- 1Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 2Mineral Nutrition Research Division, State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences (CAAS), Beijing, China
Sodium chloride (NaCl) is usually added to diets to meet the Na and Cl requirements of broilers in the Chinese poultry industry, but the optimal dietary NaCl supplemental level was not well-established. The present study was conducted to estimate the optimal dietary NaCl supplemental level of broilers fed a corn-soybean meal diet from 1 to 21 days of age. A total of 490, 1-day-old Arbor Acres male broilers were fed a NaCl-unsupplemented corn-soybean meal basal diet (control) and the basal diet supplemented with 0.10, 0.20, 0.30, 0.40, 0.50 or 0.60% NaCl for 21 days. Regression analysis was conducted to evaluate the optimal dietary NaCl level using the best fitted broken-line or asymptotic models. As dietary supplemental NaCl levels increased, average daily gain (ADG), average daily feed intake (ADFI), blood partial pressure of CO2, total CO2, base excess and anion gap, blood concentrations of HCO3, Na and Cl, serum Na concentration, jejunal villus height (VH) and tibia ash content increased linearly and quadratically (P < 0.05), while feed/gain ratio, relative weights of heart, liver and kidney, blood K concentration, serum concentrations of K, uric acid and glucose, and osmotic pressure decreased linearly and quadratically (P < 0.05). The estimates of optimal dietary NaCl levels were 0.20−0.22% based on the best fitted broken-line or asymptotic models (P < 0.0001) of ADG, ADFI and feed/gain ratio, and 0.08−0.24% based on the best fitted broken-line or asymptotic models (P < 0.0001) of blood gas indices, serum parameters, jejunal VH, tibia ash content and organ indices. These results suggested that the optimal dietary NaCl supplemental level would be 0.24% for broilers fed the corn-soybean meal diet from 1 to 21 days of age, which is lower than the current dietary NaCl supplemental level (0.30%) in the Chinese broiler production.
Introduction
Sodium (Na) and chlorine (Cl) are essential minerals for poultry, and play a critical role in regulating water-electrolyte metabolism, acid-base balance and maintaining osmotic pressure (OSM) (1–3). Optimal dietary balance of Na and Cl could improve chicken health and growth performance (4).
The current NRC recommended that both dietary Na and Cl requirements for broilers from 1 to 21 days of age are 0.20% (5), which are primarily based on the growth performance (4). Actually, 0.30% sodium chloride (NaCl) is usually added to diets to meet the Na and Cl requirements of broilers in the Chinese poultry industry. Nott and Combs (6) reported that the optimal NaCl levels in diets for broilers from 1 to 28 days of age were 0.25−0.30% based on the maximum growth. However, the growth performance parameters are usually not very sensitive in reflecting dietary Na and Cl requirements for modern fast-growing broilers. Therefore, it is necessary to reevaluate the optimal dietary NaCl supplemental level of broilers fed a conventional corn-soybean meal diet using more sensitive indices.
As important components of electrolytes in animals, Na, K, and Cl concentrations in blood can reflect the changes of electrolyte balance, and their concentrations within the normal range are important in maintaining physiological function (7–9). Oviedo-Rondón et al. (10) reported that the optimal dietary Cl supplemental level of broilers was 0.30% based on blood gas indices. Jiang et al. (11) reported that serum Na and OSM increased quadratically with increasing dietary supplemental Na and Cl levels. The Na and Cl participate in regulation of bone growth and mineralization (12). Murakami et al. (8) reported that the tibia ash content was a sensitive index for evaluating Na and Cl requirements of chicks fed a common corn-soybean basal diet, and the optimal Na and Cl requirements of broiler chicks from 1 to 21 days of age were 0.25 and 0.20%, respectively, based on the tibia ash content. Furthermore, long-term high-salt or low-salt diet could cause metabolic abnormalities and affect the growth and development of major organs in broilers (13, 14). In addition, the high-salt diet could increase the osmotic pressure of the intestinal contents and seriously affect the jejunal villus growth (15). However, it is unknown whether the above-mentioned blood gas indices, serum parameters, jejunal morphology and organ indices could be used to estimate the optimal dietary NaCl supplemental level of chicks fed the conventional corn-soybean meal diet.
Therefore, we hypothesized that blood gas indices, serum parameters, jejunal morphology and organ indices might be new and sensitive indices for evaluating the optimal dietary NaCl supplemental level of broiler chicks fed the conventional corn-soybean meal diet, and the optimal dietary NaCl supplemental level for broiler chicks estimated based on the above sensitive indices might be different from the current dietary NaCl supplemental level (0.30%) in the Chinese broiler production. The objective of the present study was to investigate the effect of dietary NaCl supplemental levels on growth performance, blood gas indices, serum parameters, jejunal morphology, tibia ash content and organ indices, and thus to select sensitive indices to evaluate the optimal dietary NaCl supplemental level for broilers fed the conventional corn-soybean meal diet from 1 to 21 days of age.
Materials and methods
Animal experiments were approved by the Yangzhou University Animal Experiments Ethics Committee, with the permit number SYXK (Su) 2021-0027.
Birds, diets, and experimental design
A total of 490, 1-day-old Arbor Acres (AA) commercial male broiler chicks were purchased from Jinghai Poultry Industry Group in Nantong, Jiangsu, China. The broilers were randomly divided into 1 of 7 groups with 7 replicate cages of 10 chicks each. All broilers were vaccinated with Newcastle disease vaccine and bronchitis combined vaccine at 7 days of age, and with infectious bursal disease virus vaccine at 14 days of age, respectively. All broilers were kept in a 24-h constant light and thermostatically controlled room equipped with stainless steel cages, feeders and plastic waterers (16). Dead birds, body weight, and feed intake were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), feed/gain ratio, and mortality from 1 to 21 days of age.
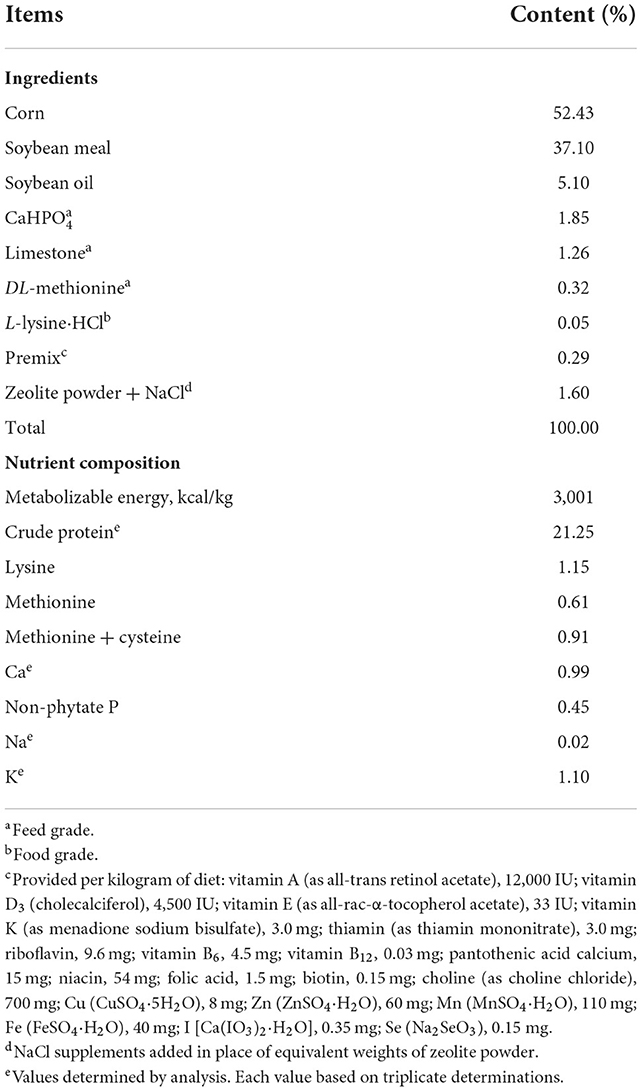
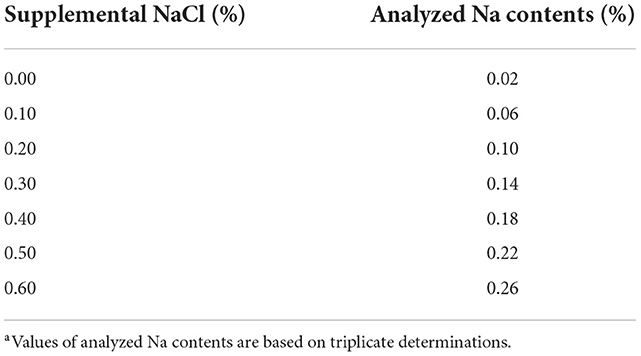
The basal corn-soybean meal diet (Table 1, containing Na: 0.02%, Cl: 0.08%) was formulated to meet or exceed the nutrient requirements (5) of starter broilers for all nutrients except for Na and Cl. Dietary treatments included the NaCl-unsupplemented corn-soybean meal basal diet (control) and the basal diet supplemented with 0.10, 0.20, 0.30, 0.40, 0.50 or 0.60% NaCl. The analyzed Na concentrations in diets are listed in Table 2.
Sample collections and preparations
The feed ingredient and diet samples were collected for analyzes of Ca, K, Na or dietary crude protein (CP) concentrations. The tap water was collected for analyzes of Ca, Na, and K concentrations (contents of these minerals in tap water were analyzed to be <44 μg/ml).
The initial weight of water was recorded and the remaining water was measured at 8:00 o'clock the next morning daily throughout the entire experimental period, and the differences between the water weight determined at the beginning and end of each measurement were used to calculate the water consumption (ml/day). Five days before the end of the experiment, all excrements in each replicate cage were collected and weighed, dried at 60°C until constant weight to determine the moisture content.
At 21 days of age, three birds from each replicate cage were chosen according to average body weight of each cage. Whole blood samples were taken into lithium heparin tubes from each of two birds via the wing veins for blood gas analysis. Another blood samples were collected from each bird into tubes without the anticoagulant, and centrifuged to harvest serum for analyzes of Na, Cl, K, glucose (GLU), and uric acid concentrations. Serum samples from three chicks in each cage were pooled into one sample in equal ratios before analyzes. The selected birds in each cage were killed by cervical dislocation, and heart, liver, spleen, lung and kidney samples were weighed and divided by living body weights to calculate organ indices. The right tibia was peeled and frozen at −20°C for analysis of the tibia ash content. The jejunal sample (2 cm in length) was collected from the midpoint of the jejunum of a chick and fixed in 4% formaldehyde for the subsequent morphological examination (17).
Measurements of Na, K, Ca, and CP concentrations
The concentrations of Na, K, and Ca in tap water, feed ingredients and diets, CP in feed ingredients or diets were determined as described previously (18, 19).
Determinations of blood gas indices and serum parameters
The whole blood samples in the lithium heparin tubes were measured with a blood gas analyzer (VS4-6163, VetStat, USA) for pH, total CO2 (TCO2), partial pressure of CO2 (PCO2), anion gap (AG), base excess (BE), and the concentrations of H, HCO3, Na, Cl, and K. An automatic biochemical analyzer (7180, Hitachi, Japan) was used to measure serum K, Na, Cl, glucose (GLU), and uric acid concentrations, and then calculated the serum OSM (20).
Measurements of tibia ash content and jejunal morphological indices
The tibia ash content was determined as described previously (21). The jejunal morphometric parameters were photographed at ×40 magnification under the computer-aided light microscope (CKX53, OLYMPUS, Japan). The total villi height (VH) and crypt depth (CD) were measured using ImageJ software, and the villi-crypt ratio (VH/CD) was calculated according to the procedure of Zhang et al. (22). The values of means from 10 to 20 adjacent and vertically orientated villi and crypts were used for further analyzes.
Statistical analyzes
The data in this study were submitted to one-way ANOVA using the general linear model of SAS 9.4 (SAS Institute Inc., Cary, NC) (23). Differences among means were tested with the LSD method. Data on the percentage of mortality of broilers were converted to arcsine for analysis. Each replicate cage was an experimental unit. The linear and quadratic responses of responsive indices to added NaCl levels were tested using orthogonal comparisons. Regression analyzes of broken-line, quadratic and asymptotic models were performed, and the best fitted models between responsive criteria and added NaCl levels were used to estimate the optimal added NaCl levels (the break point from the broken-line model or 95% of the maximum response from the asymptotic model) for broiler chicks (24). The P < 0.05 was considered to be statistically significant.
Results
Growth performance, mortality, average daily water intake, and excreta moisture content
The ADG, ADFI, average daily water intake and excreta moisture content increased linearly and quadratically (P < 0.0001), while the feed/gain ratio decreased linearly and quadratically (P < 0.0001) as dietary supplemental NaCl levels increased (Table 3). The ADG and ADFI reached a plateau at the supplemental NaCl level of 0.30%, and the feed/gain ratio reached a plateau at the supplemental NaCl level of 0.20%. The birds fed on the basal diet without the NaCl supplementation showed the highest mortality rate.
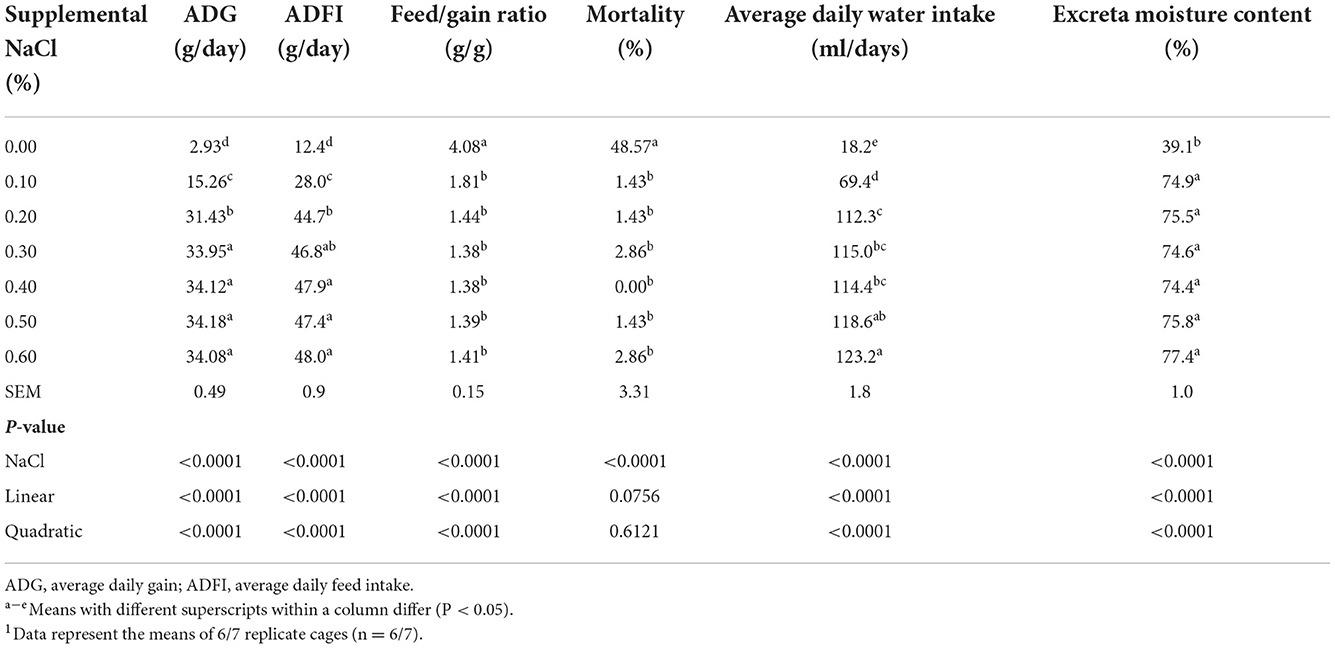
Table 3. Effect of dietary NaCl supplemental level on growth performance, mortality, average daily water intake and excreta moisture content of broilers from 1 to 21 days of age1.
Blood gas indices
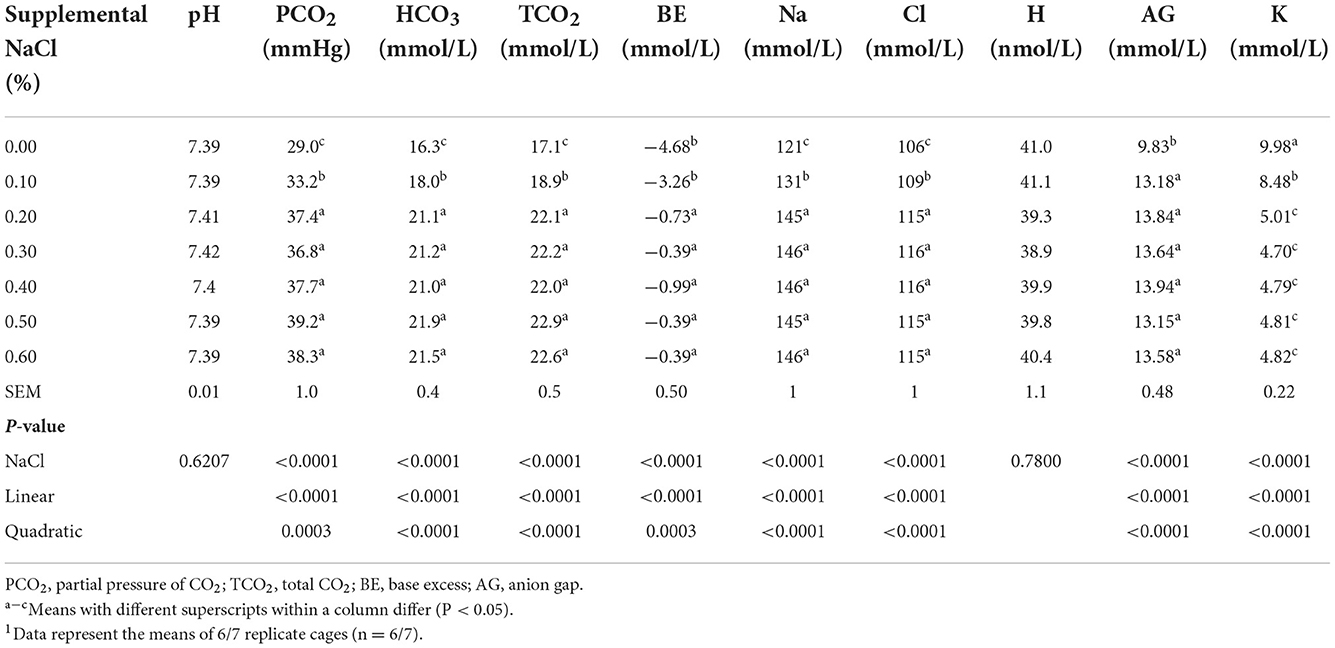
The PCO2, TCO2, BE and AG, and the concentrations of HCO3, Na, and Cl in blood increased linearly and quadratically (P < 0.0004), while the K concentration in blood decreased linearly and quadratically (P < 0.0001) with the increase of dietary NaCl levels (Table 4). The PCO2, TCO2, BE and the concentrations of HCO3, K, Na, and Cl in blood reached a plateau at supplemental NaCl levels of about 0.20−0.60%.
Serum parameters and osmotic pressure
As the levels of supplemental NaCl increased, the OSM and the concentrations of K, uric acid and GLU in serum decreased linearly and quadratically (Table 5, P < 0.0001), while the Na concentration in serum increased linearly and quadratically (P < 0.0004). The uric acid concentration in serum reached a plateau at supplemental NaCl levels of about 0.20−0.60%; the GLU concentration in serum reached a plateau at supplemental NaCl levels of about 0.20–0.50%; and the K concentration and OSM in serum reached the lowest point at the supplemental NaCl level of 0.20%.
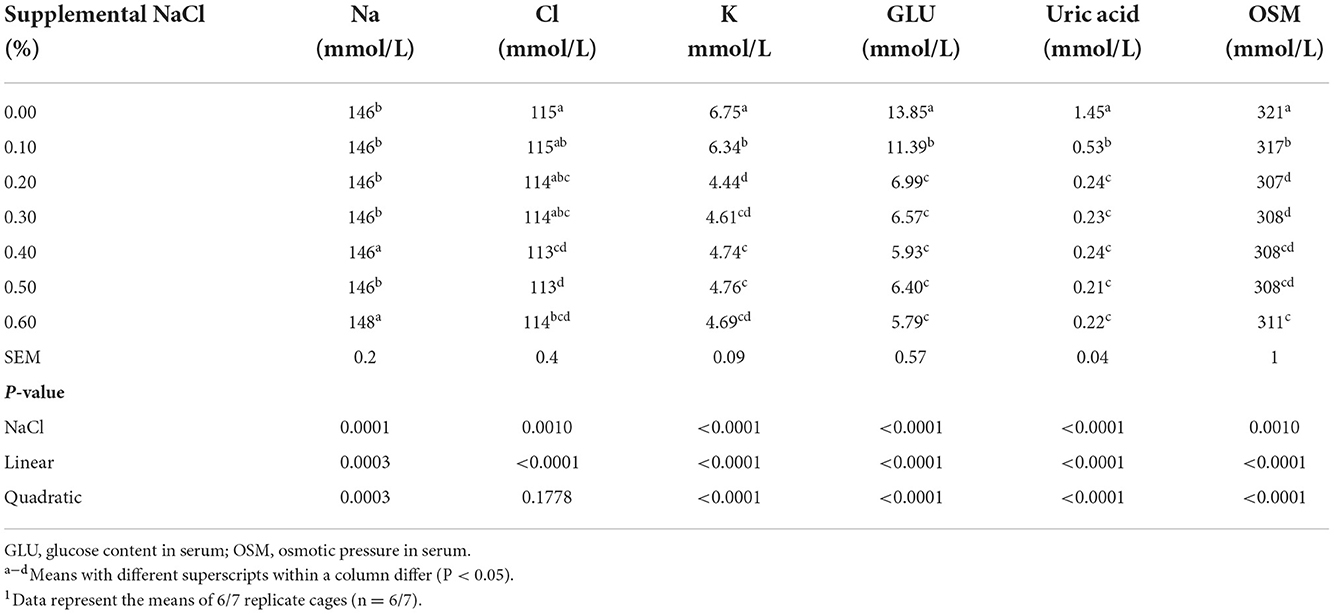
Table 5. Effect of dietary NaCl supplemental level on serum biochemical variables of broilers on day 211.
Jejunal morphological characteristics, tibia ash content, and organ indices
As the levels of supplemental NaCl increased, the jejunal CD and tibia ash content increased linearly and quadratically (Table 6, P < 0.002), but the jejunal VH only increased quadratically (Table 6, P < 0.0001). In addition, the liver and kidney indices decreased linearly and quadratically (Table 6, P < 0.0001), while the heart index only decreased quadratically (Table 6, P < 0.0001) as supplemental NaCl levels increased. The jejunal CD, tibia ash content and heart index reached a plateau at supplemental NaCl levels of about 0.20−0.50%. Liver and kidney indices reached the lowest point at the supplemental NaCl level of 0.20%, and the jejunal VH reached the highest point at the supplemental NaCl level of 0.20%.
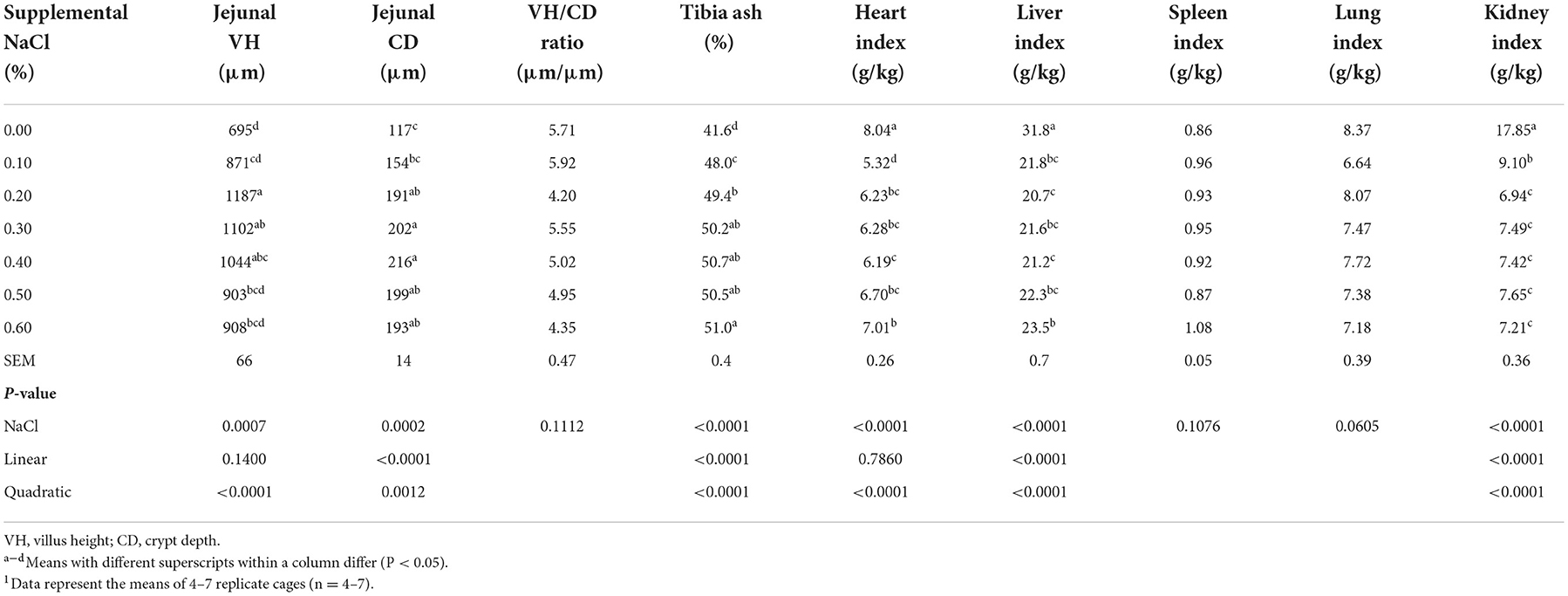
Table 6. Effect of dietary NaCl supplemental level on jejunal VH, CD, VH/CD, tibia ash content and organ indices of broilers on day 211.
Optimal dietary supplemental NaCl levels
The optimal dietary supplemental NaCl levels of broilers from 1 to 21 days of age as estimated by the non-linear regression analyzes were shown in Table 7. Based on the best fitted broken-line or asymptotic models (P < 0.0001) of the ADG, ADFI and feed/gain ratio, the optimal dietary supplemental NaCl levels were 0.20−0.22%; based on the best fitted broken-line or asymptotic models (P < 0.0001) of the blood gas parameters and serum parameters, the optimal dietary supplemental NaCl levels were 0.08−0.24%; based on the best fitted broken-line or asymptotic models (P < 0.0001) of the jejunal VH and tibia ash content, the optimal dietary supplemental NaCl level were 0.21 and 0.11%, respectively; and based on the best fitted broken-line models (P < 0.0001) of the organ indices, the optimal dietary supplemental NaCl levels were 0.09−0.12% for broilers fed the corn-soybean meal diet from 1 to 21 days of age.
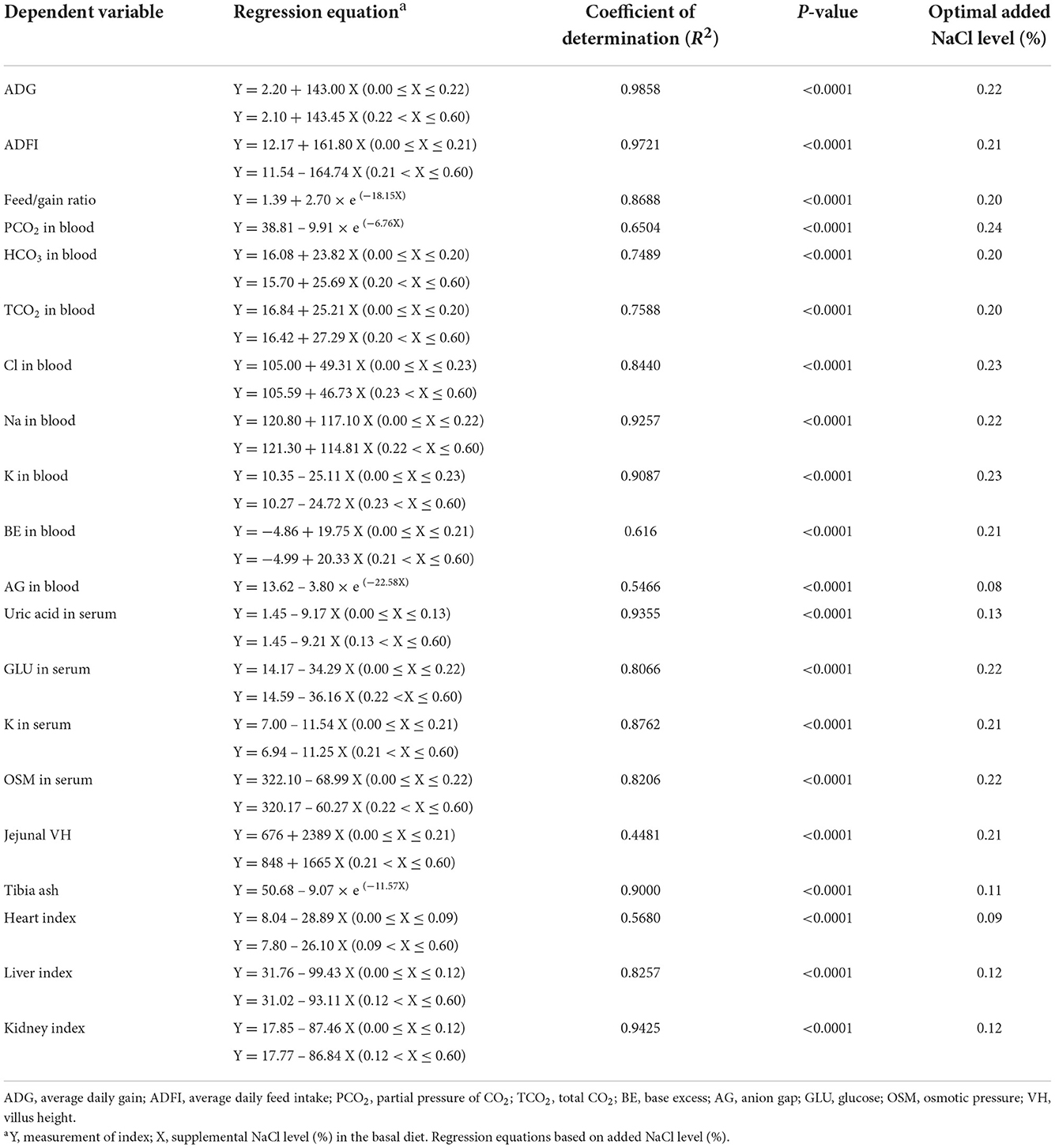
Table 7. The optimal dietary NaCl levels of chicks during 1–21 days of age as estimated based on the best fitted broken-line or asymptotic models.
Discussion
The key findings of the present study have demonstrated that the PCO2, TCO2, AG, BE, and the concentrations of HCO3, Na, Cl, and K in blood, the OSM and the concentrations of uric acid, GLU and K in serum, jejunal VH, heart, liver and kidney indices are new and sensitive criteria for estimating optimal dietary supplemental NaCl levels of broilers fed the corn-soybean meal diet from 1 to 21 days of age. Moreover, the optimal dietary supplemental NaCl levels were estimated to be 0.20−0.22% to support the best growth, 0.13−0.22% to support the serum ion balance, 0.08−0.24% to support the physiological balance in the blood, and 0.09−0.21% to support the jejunal villus, tibia and organ development of broilers fed the corn-soybean meal diet from 1 to 21 days of age based on the best fitted broken-line or asymptotic models. Therefore, it is suggested that the optimal dietary NaCl level to meet all needs of the modern and rapidly growing broilers for the above NaCl metabolisms would be 0.24%, which is lower than the current supplemental NaCl level (0.30%) in the Chinese broiler production. The above findings have supported our hypotheses and been not reported before, and also provided scientific bases for precisely adding the NaCl to the broiler diets in the Chinese broiler production.
The growth performance parameters were the early and commonly-used criteria to assess Na and Cl requirements in animals (4, 10). Previous studies demonstrated that broilers fed a diet deficient in Na and Cl decreased ADG and ADFI (2, 25). In the present study, we found that ADFI and ADG increased linearly and quadratically, while feed/gain ratio decreased linearly and quadratically with increasing dietary supplemental NaCl levels. And dietary supplemental 0.30−0.60% NaCl levels were enough to maintain normal growth for broiler chicks up to 21 days of age. Besides, in our current study, as the supplemental NaCl levels increased, the average daily water intake and excreta moisture content increased linearly and quadratically. As the water intake and the excreta moisture content could not accurately reflect the Na and Cl status of broilers, these two parameters were not suitable for evaluating the optimal dietary NaCl supplemental level of broiler chicks. In addition, broilers fed on the basal diet without the NaCl supplementation showed the highest mortality rate, which is in agreement with the previous reports (26–29), indicating that Na and Cl are essential minerals for poultry health and growth.
The Na and Cl jointly control the volume of extracellular fluid and osmotic pressure, and regulate the body fluid and acid-base balance (26, 30). The blood gas parameters are important indicators to evaluate the metabolic acid-base imbalance in the body (31, 32). A previous study showed that blood gas parameters could reflect the bioavailability of Na and Cl in broiler chicks (10). In the present study, the NaCl-unsupplemented diet (control) had an acidogenic effect on chicken metabolism, while supplemental NaCl levels significantly increased Na and Cl concentrations, and CO2 storage in blood, thus causing higher HCO3 concentration and BE in blood. Based on PCO2, TCO2, HCO3, BE, and AG parameters in blood, it was estimated that broilers fed diets containing 0.20−0.60% added NaCl could maintain acid-base balance in the body. In addition, Na and Cl are important components of blood crystalloid osmotic pressure, and the crystalloid osmotic pressure of blood could normally maintain the relative stable state of the internal environment. Salt intake could increase serum Na and uric acid concentrations, and then lead to changes in serum ionic parameters, glucose metabolism and OSM (33–36). Yu et al. (26) reported that the OSM and the concentrations of K, Na, uric acid, and GLU in the serum of broilers were affected linearly or quadratically with the increase of dietary supplemental Na and Cl levels. The present study also showed that the OSM and the concentrations of K, Na, uric acid and GLU in the serum were decreased linearly and quadratically as the levels of supplemental NaCl increased. Moreover, the above results show that the OSM and the concentrations of K, Na, uric acid and GLU in the serum are new and sensitive indices for estimating optimal dietary supplemental NaCl levels of broiler chicks, which has been not reported before. Based on the OSM and the serum K, uric acid and GLU concentrations, supplemental NaCl levels of 0.20% or more could support the OSM, serum ion and internal environment balance of broilers. Therefore, our findings along with those of others have clearly indicated that the blood gas parameters and serum biochemical indicators could be used as sensitive biomarkers for estimating optimal dietary supplemental NaCl levels of broilers. In the present study, although the serum Na concentration increased linearly and quadratically with the increase of dietary NaCl levels, the coefficient of determination R2 (0.3783) was relatively too low to evaluate the optimal dietary NaCl supplemental level of broiler chicks.
The Na and Cl balance is one part of the internal environment homeostasis that is necessary to maintain the health of the small intestine. Wang et al. (15) reported that the high-salt diet seriously affected the small intestinal villi growth in rats. In our present study, the broilers fed on the basal control diet without the NaCl addition had the lowest jejunal VH and CD. Besides, as the supplemental NaCl levels increased, the jejunal VH and CD increased linearly and quadratically. As the longer CD was unfavorable for the intestinal absorption of nutrients, it was not suitable for evaluating the optimal dietary NaCl supplemental level of broiler chicks. The Na and Cl participate in regulation of bone growth and mineralization (12). Taheri et al. (37) reported that the tibia ash content significantly increased with the increase of dietary NaCl levels. In our current study, tibia ash content increased linearly and quadratically as dietary supplemental NaCl levels increased, and reached a plateau at the supplemental NaCl level of 0.30%. The Na and Cl are mainly distributed in body fluids and soft tissues, and they are closely related to the regulation of water-salt balance in the body. Previous studies have suggested that Na intake could increase blood pressure in mice (38, 39). In addition, the long-term low salt diet increased the systolic blood pressure, cardiomyocyte size, interventricular septum thickness, renal edema and plasma angiotensin II levels, and chronically high levels of angiotensin II could lead to varying degrees of multiorgan damages (15, 40, 41). In our current study, the broilers fed with the NaCl-deficient diet had higher heart, liver and kidney indices, and they were decreased quadratically with increasing NaCl supplemental levels. Similar results were observed in the previous study of Jankowski et al. (42). These effects might be related to changes in blood pressure and angiotensin II caused by supplemental NaCl levels (40, 41), and the exact reasons need to be further investigated. Therefore, the above results demonstrated that jejunal VH and tibia ash content, heart, liver and kidney indices could be used as sensitive criteria to evaluate optimal dietary NaCl supplemental levels of broiler chicks from 1 to 21 days of age. And dietary supplemental 0.20−0.60% NaCl levels were sufficient for the normal development of tibia, jejunal villi, and organs.
The current study showed that the optimal dietary NaCl supplemental levels were 0.08−0.24% based on the best fitted broken-line or asymptotic models of new and sensitive indices of blood gas indices, serum parameters, jejunal morphology and organ indices of broilers. Although these results might not accurately reflect dietary Cl requirement of broiler chicks, the present study suggested that the optimal dietary NaCl supplemental level of 0.24% (a total 0.11% Na and 0.23% Cl with a Na/Cl ratio of 1:2.09 in the diet) could support all needs for the optimal growth, serum ion balance, blood acid-base balance, as well as jejunal villus and organ developmental needs of broilers fed a conventional corn-soybean meal diet from 1 to 21 days of age. The total 0.11% Na in the diet is less than the dietary Na requirement (0.20%), but the total 0.23% Cl is higher than the dietary Cl requirement (0.20%) as recommended by NRC (5). Therefore, further studies need be carried out to precisely reevaluate the optimal dietary Cl requirement of broilers under dietary Na/Cl ratio of 1:1 in order to minimize the excess of dietary Cl.
In conclusion, our data indicate that the PCO2, TCO2, AG, BE, and the concentrations of HCO3, Na, Cl, and K in blood, the OSM and the concentrations of uric acid, GLU and K in serum, jejunal VH, heart, liver and kidney indices can be used as new and sensitive criteria to estimate optimal dietary supplemental NaCl levels of broiler chicks. The optimal dietary NaCl supplemental level was suggested to be 0.24% for broilers fed the corn-soybean meal diet from 1 to 21 days of age, which is lower than the current dietary NaCl supplemental level (0.30%) in the Chinese broiler production.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
All experimental procedures were approved by the Animal Care Committee of the Department of Animal Science and Technology of Yangzhou University, Yangzhou, China (permit number: SYXK (Su) 2021-0027) and conducted in accordance with the guidelines of the Animal Use Committee of the Chinese Ministry of Agriculture (Beijing, China).
Author contributions
WZ: original draft preparation. DL: software. WW and BW: methodology. XC and FG: investigation. YG and LZhu: formal analysis. TL and LZha: validation and supervision. YH: review. XL: review and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Jiangsu Shuang Chuang Ren Cai program (JSSCRC2021541), the Jiangsu Shuang Chuang Tuan Dui program (JSSCTD202147), and the Initiation Funds of Yangzhou University for Distinguished Scientists.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pesti GM, Cervantes H, Bakalli RI, Bafundo KW, Garcia MN. Studies on semduramicin and nutritional responses: 3. Electrolyte balance. Poult Sci. (1999) 78:1552–60. doi: 10.1093/ps/78.11.1552
2. Walicka E, Ryś R, Koreleski J, Pietras M. Effect of sodium chloride deficiency on basal metabolism in broiler chickens. Br J Nutr. (1979) 42:547–52. doi: 10.1079/BJN19790146
3. Bohn AA, de Morais HA. A quick reference on chloride. Vet Clin North Am Small Anim Pract. (2017) 47:219–22. doi: 10.1016/j.cvsm.2016.10.008
4. Hurwitz S, Cohen I, Bar A, Bornstein S. Sodium and chloride requirements of the chick: relationship to acid-base balance. Poult Sci. (1973) 52:903–9. doi: 10.3382/ps.0520903
6. Nott H, Combs GF. Sodium requirement of the chick. Poult Sci. (1969) 48:660–5. doi: 10.3382/ps.0480660
7. Gonzalez-Esquerra R, Leeson S. Physiological and metabolic responses of broilers to heat stress-implications for protein and amino acid nutrition. World Poultry Sci J. (2006) 62:282–95. doi: 10.1079/WPS200597
8. Murakami A, Watkins S, Saleh E, England J, Waldroup P. Estimation of the sodium and chloride requirements for the young broiler chick. J Appl Poultry Res. (1997) 6:155–62. doi: 10.1093/japr/6.2.155
9. Mushtaq T, Sarwar M, Nawaz H, Mirza MA, Ahmad T. Effect and interactions of dietary sodium and chloride on broiler starter performance (hatching to twenty-eight days of age) under subtropical summer conditions. Poult Sci. (2005) 84:1716–22. doi: 10.1093/ps/84.11.1716
10. Oviedo-Rondón EO, Murakami AE, Furlan AC, Moreira I, Macari M. Sodium and chloride requirements of young broiler chickens fed corn-soybean diets (one to twenty-one days of age). Poult Sci. (2001) 80:592–8. doi: 10.1093/ps/80.5.592
11. Jiang S, Azzam M, Yu H, Fan Q, Li L, Gou Z, et al. Sodium and chloride requirements of yellow-feathered chickens between 22 and 42 days of age. Animal. (2019) 13:2183–9. doi: 10.1017/S1751731119000594
12. Araujo AC, Araújo RDS, Dourado LRB, Machado JS, Bayão GFV, Amoroso L, et al. Analysis of performance, bone characteristics, and expression of genes involved in the balance of ionic concentrations in broilers subjected to dietary electrolyte balance levels. Br Poult Sci. (2022) 63:226–34. doi: 10.1080/00071668.2021.1966754
13. Mushtaq M, Pasha T, Saima, Akram M, Mushtaq T, Parvin R, et al. Growth performance, carcass traits and serum mineral chemistry as affected by dietary sodium and sodium salts fed to broiler chickens reared under phase feeding system. Asian-Australas J Anim Sci. (2013) 26:1742–52. doi: 10.5713/ajas.2013.13266
14. Peng Y, Yang X, Li H, Iqbal M, Li A, Zhang J, et al. Salt-contaminated water inducing pulmonary hypertension and kidney damage by increasing Ang II concentration in broilers. Environ Sci Pollut Res Int. (2022) 29:1134–43. doi: 10.1007/s11356-021-13358-y
15. Wang H, Li S, Fang S, Yang X, Feng J. Betaine improves intestinal functions by enhancing digestive enzymes, ameliorating intestinal morphology, and enriching intestinal microbiota in high-salt stressed rats. Nutrients. (2018) 10:907–21. doi: 10.3390/nu10070907
17. Wang Z, Shao D, Wu S, Song Z, Shi S. Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol Environ Saf. (2022) 244:114053. doi: 10.1016/j.ecoenv.2022.114053
18. Cao S, Li T, Shao Y, Zhang L, Lu L, Zhang R, et al. Regulation of bone phosphorus retention and bone development possibly by related hormones and local bone-derived regulators in broiler chicks. J Anim Sci Biotechnol. (2021) 12:88. doi: 10.1186/s40104-021-00610-1
19. AOAC. Official Methods of Analysis. 15th ed. Arlington, VA: Association of Official Analytical Chemist (1990).
20. Worthley LI, Guerin M, Pain RW. For calculating osmolality, the simplest formula is the best. Anaesth Intensive Care. (1987) 15:199–202. doi: 10.1177/0310057X8701500214
21. Shao Y, Sun G, Cao S, Lu L, Zhang L, Liao X, et al. Bone phosphorus retention and bone development of broilers at different ages. Poult Sci. (2019) 98:2114–21. doi: 10.3382/ps/pey565
22. Zhang Q, Zhang S, Wu S, Madsen MH, Shi S. Supplementing the early diet of broilers with soy protein concentrate can improve intestinal development and enhance short-chain fatty acid-producing microbes and short-chain fatty acids, especially butyric acid. J Anim Sci Biotechnol. (2022) 13:97. doi: 10.1186/s40104-022-00749-5
23. Liu G, Sun G, Liao X, Huang J, Guo M, Zhang L, et al. Effect of dietary supplementation of pyrroloquinoline quinone disodium on growth performance, meat quality and antioxidative ability of broilers. J Integr Agr. (2020) 19:1850–6. doi: 10.1016/S2095-3119(19)62851-0
24. Hu Y, Chen Z, Lu L, Zhang L, Liu T, Luo X, et al. Determination of dietary copper requirement by the monoamine oxidase activity in kidney of broilers from 1 to 21 days of age. Anim Nutr. (2022) 8:227–34. doi: 10.1016/j.aninu.2021.05.013
25. Watkins SE, Fritts CA, Yan F, Wilson ML, Waldroup PW, Watkins SE, et al. The interaction of sodium chloride levels in poultry drinking water and the diet of broiler chickens. J Appl Poultry Res. (2005) 14:55–9. doi: 10.1093/japr/14.1.55
26. Yu H, Jiang SQ, Liu M, Jiang ZY, Li L, Gou ZY, et al. Sodium and chlorine requirements of yellow-feathered broilers aged from 1 to 21 days. Chin J Anim Nutr. (2017) 29:786–97. doi: 10.3969/j.issn.1006-267x.2017.03.008
27. Damron BL, Johnson WL, Kelly LS. Utilization of sodium from sodium bicarbonate by broiler chicks. Poult Sci. (1986) 65:782–5. doi: 10.3382/ps.0650782
28. Mushtaq M, Pasha T, Mushtaq T, Parvin R. Electrolytes, dietary electrolyte balance and salts in broilers: an updated review on growth performance, water intake and litter quality. World Poultry Sci J. (2013) 69:789–802. doi: 10.1017/S0043933913000810
29. Vieira SL, Penz AM, Pophal S, Godoy de Almeida J. Sodium requirements for the first seven days in broiler chicks. J Appl Poult Res. (2003) 12:362–70. doi: 10.1093/japr/12.3.362
30. Mahmud A, Hayat Z, Khan M, Muhammad Z, Khalique A, Younus M. Comparison of source and levels of sodium in broilers under low temperature conditions. Pak J Zool. (2010) 42:383–8.
31. Havlin J, Matousovic K, Schück O. Sodium-chloride difference as a simple parameter for acid-base status assessment. Am J Kidney Dis. (2017) 69:707–8. doi: 10.1053/j.ajkd.2016.12.019
32. Bournazel M, Duclos MJ, Lecompte F, Guillou D, Peyronnet C, Quinsac A, et al. Effects of dietary electrolyte balance and calcium supply on mineral and acid-base status of piglets fed a diversified diet. J Nutr Sci. (2020) 9:e18. doi: 10.1017/jns.2020.10
33. Forman JP, Scheven L, De Jong PE, Bakker SJ, Curhan GC, Gansevoort RT. Association between sodium intake and change in uric acid, urine albumin excretion, and the risk of developing hypertension. Circulation. (2012) 125:3108–16. doi: 10.1161/CIRCULATIONAHA.112.096115
34. Hou L, Zhang M, Han W, Tang Y, Xue F, Liang S, et al. Influence of salt intake on association of blood uric acid with hypertension and related cardiovascular risk. PLoS ONE. (2016) 11:e0150451. doi: 10.1371/journal.pone.0150451
35. Kanbay M, Aslan G, Afsar B, Dagel T, Siriopol D, Kuwabara M, et al. Acute effects of salt on blood pressure are mediated by serum osmolality. J Clin Hypertens. (2018) 20:1447–54. doi: 10.1111/jch.13374
36. Pawłowska J, Sosnówka-Czajka E, Skomorucha I. Effect of the in ovo injection site of electrolytes on some biochemical blood parameters and quality of layer chicks. Animals. (2022) 21:532. doi: 10.3390/ani12040532
37. Taheri HR, Nasiri H, Ahmadkhani R. Which source and level of dietary sodium is appropriate for broiler chickens reared in a high-altitude area? J Anim Physiol Anim Nutr. (2019) 103:1090–8. doi: 10.1111/jpn.13096
38. Kitada K, Daub S, Zhang Y, Klein J, Nakano D, Pedchenko T, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest. (2017) 127:1944–59. doi: 10.1172/JCI88532
39. Reddy V, Sridhar A, Machado RF, Chen J. High sodium causes hypertension: evidence from clinical trials and animal experiments. J Integr Med. (2015) 13:1–8. doi: 10.1016/S2095-4964(15)60155-8
40. Okamoto C, Hayakawa Y, Aoyama T, Komaki H, Minatoguchi S, Iwasa M, et al. Excessively low salt diet damages the heart through activation of cardiac (pro) renin receptor, renin-angiotensin-aldosterone, and sympatho-adrenal systems in spontaneously hypertensive rats. PLoS ONE. (2017) 12:e0189099. doi: 10.1371/journal.pone.0189099
41. Wang J, Deng Y, Zou X, Luo H, Jose PA, Fu C, et al. Long-term low salt diet increases blood pressure by activation of the renin-angiotensin and sympathetic nervous systems. Clin Exp Hypertens. (2019) 41:739–46. doi: 10.1080/10641963.2018.1545850
Keywords: sodium chloride, optimal level, blood gas, serum parameters, jejunal morphology, organ index, broiler
Citation: Zhang W, Wu B, Wu W, Cui X, Li D, Gao F, Li T, Zhu L, Geng Y, Zhang L, Hu Y and Luo X (2022) An optimal dietary sodium chloride supplemental level of broiler chicks fed a corn-soybean meal diet from 1 to 21 days of age. Front. Vet. Sci. 9:1077750. doi: 10.3389/fvets.2022.1077750
Received: 23 October 2022; Accepted: 22 November 2022;
Published: 06 December 2022.
Edited by:
Shourong Shi, Poultry Institute (CAAS), ChinaReviewed by:
Guohua Liu, Feed Research Institute (CAAS), ChinaHouqiang Luo, Wenzhou Vocational College of Science and Technology, China
Lei Wu, Nanjing Agricultural University, China
Copyright © 2022 Zhang, Wu, Wu, Cui, Li, Gao, Li, Zhu, Geng, Zhang, Hu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Hu, aHV5dW5AeXp1LmVkdS5jbg==; Xugang Luo, d2x5c3pAMjYzLm5ldA==
 Weiyun Zhang
Weiyun Zhang Bingxin Wu1
Bingxin Wu1 Tingting Li
Tingting Li Yun Hu
Yun Hu