
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci. , 21 December 2022
Sec. Veterinary Infectious Diseases
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1058844
This article is part of the Research Topic Bacterial Diseases In Poultry: Biology, Virulence And Prevention In The Age Of Reduced Antibiotic Use View all 9 articles
In order to prevent pullorum disease and fowl typhoid in breeders, the use of oregano essential oil (OEO) was tested for the prevention and treatment of infections of multidrug-resistant Salmonella pullorum (SP) and Salmonella gallinarum (SG) in commercial Yellow-chicken breeders. In the challenge-protection experiment, commercial Hongguang-Black 1-day-old breeder chicks were randomly divided into four groups, including A (challenged, preventive dose), B (challenged, treatment dose), C (challenged, untreated), and D (unchallenged, untreated). Group A was supplemented with 200 μL/L OEO in the drinking water during the whole trial (1-35 days of age) and group B was supplemented with 400 μL/L OEO during 8–12 days of age, while groups C and D were kept as untreated controls. At 7 days of age, birds of groups A, B, and C were divided into two subgroups with equal number of birds (A1-A2, B1-B2, and C1-C2), and then subgroups A1, B1, and C1 were challenged with SP, while subgroups A2, B2, and C2 were challenged with SG. Clinical symptoms and death were observed and recorded daily. Every week during the experiment, serum antibodies against SP and SG of all the groups were detected by the plate agglutinate test (PAT). At the age of 35 days, all birds were weighed and necropsied, lesions were recorded and the challenging pathogens were isolated. The results showed that the positive rates of SP and SG isolation in groups A1, A2 and B1, B2 were significantly lower (P < 0.05) than those of groups C1 and C2, respectively, while groups A1 and A2 were slightly lower (P > 0.05) than those of groups B1 and B2. The average body weight (BW) of groups A1 and A2 were significantly higher (P < 0.05) than those of groups B1, B2 and C1, C2, respectively, but there was no significant difference (P > 0.05) with that of group D. The r-value between PAT positive and the recovery rates of Salmonella was 0.99, which means they are highly positively correlated. The results of this study demonstrated that the prevention dose (200μL/L) and the treatment dose (400 μL/L) of OEO supplemented in the drinking water could all effectively decrease infections of SP and SG and that the effect of the prevention was greater than that of the treatment and finally that the prevention could also significantly reduce the BW decline of birds challenged with SP and SG.
Pullorum disease (PD) and fowl typhoid (FT), respectively caused by Salmonella pullorum (SP) and Salmonella gallinarum (SG), are two major avian salmonellosis diseases that seriously harm the health of the breeders of the local, so called Yellow-chickens (1, 2). Yellow-chickens, the main breed of broiler production and supply in China, are becoming more popular with consumers because of the delicious flavor of the meat. This breed dominates other broilers in southern China by 5 billion birds annually and are produced in the free-range style (3, 4). PD and FT can reduce the hatching ability and bring serious economic losses to the poultry industry (5). Interestingly, after infection with SP and SG, some infected birds can recover from PD and FT and some adult birds may not show clinical disease symptoms. However, they are still the carriers of SP and SG and then become a repository of Salmonella to contaminate other healthy birds through horizontal and vertical transmission (6, 7). PD and FT have been mostly eradicated from commercial poultry flocks in the developed countries but they are still prevalent in the developing countries, especially in the Yellow-chickens (8), due to the style of husbandry, incomplete eradication and inadequate biosecurity (9, 10).
Traditionally, antibiotics have been used to control commercial poultry infection by SP and SG and the isolation of Salmonella with multidrug resistance (MDR) has been increasing dramatically in recent years. That presents a threat to both the poultry industry and to public health (11, 12). Reducing antibiotic usage is an inevitable trend, and added antibiotics have been banned in the feed in China since 2020 (13). Therefore, there has been increasing interest in the development of new, effective and nontoxic antimicrobial compounds as an alternative to traditional antibiotic use.
Currently, some studies found that plant essential oils are a potentially useful source of antimicrobial compounds. This is attributed to their availability, fewer side effects and their ability to synthesize aromatic substances, the majority of which are phenols or oxygen-substituted derivatives (14). Oregano essential oil (OEO), an extract of oregano, has been found to be amongst the most effective antimicrobial and antioxidant natural agents (15). OEO is composed of a variety of aromatic compounds including carvacrol, thymol, γ-terpinene, p-cymene, linalool, β-myrcene and so on (16). Especially, the carvacrol and thymol, the two main phenols of OEO, are principally responsible for the antimicrobial activity of the oil and there is no problem with drug-residues and drug-resistance while using this product (17). These aromatic compounds can penetrate the cell membranes of pathogenic bacteria, change the permeability of the mitochondrial membranes and prevent mitochondria from absorbing oxygen. So, the growth of bacteria is inhibited because the pathogenic cells suffocated and died (18). Research has found that OEO, as a feed additive, can replace antibiotics, maintain animal growth performance and improve disease resistance, which allows oregano to be potentially used extensively in animal husbandry (19). The dietary supplementation with OEO can inhibit or kill harmful intestinal pathogens and improve broiler chicken production performance (20). Mathlouthi found that OEO supplementation could inhibit the growth of Escherichia coli, Salmonella indiana, Listeria innocua, Staphylococcus aureus and Bacillus subtilis (21). In addition to its antimicrobial and antioxidant effects, OEO also has anticoccidial and antiparasitic efffects. These efffects were demonstrated by both the studies of M. Bozkurt and M. Mohiti-Asli et al. (22, 23).
However, no study to date has reported on the prevention and treatment of PD and FT, the most important salmonellosis in Yellow-chicken commercial breeders, by using OEO. Therefore, this study aims to evaluate the prevention and treatment effects of OEO against the challenge of SP and SG in commercial Yellow-chicken breeders.
The live animals described in this study were treated according to the National Guidelines to Humanitarian Governance of Laboratory Animals Welfare (National Development and Reform Commission of the People's Republic of China, 2006) and the animal experiment was approved by the Animal Welfare and the Animal Experimental Ethical Committee of Guangxi University (No. 2020-gxu-097). All the experimental procedures were conducted in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. The animals were sacrificed by carbon dioxide narcosis.
The OEO product, composed of thymol and carvacrol in the concentrations of 5.1 and 0.12% (w/w), was kindly provided by Shanghai Huihai Huamao Industrial Co., Ltd., China. 1-day-old breeder chicks of Hongguang-Black, negative for SP and SG, from the grandparent flock, were kindly provided by Guangxi Hongguang Agricultural and Animal Husbandry Ltd., China. Plate agglutination test (PAT) antigens of SP and SG were purchased from Beijing Zhonghai Biotech Co. Ltd., China. Salmonella A to F group-specific diagnostic sera and Salmonella serotype-specific monovalent sera were purchased from the Ningbo Tianrun Bio-pharmaceutical Co. Ltd., China. The isolates of multidrug-resistant SP and SG (The MDR spectrum were 17 and 12 antimicrobials, respectively) used for the challenge were provided by the Institute for Poultry Science and Health, Guangxi University, China.
Fifty-five 1-day-old parent-stock breeder chicks of Hongguang-Black chickens were divided into five groups (A–E) with 10 birds per group, plus a group F with five birds, which served as the unchallenged and untreated control. The birds have free access to feed and water during the whole experiment and the diet was the commercial feed without any antibiotics or additives. Before the experiment, we collected serum from each 1 day old chicks for the plate agglutination test using Salmonella A to F group-specific diagnostic sera and Salmonella serotype-specific monovalent sera. At the age of 7 days, birds in groups A to E were divided into two subgroups, equal in number, designated groups A1 to E1 and A2 to E2. Groups A1 to E1 were orally challenged with 2 × 106 CFU/mL, 2 × 107 CFU/mL, 2 × 108 CFU/mL, 2 × 109 CFU/mL and 2 × 1010 CFU/mL of SP in 0.5 mL-volume/bird respectively, while groups A2 to E2 were orally challenged with 2 × 106 CFU/mL, 2 × 107 CFU/mL, 2 × 108 CFU/mL, 2 × 109 CFU/mL and 2 × 1010 CFU/mL of SG in 0.5 mL-volume/bird respectively. The dose of challenge and detection index refers to the previous procedures of our laboratory (24). More than 80% infective dose of Salmonella infection in birds was determined, which referred to Guiding Principles for Clinical Efficacy Evaluation of Antimicrobial Drugs II and III (China Ministry of Agriculture Proclamation No. 1247) (25). It is pointed out in the guideline that the infection rate of 80% in the artificial infection model is considered to be successful in the construction of the model and more in line with the actual situation.
In this experiment, 105 1-day-old breeder chicks of Hongguang-Black chickens were randomly divided into groups A (challenged, preventive dose), B (challenged, treatment dose) and C (challenged, untreated), with 30 birds each, and D (unchallenged, untreated) with 15 birds (Supplementary Table 1). The birds of each group were housed separately in the isolators. The birds were raised according to the model established by the laboratory and throughout the entire experiment they had free access to feed and water (24).
During the entire trial of 35 days, all groups were fed the same feed as that used in the prior experiment of the infection dose. In the drinking water, 200 μL/L of OEO was added in group A during the whole trial, 400 μL/L of OEO was added in group B during the ages of 8–12 days, while no supplement was added in groups C and D. At the age of 7 days, birds in groups A, B, and C were divided into two subgroups respectively, equal in number, and designated as A1, A2, B1, B2, C1, and C2. Groups A1, B1, and C1 were orally challenged with 0.5 mL-volume/bird of the suspension containing 1 × 108 CFU of SP, and groups A2, B2, and C2 were orally challenged with 0.5 mL-volume/bird of the suspension containing 1 × 109 CFU of SG. The infective doses of SP and SG were determined by the prior experiment of the infective dose.
The clinical symptoms and the pathological changes of the possible dead birds during the experiment were observed and recorded daily. At the end of the experiment, at the age of 35 days, the live BW of all the birds in each group was determined and recorded.
PAT was performed before challenge, and all the birds were negative. At 1, 2, 3, and 4 weeks post-challenge, the serum antibodies against SP and SG of the birds were detected by PAT with the antigens of SP and SG according to the described method and the positive rates of the groups were calculated (26).
At the end of the experiment at the 35 days of age, all surviving birds were euthanized and their organs were excised aseptically. The gross lesions were observed and recorded. The cloaca swabs, cecum, and the mixture of visceral parenchyma organs including heart, liver and spleen were sampled, respectively, for the isolation of the challenge Salmonella according to the routine of China National Food Safety Standard Methods for Food Microbiological Examination-Salmonella (GB/T4789.4-2016).
The Microsoft Excel function and the Statistical Program for Social Sciences (SPSS) 22.0 statistical software were used to process the test data. Factor analysis of variance and chi-square test were used to test the difference between groups. P < 0.05 indicated that the difference was significant, while P > 0.05 was not. Different letters indicate that the difference was significant in the Figures 2–4 (P < 0.05), while the same letters were not. The correlation coefficient r-value was calculated by the CORREL formula used to evaluate the correlation between PAT positive and the recovery rate of Salmonella. The range of the r-value from −1 to 1 (r > 0 is a positive correlation, r < 0 is negative correlation, and r = 0 is irrelevant). The greater the absolute r-value is, the higher the correlation is.
According to the results of the experiment of the infection dose (Table 1), 80% of birds were infected by the challenge with 2 × 108 CFU/mL of SP and 2 × 109 CFU/mL of SG, and there was no bird death during the whole trial. The infective doses of SP and SG were 2 × 108 CFU/mL and 2 × 109 CFU/mL.
According to clinical reports, the typical clinical symptoms of chicks were weakness, depressed appetite, poor growth, wet vent with chalky white droppings after S. pullorum and S. gallinarum infection. Birds in groups C1 and C2 showed weakness, ruffled feathers, loss of appetite, poor growth, and white diarrhea (Supplementary Figures 1A, B). The birds in A, B and C groups had the wet vent of chalky white material, and the number of birds in group C was the highest. At the necropsy of the birds, livers with necrotic white foci, as well as the softened heart with pericardial effusion containing yellow cellulose exudates, and granuloma of the heart could be observed in group C (Figures 1a–c); Livers with no lesions could be observed in group D (Figure 1d).
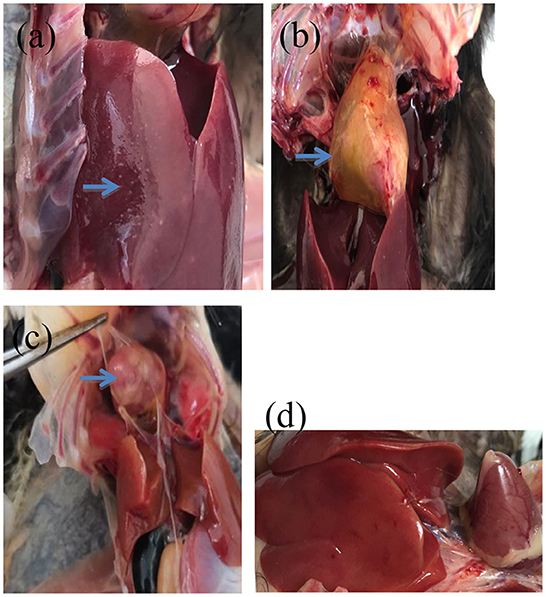
Figure 1. The anatomic lesions of the challenged birds. (a) Liver with necrotic white foci on the surface (arrow). (b) Heart with pericardial effusion containing yellow cellulose exudates (arrow). (c) Heart with granuloma (arrow). (d) Blank control.
In the challenge-protection experiment, the average BW of birds in groups A1 and A2 were significantly greater (P < 0.05) than those of groups B1, B2 and C1, C2, respectively. Those of groups B1 and B2 were slightly greater (P > 0.05) than those of groups C1 and C2, respectively. And that of group D was significantly greater (P < 0.05) than those of groups B and C, but no significant difference (P > 0.05) was observed between groups D and A, even though that of group D was slightly greater than those of groups A1 and A2 (Figure 2).
In the challenge-protection experiment, as shown in Table 2, the PAT positive rate of group A1 was significantly lower (P < 0.05) than that of group C1 at the end of the experiment (4 week post-challenge = 35 days of age), and no significant difference (P > 0.05) was found between groups A1 and B1, B1 and C1, even though that of group B1 was slightly higher than that of group A1 and was slightly lower than that of group C1. Also, the PAT positive rate of group A1 was found begun to be decreased from 3 weeks post-challenge and to become significantly decreased at 4 weeks post-challenge.
Also, the PAT positive rates of groups A2 and B2 were significantly lower (P < 0.05) than that of group C2 at the end of the experiment, but no significant difference (P > 0.05) was observed between groups A2 and B2, even though that of group B2 was slightly higher than that of group A2. Also, the PAT positive rate of group A2 began to decrease from 2 weeks post-challenge and became significantly decreased at 4 weeks post-challenge. And the same trend was found in group B2 also.
As shown in Figure 3, the total positive rates of Salmonella in groups A1 and B1 were significantly lower (P < 0.05) than that of group C1, and no significant difference (P > 0.05) was observed between groups A1 and B1. Similar results were found in the groups of the SG challenge-protection experiment. No Salmonella was isolated in group D.
In both the SP and SG challenge-protection experiments, the positive rates of the mixture samples of the visceral parenchyma organs were significantly higher (P < 0.05) than that of cloaca swab, and slightly higher (P > 0.05) than that of the cecum (Figure 4).
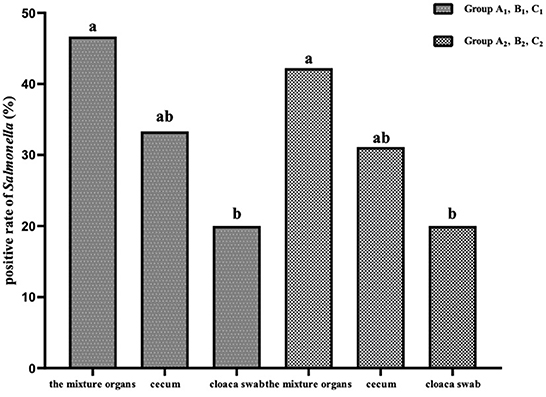
Figure 4. The positive rates of Salmonella in different organs in the challenge-protection experiment.
As shown in Table 3, the total positive rate of PAT was higher than or equal to the recovery rate of the challenged Salmonella, and a group with a higher positive rate of PAT that also had a higher recovery rate of Salmonella. The r-value between these two tests was 0.99, which means they are highly positively correlated.
FT and PD caused by SG and SP, respectively, are two serious septicemia diseases that greatly endanger the poultry industry and remain of major economic significance in many parts of the world (27). In this experiment, the birds manifested somnolescence, weakness, depressed appetite, poor growth, wet vent with chalky white droppings and livers with necrotic white foci, which are consistent with previous research (7). There was no bird death in the whole experiment, which was the same as in our previous study (24) and in the literature (28). Unsurprisingly, compared with other groups, no obvious clinical symptoms were observed in groups A1 and A2, indicating that there were better preventive effects of OEO against the challenges of SP and SG. To further understand the effects of OEO on SP and SG colonization in the challenged birds, the isolation and identification of the SP and SG in the internal organs and the intestine of birds were performed. The positive rates of isolation in groups C1 and C2 were significantly higher (P < 0.05) than those of groups A1 and A2 and B1 and B2, respectively, at the end of the experiment. The positive rates of SP and SG in groups A1 and A2 were slightly lower (P > 0.05) than those of groups of B1 and B2, respectively. The results showed that OEO could effectively prevent and treat the infection of SP and SG in chicks, and that the effect of prevention (supplemented during the whole experiment) was better than that of the treatment (supplemented during 1–5 days post-challenge). In addition, early studies have shown that the isolation rate in the liver was the highest, followed by the cecum and cloaca swabs (24, 29). In this study, the results showed that the positive rates of SP and SG in the mixture sample of the visceral parenchyma organs, which including heart, liver and spleen, was significantly higher (P < 0.05) than that of the cloaca swabs, and the positive rate of cecum was slightly higher (P > 0.05) than that of the cloaca swabs and was slightly lower (P > 0.05) than that of the mixture sample of the visceral parenchyma organs. These results were coincident with the previous studies (29). After the challenge with Salmonella, the liver, spleen and cecum are usually the main infected organs, accompanied by white foci of necrosis in the liver (30). The positive rate of cecum was lower than that of the mixture sample of the visceral parenchyma organs, which may be due to the intestinal antibacterial effect of OEO. Some research suggests that the barrier and antioxidant capacity of the intestine was improved by the oral administration of OEO, which may aide in gastrointestinal function without growth-promoting antibiotics (31). So the OEO used in this study may help to reduce the probability of Salmonella colonizing the intestine and may subsequently prevent the invasion of the pathogen into the internal organs.
In China, especially in Yellow-chickens, SP and SG were the most common serotypes of Salmonella infection (32). Therefore, it is important to control infection of SP and SG through implementation of an eradication program. The PAT is widely used on farms and on-site testing for eradication at the pre-maturation and the maturation stages of the breeders, being simpler and less costly than the isolation and identification of the bacteria (26, 29). In this study, at the end of the experiment (4 weeks post-challenge), the PAT positivity rates of groups A1 and A2 were lower than those of all other groups in the same challenged strains, and were significantly lower (P < 0.05) than those of groups C1 and C2, respectively. These results indicated that the preventive supplementation of OEO in the drinking water had a significant effect on the prevention of SP and SG in the birds. Meanwhile, the PAT positivity rates of groups A1 and B1 were slightly higher (P > 0.05) than those of groups A2 and B2, respectively, indicating that the effects of OEO on SG was better than those on SP. Interestingly, although the positivity numbers of serum PAT were the same or were more than those of the bacteria isolation in all the groups, there was still a high positive correlation between the results of these two tests. A few birds with the positive results of PAT might not be positive for the bacteria isolation and also a few birds with the negative results of PAT could be positive for the bacteria isolation. The two methods were not being completely equivalent, indicating that the PAT has certainly reference value for detecting Salmonella infection, but deviations may exist. Therefore, it is not possible to rely solely on the PAT for the detection of the positive birds. The positive birds should be confirmed by bacteriologic examination of 1 or more reactors (7). In routine testing, it is necessary to use the national standard method of bacteria isolation, for the detection of positive birds.
The published results highlight the bactericidal activity of OEO authorized in animal health as food supplements (33). OEO has the ability to improve the growth performance of poultry, increase the weight gain, improve the absorption capacity for nutrients, enhance the immune ability and reduce the infection of bacteria and diseases (19). In this study, at the end of the challenge-protection experiment (35 days of age), the BW of birds in group C was significantly lower (P < 0.05) than those in other groups, which may be due to the infection of Salmonella which can cause a severe inflammatory reaction in the intestinal mucosa of birds and can then lead to the decreased function of digestion and absorption and to the excessive requirement of nutrients in the process of resisting infection and then to poor growth performances (34). The BW of group A supplemented with 200 μL/L of OEO was significantly improved (P < 0.05) compared to that of group C and there was no significant difference (P > 0.05) compared with group D (the blank control). The BW of groups B1 and B2 were slightly greater (P > 0.05) than those of groups C1 and C2, respectively. It is inferred that the supplementation of OEO in the drinking water could help to improve the BW decline in birds due to Salmonella infection, and also revealed that the effect of the preventive supplementation was better than that of the treatment supplementation, even though the dose of the later was double that of the former. At the same time, compared with the effect of the treatment dose, the effect of the prevention dose was not only better in improving the BW decline caused by the infections of SP and SG, but also decreased the positive rates of the Salmonella isolation and the PAT. This might be related to the longer time of adding OEO in the groups of the preventive supplementation and the use of OEO before the bacteria challenges. Researchers have found that OEO can significantly promote the absorption of nutrients and regulate the balance of intestinal microflora (35, 36). Therefore, the preventive supplementation of OEO in the drinking water during the whole experiment could increase the BW gain performance of the challenged birds.
The OEO used in this study is mainly composed of thymol and carvacrol in the concentrations of 5.1 and 0.12% (w/w), respectively. Previous studies showed that the addition of a mixture of thymol and carvacrol can improve the production performance and antioxidant enzyme activity, delay the lipid oxidation, enhance the digestive enzyme activity and improve the immune response of broilers (37). Meanwhile, carvacrol and thymol, as the two main phenols of OEO, are principally responsible for the antimicrobial activity of the oil and there is no problem of a drug-residue issue in the products after using (16, 17). OEO can effectively kill bacteria without becoming resistant to them (38). Carvacrol and thymol can disturb the membrane integrity, increase membrane permeability and cause the leakage of protons and potassium, finally leading to the loss of membrane potential of the bacteria (39). So, the growth of bacteria is inhibited because of the depletion of the ATP pool and collapse of the proton motive force subsequently leading to the pathogen cells death (40). Therefore, this experiment proved that carvacrol and thymol might play an important role in the prevention of Salmonella (SP and SG) with OEO.
In summary, the supplementation of OEO in the drinking water could effectively reduce the infection of Salmonella and its effect on SG was better than that on SP. Further, OEO could significantly improve the BW decline in birds infected by SP and SG. Also, the effect of the preventive supplementation was better than that of the treatment supplementation in the commercial Yellow-chickens.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Animal Welfare and the Animal Experimental Ethical Committee of Guangxi University (protocol code 2020-gxu-097).
Conceptualization and methodology: ZX and CW; Software, formal analysis, and investigation: CW and ZX; Resources and data curation: CW, CZ, and WC; Writing–original draft preparation: ZX, CW, CZ, and CL; Writing–review & editing and visualization, supervision, project administration, and funding acquisition: PW. All authors have read and agreed to the published version of the manuscript.
This work was supported by the Natural Science Foundation of China (32160835) and the Guangxi Program for Modern Agricultural Industry Technical System Construction–Chicken Industry (nycytxgxcxtd-19-03).
The manuscript was kindly reviewed by Dr. Richard Roberts, Aurora, CO, United States.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1058844/full#supplementary-material
1. Soria MC, Soria MA, Bueno DJ. Comparison of 2 culture methods and PCR assays for Salmonella detection in poultry feces. Poult Sci. (2012) 91:616–26. doi: 10.3382/ps.2011-01831
2. Xiong D, Song L, Geng S, Tao J, An S, Pan Z, et al. One-step PCR detection of Salmonella pullorum/gallinarum using a novel target: the flagellar biosynthesis gene flhB. Front Microbiol. (2016) 7:1863. doi: 10.3389/fmicb.2016.01863
3. Shi MY Li M, Wang PK, Wang WW Li HJ, Gao YL, et al. An outbreak in three-yellow chickens with clinical tumors of high mortality caused by the coinfection of reticuloendotheliosis virus and Marek's disease virus: a speculated reticuloendotheliosis virus contamination plays an important role in the case. Poult Sci. (2021) 100:19–25. doi: 10.1016/j.psj.2020.09.034
4. Wei P. Challenge and development opportunity of quality chicken industry in China: a review about 9 issues on the topic. Chin Poult. (2019) 41:1–6.
5. Hu C, Dou W, Zhao G. Enzyme immunosensor based on gold nanoparticles electroposition and Streptavidin-biotin system for detection of S. pullorum and S gallinarum. Electrochimica Acta. (2014) 117:239–45. doi: 10.1016/j.electacta.2013.11.132
6. Berchieri A, Murphy CK, Marston K, Barrow PA. Observations on the persistence and vertical transmission of Salmonella enterica, Serovars Pullorum, and Gallinarum in chickens: effect of bacterial and host genetic background. Avian Pathol. (2001) 30:221–31. doi: 10.1080/03079450120054631
7. Swayne DE, Boulianne M, Logue CM, McDougald LR, Suarez DL, Nair V. Disease of Poultry. New York, NY: John Wiley and Sons, Inc, p. 719–37. (2018).
8. Kang MS, Kwon YK, Jung BY, Kim A, Lee KM, An BK, et al. Differential identification of Salmonella enterica subsp. Enterica serovar, Gallinarum biovars, Gallinarum, and Pullorum based on polymorphic regions of glgC and speC genes. Vet Microbiol. (2011) 147:181–5. doi: 10.1016/j.vetmic.2010.05.039
9. Wei P, Cui ZZ. Prevention and control of avian leukosis and pullorum in local chicken breeds. Chin Poult. (2015) 37:1–4. doi: 10.16372/j.issn.1004-6364.2015.09.001
10. Wang HY, Xu JF, Mo GD, Hou XJ, Ge ZH, Wei P. Comparison of purified antigens of pullorum and typhoid in breeder farms and determination of detection methods. Chin Poult. (2014) 36:52–3. doi: 10.16372/j.issn.1004-6364.2014.02.016
11. Hou XJ, Wu KM, Mo GD, Liu SH, Yan TJ, Jiao PT, et al. Evaluation of drug-resistant phenotypes and genes in foodborne Salmonella isolates. Food Sci. (2016) 37:166–70. doi: 10.7506/spkx1002-6630-201619028
12. Xu ZH, Wang M, Zhou CY, Gu GM, Liang JZ, Hou XJ, et al. Prevalence and antimicrobial resistance of retail-meat-borne Salmonella in southern China during the years 2009–2016: the diversity of contamination and the resistance evolution of multidrug-resistant isolates. Int J Food Microbiol. (2020) 333:108790. doi: 10.1016/j.ijfoodmicro.2020.108790
13. Hu YJ, Cowling BJ. Reducing antibiotic use in livestock, China. Bull World Health Organ. (2020) 98:360–1. doi: 10.2471/BLT.19.243501
14. Sakkas H, Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J Microbiol Biotechnol. (2017) 27:429–38. doi: 10.4014/jmb.1608.08024
15. Rodriguez-Garcia I, Silva-Espinoza BA, Ortega-Ramirez LA, Leyva JM, Siddiqui MW, Cruz-Valenzuela MR, et al. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit Rev Food Sci Nutr. (2016) 56:1717–27. doi: 10.1080/10408398.2013.800832
16. Leyva-Lopez N, Gutierrez-Grijalva EP, Vazquez-Olivo G, Heredia JB. Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules. (2017) 22:989. doi: 10.3390/molecules22060989
17. Govaris A, Solomakos N, Pexara A, Chatzopoulou PS. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol. (2010) 137:175–80. doi: 10.1016/j.ijfoodmicro.2009.12.017
18. Griffin S, Grant WS, Markham J. Determination of octanol–water partition coefficient for terpenoids using reversed-phase high-performance liquid chromatography. J Chromatography. (1999) 864:221–8. doi: 10.1016/S0021-9673(99)01009-2
19. Hu Y, Zhang Y, Song Z, Zhang H, Tian W, He X. Advances in biological functions of oregano oil and its application in livestock production. Chin J Anim Sci. (2021) 57:1–5. doi: 10.19556/j.0258-7033.20200723-05
20. Tzora A, Giannenas I, Karamoutsios A. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. J Poult Sci. (2017) 54:218–27. doi: 10.2141/jpsa.0160071
21. Mathlouthi N, Bouzaienne T, Oueslati I, Recoquillay F, Hamdi M, Urdaci M, et al. Use of rosemary, oregano, and a commercial blend of essential oils in broiler chickens: in vitro antimicrobial activities and effects on growth performance. J Anim Sci. (2012) 90:813–23. doi: 10.2527/jas.2010-3646
22. Mohiti-Asli M, Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev Vet Med. (2015) 120:195–202. doi: 10.1016/j.prevetmed.2015.03.014
23. Bozkurt M Ege G Aysul N Akşit H Tüzün AE Küçükyilmaz K . Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult Sci. (2016) 95:1858–68. doi: 10.3382/ps/pew077
24. Zhou CY, Liang JZ, Jiang WW, He X, Liu SH, Wei P. The effect of a selected yeast fraction on the prevention of pullorum disease and fowl typhoid in commercial breeder chickens. Poult Sci. (2020) 99:101–10. doi: 10.3382/ps/pez567
25. Services MOAV. Guiding Principles for Clinical Efficacy Evaluation of Antimicrobial Drugs II and III. Guidelines for Safe Pharmacological Testing of Veterinary Chemicals (China Ministry of Agriculture Proclamation No. 1247). (2009).
26. Gast RK. Detecting infections of chickens with recent Salmonella pullorum isolates using standard serological methods. Poult Sci. (1997) 76:17–23. doi: 10.1093/ps/76.1.17
27. Barrow PA, Freitas Neto OC. Pullorum disease and fowl typhoid–new thoughts on old diseases: a review. Avian Pathol. (2011) 40:1–13. doi: 10.1080/03079457.2010.542575
28. Haider MG, Chowdhury EH, Khan MAHNA, Hossain MT, Rahman MS, Song HJ, et al. Experimental pathogenesis of Pullorum disease with the local isolate of Salmonella enterica serovar enterica subspecies Pullorum in Pullets in Bangladesh. Korean J Poult Sci. (2009) 35:341–50. doi: 10.5536/KJPS.2009.35.4.341
29. Gast RK, Beard CW. Isolation of Salmonella enteritidis from internal organs of experimentally infected hens. Avian Dis. (1990) 34:991–3. doi: 10.2307/1591394
30. Shivaprasad HL. Fowl typhoid and pullorum disease. Rev Sci Tech Off Int Epiz. (2000) 19:405–24. doi: 10.20506/rst.19.2.1222
31. Ding X, Wu X, Zhang K, Bai S, Wang J, Peng H, et al. Dietary supplement of essential oil from oregano affects growth performance, nutrient utilization, intestinal morphology and antioxidant ability in Pekin ducks. J Anim Physiol Anim Nutr. (2020) 104:1067–74. doi: 10.1111/jpn.13311
32. Wang X, Wang H, Li T, Liu F, Cheng Y, Guo X, et al. Characterization of Salmonella spp. isolated from chickens in Central China. BMC Vet Res. (2020) 16:299. doi: 10.1186/s12917-020-02513-1
33. Solarte AL, Astorga RJ, de Aguiar FC, Tarradas C, Luque I, Gomez-Gascon L, et al. Reduced susceptibility of Salmonella typhimurium strains to oregano essential oil and enrofloxacin: an in vitro assay. Foodborne Pathog Dis. (2020) 17:29–34. doi: 10.1089/fpd.2019.2635
34. Wang LC, Zhang TT, Wen C, Jiang ZY, Wang T, Zhou YM. Protective effects of zinc-bearing clinoptilolite on broilers challenged with Salmonella pullorum. Poult Sci. (2012) 91:1838–45. doi: 10.3382/ps.2012-02284
35. Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. (2008) 86:E140–148. doi: 10.2527/jas.2007-0459
36. Dandan H, Xu Z, Jia C, Xiaonan X, Dongyu L, Le W, et al. Effects of oil origanum on performance and immunity of laying hens. Chin J Vet Sci. (2017) 37:1121–7. doi: 10.16303/j.cnki.1005-4545.2017.06.26
37. Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult Sci. (2013) 92:2059–69. doi: 10.3382/ps.2012-02685
38. Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbio. (2004) 94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022
39. Xu J, Zhou F, Ji BP, Pei RS, Xu N. The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol. (2008) 47:174–9. doi: 10.1111/j.1472-765X.2008.02407.x
Keywords: oregano essential oil, S. pullorum, S. gallinarum, body weight, antibiotic alternatives
Citation: Xu Z, Wang C, Li C, Wang M, Chen W, Zhou C and Wei P (2022) The effect of oregano essential oil on the prevention and treatment of Salmonella pullorum and Salmonella gallinarum infections in commercial Yellow-chicken breeders. Front. Vet. Sci. 9:1058844. doi: 10.3389/fvets.2022.1058844
Received: 30 September 2022; Accepted: 02 December 2022;
Published: 21 December 2022.
Edited by:
Thi Thu Hao Van, RMIT University, AustraliaReviewed by:
Ilias Giannenas, Aristotle University of Thessaloniki, GreeceCopyright © 2022 Xu, Wang, Li, Wang, Chen, Zhou and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Wei,  cGluZ3dlaThAMTI2LmNvbQ==
cGluZ3dlaThAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.