- 1Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
- 2Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia
Third-world countries have a higher prevalence of food-related disorders than developed nations. Millions of people in underdeveloped countries are seriously at risk from the potential water supply contamination with protozoan diseases. Toxoplasma gondii is one of the important protozoans causing diseases in livestock and humans. Despite the standard tests for diagnosing this parasite and different treatment methods, the spread of these parasites is uncontrollable and rising every year due to other management disorders. In this review, we summarize etiopathogenesis and prevalence in Pakistan. We looked for papers reporting the seroprevalence of T. gondii in people and animals between 2000 and 2022 in different databases: PubMed, Google Scholar, ScienceDirect, Scopus, and Web of Science. Data on the seroprevalence of T. gondii in Pakistan's domestic animals (sheep and goats, horses, donkeys, mules, cattle, and buffaloes), domestic pets (cats and dogs), poultry and rodents, and humans were gathered. According to the findings, sheep had an estimated pooled seroprevalence of T. gondii that varied from 11.20 to 26.50 %, and goats from 24.50 to 38.40%. Whereas in buffalo the opposite trend was followed, and the prevalence was observed is 0% in 2022, in horses, donkeys, and mules, only one study was reported according to which a high prevalence was observed in mules (28.60%) followed by donkeys (23.50%) and horses (23.50%), in cats 38.5% prevalence was observed in a recent study and in dogs 28.43% observed, and in humans from 22 to 60%. Human beings are found to be the most affected species showing high prevalence among all. According to our findings, animals and pets not only serve as a reservoir for the parasite but also serve as a direct route for human infection with T. gondii. The diagnostic techniques used in the observed studies were mostly serological testing whereas only a few studies have only been observed with molecular testing. To know the exact pattern of the disease for its control, the trend of molecular and advanced testing should be adopted as it is more reliable. Moreover, to decrease the transmission chances of T. gondii to humans, it is crucial to manage T. gondii infections in non-human species.
1. Introduction
Infections caused by food and water have attracted a lot of attention recently. The term “foodborne sickness” refers to a set of diseases that develop after consuming microbially or chemically contaminated food. Even contaminated water, utensils, as well as the hands of the diner can spread the disease. Third-world countries have a higher prevalence of food-related disorders than developed nations. Most people in the world still lack access to clean water and sanitary facilities, and households in rural areas where untreated water used for drinking, cooking, washing fruits, bathing, and swimming expose them to various pathogens including protozoan parasites (1, 2). Millions of people in underdeveloped countries are seriously at risk from the potential water supply contamination with protozoan diseases. There are many basic signs of food-related diseases, and gastrointestinal dysfunction is commonly used to diagnose them.
Parasites are capable of causing an acute, chronic, and debilitating type of diseases (3–6). In nature, parasitic protozoa may be found almost everywhere. In both developed and developing nations, they are accountable for epidemics and chronic poverty (7). Since certain parasites are zoonotic in nature and consequently exist in animals, their food and water prevalence should be considered a public health problem (8). The prevalence of food- and waterborne parasites has increased throughout time due to several past disease outbreaks linked to parasites. The World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) published their worldwide risk rating of foodborne parasites (FBPs) in 2014 (2). It was followed in 2015 as a global burden related to foodborne pathogens (9). Despite being acknowledged as significant foodborne pathogens, parasites are still underappreciated when compared to bacterial and viral foodborne pathogens (10).
Toxoplasma spp is one of the important protozoans causing disease in livestock and humans (11–13). Across the world, this parasite has posed a serious threat. Despite the standard test for diagnosing this parasite and different treatment methods, the spread of these parasites is uncontrollable due to the other management disorders (14). This review summarizes etiopathogenesis, epidemiology in Pakistan from 2000 to 2022, and preventive measures for zoonotic toxoplasmosis.
2. Material and methods
2.1. Search technique
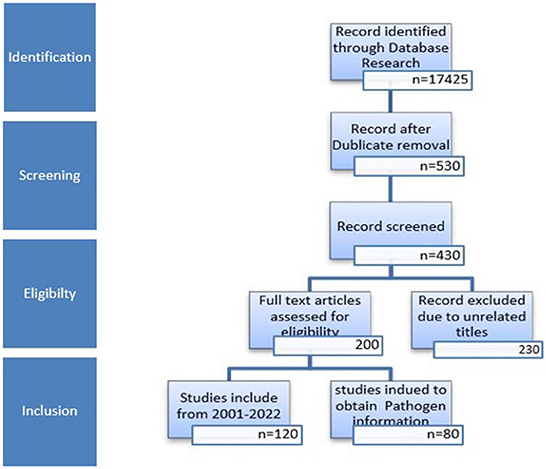
We searched databases (PubMed, Embase, Google Scholar, ScienceDirect, Scopus, ProQuest, and Web of Science) for articles reporting the seroprevalence of T. gondii in Pakistan from 2000 to 2022 to conduct this systematic review. The searches were limited to English-language articles. Electronic searches mostly employed the MeSH keywords (Human, Animal) AND (Toxoplasma gondii OR toxoplasmosis; Prevalence and seroprevalence OR serology).
For management, the citations were observed keenly. The final article choice was made after screening each article's title and abstract by eliminating duplicate records.
2.2. Criteria for inclusion and exclusion
Using the titles as a guide, references were screened, and unnecessary and duplicate references were removed. A flow chart of the article identification, screening, eligibility, and inclusion criteria is shown in Figure 1. The last search was conducted on October 6th, 2022.
3. Toxoplasmosis
Toxoplasma gondii is a member of the phylum Apicomplexa, which is made up of a variety of protists, most of which are intracellular parasites capable of inflicting potentially life-threatening diseases in both humans and animals. Given its ability to infect virtually all warm-blooded vertebrates, T. gondii is the most prevalent. It is also thought to infect about one-third of the world's population of humans (15). According to the nation or region under consideration, seropositivity rates in the human population vary from < 10 to over 90%, partly due to local socio-economic conditions and population patterns (16). For instance, there is a more significant incidence in continental Europe, South America, and the United Kingdom than in the United States or the United Kingdom. Both wild and domestic animals have high seroprevalence, making them essential T. gondii reservoirs and sources for human contamination through meat intake (17). In addition to being an issue for human contamination, toxoplasmosis in farm animals has a significant negative impact on the livestock (on milk production and reproductive performance), which results in a high cost for the industry (18).
Only one species has been identified for the genus Toxoplasma, yet many clonal lineages have varying degrees of pathogenicity. Four primary clonal types I, II, III, and XII dominate the population pattern of T. gondii in Europe and North America (19–21). The most common strains in a wild and domestic context in Europe are type II (and type III, though to a lesser extent) (22, 23). Domestic isolates from North America are comparable to those from Europe (types II and III), while in the wild, strains from type XII prevail (24, 25). More contrast exists in other regions of the world. For instance, South America has a lot more genetic variety (26, 27), which suggests that recombination occurs more frequently there. Following T. gondii infection, the host's type, genetic makeup, and of course, the host's immune status all play a role in the development of the disease. Some species appear to be innately resistant to T. gondii infection. In contrast, others are highly susceptible, partly due to variables like their habitat's closeness to the parasite's definitive hosts (28). However, the host immune system and how parasite factors affect it continue to be one of the most critical factors affecting susceptibility to T. gondii (29–32).
4. Life cycle and routes of transmission of Toxoplasma
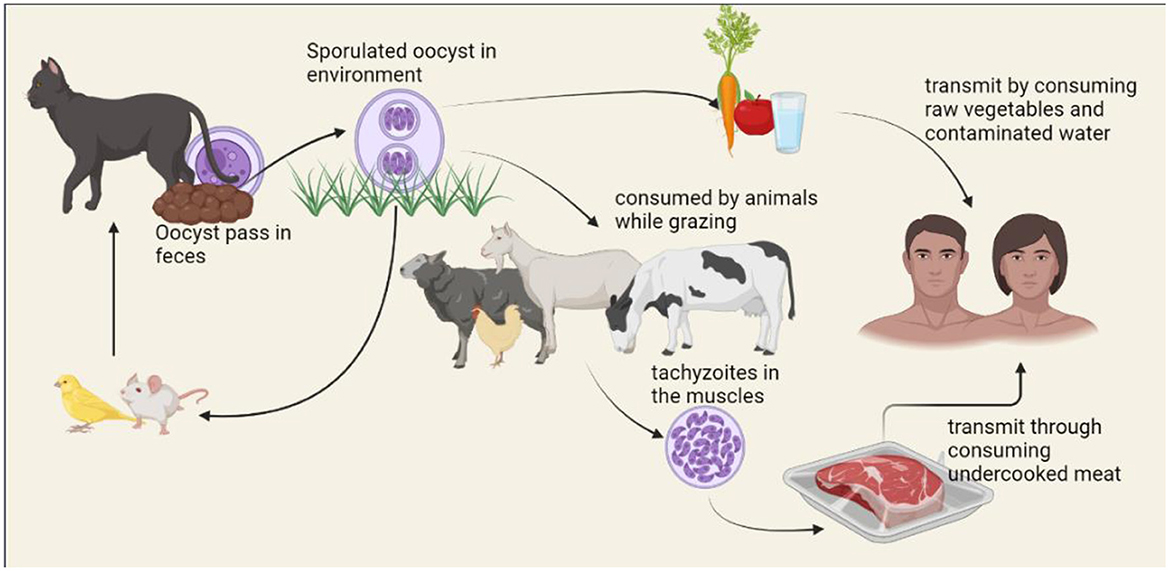
The life cycle of T. gondii includes both, asexual replication in a range of vertebrate hosts (intermediate hosts) and sexual replication in felids (definitive hosts). Felids consume the T. gondii by consuming encysted bradyzoites on infected intermediate hosts. Under the influence of digestive enzymes and acid, bradyzoites are liberated from cysts and enter the small intestine's epithelial cells. Although the parasite may spread throughout the body of the final host and cause clinical symptoms, this is uncommon (33). More typically, bradyzoites transform into schizonts in the intestine before reaching the merozoite stage (34). Merozoites differentiate into male and female gametes after a few cycles of asexual division. After that, male and female gametes combine to form diploid oocysts, which are enclosed in a solid, impenetrable wall. The millions of them contaminate the ecosystem that the felids excrete. The oocysts are resilient and survive in the environment, allowing them to spread (35, 36). Intermediate hosts consume sporulated oocysts by drinking or eating contaminated water and foods. Invading sporozoites quickly transform into the tachyzoite form inside a transitory parasitophorous vacuole (PV) that stays in the host cells (37). Tachyzoites are proliferative forms of toxoplasmosis that spread throughout the body and cause acute symptoms. They can move between tissues via blood vessels or the lymphatic system. At least when felids can prey on the intermediate host, this ensures parasite transmission to the final host to finish the cycle. Even when intermediate hosts aren't the felids who are often their prey, the parasites can still spread to new intermediate hosts through carnivory, keeping the parasite transmission cycle going without the necessity for sexual reproduction. The life cycle of T. gondii is shown in Figure 2.
Human infection can occur through food consumption, such as raw or undercooked meat containing cysts or vegetables, fruits, or water that has sporulated oocysts (38). To prevent foodborne toxoplasmosis, it is crucial to wash produce, cook meat properly, and adequately treat sewage or water (39). Congenital transfer of tachyzoites from a woman who is mainly infected to the growing fetus through the placenta is one of the alternative ways of transmission (40).
Congenital toxoplasmosis must be managed with precautions that restrict the mother's exposure to established transmission channels while pregnant and with quick identification and treatment beginning following infection. Although uncommon, blood transfusion (41) or organ transplantation (42) from sick donors are potential sources of contamination in people.
5. Clinical manifestations of toxoplasmosis
In immunocompetent people, toxoplasmosis can cause a minor, self-limiting disease or remain unnoticed in most cases (43). In pregnant women, congenital toxoplasmosis may develop because, during the parasite's dissemination phase, the parasite passes through the placenta and infect the growing fetus. Depending on the gestational stage at the time of maternal infection, it might result in varying degrees of neurological, ophthalmic, or systemic damage. For instance, a maternal illness in the first trimester may result in more severe symptoms (44). Although some of them can also happen later in life, hydrocephalus, mental retardation, epilepsy, and blindness are the most significant sequelae for newborns (45).
Even though acute acquired infection can occur, immunodeficiency in adults can also result in severe toxoplasmosis.
People who have impaired immune systems or immunosuppression [such as those with HIV (46), cancer patients (47), or transplant recipients] are particularly vulnerable. Toxoplasmic encephalitis may be the most severe outcome because it causes significant tissue damage and inflammation when toxoplasmosis from parasites ensconced in the central nervous system returns (48). Left untreated, this cerebral toxoplasmosis can be potentially fatal and frequently manifest as headache, fever, ataxia, or seizures. Acute toxoplasmosis has harmful effects, but chronic toxoplasmosis—the parasite's long-term persistence in the body as tissue cysts—may also have significant effects on behavioral changes and psychiatric problems, especially because it affects the central nervous system (49).
6. Prevalence of toxoplasmosis in humans and animals of Pakistan
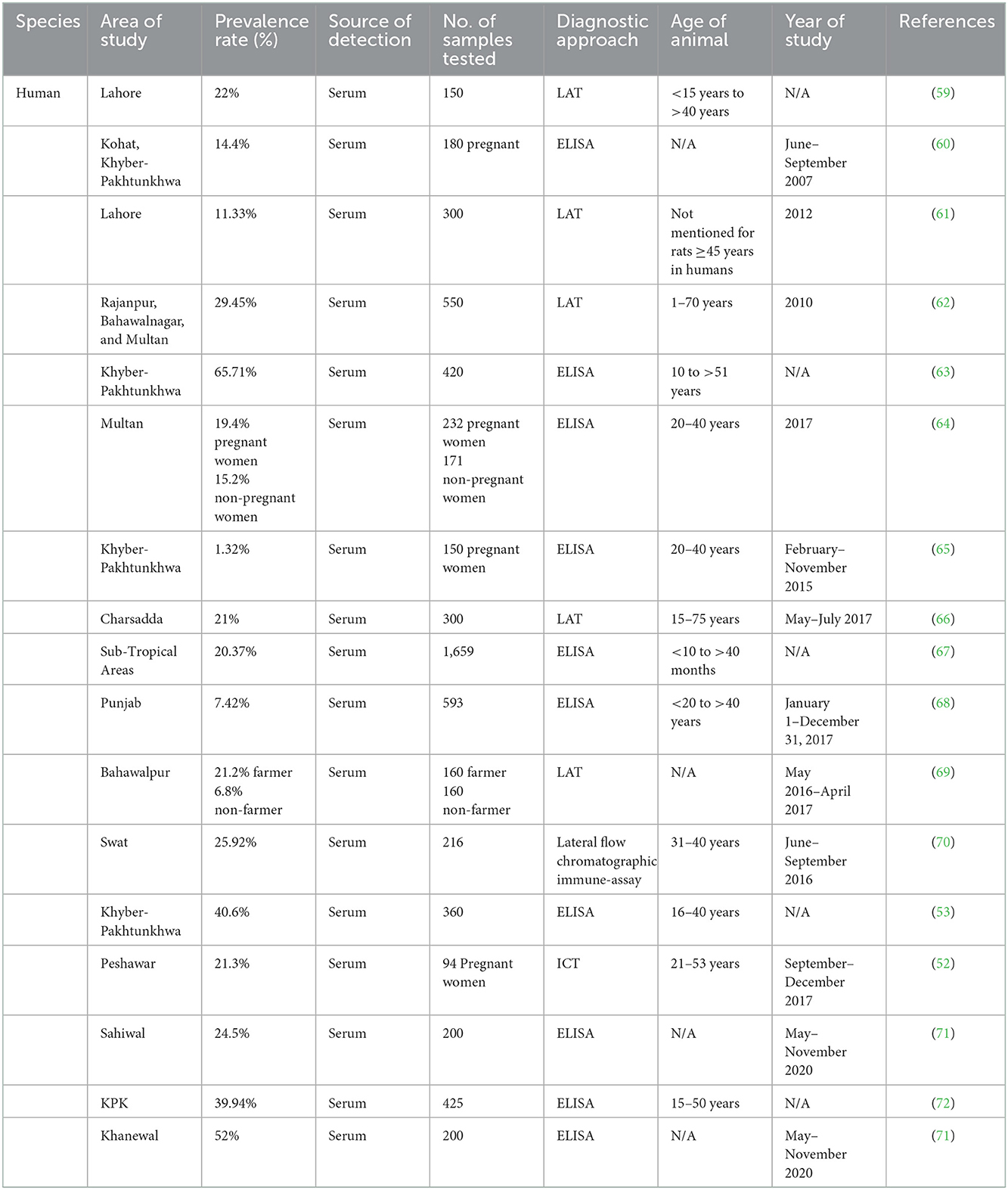
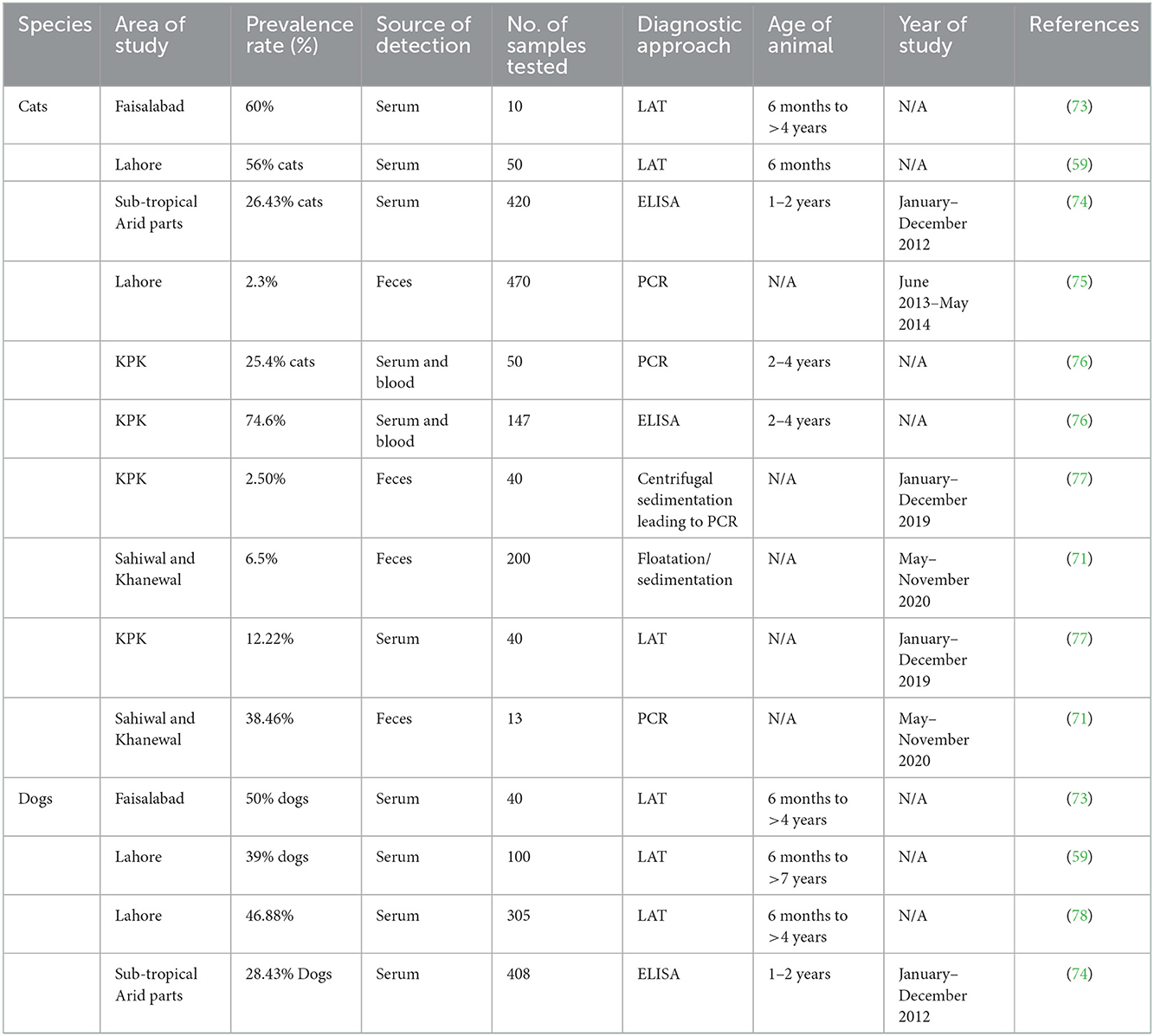
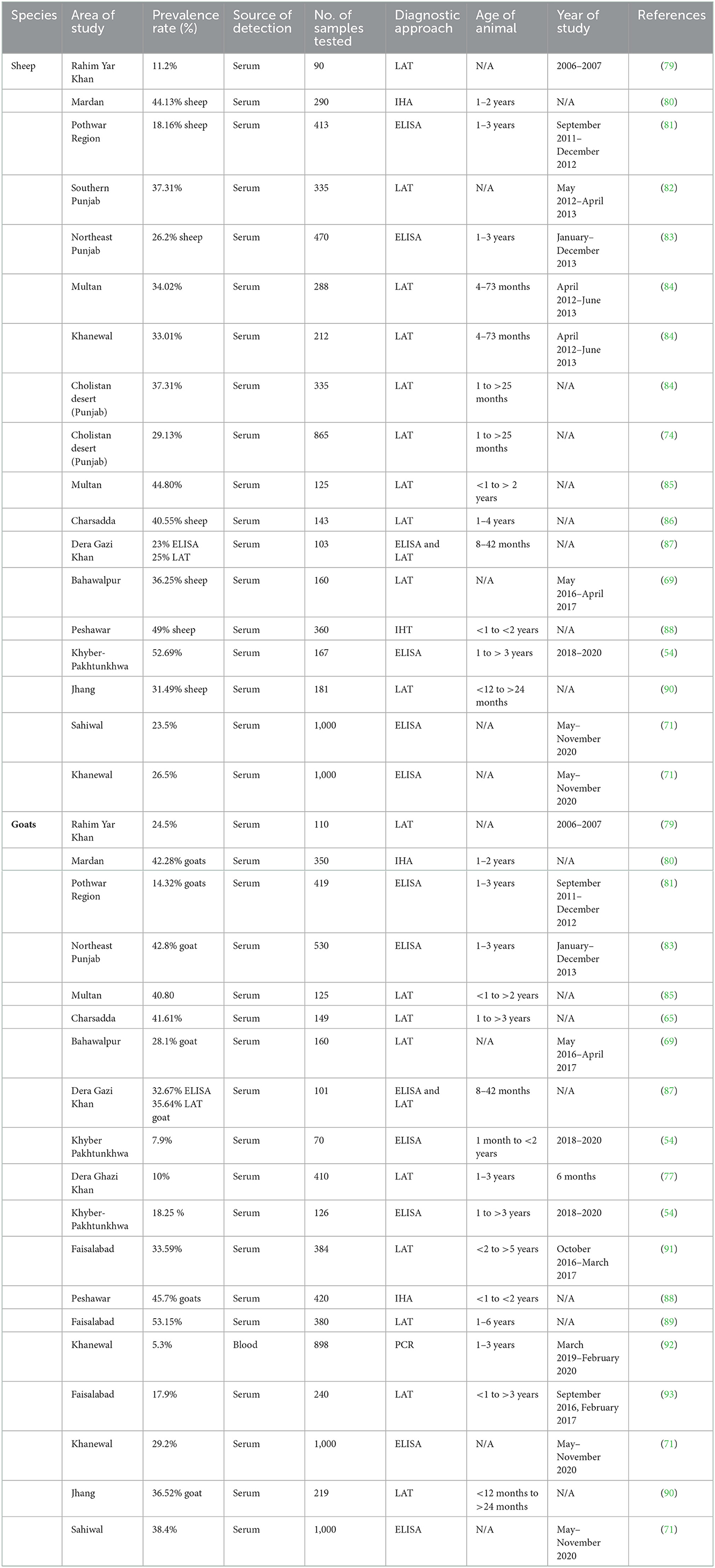
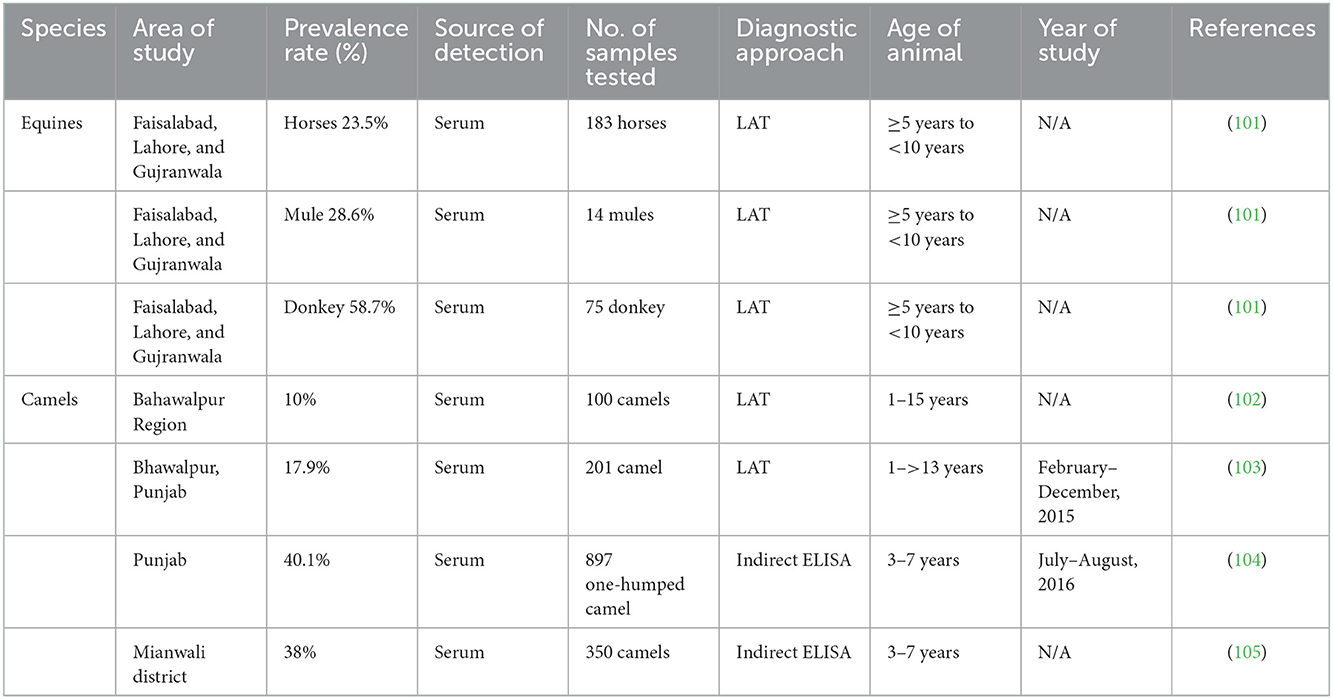
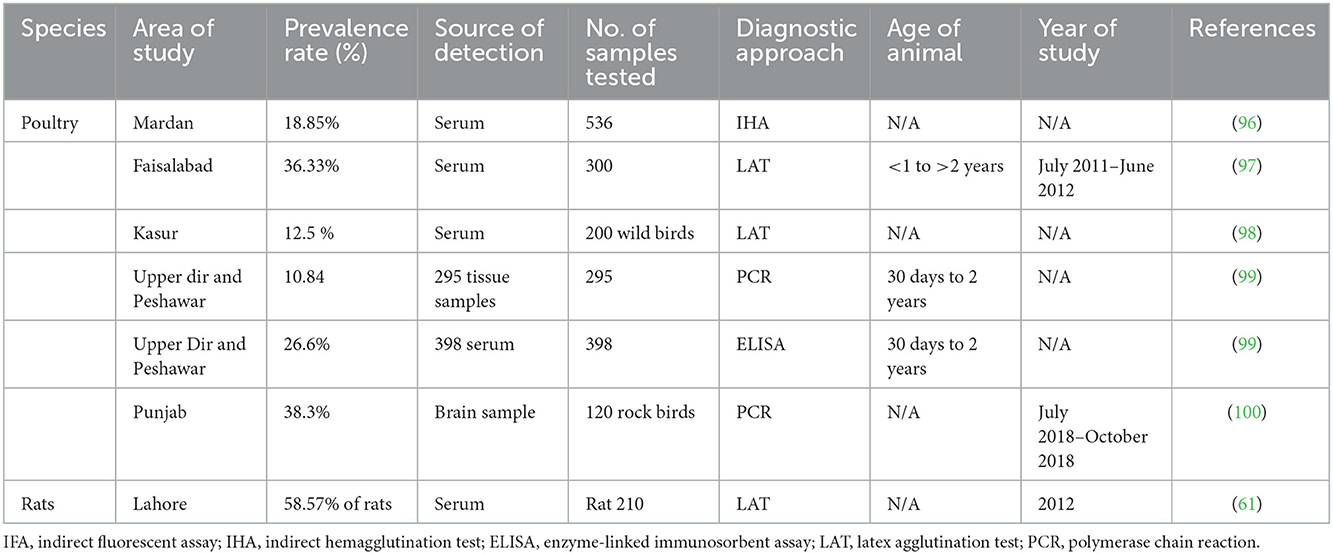
Human studies that assessed the seroprevalence of T. gondii among the various individual groups listed in Table 1 have been published in Pakistan. Seroprevalence of T. gondii in dogs and cats, small ruminants, large ruminants, equines and camels, and poultry are mentioned in Tables 2–6, respectively. Pregnant women's seroprevalence has received the majority of attention in research (50–52), followed by patients with illnesses (53), The sociodemographic information, epidemiological profile, potential risk factors for transmitting the T. gondii infection, and the source of detection and diagnostic approach is also the focus of these investigations. Variable seroprevalence levels have been observed with a rise in percentage between 2001 and 2022.
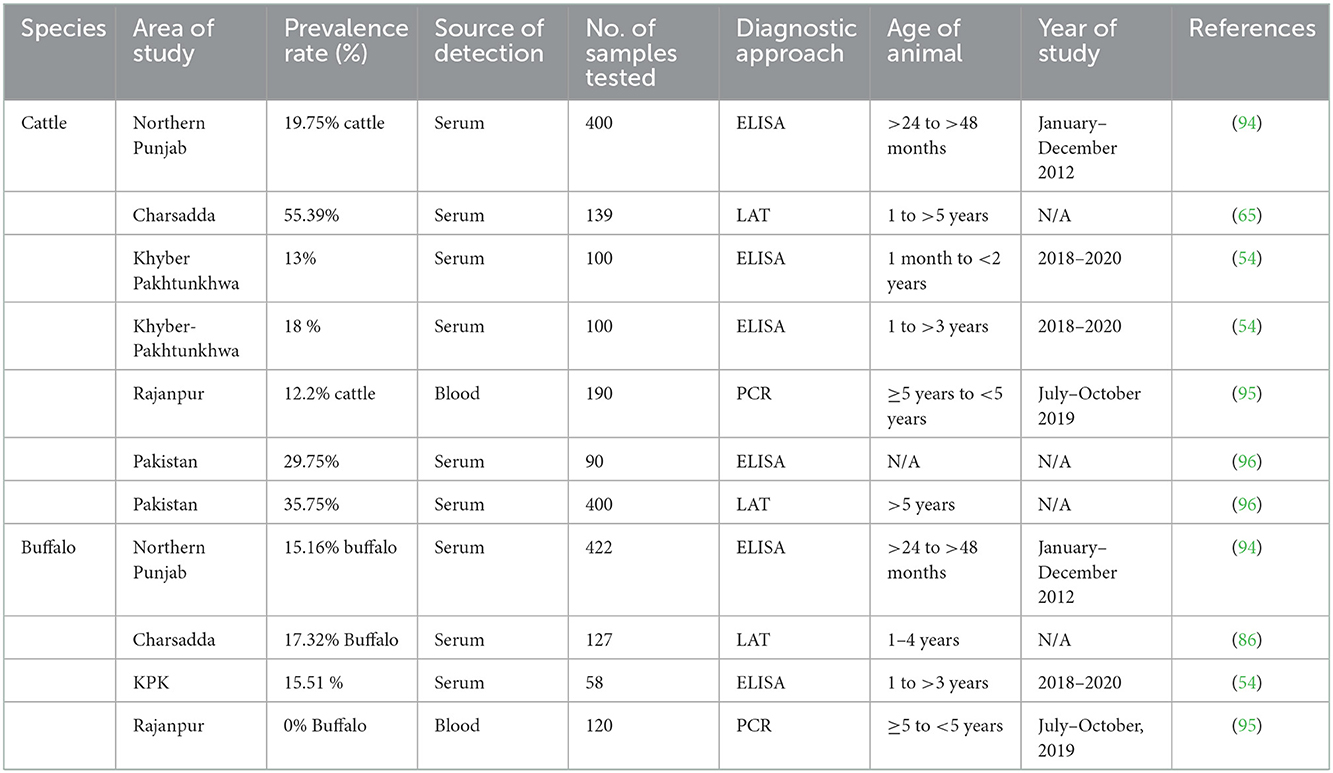
Table 4. Reported prevalence of Toxoplasma gondii in large ruminants (cattle and buffalo) in Pakistan.
The procedure used in the publications is often the collection of a blood sample from the population and testing it for anti-toxoplasma antibodies in the sera. The ELISA, latex agglutination (LA) test, and other serological assays were often employed (54, 55). Although their sensitivity and specificity varied, commercial test kits were employed, and the results were occasionally inconclusive.
In the food chain, which serves as a source of nutrition for humans and other animals, animals play a crucial role. The bradyzoite cyst is present in the body tissues of animals, and the parasite then transmits to new hosts by eating a raw or undercooked piece of the infected tissues (56). This situation raises the risk of zoonotic infection by foodborne pathogens since a particular group of humans, such as hunters, butchers, and consumers may get infected by ingesting domestic or wild meat (57). Only wild and domestic cats excrete the oocyst infective stage, which may infect humans when eaten in tainted food, water, or vegetables, which is especially noteworthy (58).
According to an analysis of the records currently available over the previous 20 years (2001–2022), domestic animals in Pakistan have a relatively high and rising seroprevalence rate of T. gondii as a zoonotic infection, except for buffalo, cats, and dogs. The comparison is shown in Figure 3. Although the prevalence level may have decreased and increased in different species, caution is advised.
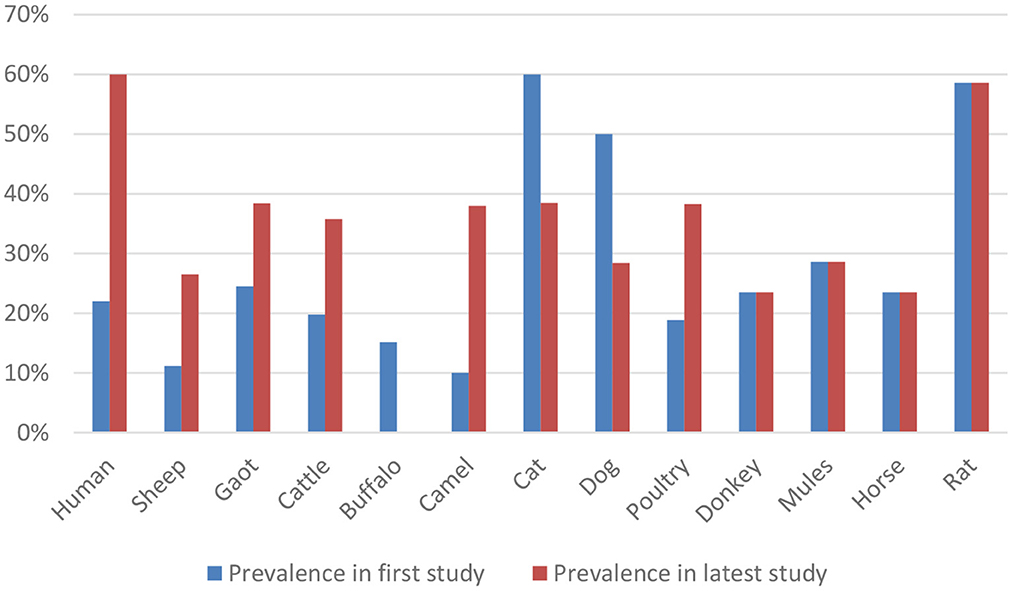
Figure 3. Comparison of prevalence rate of Toxoplasma gondii between the first and the latest study.
All the data mentioned in the previous study of Figure 1 is collected from the year 2022 except for equines, dogs, and rats because the previous study performed in equine was in the year 2015, and for rat, it was in 2012. Whereas in dogs, the last study was performed in 2014 in Pakistan.
7. Methods used for the detection of T. gondii
Examining the levels of immunoglobulin G (IgG), immunoglobulin M (IgM), and IgG avidity in a sample—typically serum from the blood of a particular host population—the serological test assesses the antibodies and calculates the seroprevalence of infection. Although it is the simplest and most straightforward test, it frequently yields false-positive or false-negative findings (106). Most of the studies in Pakistan have been diagnosed through Latex Agglutination Test (LAT). Cd4 mentioned in Table 1. The tests using molecular methods are reliable, perceptive, and accurate (107). Few studies have been reported and mentioned in Table 1, which have been diagnosed with Molecular methods. They use a variety of samples to find a specific gene of interest that is unique to this particular organism. Numerous techniques are routinely used, including loop-mediated amplification (LAMP), quantitative PCR, and traditional polymerase chain reaction (PCR) (108). This technique is seldom employed in histological procedures. It is primarily concerned with identifying the bradyzoite stage in. tissues such as the heart, liver, and brain. The bradyzoite stage is primarily detected in tissues, including the heart, liver, and brain (109). Before being examined under a microscope, such tissues are mounted on a glass slide and stained with hematoxylin and eosin (H&E). Another method of evaluating suspected samples, such as cat feces, liver, lung, and brain homogenates of intermediate hosts by inoculation and then testing the animal for the presence of an infection, is bioassay/in vivo using an animal model (mice/rat) (110). The test is costly and time-consuming, but it is an accurate approach to assessing the sustainability and pathogenicity of the various strains. Through the establishment of an enclosed environment where suspected specimens, such as blood, are cultured in a medium, the in vitro/tissue culture technique removes the usage of animals (111). Microscopy is used to assess the sample's motility or viability for the tissue culture endpoint. Most intuitive findings that can identify the parasite's morphology nevertheless rely heavily on microscopy as their foundation. Other tests like tissue culture and histology consistently rely on it because of its adaptability (112).
8. Comparison of serological techniques for T. gondii antibody detection in Pakistan
All studies employed convenient sampling to gather data, and two serological tests—the Latex Agglutination Test (LAT) and Enzyme-Linked Immunosorbent Assay (ELISA)—are mostly used to assess the outcomes based on the detection of IgG, IgM, and avidity test of T. gondii antibodies (Table 1) (96, 99). Most studies did not follow established procedures for collecting and processing specimens, and most did not have information on the control group. However, since a different company produced each ELISA and LAT test, it was challenging to evaluate and confirm each assay's specificities and sensitivities. Except for a few recent studies, further PCR validation of the data was not done (75, 77, 95, 100). Because of differences in the experimental design and the commercial kits utilized, some of the results are thus disputed.
9. Prevention of toxoplasmosis
The foundation for the current strategies employed to control T. gondii infection has been supplied by the exponential growth in our understanding of T. gondii biology, epidemiology, and ecology during the past few decades. Limiting contact with available transmission channels and minimizing exposure to the parasite's infectious phases are the main goals of preventative interventions.
As previously indicated, people contract T. gondii either by eating or drinking raw or undercooked meat with parasite cysts on it or by drinking water contaminated with oocysts deposited in cat feces. Additionally, eating raw shellfish can result in illness (106).
Therefore, seronegative should only consume fully cooked meat, refrain from consuming raw shellfish, carefully wash their hands after coming into contact with raw meat, avoid gardening and soil handling without gloves, and thoroughly clean fruits and vegetables.
People who take care of the litter box should make it a habit to wear disposable gloves and wash their hands thoroughly with antiseptic. Seronegative should refrain from adopting or handling stray cats, and cats should stay indoors whenever feasible. They should also not be fed raw or undercooked meat. The suggestions mentioned above for preventing T. gondii infection also apply to people in other particular at-risk groups. Soon after HIV diagnosis, standardized guidelines advise testing all for serological signs of prior T. gondii infection (107).
Primary prophylaxis should be given to those who are also seropositive for T. gondii and have peripheral blood CD4 T cell levels of 100/L (107). People receiving cART who have had more than 200 CD4 T cells/L for 3 months can stop using primary prophylaxis without risk. When they reach more than 200 CD4 T cells/L for 6 months, PLWH who have undergone effective therapy for TE and are getting cART can stop receiving maintenance treatment (107). It is important to remember that despite these precautions, T. gondii infection cannot be entirely avoided.
10. Conclusion
Infectious diseases of animals including parasitic infestations pose significant threats to health and productivity potential of animals (113–116) which leads to heavy economic losses (117–122). Parasitic infections lead to chronic and debilitating types of diseases and have zoonotic implications as well (3, 123–126). Results of the current evaluation on toxoplasmosis research in Pakistan from 2001 to 2022 revealed little information on animal seroprevalence in cases of humans, cattle, buffaloes, sheep, goats, cats, dogs, camels, and horses. In Pakistan, the seroprevalence among human females is rising. The frequency of toxoplasmosis in cattle, mainly chicken intended for human consumption, is also little understood. The T. gondii strain prevalent in Pakistan from HIV patients, pregnant women, livestock, and domestic cats has not yet been genetically characterized. Alarming reports of toxoplasmosis in the KPK population have been observed in humans. Despite the widespread occurrence and severe effects of toxoplasmosis, which are mostly seen in immunocompromised patients, there are significant flaws in the present control programs, particularly in the diagnostic resources available. The prevalence throughout the country is increasing every year. The majority of diagnostic procedures also frequently misdiagnose the illness in endemic regions. It is necessary to create molecular approaches that are sensitive, specific, straightforward to use, affordable, and high throughput because early detection is the most effective way to combat the illness. Researchers, healthcare professionals, veterinary professionals, and politicians can benefit from the current review on toxoplasmosis. Therefore, there is an urgent need to inform and educate the public about the risk factors for toxoplasmosis infection in humans and animals. That may be accomplished by running health-related advertisements and educational campaigns in regional newspapers, television, radio, and, more recently, social media platforms.
Data availability statement
All data supporting the conclusions of this article are included within the article.
Author contributions
WQ and AA worked on the development of this unique title of review, planned, designed, and structured the layout of the article. WQ wrote the article. AA reviewed the article. All authors finally approved this review article.
Acknowledgments
The researcher would like to thank the Deanship of Scientific Research, Qassim University, Saudi Arabia, for funding the publication of this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Javed A. Foodborne health issues and their relevance to Pakistani Society. Am Acad Sci Res J Eng Technol Sci. (2016) 26:235–51. Available online at: https://asrjetsjournal.org/index.php/American_Scientific_Journal/article/view/2442
2. World Health Organization. Multicriteria-based ranking for risk management of food-born parasites. Microbiological Risk Assessment Series, 23. Rome, Italy: FAO, World Health Organization (2014).
3. Strbac F, Bosco A, Amadesi A, Rinaldi L, Stojanović D, Simin N, et al. Ovicidal potential of five different essential oils to control gastrointestinal nematodes of sheep. Pak Vet J. (2020) 41:353–8. doi: 10.29261/pakvetj/2021.026
4. Alvi MA, Ohiolei JA, Saqib M, Li L, Tayyab MH, Alvi AA, et al. Echinococcus granulosus (sensu stricto)(G1, G3) and E. ortleppi (G5) in Pakistan: phylogeny, genetic diversity and population structural analysis based on mitochondrial DNA. Parasit Vectors. (2020) 13:347. doi: 10.1186/s13071-020-04199-8
5. Kandeel M, Akhtar T, Zaheer T, Ahmad S, Ashraf U, Omar M, et al. Anti-parasitic applications of nanoparticles: a review. Pak Vet J. (2022) 42:2074–7764. doi: 10.29261/pakvetj/2022.040
6. Mahmood Q, Younus M, Sadiq S, Iqbal S, Idrees A, Khan S, et al. Prevalence and associated risk factors of cystic echinococcosis in food animals–a neglected and prevailing zoonosis. Pak Vet J. (2022) 42:59–64. doi: 10.29261/pakvetj/2022.008
7. Al-Malki ES. Toxoplasmosis: stages of the protozoan life cycle and risk assessment in humans and animals for an enhanced awareness and an improved socio-economic status. Saudi J Biol Sci. (2021) 28:962–9. doi: 10.1016/j.sjbs.2020.11.007
8. Thompson RA. Parasite zoonoses and wildlife: one health, spillover and human activity. Int J Parasitol. (2013) 43:1079–88. doi: 10.1016/j.ijpara.2013.06.007
9. Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. (2015) 12:e1001920. doi: 10.1371/journal.pmed.1001920
10. Trevisan C, Torgerson PR, Robertson LJ. Foodborne parasites in Europe: present status and future trends. Trends Parasitol. (2019) 35:695–703. doi: 10.1016/j.pt.2019.07.002
11. Leung AK, Leung AA, Wong AH, Sergi CM, Kam JK. Giardiasis: an overview. Recent Pat Inflamm Allergy Drug Discov. (2019) 13:134–43. doi: 10.2174/1872213X13666190618124901
12. Almeria S, Dubey JP. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res Vet Sci. (2021) 135:371–85. doi: 10.1016/j.rvsc.2020.10.019
13. Gorcea M, Neculicioiu V, Junie L. Cryptosporidium and Giardia–an overview. Sci Parasitol. (2020) 21:18–24. Available online at: https://www.researchgate.net/profile/Lia-Junie/publication/340633629_Cryptosporidium_and_Giardia_-an_ove rview/links/5e95f660a6fdcca789159556/Cryptosporidium-and-Giardia-an-overvi ew.pdf
14. Siwila J, Mwaba F, Chidumayo N, Mubanga C. Food and waterborne protozoan parasites: the African perspective. Food Waterborne Parasitol. (2020) 20:e00088. doi: 10.1016/j.fawpar.2020.e00088
15. Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. (2012) 25:264–96. doi: 10.1128/CMR.05013-11
16. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. (2009) 39:1385–94. doi: 10.1016/j.ijpara.2009.04.003
17. Schlüter D, Däubener W, Schares G, Groß U, Pleyer U, Lüder C. Animals are key to human toxoplasmosis. Int J Med Microbiol. (2014) 304:917–29. doi: 10.1016/j.ijmm.2014.09.002
18. Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Su C. Economic and public health importance of Toxoplasma gondii infections in sheep: 2009–2020. Vet Parasitol. (2020) 286:109195. doi: 10.1016/j.vetpar.2020.109195
19. Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. (1995) 172:1561–66. doi: 10.1093/infdis/172.6.1561
20. Lorenzi H, Khan A, Behnke MS, Namasivayam S, Swapna LS, Hadjithomas M, et al. Local admixture of amplified and diversified secreted pathogenesis determinants shapes mosaic Toxoplasma gondii genomes. Nat Commun. (2016) 7:10147. doi: 10.1038/ncomms10147
21. Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM, Sibley LD. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol. (2011) 41:645–55. doi: 10.1016/j.ijpara.2011.01.005
22. Ajzenberg D, Year H, Marty P, Paris L, Dalle F, Menotti J, et al. Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J Infect Dis. (2009) 199:1155–67. doi: 10.1086/597477
23. Verma SK, Ajzenberg D, Rivera-Sanchez A, Su C, Dubey JP. Genetic characterization of Toxoplasma gondii isolates from Portugal, Austria and Israel reveal higher genetic variability within the type II lineage. Parasitology. (2015) 142:948–57. doi: 10.1017/S0031182015000050
24. Jiang T, Shwab EK, Martin RM, Gerhold RW, Rosenthal BM, Dubey JP, et al. A partition of Toxoplasma gondii genotypes across spatial gradients and among host species, and decreased parasite diversity towards areas of human settlement in North America. Int J Parasitol. (2018) 48:611–9. doi: 10.1016/j.ijpara.2018.01.008
25. Khan A, Fux B, Su C, Dubey JP, Darde ML, Ajioka JW, et al. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Nat Acad Sci. (2007) 104:14872–7. doi: 10.1073/pnas.0702356104
26. Lehmann T, Marcet PL, Graham DH, Dahl ER, Dubey JP. Globalization and the population structure of Toxoplasma gondii. Proc Nat Acad Sci. (2006) 103:11423–8. doi: 10.1073/pnas.0601438103
27. Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML, et al. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Nat Acad Sci. (2012) 109:5844–9. doi: 10.1073/pnas.1203190109
28. Innes EA. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis. (1997) 20:131–8. doi: 10.1016/S0147-9571(96)00038-0
29. Mukhopadhyay D, Arranz-Solís D, Saeij JPJ. Influence of the host and parasite strain on the immune response during Toxoplasma infection. Front Cell Infect Microbiol. (2020) 10:580425. doi: 10.3389/fcimb.2020.580425
30. Ihara F, Nishikawa Y. Toxoplasma gondii manipulates host cell signaling pathways via its secreted effector molecules. Parasitol Int. (2021) 83:102368. doi: 10.1016/j.parint.2021.102368
31. Tomita T, Guevara RB, Shah LM, Afrifa AY, Weiss LM. Secreted effectors modulating immune responses to Toxoplasma gondii. Life. (2021) 11:988. doi: 10.3390/life11090988
32. Hakimi M-A, Olias P, Sibley LD. Toxoplasma effectors targeting host signaling and transcription. Clin Microbiol Rev. (2017) 30:615–45. doi: 10.1128/CMR.00005-17
33. Calero-Bernal R, Gennari SM. Clinical toxoplasmosis in dogs and cats: an update. Front Vet Sci. (2019) 6:54. doi: 10.3389/fvets.2019.00054
34. Speer CA, Dubey JP. Ultrastructural differentiation of Toxoplasma gondii schizonts (types B to E) and gamonts in the intestines of cats fed bradyzoites. Int J Parasitol. (2005) 35:193–206. doi: 10.1016/j.ijpara.2004.11.005
35. Shapiro K, Bahia-Oliveira L, Dixon B, Dumètre A, de Wit LA, VanWormer E, et al. Environmental transmission of Toxoplasma gondii: oocysts in water, soil and food. Food Waterborne Parasitol. (2019) 15:e00049. doi: 10.1016/j.fawpar.2019.e00049
36. Torrey EF, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol. (2013) 29:380–4. doi: 10.1016/j.pt.2013.06.001
37. Tilley M, Fichera ME, Jerome ME, Roos DS, White MW. Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains only a subset of dense-granule proteins. Infect Immun. (1997) 65:4598–605. doi: 10.1128/iai.65.11.4598-4605.1997
38. Belluco S, Simonato G, Mancin M, Pietrobelli M, Ricci A. Toxoplasma gondii infection and food consumption: a systematic review and meta-analysis of case-controlled studies. Crit Rev Food Sci Nutr. (2018) 58:3085–96. doi: 10.1080/10408398.2017.1352563
39. Thebault A, Kooh P, Cadavez V, Gonzales-Barron U, Villena I. Risk factors for sporadic toxoplasmosis: a systematic review and meta-analysis. Microb Risk Anal. (2021) 17:100133. doi: 10.1016/j.mran.2020.100133
40. Borges M, Silva TM, Brito C, Teixeira N, Roberts CW. How does toxoplasmosis affect the maternal-foetal immune interface and pregnancy? Parasite Immunol. (2019) 41:e12606. doi: 10.1111/pim.12606
41. Foroutan-Rad M, Majidiani H, Dalvand S, Daryani A, Kooti W, Saki J, et al. Toxoplasmosis in blood donors: a systematic review and meta-analysis. Transfus Med Rev. (2016) 30:116–122. doi: 10.1016/j.tmrv.2016.03.002
42. Dard C, Marty P, Brenier-Pinchart M-P, Garnaud C, Fricker-Hidalgo H, Pelloux H, et al. Management of toxoplasmosis in transplant recipients: an update. Expert Rev Anti Infect Ther. (2018) 16:447–60. doi: 10.1080/14787210.2018.1483721
43. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
44. Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet. (1999) 353:1829–33. doi: 10.1016/S0140-6736(98)08220-8
45. McAuley JB. Congenital toxoplasmosis. J Pediatric Infect Dis Soc. (2014) 3:S30–35. doi: 10.1093/jpids/piu077
46. Wang Z-D, Wang S-C, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. (2017) 4:e177–88. doi: 10.1016/S2352-3018(17)30005-X
47. Ali MI, Wahab WMAE, Hamdy DA, Hassan A. Toxoplasma gondii in cancer patients receiving chemotherapy: seroprevalence and interferon gamma level. J Parasit Dis. (2019) 43:464–71. doi: 10.1007/s12639-019-01111-9
48. Blanchard N, Dunay IR, Schlüter D. Persistence of Toxoplasma gondii in the central nervous system: a fine-tuned balance between the parasite, the brain and the immune system. Parasite Immunol. (2015) 37:150–8. doi: 10.1111/pim.12173
49. Tyebji S, Seizova S, Hannan AJ, Tonkin CJ. Toxoplasmosis: a pathway to neuropsychiatric disorders. Neurosci Biobehav Rev. (2019) 96:72–92. doi: 10.1016/j.neubiorev.2018.11.012
50. Nogareda F, Le Strat Y, Villena I, De Valk H, Goulet V. Incidence and prevalence of Toxoplasma gondii infection in women in France, 1980–2020: model-based estimation. Epidemiol Infect. (2014) 142:1661–70. doi: 10.1017/S0950268813002756
51. Khan F, Rooman M, Rehman H, Rab A, Khan A, Rehman A, et al. Prevalence of Toxoplasma gondii antibodies among pregnant women in District Bannu, Khyber Pakhtunkhwa, Pakistan. Int J Biosci. (2018) 12:235−9. doi: 10.12692/ijb/12.5.233-237
52. Ullah A, Bibi Y, Mohammad S, Ullah F, Fatima N, Shuaib SL, et al. Prevalence of toxoplasmosis in pregnant women. Pak J Med Health Sci. (2022) 16:857–857. doi: 10.53350/pjmhs22166857
53. Rehman F, Shah M, Ali A, Rapisarda AMC, Cianci A. Seroprevalence and risk factors of toxoplasma gondii infection in women with recurrent fetal loss from the province of khyber Pakhtunkhwa, Pakistan. J Neonatal Perinatal Med. (2021) 14:115–21. doi: 10.3233/NPM-190323
54. Ali A, Omer T, Ullah A, Haleem A, Naseem M, Ullah M, et al. Epidemiological survey of Toxoplasma gondii and associated risk factors in ruminant species of the Khyber Pakhtunkhwa Province of Pakistan. J Parasitol Res. (2021) 2021:1–8. doi: 10.1155/2021/6653239
55. Kamal A, Din JU, Kamil A, Khan MT, Bibi H, Hussain A, et al. Seroprevalence of Toxoplasma gondii in sheep and buffalo of District Charsadda, Khyber Pakhtunkhwa, Pakistan. Int J Biosci. (2019) 14:497–502. doi: 10.12692/ijb/14.3.482-487
56. Zolfaghari Emameh R, Purmonen S, Sukura A, Parkkila S. Surveillance and diagnosis of zoonotic foodborne parasites. Food Sci Nutr. (2018) 6:3–17. doi: 10.1002/fsn3.530
57. Todd ECD. Foodborne diseases: overview of biological hazards and foodborne diseases. Encycl Food Saf. (2014) 221:221–42. doi: 10.1016/B978-0-12-378612-8.00071-8
58. Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasites Vectors. (2021) 14:1–12.
59. Shahzad A, Khan MS, Ashraf K, Avais M, Pervez K, Khan JA, et al. Sero-epidemiological and haematological studies on toxoplasmosis in cats, dogs and their owners in Lahore, Pakistan. J Protozool Res. (2006) 16:60–73. Available online at: http://thejaps.org.pk/docs/v-22-1/2.pdf
60. Khan SN, Khan S, Ayaz S, Jan AH, Jehangir S, Attaullah S, et al. Seroprevalance and risk factors of toxoplasmosis among pregnant women in District Kohat, Khyber Pakhtunkhwa, Pakistan. World Appl Sci J. (2011) 14:1032–6. Available online at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=6bf1ae03cea23965f35de85b314950d27cb28ced
61. Ahmad MS, Maqbool A, Mahmood-ul-Hassan M, Mushtaq-ul-Hassan M, Anjum AA. Prevalence of Toxoplasma gondii antibodies in human beings and commensal rodents trapped from Lahore, Pakistan. J Anim Plant Sci. (2012) 22:51–3.
62. Tasawar Z, Aziz F, Lashari MH, Shafi S, Ahmad M, Lal V, et al. Seroprevalence of Human toxoplasmosis in southern Punjab, Pakistan. Pak J Life Soc Sci. (2012) 10:48–52. Available online at: https://www.faunajournal.com/archives/2018.v5.i6.A.553/seroprevalence-of-toxoplasma-gondii-infection-in-cows-and-goats-of-district-charsadda-khyber-pakhtunkhwa-pakistan
63. Khan MZ, Rahman SU, Gul N, Khan AA. Toxoplasmosis; seroprevalence, comparative analysis of diagnostic techniques and identification of risk factors in humans in Malakand Agency, Khyber Pakhtunkhwa, Pakistan. Int J Biosci. (2014) 5:1–6. doi: 10.12692/ijb/5.4.1-6
64. Nazir MM, Akhtar M, Maqbool A, Waheed A, Sajid MA, Ali MA, et al. Antibody prevalence an d risk factors for Toxoplasma gondii infection in women from Multan, Pakistan. Zoonoses Public Health. (2017) 64:537–42. doi: 10.1111/zph.12336
65. Khan MT, Din JU, Ali S, Kamal A, Hussain A, Yar A, et al. Seroprevalence of toxoplasma gondii infection in cows and goats of district Charsadda, Khyber Pakhtunkhwa, Pakistan. Int J Fauna Biol Stud. (2018) 5:18–22.
66. HasnainJan MH, Faisal S, Abid Kamal MT, Khan KA, Muhammad W. Sero-epidemiology of human Toxoplasma gondii infection among male population in Charsadda, KPK, Pakistan (2018).
67. Ahmad N, Khan IA, Iqbal Z, Naseem AA, Kayani AR, Afshan K, et al. Seroepidemiology of toxoplasmosis in human population with reference to its zoonotic potential in sub-tropical areas of Pakistan. Pak Vet J. (2019) 39:2074–7764. doi: 10.29261/pakvetj/2019.017
68. Ali S, Amjad Z, Khan TM, Maalik A, Iftikhar A, Khan I, et al. Occurrence of Toxoplasma gondii antibodies and associated risk factors in women in selected districts of Punjab province, Pakistan. Parasitology. (2020) 147:1133–9. doi: 10.1017/S0031182020000967
69. Ahmad S, Hanif W, Younas S. Toxoplasma gondii infection in sheep, goats and farmers from Bahawalpur (Pakistan). Biologia. (2020) 66:125–31.
70. Ullah N, Nawaz D, Shah M, Rasool A, Akbar F, Israr M, et al. Prevalence of Toxoplasma Gondii in Women Population in Swat, Pakistan. Biomed J Sci Tech Res. (2020) 30:23247–51. doi: 10.26717/BJSTR.2020.30.004926
71. Abdul Hafeez M, Mehdi M, Aslam F, Ashraf K, Aleem MT, Khalid AR, et al. Molecular characterization of Toxoplasma gondii in cats and its zoonotic potential for public health significance. Pathogens. (2022) 11:437. doi: 10.3390/pathogens11040437
72. Afshan K, Baseer S, Kiran S, Narjis G, Firasat S. Seroprevalence of Toxoplasma gondii in pregnant and non-pregnant women of Khyber Pakhtunkhwa, Pakistan. (2022). doi: 10.17582/journal.pjz/20220214060241
73. Ahmad F, Maqbool A, Mahfooz A, Hayat S. Serological survey of Toxoplasma gondii in dogs and cats. Pak Vet J. (2001) 21:31–5.
74. Ahmad S, Tasawar Z. Seroprevalence of Toxoplasmosis in small ruminants from Cholistan desert and agricultural areas of Rahim Yar Khan and Rajan Pur (Punjab) Pakistan. Pak J Zool. (2016) 48.
75. Nabi H, Rashid MI, Islam S, Bajwa AA, Gul R, Shehzad W, et al. Prevalence of Toxoplasma gondii oocysts through Copro-PCR in cats at Pet Center (UVAS), Lahore, Pakistan. J Pak Med Assoc. (2018) 68:115–8.
76. Majid A, Ahmad N, Haleem S, Zareen S, Taib M, Khan S, et al. Detection of toxoplasmosis in pets and stray cats through molecular and serological techniques in Khyber Pakhtunkhwa, Pakistan. BMC Vet Res. (2021) 17:1–7. doi: 10.1186/s12917-021-03064-9
77. Raza H, Tipu MY, Ahmad A, Umar T, Suleman M. Asfa sakhawat, Sara Attique and Ziaur Rehman. Screening of toxoplasmosis in cats through serology and PCR from selected districts of Khyber Pakhtunkhwa Pakistan. Pure Appl Biol. (2022) 12: 78–86. doi: 10.19045/bspab.2023.120009
78. Jadoon A, Akhtar T, Maqbool A, Anjum AA, Ajmal A. “Seroprevalence of Toxoplasma gondii in Canines.” J Anim Plant Sci. (2009) 19:179–81. Available online at: http://thejaps.org.pk/docs/19-no-4-2009/Jadoon.pdf
79. Ramzan M, Akhtar M, Muhammad F, Hussain I, Hiszczyńska-Sawicka E, Haq AU, et al. Seroprevalence of Toxoplasma gondii in sheep and goats in Rahim Yar Khan (Punjab), Pakistan. Trop Anim Health Prod. (2009) 41:1225–9. doi: 10.1007/s11250-009-9304-0
80. Shah M, Zahid M, Asmat P, Alam A, Sthanadar A. Seroprevalence of Toxoplasma gondii in goats and sheep of district Mardan, Pakistan. Int J Biosci. (2013) 7:90–7.
81. Ahmad N, Iqbal Z, Mukhtar M, Mushtaq M, Khan KM, Qayyum M, et al. Seroprevalence and associated risk factors of toxoplasmosis in sheep and goats in Pothwar region, Northern Punjab, Pakistan. Pak J Zool. (2015) 1:161–7. Available online at: https://www.zsp.com.pk/pdf47/161-167%20(22)%20PJZ-1969-14%206-1-15%20%20Revised%20Manuscript%20(2)%20(3)
82. Ahmad S, Tasawar Z. Seroprevalence of Toxoplasma gondii in four ovine breeds of Cholistan desert of Pakistan. Pak J Life Soc Sci. (2015) 2:91–6.
83. Ahmed H, Malik A, Arshad M, Mustafa I, Khan MR, Afzal MS, et al. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in North-Eastern Region of Pakistan. Korean J Parasitol. (2016) 54:439. doi: 10.3347/kjp.2016.54.4.439
84. Hanif M, Tasawar Z. Seroprevalence and risk factors associated with toxoplasmosis in sheep in Multan and Khanewal districts of Punjab (Pakistan). J Anim Plant Sci. (2016) 26:1620–7. Available online at: http://www.thejaps.org.pk/docs/v-26-06/12.pdf
85. Ullah MZ, Awais MM, Akhtar M, Anwar MI, Navid MT, Khan I, et al. Seroprevalence, associated risk factors and hematological impacts of toxoplasmosis in small ruminants of Multan, Punjab-Pakistan. Trop Biomed. (2018) 35:1028–40. Available online at: https://europepmc.org/article/med/33601850
86. Khan FU, Hussain N. NH serological and molecular based diagnosis of toxoplasma gondii in galliformes by using ToxPK1 gene. J Scient Res Med Biol Sci. (2020) 1:116–22.
87. Lashari MH, Farooq U, Mubeen S, Hassan W, Azhar MF, Shahida S, et al. Seroprevalence of Toxoplasma gondii and associated hematological alterations in small ruminants of DG Khan district of Southern Punjab, Pakistan. Arq Med Vet Zootec. (2020) 72:1698–704. doi: 10.1590/1678-4162-11723
88. Yousaf A, Soomro AG, Subhani A, Fazilani SA, Jan MN, Babar A, et al. Detection of Toxoplasma Gondii Infection in Goats and Sheep using the Indirect Haemagglutination Test in Peshawar, Kyber Pakhtunkhwa-Pakistan. J. Vet. Med. Anim. Sci. (2021) 4:1087.
89. Yousaf A, Tabbasum R, Awais T, Sakhawat A, Khan S, Bhutto AL, et al. Prevalence of Toxoplasma Gondii in domestic breeds of goats in Faisalabad, Punjab. Anim Vet Sci. (2021) 9:145–8. doi: 10.11648/j.avs.20210905.14
90. Zafar MA, Shafique M, Zahoor MA, Saqalein M, Aslam B, Arshad MI, et al. Evaluation of seroprevalence and associated risk factors of Toxoplasmosis in sheep and goats in District Jhang-Pakistan. J Hell Vet Med Soc. (2022) 73:3881–8. doi: 10.12681/jhvms.26205
91. Saeed MS, Randhawa UA, Ahmad I, Ullah MI, Gul ST, Hassan MM, et al. Seroprevalence and risk factors of toxoplasmosis in beetal goats in district Faisalabad and its association with reproductive problems. Pak J Agric Sci. (2021) 58:2067–80. doi: 10.21162/PAKJAS/21.1555
92. Aziz MN, Iqbal RK, Irfan M, Parveen A, Asif M, Ozubek S, et al. First report on molecular epidemiology, seasonality and phylogeny of Toxoplasma gondii infecting goats from Khanewal district in Punjab, Pakistan. Acta Trop. (2022) 228:106304. doi: 10.1016/j.actatropica.2022.106304
93. Rafique A, Nasir S, Ashraf A, Nawaz Z, Zahid FM, Abbas A, et al. Sero-surveillance and risk factors analysis of caprine toxoplasmosis in Faisalabad Punjab, Pakistan. Pak Vet J. (2022) 42:129–35.
94. Ahmad N, Qayyum M. Seroprevalence and risk factors for toxoplasmosis in large ruminants in northern Punjab, Pakistan. J Infect Dev Ctries. (2014) 8:1022–8. doi: 10.3855/jidc.4405
95. Taalay I, Iqbal RK, Asif M, Ahmad A, Amjad M, Anwar FN, et al. Molecular survey of Toxoplasma gondii in cattle and buffaloes and phylogenetic position of Pakistani isolates based on ITS-1 gene. Comp Immunol Microbiol Infect Dis. (2022) 84:101782. doi: 10.1016/j.cimid.2022.101782
96. Akbar H, Shabbir MZ, Ullah U, Rashid MI. Serological investigation of bovine toxoplasmosis using commercial and indigenous ELISA kits while validating cattle toxo IgG ELISA Kit. Animals. (2022) 12:2067–80. doi: 10.3390/ani12162067
97. Awais MA, Ahmed A, Muhammad M, Muhammad A, Saleemi K, Ashraf K, et al. Seroprevalence of Toxoplasma gondii in the Backyard chickens of the rural areas of Faisalabad Punjab Pakistan. Int J Agric Biol. (2014) 16.
98. Naveed A, Ali S, Ahmed H, Simsek S, Rizwan M, Kaleem I, et al. Seroprevalence and risk factors of Toxoplasma gondii in wild birds of Punjab Province, Pakistan. J Wildl Dis. (2019) 55:129–35. doi: 10.7589/2017-09-228
99. Khan MB, Khan S, Rafiq K, Khan SN, Attaullah S, Ali I, et al. Molecular identification of Toxoplasma gondii in domesticated and broiler chickens (Gallus domesticus) that possibly augment the pool of human toxoplasmosis. PLoS ONE. (2020) 15:e0232026. doi: 10.1371/journal.pone.0232026
100. Tayyub M, Ali S, Javid A, Imran M. Molecular detection of Toxoplasma gondii and Neospora caninum in rock pigeons (Columba livia) in Punjab, Pakistan. Parasitol Res. (2022) 121:1499–505. doi: 10.1007/s00436-022-07494-8
101. Saqib M, Hussain MH, Sajid MS, Mansoor MK, Asi MN, Fadya A, et al. Sero-epidemiology of equine toxoplasmosis using a latex agglutination test in the three metropolises of Punjab, Pakistan. Trop Biomed. (2015) 32:276–85.
102. Chaudhry UN, Ali AA, Ashraf S, Khan MT, Nadeem SM, Ashraf K, et al. Seroprevalence of Toxoplasma gondii infection in camels (Camelus dromedarius) in and around Bahawalpur region of Pakistan. J Inf Mol Biol. (2014) 2:16–8. doi: 10.14737/jimb.2307-5465/2.1.16.18
103. Lashari MH, Ghouri MT, Akhtar MS, Kamran Z, Chaudhari MS, Ayaz M, et al. Hematological and biochemical alterations associated with toxoplasmosis in dromedaries (Camelus dromedarius) habitating in Cholistan desert of Bahawalpur, Punjab, Pakistan. J Anim Plant Sci. (2018) 28.
104. Fatima T, Mehnaz S, Wang M, Yang J, Sajid MS, Shen B, et al. Seroprevalence of Toxoplasma gondii in one-humped camels (Camelus dromedarius) of Thal and Cholistan deserts, Punjab, Pakistan. Parasitol Res. (2019) 118:307–16. doi: 10.1007/s00436-018-6124-z
105. Shehzad A, Masud A, Fatima T, Khan FM, Rehman S, Effendi MH, et al. Seroprevalence of Toxoplasma gondii and associated alterations in hematology and serum biochemistry of one-humped camels (Camelus dromedarius) in Pakistan. Veterinary World. (2022) 15:110. doi: 10.14202/vetworld.2022.110-118
106. Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. (2009) 49:878–84. doi: 10.1086/605433
107. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. (2009) 58:1–207. quiz CE1–4. doi: 10.1037/e537722009-001
108. Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C. An overview of foodborne pathogen detection: in the perspective of biosensors. Biotechnol Adv. (2010) 28:232–54. doi: 10.1016/j.biotechadv.2009.12.004
109. Cerutti A, Blanchard N, Besteiro S. The bradyzoite: a key developmental stage for the persistence and pathogenesis of toxoplasmosis. Pathogens. (2020) 9:234. doi: 10.3390/pathogens9030234
110. Schares G, Koethe M, Bangoura B, Geuthner AC, Randau F, Ludewig M, et al. Toxoplasma gondii infections in chickens–performance of various antibody detection techniques in serum and meat juice relative to bioassay and DNA detection methods. Int J Parasitol. (2018) 48:751–62. doi: 10.1016/j.ijpara.2018.03.007
111. Nyholt D. Novel insights into host-parasite interactions informed by the ≪in vitro≫ study of serum biomarkers: case of Chagas' disease and apolipoprotein AI. (2008).
112. Aldebert D, Hypolite M, Cavailles P, Touquet B, Flori P, Loeuillet C, et al. Development of high-throughput methods to quantify cysts of Toxoplasma gondii. Cytometry Part A. (2011) 79:952–8. doi: 10.1002/cyto.a.21138
113. Ismael SMM, Salem SA, Elshahidy MS. Isolation and molecular characterization of circulating foot and mouth disease virus in Egypt during 2018-2020. Int J Vet Sci. (2021) 10:162–71. doi: 10.47278/journal.ijvs/2021.046
114. Nasr EA, Fawzy RE, Marian GS, Abbas AM, Khalifa E. Using of gamma interferon γIFN and multiplex PCR (m-PCR) for detection of bovine tuberculosis in dairy herds in Egypt. Int J Vet Sci. (2021) 10:229–33. doi: 10.47278/journal.ijvs/2021.035
115. Osman SA, Tharwat M, Saeed EMA. An outbreak of epidemiological, clinical and treatment outcomes of ovine listeriosis in Qassim region, Saudi Arabia. Int J Vet Sci. (2021) 10:312–6. doi: 10.47278/journal.ijvs/2021.060
116. Ali S, IjazM, Ahmed A, AzizMU, NaveedM, JavedMU, et al. Prevalence and associated risk factors of bovine babesiosis in Lahore, Pakistan. Agrobiol Records. (2020) 2:17–23. doi: 10.47278/journal.abr/2020.007
117. Zaman MA, Mehreen U, Qamar W, Qamar MF, Kashif M, Shahid Z, et al. Brief account of bovine theileriosis prevalence in some South Asian countries. Agrobiol Records. (2020) 2:38–48. doi: 10.47278/journal.abr/2020.010
118. Sharif M, Tunio SA, Bano S. Synergistic effects of Zinc oxide nanoparticles and conventional antibiotics against methicillin resistant Staphylococcus aureus. Adv Life Sci. (2021) 8:167–71.
119. Reshetnikova TI, Zenkin AS, Krylova TG. Experimental use of the Triazavirin antiviral medication in conditions of group administration at the pig-breeding unit. Adv Life Sci. (2021) 8:381–6.
120. Özcan U, Sezener MG, Sayilkan BU, Ergüden VE, Küllük E, Yaman SA, et al. New aspect in neonatal calf diarrhea: presence of Escherichia coli CS31A at unexpected ratio. Kafkas Univ Vet Fak Derg. (2021) 27:133–4.
121. Özdemir O, Ortatatli M, Terzi F, Hatipoglu FH, Çiftçi MK, Ateş MB, et al. The usability of cytological and immunocytological methods for rapid diagnosis of encephalitic listeriosis in ruminants. Kafkas Univ Vet Fak Derg. (2021) 27:225–33.
122. Aksel EG, Akçay A, Arslan K, Sohel MH, Güngör G, Akyüz B, et al. The effects of MBL1 gene polymorphism on subclinical mastitis in Holstein cows. Kafkas Univ Vet Fak Derg. (2021) 27:389–95.
123. Selim A, Abdelhady A. Alahadeb. Prevalence and molecular characterization of Ehrlichia canis in Egyptian DOGS. Pak Vet J. (2021) 41:117–21. doi: 10.29261/pakvetj/2020.061
124. Jalil PJ, Shnawa BH, Hammad SM. Silver nanoparticles: green synthesis, characterization, blood compatibility and protoscolicidal efficacy against Echinococcus granulosus. Pak Vet J. (2021) 41:393–9. doi: 10.29261/pakvetj/2021.039
125. Wajiha, Qureshi NA. In vitro anticoccidial, antioxidant activities and biochemical screening of methanolic and aqueous leaves extracts of selected plants. Pak Vet J. (2021) 41:57–63. doi: 10.29261/pakvetj/2020.071
Keywords: Toxoplasma, life cycle, transmission, symptoms, prevalence, diagnostic methods/prevention
Citation: Qamar W and Alsayeqh AF (2023) A review of foodborne Toxoplasma gondii with a special focus on its prevalence in Pakistan from 2000 to 2022. Front. Vet. Sci. 9:1080139. doi: 10.3389/fvets.2022.1080139
Received: 25 October 2022; Accepted: 07 December 2022;
Published: 18 January 2023.
Edited by:
Kun Li, Nanjing Agricultural University, ChinaReviewed by:
Shahbaz Ul Haq, Lanzhou Institute of Husbandry and Pharmaceutical Sciences (CAAS), ChinaMuhammad Ehsan, Islamia University of Bahawalpur, Pakistan
Qaisar Tanveer, University of Edinburgh, United Kingdom
Copyright © 2023 Qamar and Alsayeqh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah F. Alsayeqh,  YS5hbHNheWVxaEBxdS5lZHUuc2E=
YS5hbHNheWVxaEBxdS5lZHUuc2E=
 Warda Qamar
Warda Qamar Abdullah F. Alsayeqh
Abdullah F. Alsayeqh