Moneim, Egyptian Atomic Energy Authority, EgyptCopyright © 2023 Li, Zhou, Li, Luo, Wang, Qin, Xu, Lu and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingzhou Tian,  dGlhbnhpbmd6aG91QHllYWgubmV0;
dGlhbnhpbmd6aG91QHllYWgubmV0;  eHp0aWFuM0BnenUuZWR1LmNu
eHp0aWFuM0BnenUuZWR1LmNu
 Jiaxuan Li1
Jiaxuan Li1 Qingyuan Luo
Qingyuan Luo Xingzhou Tian
Xingzhou Tian