- 1Department of Microbiology, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq
- 2Department of Basic Sciences, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq
Theileriosis, the hemoprotozoan infection, is an endemic condition in tropical and subtropical areas. In this study, conventional PCR analysis was applied to detect the natural infection of native sheep with theileriosis and estimate its effect on hemato-biochemical parameters. The study was carried out in five regions of Sulaimani province, northern Iraq. From May to October 2021, a total of 360 blood samples were collected randomly from the jugular vein of sheep belonging to 23 flocks with a history of tick infestations. After PCR for theileriosis, the hematobiochemical parameters were evaluated by an automatic analyzer using commercial kits. The PCR results represented that 71.7% of the examined sheep were infected with Theileria parasites. The highest prevalence rate (74.6%) was reported in Said Sadiq, and the lowest prevalence (69.5%) was from Bazian. The infection rates in Mawat, Qaradagh, and Sharazoor were 73.1, 70.3, and 71.8%, respectively. The hemogram data revealed a significant decrease in erythrocyte count, hemoglobin concentration, and hematocrit values. Erythrocyte indices also showed significant increases in MC, MCH, and MCHC levels, but no significant differences were detected between the counting of leukocytes, lymphocytes, and granulocytes. Biochemical analysis revealed a significant decrease in total protein, albumin, and creatinine levels with a significant increase in urea and AST levels in infected sheep with theileriosis. Alteration in hemato-biochemical parameters from infected animals can outline the impact of theileriosis on body metabolism and blood factors in naturally infected sheep.
Introduction
Theileriosis is a hemoprotozoan disease caused by Apicomplexan parasites of the genus Theileria. Infection with Theileria spp. is considered an important devastating disease of small ruminants, appearing in subacute, acute, or chronic forms (1). Theileriosis is associated with substantial economic losses in livestock due to high morbidity and mortality, especially in tropical and subtropical areas (2, 3). Several species cause theileriosis in ovines with various pathogenicities. T. lestoquardi, T. luwenshuni, and T. uilenbergi produce malignant infections, while T. ovis, T. separata, and T. recondita produce mild-type infections. Also, natural infection with T. annulata in sheep has been reported worldwide (4). Other species of sheep have also been reported, including Theileria sp. OT1, Theileria sp. OT3, and Theileria sp. MK (5, 6). The parasite was transmitted through vector ticks belonging to the genera Rhipicephalus, Hyalomma, and Haemaphysalis (7). As a common vector-borne livestock infection, Theileria infects leukocytes and erythrocytes (8).
Theileriosis occurs after establishing schizont stages within the cytoplasm of susceptible host lymphocytes, with subsequent development of merozoites invading the erythrocytes (9). Other stages of the parasite's life cycle occur in the tick's gut, leading to the development of infective sporozoites (10). Previous studies represented that various factors play a role in the incidence and dissemination of ovine theileriosis (11, 12). The main dispersing factor associated with the prevalence of theileriosis is the existence of susceptible hosts and ectoparasitic vectors (13, 14). The dormant stage of infection is vital in disease epidemiology because chronic carriers act as reservoirs for tick-borne infection. Molecular techniques enable the sensitive and specific detection of pathogens (6). Furthermore, changes in blood biomarker profiles can provide valuable information on the severity of the infection and help measure the prognosis of the disease and determine the applied therapy (15, 16).
Blood and biochemical profiles, including total proteins, globulins, albumin, glucose, bilirubin, cholesterol, urea, and creatinine, can be helpful in disease diagnosis, and serum levels of ALT and AST enzymes give essential evidence about the health status of muscular and liver tissues. These hematobiochemical data effectively distinguish between healthy and diseased animals in veterinary studies (14). Hematological parameters, such as total erythrocyte count, hematocrit, hemoglobin concentration, mean corpuscular volume, mean corpuscular hemoglobin concentration, and total and differential leukocyte count, provide vital information on the health status of the animal (17).
Detection of ovine theileriosis and verification have been performed adequately through different diagnostic methods, including PCR and sequencing of amplified DNA products. In contrast, there was little about its effects on hemato-biochemical parameters. Accordingly, the study was designed to define the impact of natural Theileria infection on body metabolism and blood factors in naturally infected sheep.
Materials and methods
Study areas and sampling
The study was carried out in the Sulaimani province and its surrounding districts in the northern region of Iraq, namely, Bazian, Mawat, Qaradagh, Said Sadiq, and Sharazoor (Figure 1). The number of sheep in the included districts was estimated at 60,000 sheep. From May to October 2021, blood samples were randomly collected from 360 native sheep within 23 herds with a history of tick infestations. The sample size accounted for about 0.6% of the sheep in the study area, calculated based on a 95% confidence interval and a 5% error margin. The age of the included animals was more than 1 year, and of both sexes. About 7 mL of blood was drawn aseptically from the jugular vein. Two mL of blood were placed in an anticoagulant tube (EDTA) for hematological evaluation and molecular detection, and the rest in free anticoagulant tubes for serum collection. The samples were kept at a low temperature until transported to the laboratory, then processed, and the obtained sera were collected in Eppendorf and stored at −20°C until used for biochemical analysis. After hematological estimation, the remaining blood samples were stored similarly in a deep freezer for DNA extraction.
Laboratory examination
Molecular study
The genomic DNA was extracted from whole blood samples using a DNA extraction kit from GeNet Bio (South Korea). Following the manufacturer's instructions, the extracted DNA concentration (ng/mL) was measured using a NanoDrop spectrophotometer and stored at −20°C until used. By applying a conventional PCR assay, the Theileria parasite was detected targeting the 18S rRNA gene, using a previously designed specific oligonucleotide primer set with a 1098 bp portion of the gene (F: 5′-AGTTTCTGACCTATCAG-3′ and R: 5′-TTGCCTTAAACTTCCTTG-3′) described by Allsopp et al. (18).
For the PCR assay, the GeNet Bio green master mix from South Korea was used to screen all extracted DNA samples in a total volume of 20 μL. Amplification was achieved in a programmable thermocycler (Prime, UK) with the reactions starting with predenaturation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min. The final extension was carried out at 72°C for 7 min, then ended by cooling at 4°C. After amplification, 7 μL of PCR products were stained with ethidium bromide and run on a 1.5% agarose gel in TBE buffer for 80 min with 200 mA and 90 volts. After that, the specific band was visualized using a UV illuminator. The amplified positive samples were compared to the 1098 bp of the 18S rRNA gene.
Hematological studies
The hematological parameters studied included total erythrocyte count (millions/μL), hemoglobin concentration (Hb, g/dL), and hematocrit value (HCT, %). Several erythrocytic indices were also estimated, including Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC), Total Leukocytes Counts (TLC), Lymphocytes (LYM), and Granulocytes (GRA) using a hematology analyzer for infected and healthy animals (19).
Biochemical study
Sera from infected and uninfected animals were analyzed for biochemical parameters via an auto-analyzer (Alpha Classic, Sweden). The studied biochemical profiles, including total serum protein (TSP), serum albumin (SA), blood urea nitrogen (BUN), serum creatinine (CRE), and Aspartate Transaminase (AST), were estimated through spectrophotometry using commercial test kits (20).
Statistical analysis
Data analysis was performed through the Statistical Package for Social Sciences (SPSS version 24.0, IBM, USA). A comparison of the hematobiochemical parameters between infected and uninfected sheep was made using an independent samples t-test. A probability level of <0.05 was considered significantly different.
Results
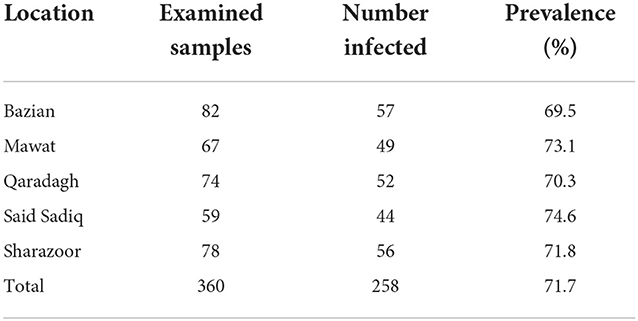
The data of the present study revealed that of 360 native sheep examined, 71.7% were positive for Theileria protozoa. The PCR-based diagnosis of Theileria revealed the highest prevalence rate in Said Sadiq (74.6%) and the lowest prevalence in Bazian (69.5%), as shown in Figure 2 and Table 1.
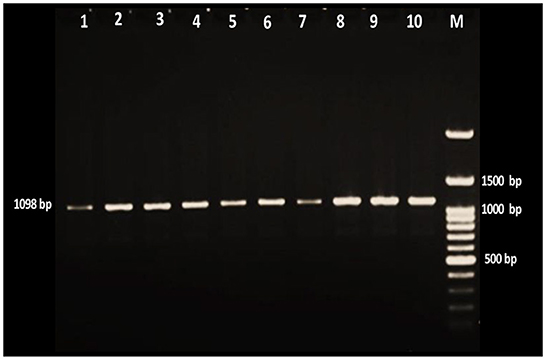
Figure 2. Gel electrophoresis of amplified Theileria positive products with ethidium bromide stain. Lane M is a 100-bp DNA ladder, and lanes 1–10 were positive samples for the Theileria parasite.
The hematological profiles of infected sheep represented a significant decrease in total erythrocyte count, hemoglobin, and hematocrit. Furthermore, the erythrocytic indices of MCV, MCH, and MCHC recorded significantly higher values. Furthermore, a decrease in total leukocytes, lymphocytes, and granulocytes was nonsignificant (P > 0.05).
Serum concentrations of total protein, albumin, and creatinine decreased significantly in infected sheep. On the contrary, significant increases in AST and urea concentrations were recorded in animals infected with Theileria (Table 2).
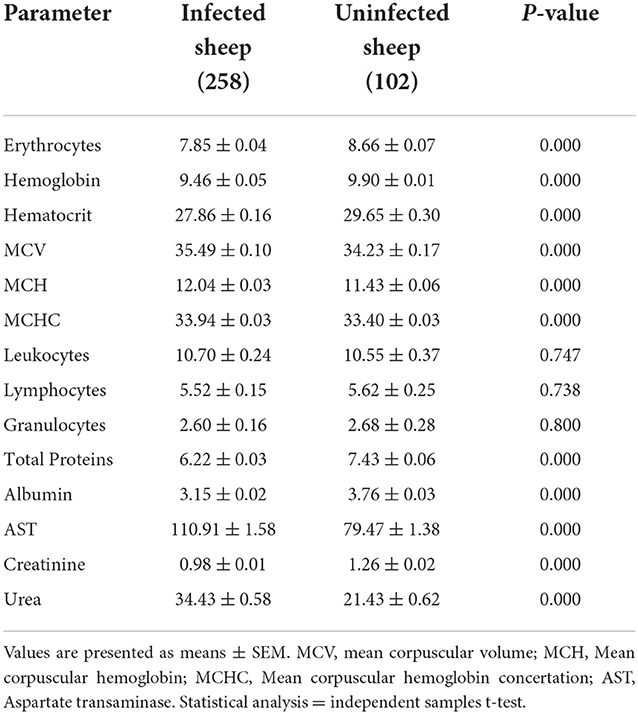
Table 2. Hematological and biochemical parameters of infected and uninfected sheep with Theileria spp.
Discussion
Ovine theileriosis represents the endemic condition in the study area with a significant impact on the livestock industry. The present study showed that the prevalence of theileriosis in native sheep was 71.7%, in agreement with the study data reported by Abdullah and Ali (6) 5 years earlier, with a prevalence rate of 76.4% in the Sulaimani province. A lower prevalence than the current study has been reported in other locations. Infection rates of 62.5% in Iran, 54.4% in Oman, and 53.0% in Pakistan were found by Zarei et al. (21), Al-Hamidhi et al. (17), and Shahzad et al. (22), respectively. In other studies, Nangru et al. (23) and Riaz and Tasawar (14) reported 44% in India and 39.5% in Pakistan.
In the current study, samples were collected from small ruminant flukes with a history of tick infestation. Such a high prevalence rate indicated that tick infestation is a chief factor for the persistence of theileriosis in flukes. The significantly higher prevalence of theileriosis in tick-infested animals compared to tick-free sheep was reported by Ullah et al. (24). The highest prevalence of Theileria spp. was recognized in Said Sadiq, while the lowest was in Bazian based on PCR. The results indicated different prevalence rates of ovine theileriosis at different sampling sites. Different factors such as husbandry practices, nutritional deficiencies, breed, sex, flock size, climatic conditions (humidity, temperature), and contagious infections directly affect health status. Sheep infections with Theileria spp. are associated with the intra-leukocyte schizont and intra-erythrocyte piroplasm stages, which affect the hemato-biochemical changes, and the severity of these changes is related to the virulence of the parasite strain, the infectious dose, the animal breed, and immune status (25).
The present study results show that Theileria infection in ovine led to alterations in hemato-biochemical parameters. The hemogram showed a significant decrease in total erythrocyte count, hemoglobin concentration, and hematocrit value. Furthermore, significantly higher values of the MCV, MCH, and MCHC were recorded from PCR-positive samples. These findings agreed with previous studies (24, 26). Infection with Theileria spp. results in increased oxidative stress in infected animals, leading to increased fragility of erythrocytes due to membrane lysis and a lower concentration of hemoglobin (27). The reduction of erythrocyte counts might be associated with phagocytosis of the infected cells by an endothelial system of the spleen, lymph nodes, and other organs (28). The total leukocytes, lymphocytes, and granulocyte counts did not differ between the infected and uninfected sheep. Al-Hamidhi, Elshafie, Yaghfoori, Morrison, Johnson and Babiker (17) observed the same finding, while Shahzad (21) reported a significant decrease in WBCs, monocytes, and lymphocytes. A decrease in total cell count is presented by clinical signs like hemolytic anemia, dyspnea, and lethargy (27).
The biochemical analysis showed significantly lower levels of total protein, albumin, and creatinine concentrations with a significant increase in blood urea levels and serum AST enzyme activities of animals infected with Theileria. In agreement with the study findings (16, 22), also recognized that Theileria parasites cause a significant decrease in protein concentration. The reduction in serum total protein content is due to hypoalbuminemia caused by decreased albumin synthesis, which is the direct or indirect effect of the parasite on hepatocytes, leading to liver failure. Decreased dietary protein intake and diarrhea have also been related factors. Hypoproteinemia and hypoalbuminemia might also result from the accumulation of extravascular proteinaceous fluid after the lymph nodes are affected (29).
A significant decrease in serum creatinine concentration in infected animals was consistent with previous reports by Shahzad et al. (22) in sheep infected with theileriosis. In contrast (30), reported a nonsignificant decrease in creatinine concentration from infected sheep with theileriosis. Furthermore, current data contradict what was reported by Col and Uslu (16) and (31), with a reverse pattern of a nonsignificant increase in serum creatinine levels in cattle and sheep. The concentration of blood urea in the current study was significantly increased, following the findings of Baghshani et al. (30) and Al-Fetly (31). However, Riaz and Tasawar (14) have reported a nonsignificant increase in urea levels among infected sheep. Increased urea concentrations in animals infected with Theileria could be related to kidney damage (16) and strongly associated with the level of parasitemia (14).
The measurement of enzyme concentration is another laboratory trial for disease evaluation. An increase in serum enzyme activity indicates the occurrence of necrosis or destruction in the liver or muscle tissues (32). Detection of AST and ALT serum concentrations provides a significant indication for determining the physiological function of the liver. An increase in the level of AST concentration was significant in Theileria-infected sheep compared with uninfected. Other researchers have reported similar findings (19, 30, 32). This rise in AST levels might be associated with hepatic dysfunction or muscular distress (16). Furthermore, the toxic metabolites of Theileria spp. have harmful effects on liver cells, which could raise the level of enzymes (33).
Conclusions
The study data denoted a high prevalence of Theileria infection among sheep, confirming the endemicity of the disease in the studied area. Theileria spp. are dominant hemoparasites in the study area, confirming the high population of transmitter vector ticks for different Theileria spp. Under traditional husbandry conditions, subclinically infected animals remain a source of tick infection. The annual costs of animal loss and treatment should be considered. Alteration in some hematological and biochemical profiles of infected sheep reveals the influence of theileriosis on metabolic disturbances due to its harmful effects on the body's organs and systems. Hence, a reduction in animal production might result from chronic infection with theileriosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee at the College of Veterinary Medicine, University of Sulaimani. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
SA conducted the design, methodology, and writing of the manuscript. HD and NS participated in the data interpretation and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank the veterinarians and owners who facilitated blood sampling from animals in all districts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ullah N, Durrani AZ, Avais M, Nisar A, Ullah S, Khan MS, et al. Prevalence, risk factors and host biomarkers of ovine theileriosis. Pak J Zool. (2018) 50:1211–6. doi: 10.17582/journal.pjz/2018.50.4.1211.1216
2. El Damaty HM, Yousef SG, El-Balkemy FA, Nekouei O, Mahmmod YS, Elsohaby I. Seroprevalence and risk factors of tropical theileriosis in smallholder asymptomatic large ruminants in Egypt. Front Vet Sci. (2022) 9:1004378. doi: 10.3389/fvets.2022.1004378
3. Zhao HX, Li X, Liu JL, Guan GQ, Luo JX. Changes in TFG gene expression in bovine leucocytes transformed by Theileria annulata. Front Vet Sci. (2022) 9:997294. doi: 10.3389/fvets.2022.997294
4. Mans BJ, Pienaar R, Latif AA. A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl. (2015) 4:104–18. doi: 10.1016/j.ijppaw.2014.12.006
5. Goudarzi G, Tavakoli M, Ezatpour B, Kooshki H, Hosseini-Chegeni A. Molecular detection of Theileria ovis (Apicomplexa: Theileriidae), Anaplasma ovis (Rickettsiales: Anaplasmataceae), and Mycoplasma sp. (Tenericutes: Mycoplasmataceae) from sheep blood in western Iran. Compar Clin Pathol. (2019) 28:1661–6. doi: 10.1007/s00580-019-02995-y
6. Abdullah S, Ali S. Molecular investigation and phylogeny of Theileria spp. from naturally infected sheep and the first report of Theileria sp. OT3 in Sulaymaniyah governorate/Iraq. Pol J Vet Sci. (2021) 24:201–9. doi: 10.24425/pjvs.2021.136809
7. Gargano V, Blanda V, Gambino D, La Russa F, Di Cataldo S, Gentile A, et al. Serological survey and molecular characterization of Theileria annulata in Sicilian cattle. Pathogens. (2021) 10:101. doi: 10.3390/pathogens10020101
8. Jenkins C. Bovine theileriosis in Australia: a decade of disease. Microbiol Aust. (2018) 39:215–9. doi: 10.1071/MA18067
9. Elati K, Zweygarth E, Mhadhbi M, Darghouth MA, Nijhof AM. Cultivation, cryopreservation and resuscitation of Theileria annulata transformed cells in serum-free media. Front Vet Sci. (2022). doi: 10.3389/fvets.2022.1055022
10. Watts J, Playford M, Hickey K. Theileria orientalis: a review. N Z Vet J. (2016) 64:3–9. doi: 10.1080/00480169.2015.1064792
11. Gebrekidan H, Hailu A, Kassahun A, Rohoušová I, Maia C, Talmi-Frank D, et al. Theileria infection in domestic ruminants in northern Ethiopia. Vet Parasitol. (2014) 200:31–8. doi: 10.1016/j.vetpar.2013.11.017
12. Rjeibi M, Darghouth M, Rekik M, Amor B, Sassi L, Gharbi M. First molecular identification and genetic characterization of Theileria lestoquardi in sheep of the Maghreb region. Transbound Emerg Dis. (2016) 63:278–84. doi: 10.1111/tbed.12271
13. Fatima M, Saeed S, Shaikh RS, Ali M, Iqbal F. A study on molecular detection of Theileria lestoquardi by PCR amplification in apparently healthy small ruminants from five districts of Southern Punjab. Pak J Zool. (2015) 47:441–6.
14. Riaz M, Tasawar Z, A. study on molecular diagnosis of Theileria species infection by PCR amplification in sheep and goats in Multan, Pakistan. Biol Sci PJSIR. (2017) 60:36–45. doi: 10.52763/PJSIR.BIOL.SCI.60.1.2017.36.45
15. Roubies N, Panousis N, Fytianou A, Katsoulos PD, Giadinis N, Karatzias H. Effects of age and reproductive stage on certain serum biochemical parameters of Chios sheep under Greek rearing conditions. J Vet Med SerA. (2006) 53:277–81. doi: 10.1111/j.1439-0442.2006.00832.x
16. Col R, Uslu U. Changes in selected serum components in cattle naturally infected with Theileria annulata. Bull Vet Ins Pulawy. (2007) 51:15–8.
17. Al-Hamidhi S, Elshafie EI, Yaghfoori S, Morrison WI, Johnson EH, Babiker HA, et al. comparative study of single Theileria lestoquardi and mixed infections with Theileria ovis. Parasit Vectors. (2021) 14:1–10. doi: 10.1186/s13071-021-04864-6
18. Allsopp B, Baylis H, Allsoppi M, Cavalier-Smith T, Bishop RP, Carrington D, et al. Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology. (1993) 107:157–65. doi: 10.1017/S0031182000067263
19. Mahmoud M, Al-Dhalimy A, Al-Dujaily A. Study of hematological and biochemical changes in sheep and goats infected with theileriosis AT-Najaf province, Iraq. Biochem Cell Arch. (2019) 19:803–6. doi: 10.35124/bca.2019.19.1.803
20. Ismael AB, Swelum AA, Khalaf AF, Abouheif MA. Clinical, haematological and biochemical alterations associated with an outbreak of theileriosis in dromedaries (Camelus dromedarius) in Saudi Arabia. Pak Vet J. (2014) 34:209–13.
21. Zarei F, Ganjali M, Nabavi R. Identification of Theileria species in sheep and vector ticks using PCR method in Zabol, Eastern Iran. J Arthropod Borne Dis. (2019) 13:76–82. doi: 10.18502/jad.v13i1.934
22. Shahzad S, Tipu M, Aslam A, Hussain T, Ali Z, Shelly S, et al. Comparative hemato-biochemical study on theileriosis in naturally infected Punjab Urial (Ovis vignei punjabiensis) and domestic sheep (Ovis aries) in Pakistan. J Anim Plant Sci. (2015) 25:472–6.
23. Nangru A, Maharana BR, Vohra S, Kumar B, Ganguly A. Molecular detection and differentiation of different theileria species in naturally infected goats using nested PCR–RFLP: a first report from Northern India. Acta Parasitol. (2022) 67:997–1006. doi: 10.1007/s11686-022-00553-1
24. Ullah N, Durrani AZ, Ullah S, Ullah S, Shah MK, Khan AZ, et al. study on potential factors and physiological biomarkers associated with the occurrence of ovine theileriosis. Small Rumin Res. (2018) 168:32–8. doi: 10.1016/j.smallrumres.2018.09.010
25. Ayadi O, Gharbi M, Benchikh-Elfegoun MC. Haematological and biochemical indicators of tropical theileriosis diseased cattle in wilaya of Sétif (North East Algeria). J Parasitic Dis. (2017) 41:538–42. doi: 10.1007/s12639-016-0846-6
26. Shah S, Khan M, Rahman H. Epidemiological and hematological investigations of tick-borne diseases in small ruminants in Peshawar and Khyber Agency. Pakistan J Adv Parasitol. (2017) 4:15–22. doi: 10.17582/journal.jap/2017/4.1.15.22
27. Grewal A, Ahuja C, Singha S, Chaudhary K. Status of lipid peroxidation, some antioxidant enzymes and erythrocytic fragility of crossbred cattle naturally infected with Theileria annulata. Vet Res Commun. (2005) 29:387–94. doi: 10.1007/s11259-005-4682-x
28. Nazifi S, Razavi S, Kianiamin P, Rakhshandehroo E. Evaluation of erythrocyte antioxidant mechanisms: antioxidant enzymes, lipid peroxidation, and serum trace elements associated with progressive anemia in ovine malignant theileriosis. Parasitol Res. (2011) 109:275–81. doi: 10.1007/s00436-010-2248-5
29. Homer MJ, Aguilar-Delfin I, Telford III SR, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. (2000) 13:451–69. doi: 10.1128/CMR.13.3.451
30. Baghshani H, Razmi GR, Yaghfouri S, Ahmadi Dezaki A. Investigation of selected biochemical parameters in sheep naturally infected with theileriosis. Comp Clin Path. (2012) 21:1417–20. doi: 10.1007/s00580-011-1308-2
31. Al-Fetly DRH. Detection of Theileria spp. in blood samples and estimation of haematological and biochemical changes in sheep in Al-Diwaniya province. Kufa J Vet Med Sci. (2012) 3:45–53.
Keywords: Theileria, sheep, blood, PCR, Sulaimani
Citation: Abdullah SH, Dyary HO and Saeed NM (2022) Molecular detection of Theileria spp. in native sheep and estimation of hemato-biochemical parameters from Sulaimani province/Iraq. Front. Vet. Sci. 9:1059599. doi: 10.3389/fvets.2022.1059599
Received: 01 October 2022; Accepted: 28 November 2022;
Published: 16 December 2022.
Edited by:
Khalid Mehmood, Islamia University of Bahawalpur, PakistanReviewed by:
Adel Talib Al-Saeed, University of Duhok, IraqMuhammad Fakhar-e-Alam Kulyar, Huazhong Agricultural University, China
Nadia Sultan Alhayali, University of Mosul, Iraq
Copyright © 2022 Abdullah, Dyary and Saeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shadan Hassan Abdullah, c2hhZGFuLmFiZHVsbGFoQHVuaXZzdWwuZWR1Lmlx
 Shadan Hassan Abdullah
Shadan Hassan Abdullah Hiewa Othman Dyary
Hiewa Othman Dyary Nahla Mohammed Saeed1
Nahla Mohammed Saeed1