
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 29 November 2022
Sec. Parasitology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1053270
This article is part of the Research Topic Pathobiology, Epidemiology and Control of Protozoan Diseases of Veterinary Importance View all 8 articles
 Hui Cao1†
Hui Cao1† Wen-Bin Zheng1†
Wen-Bin Zheng1† Yu Wang1
Yu Wang1 Wen-Wei Gao1
Wen-Wei Gao1 Qing Liu1
Qing Liu1 Xing-Quan Zhu1,2
Xing-Quan Zhu1,2 Yu-Ping Lei3
Yu-Ping Lei3 Bayaer Tumen3*
Bayaer Tumen3* Hong-Yu Song1*
Hong-Yu Song1*Neospora caninum is an obligate intracellular parasitic protozoan that can cause abortions in cattle and pose considerable economic losses to the cattle industry. As a major livestock province, little is known of N. caninum infection in cattle in Shanxi Province, north China. In order to investigate the seroprevalence of N. caninum in cattle in Shanxi Province, 978 cattle serum samples were collected from 11 cities in three representative geographical locations in Shanxi Province, and the N. caninum-specific IgG antibodies were examined using an indirect enzyme linked immunosorbent assay (ELISA) kit commercially available. The results showed that 133 of the 978 examined cattle serum samples (13.60%, 95% CI = 11.45–15.75) were positive for N. caninum antibodies, and the seroprevalence in different cities ranged from 0 to 78.89%. The geographical location and management mode were the risk factors associated with N. caninum infection in cattle herds in Shanxi Province. Cattle in Northern and Central Shanxi Province as well as cattle whose management mode is that of large-scale cattle farming companies are more susceptible to N. caninum infection. This was the first large-scale survey of N. caninum seroprevalence and assessment of associated risk factors in cattle in Shanxi Province, which provided baseline information for the prevention and control of N. caninum infection in cattle in Shanxi Province, north China.
Neospora caninum is an obligate intracellular parasitic protozoan that has a complex life cycle and infects a number of warm-blooded animals (1, 2). N. caninum can complete sexual reproduction in canids which is the definitive hosts of this parasite; while many important domestic and wild animals, such as cattle, sheep and camels, can serve as intermediate hosts (3–5). Because dogs often can move freely in the cattle herds, the risk of cattle infection with N. caninum is high, and the seroprevalence of N. caninum can be as high as 90% in some cattle herds (6, 7). N. caninum infection can cause abortion, stillbirths in cattle or neurological disorders in newborn calves (8, 9). The infection has considerable negative economic impacts on the cattle industry, due to abortions, premature cow culling and reduced milk production (10, 11).
Neosporosis is distributed worldwide, which has attracted the attention by the global livestock industry (12, 13). According to reports, the seroprevalence of N. caninum was 21.03% in dairy cows in Northern Greece, and 26.20% in cattle in Mongolia (14, 15). Likewise, neosporosis in cattle has also been widely reported in China. A recent review showed that the average seroprevalence of N. caninum was 13.69% in cattle herds in China, and the prevalence of N. caninum infection in cattle has been reported in many provinces of China (16). But so far, information on N. caninum infection in cattle in Shanxi Province is very limited, only two articles mentioned that the seroprevalence of N. caninum in cattle in Shanxi Province was 23.08% (6/26). Since these two studies used a very small number of samples (n = 26) from Shanxi and were published in 2007 (17, 18), these preliminary results are not sufficient for the correct assessment of N. caninum infection in cattle in Shanxi Province. The latest data from the Shanxi Provincial Bureau of Statistics, China showed that approximately 1,174,300 cattle are raised in Shanxi Province (19). Considering the economic importance of neosporosis in cattle industry and the lack of specific drugs and vaccines against N. caninum, it is particularly important to conduct an epidemiological survey of N. caninum infections and assess the risk factors associated with its seroprevalence in cattle in Shanxi Province.
Therefore, in order to investigate the seroprevalence of N. caninum infection in cattle in Shanxi Province, the indirect enzyme linked immunosorbent assay (ELISA) was used to examine anti-N. caninum antibodies in cattle serum samples collected from 11 cities of Shanxi Province, and to analyze the potential risk factors of N. caninum infection, with the objective of providing baseline data for executing effective measures to prevent and control N. caninum infection in cattle in Shanxi Province.
This study covered 40 cattle farms in 11 administrative cities in Shanxi Province, including three cities in Northern Shanxi, four cities in Central Shanxi and four cities in Southern Shanxi. Shanxi Province (34°36'-40°44'N, 110°15'-114°32'E) is located in north China and has a temperate continental monsoon climate with four distinct seasons and large temperature differences between the north and the south, with an average annual temperature of 3°C~14°C (Figure 1).
Figure 1. Map showing the geographical locations in Shanxi Province, north China. Yellow, pink and green colors represent the geographical location of Northern Shanxi (Datong, Shuozhou, Xinzhou), Central Shanxi (Taiyuan, Lvliang, Jinzhong, Yangquan) and Southern Shanxi (Changzhi, Jincheng, Linfen, Yuncheng), respectively. Red star mark: Beijing, the capital of China. Double circles: Eleven cities in Northern, Central and Southern Shanxi.
978 serum samples were randomly collected from cattle in 40 cattle farms in 11 cities in three geographical locations of Shanxi Province by blind sampling in November 2020; among which 267, 356 and 355 samples were collected from Northern Shanxi Province (Datong, Shuozhou and Xinzhou), Central Shanxi Province (Taiyuan, Lvliang, Jinzhong and Yangquan), and Southern Shanxi Province (Changzhi, Jincheng, Linfen and Yuncheng), respectively (Figure 1). Of the 978 cattle serum samples, 563 samples were from cattle in household cattle farms, 100 samples from cattle farming cooperatives and 315 samples from large-scale cattle farming companies. After collecting blood sample from each cattle, the serum was separated and transported to the laboratory, and stored at−40°C until analysis.
The presence of N. caninum-specific IgG antibodies was detected using a commercial indirect ELISA kit (ID Screen® Neospora caninum Indirect, Innovative Diagnostics, France), according to the manufacturer's specifications. Briefly, the reagents and serum samples were returned to room temperature (20°C), and then were shaken gently. The optical densities (ODs) of each sample were measured at 450 nm using an absorbance reader (CMax Plus, Molecular Devices, USA). The results were considered valid when the mean ODs of the positive control (ODPC) is >0.350, and the ratio between the ODPC and the ODs of the negative control (ODNC) is >3. The value of S/P% per sample is calculated by [(OD sample-ODNC)/ODPC-ODNC] × 100%. Samples with S/P% ≥ 50% or ≤ 40% were considered positive or negative, respectively, and 40 < S/P% < 50% were considered as “suspicious samples” and were retested. The samples that were negative or positive after retesting were considered as negative or positive samples, respectively; while the samples that were still suspicious after retesting were considered as positive samples.
Chi-square test was performed on the seroprevalence and potential risk factors (geographical location and management mode) of N. caninum infection in cattle herds in Shanxi Province using SPSS 26.0 software (Chicago, USA). The odds ratios (ORs) and their 95% confidence interval (95% CI) of each risk factor were analyzed in this study. P < 0.05 was considered statistically significant.
In this study, 133 (13.60%, 95% CI = 11.45–15.75) of the examined 978 cattle serum samples from the 40 cattle farms were detected to be positive for anti-N. caninum antibodies by ELISA, with seroprevalence in different cities ranging from 0 to 78.89% (Table 1). Among the 133 positive cattle serum samples, 47 samples were from Northern Shanxi (17.60%, 95% CI = 13.03–22.17), 86 samples were from Central Shanxi (24.16%, 95% CI = 19.71–28.60), and 0 sample was from Southern Shanxi (0%); 54 samples were from household cattle farms (9.59%, 95% CI = 7.16–12.02), 0 sample was from cattle farming cooperatives (0%), and 79 samples were from large-scale cattle farming companies (25.08%, 95% CI = 20.29–29.87; Table 2).
Regarding the risk factors associated with N. caninum seroprevalence in cattle in Shanxi Province, the results of the statistics are summarized in Table 2. Geographical location (P < 0.001) and management mode (P < 0.001) were revealed as risk factors for N. caninum infection in cattle herds in Shanxi Province. Considering the geographical locations of the cattle farms, the seroprevalence of N. caninum in cattle was higher in Central Shanxi than in Northern Shanxi and the difference between them was statistically significant (OR = 1.49; P < 0.001). Notably, cattle herds in Southern Shanxi were free from N. caninum infections; even after excluding Southern Shanxi, the seroprevalence of N. caninum in cattle was higher in Central Shanxi than in Northern Shanxi and the difference between them was statistically significant (OR = 1.49; P < 0.05; Supplementary Table 1). When considering the different management modes of the cattle farms, the seroprevalence of N. caninum was higher in cattle in large-scale cattle farming companies than in household cattle farms and the difference between them was statistically significant (OR = 3.16; P < 0.001). After excluding cattle farming cooperatives, the prevalence of N. caninum was higher in cattle in large-scale cattle farming companies than in household cattle farms and the difference between them was extremely significant statistically (OR = 3.16; P < 0.001; Supplementary Table 1). In addition, to explore the relationship between regions and management modes, the seroprevalence for the three management modes between different cities in each region was calculated in Table 3. The results showed that the N. caninum seroprevalence was higher in cattle in large-scale cattle farming companies in Northern Shanxi and Central Shanxi, while cattle in household cattle farms had a higher prevalence if they were in Taiyuan, Central Shanxi, and lower in other household cattle farms; while cattle farming cooperatives were unique to Central Shanxi and all had zero N. caninum seroprevalence (Table 3).
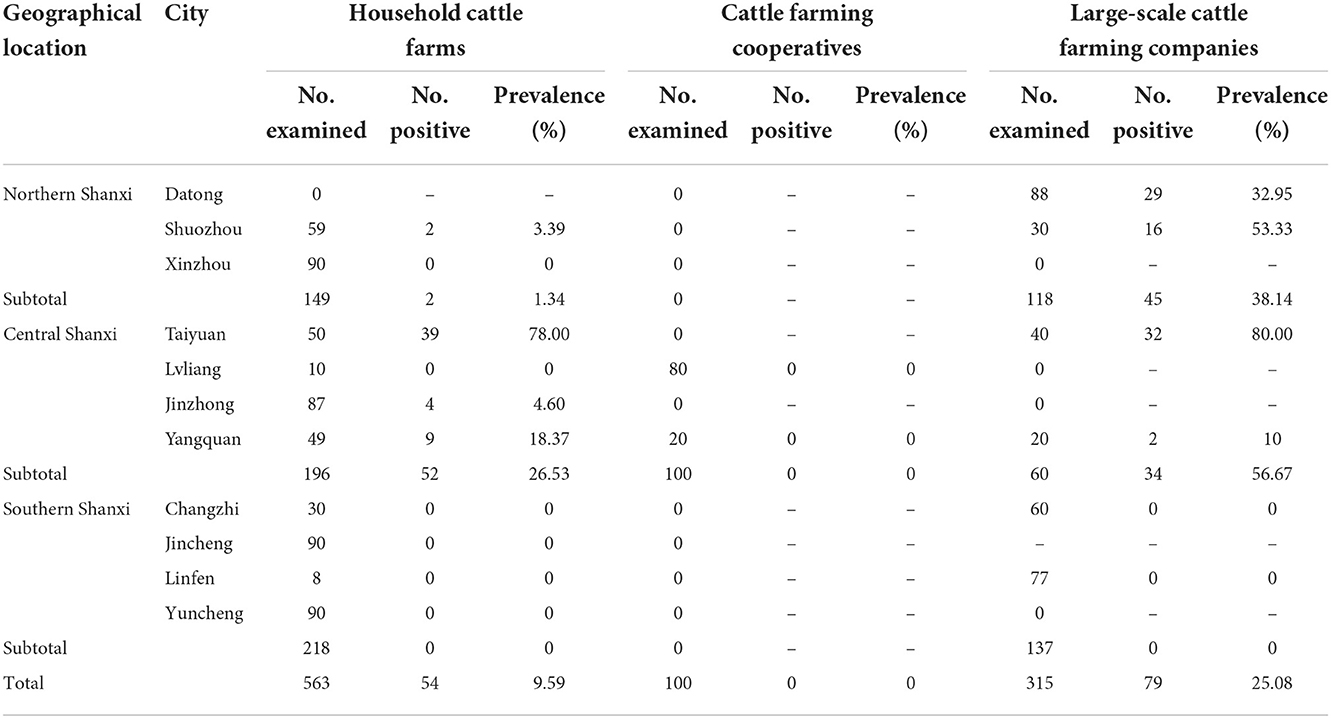
Table 3. The seroprevalence of N. caninum in cattle for the three management modes between different cities in each region in Shanxi Province.
N. caninum has been discovered for more than 30 years since the late 1980s (20). Due to its global distribution and efficient transmission, it seriously affects the cattle industry and leads to significant economic losses. Therefore, the prevention and control of N. caninum in cattle is very important. Vaccination has been recognized as the most desirable option to control the disease in cattle herds, however, there are no commercially available vaccines for the prevention of N. caninum (21). Therefore, the impact of the disease can only be reduced through biosecurity and management measures, and differentiating between infected and uninfected animals becomes the basis for disease management. Many diagnostic methods have been developed, such as PCR assays for detection of parasite DNA and serological tests for specific antibodies (8). N. caninum infection is usually diagnosed by serology in live cattle, indirect diagnoses through serological testing have great advantages, and ELISAs are used for high-throughput screening (11). Previous studies have shown that serological detection of N. caninum infection is accurate in examining tachyzoite-specific antibodies in cattle, the commercially available kits also have good test agreement and share high performance in terms of specificity and sensitivity (11, 22). This study therefore investigated the seroprevalence of N. caninum infection in cattle in Shanxi Province using a commercial indirect ELISA kit.
The seroprevalence of N. caninum in cattle varies considerably between different countries, as well as different regions and cattle farms in the same country; for example, the seroprevalence of N. caninum in Serbia and Iranian cattle herds was 7.20 and 23.60%, respectively (23, 24). The seroprevalence of N. caninum in cattle herds detected in different regions of Brazil ranged from 9.1 to 97.2% (25, 26). Mongolia and Vietnam border on the north and south of China, respectively. In Mongolia, the overall seroprevalence of N. caninum in cattle was 26.2%, sex was revealed as an important risk factor of N. caninum infection, and the N. caninum seroprevalence may be affected by climate and geographical conditions (15). In Southern Vietnam, the N. caninum seroprevalence varied between 16 and 53% in the state herds, and the prevalence of N. caninum was higher in the herds that had imported cows than in the herds that only had locally bred cows (27). In China, the average seroprevalence of N. caninum was 13.69% in cattle herds, and the presence or absence of pregnancy and the number of pregnancies is considered as risk factors (16). In the present survey, the average seroprevalence of N. caninum in cattle herds in Shanxi was 13.60% detected by ELISA, which was similar to the average seroprevalence in China. By comparing seroprevalence of N. caninum in cattle in Shanxi Province with that in neighboring provinces, we found that the seroprevalence of N. caninum in cattle in Shanxi Province was similar to that in Inner Mongolia Autonomous Region (IMAR) (15.88%) (28), but lower than that in Henan Province (41.20%) (29) and Hebei Province (37.34%) (30). Differences in seroprevalence of N. caninum may be related to specific characteristics of each region, such as climatic conditions, production systems, the presence of definitive hosts and herds health management, or to different study designs and diagnostic methods (31, 32).
The distance between the Northern and Southern Shanxi Province is more than 680 kilometers, with the large temperature differences and different local conditions and customs, which may be related to different N. caninum seroprevalence among cattle herds. This study showed that the seroprevalence of N. caninum in cattle in different cities of Shanxi Province varied from 0% in six cities to 32.95% in Datong in Northern Shanxi and 78.89% in Taiyuan in Central Shanxi. The geographical location was revealed as a risk factor for N. caninum infection in cattle in Shanxi Province (P < 0.001; Table 2). Studies have shown that sex, age, whether or not the cattle are pregnant, and the management mode of the farm are potential risk factors for N. caninum seroprevalence in cattle (16, 33). Unfortunately, the collection of cattle serum samples in the present study was done by blind sampling, thus data about the sex and age of the sampled cattle, and whether or not the cattle sampled was pregnant, were not available; so we explored whether management mode was a risk factor for N. caninum infection in cattle in Shanxi Province.
The results showed that cattle in household cattle farms (9.59%, 95% CI = 7.16–12.02) and large-scale cattle farming companies (25.08%, 95% CI = 20.29–29.87) had a higher seroprevalence of N. caninum than that in cattle farming cooperatives (0%). Compared to household cattle farms, the risk of N. caninum infection was more than three times higher (OR = 3.16, 95% CI = 2.16–4.61) for cattle in large-scale cattle farming companies. These results suggested that the management mode was a risk factor for N. caninum infection in cattle in Shanxi Province (P < 0.001; Table 2). Cattle in large-scale cattle farming companies may have higher N. caninum prevalence and wide distribution if sporulated oocysts are present in the breeding environment due to high breeding density, intensive management and mode of transmission routes (horizontal or vertical transmission). Therefore, we suggest that large-scale cattle farming companies should optimize the breeding environment, eliminate positive cattle in time and block its transmission in the herds. While household cattle farms are profit-oriented, the farm owners will be more responsible in the breeding process. However, household cattle farms have certain disadvantages in declaring subsidies and other aspects due to their small scale, so it may be difficult to carry out intervention measures due to limited funding, thus leading to the presence of N. caninum infection in cattle in household cattle farms, but the prevalence is not high and varies among household cattle farms. In addition, cattle farming cooperatives are favorably supported by governments of different levels with higher subsidy funds, thus the animal feeding management, medication and immunization procedures are more formal, which may be the reason for the low prevalence of N. caninum infection in cattle in cattle farming cooperatives.
Interestingly, by comparing the seroprevalence of N. caninum in cattle in three management modes in different cities of different regions (Table 3), we found that even though cattle in Central Shanxi (24.16%) has a higher seroprevalence of N. caninum than in Northern Shanxi (17.60%), most of the positive serum samples were from either household cattle farms or large-scale cattle farming companies from Taiyuan (71/86, 82.56%), where a large population (approximately 5 million), a booming pet market and ubiquitous presence of stray dogs may be responsible for the high rate of N. caninum infection in local cattle herds. Among the Northern Shanxi, the seroprevalence of N. caninum was higher in cattle in large-scale cattle farming companies, accounting for 95.74% (45/47) of the N. caninum-positive cattle serum samples in the whole Northern Shanxi, which may be due to that Datong and Shuozhou directly border the Inner Mongolia grassland, and its living habits are closer to the IMAR. IMAR is a pastoral area with a large number of shepherd dogs, so it is easier for N. caninum to spread in cattle herds, and the seroprevalence of N. caninum in IMAR cattle herds was 15.88% (28). Therefore, we suggest that the prevention and control of N. caninum on cattle farms in Taiyuan, Datong and Shuozhou should be strengthened and the government should increase the proportion of cattle farming cooperatives. In addition, future large-scale investigations should be conducted in cattle in Southern Shanxi to reveal the prevalence of N. caninum in cattle, so as to lay the foundation for the control of N. caninum on cattle farms.
The average seroprevalence of N. caninum in cattle in Shanxi Province was 13.60% (133/978), and geographical location and management mode were revealed as the risk factors related to N. caninum infection in cattle herds in Shanxi Province, north China. This was the first report of a large-scale investigation of the seroprevalence and risk factors for N. caninum in cattle herds in Shanxi Province, which not only extended the N. caninum seroprevalence data in cattle, but also provided baseline data to prevent and control N. caninum infection in cattle in Shanxi Province.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Experimental Animal Ethics Committee of Shanxi Agricultural University (Approval No. 2019IACUCSXAU002A01).
BT and H-YS conceived and designed the experiments. HC and W-BZ performed the experiments, analyzed the data, and wrote the paper. YW, Y-PL, and QL participated in the collection of serum samples. W-WG and QL participated in the implementation of the study. H-YS, QL, BT, and X-QZ critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Project support was provided by Fund for Shanxi 1331 Project (Grant No. 20211331-13), the Special Research Fund of Shanxi Agricultural University for High-level Talents (Grant No. 2021XG001), and the Yunnan Expert Workstation (Grant No. 202005AF150041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1053270/full#supplementary-material
1. Sokol-Borrelli SL, Coombs RS, Boyle JP. A comparison of stage conversion in the coccidian apicomplexans Toxoplasma gondii, Hammondia hammondi, and Neospora caninum. Front Cell Infect Microbiol. (2020) 10:608283. doi: 10.3389/fcimb.2020.608283
2. Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. (2007) 20:323–67. doi: 10.1128/CMR.00031-06
3. Amdouni Y, Rjeibi MR, Awadi S, Rekik M, Gharbi M. First detection and molecular identification of Neospora caninum from naturally infected cattle and sheep in North Africa. Transbound Emerg Dis. (2018) 65:976–82. doi: 10.1111/tbed.12828
4. Gondim LF. Neospora caninum in wildlife. Trends Parasitol. (2006) 22:247–52. doi: 10.1016/j.pt.2006.03.008
5. Alves Sinnott F, Da Silva Leal K, De Oliveira Silva MT, Barros de. Pinho R, Pappen F, da Rosa Farias NA, et al. An indirect ELISA for neosporosis: associating recombinant Neospora caninum proteins NcSRS2 and NcSAG1. Vet Parasitol. (2020) 281:109101. doi: 10.1016/j.vetpar.2020.109101
6. Olum MO, Mungube EO, Njanja J, Kidali J, Njenga E, Maichomo M, et al. Seroprevalence of canine neosporosis and bovine viral diarrhoea in dairy cattle in selected regions of Kenya. Transbound Emerg Dis. (2020) 67:154–8. doi: 10.1111/tbed.13429
7. Dubey JP, Schares G. Neosporosis in animals-the last five years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
8. Sinnott FA, Monte LG, Collares TF, Silveira RM, Borsuk S. Review on the immunological and molecular diagnosis of neosporosis (years 2011–2016). Vet Parasitol. (2017) 239:19–25. doi: 10.1016/j.vetpar.2017.04.008
9. McAllister MM. Diagnosis and control of bovine neosporosis. Vet Clin North Am Food Anim Pract. (2016) 32:443–63. doi: 10.1016/j.cvfa.2016.01.012
10. Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle-the billion dollar question. Int J Parasitol. (2013) 43:133–42. doi: 10.1016/j.ijpara.2012.10.022
11. Guido S, Katzer F, Nanjiani I, Milne E, Innes EA. Serology-based diagnostics for the control of bovine neosporosis. Trends Parasitol. (2016) 32:131–43. doi: 10.1016/j.pt.2015.11.014
12. Basso W, Schares S, Minke L, Bärwald A, Maksimov A, Peters M, et al. Microsatellite typing and avidity analysis suggest a common source of infection in herds with epidemic Neospora caninum-associated bovine abortion. Vet Parasitol. (2010) 173:24–31. doi: 10.1016/j.vetpar.2010.06.009
13. Khan A, Fujita AW, Randle N, Regidor-Cerrillo J, Shaik JS, Shen K, et al. Global selective sweep of a highly inbred genome of the cattle parasite Neospora caninum. Proc Natl Acad Sci U S A. (2019) 116:22764–73. doi: 10.1073/pnas.1913531116
14. Lefkaditis M, Mpairamoglou R, Sossidou A, Spanoudis K, Tsakiroglou M. Neospora caninum, a potential cause of reproductive failure in dairy cows from northern Greece. Vet Parasitol Reg Stud Reports. (2020) 19:100365. doi: 10.1016/j.vprsr.2019.100365
15. Pagmadulam B, Myagmarsuren P, Fereig RM, Igarashi M, Yokoyama N, Battsetseg B, et al. Seroprevalence of Toxoplasma gondii and Neospora caninum infections in cattle in Mongolia. Vet Parasitol Reg Stud Reports. (2018) 14:11–7. doi: 10.1016/j.vprsr.2018.08.001
16. Ying Z, Zhu ZF, Yang X, Liu J, Liu Q. Prevalence and associated risk factors of Neospora caninum infection among cattle in mainland China: a systematic review and meta-analysis. Prev Vet Med. (2022) 201:105593. doi: 10.1016/j.prevetmed.2022.105593
17. Liu J, Yu J, Wang M, Liu Q, Zhang W, Deng C, et al. Serodiagnosis of Neospora caninum infection in cattle using a recombinant tNcSRS2 protein-based ELISA. Vet Parasitol. (2007) 143:358–63. doi: 10.1016/j.vetpar.2006.08.034
18. Yu J, Xia Z, Liu Q, Liu J, Ding J, Zhang W. Seroepidemiology of Neospora caninum and Toxoplasma gondii in cattle and water buffaloes (Bubalus bubalis) in the people's republic of China. Vet Parasitol. (2007) 143:79–85. doi: 10.1016/j.vetpar.2006.07.031
19. Shanxi Provincial Bureau of Statistics. Available online at: http://tjj.shanxi.gov.cn/tjsj/tjnj/nj2021/zk/indexch.htm [9-11, cattle at Year (2020)-end: http://tjj.shanxi.gov.cn/tjsj/tjnj/nj2021/zk/html/09-11.jpg]
20. Reichel MP, Wahl LC, Ellis JT. Research into Neospora caninum-what have we learnt in the last thirty years? Pathogens. (2020) 9:505. doi: 10.3390/pathogens9060505
21. Goodswen SJ, Kennedy PJ, Ellis JT. Discovering a vaccine against neosporosis using computers: is it feasible? Trends Parasitol. (2014) 30:401–11. doi: 10.1016/j.pt.2014.06.004
22. Alvarez-García G, García-Culebras A, Gutiérrez-Expósito D, Navarro-Lozano V, Pastor-Fernández I, Ortega-Mora LM. Serological diagnosis of bovine neosporosis: a comparative study of commercially available ELISA tests. Vet Parasitol. (2013) 198:85–95. doi: 10.1016/j.vetpar.2013.07.033
23. Klun I, Cirković V, Maletić M, Bradonjić S, Djurković-Djaković O. Seroprevalence of Neospora caninum infection and associated risk factors in dairy cattle in Serbia. Parasitol Res. (2019) 118:1875–83. doi: 10.1007/s00436-019-06307-9
24. Ansari-Lari M. Bovine neosporosis in Iran: a systematic review and meta-analysis. Prev Vet Med. (2020) 176:104913. doi: 10.1016/j.prevetmed.2020.104913
25. Azevedo Filho PCG, Ribeiro-Andrade M, Santos JFD, Reis ACD, Pinheiro Júnior JW, Valença S, et al. Neospora caninum infection in cattle in the state of Amazonas, Brazil: seroprevalence, spatial distribution and risk factors. Rev Bras Parasitol Vet. (2021) 30:e020820. doi: 10.1590/s1984-296120201083
26. Reichel MP, McAllister MM, Nasir A, Moore DP. A review of Neospora caninum in water buffalo (Bubalus bubalis). Vet Parasitol. (2015) 212:75–9. doi: 10.1016/j.vetpar.2015.08.008
27. Duong MC, Alenius S, Huong LT, Björkman C. Prevalence of Neospora caninum and bovine viral diarrhoea virus in dairy cows in Southern Vietnam. Vet J. (2008) 175:390–4. doi: 10.1016/j.tvjl.2006.01.016
28. Sun WW, Meng QF, Cong W, Shan XF, Wang CF, Qian AD. Herd-level prevalence and associated risk factors for Toxoplasma gondii, Neospora caninum, Chlamydia abortus and bovine viral diarrhoea virus in commercial dairy and beef cattle in eastern, northern and northeastern China. Parasitol Res. (2015) 114:4211–8. doi: 10.1007/s00436-015-4655-0
29. Qian W, Wang T, Yan W, Zhang M, Han L, Xue R, et al. Seroprevalence and first multilocus microsatellite genotyping of Neospora caninum in dairy cattle in Henan, central China. Vet Parasitol. (2017) 244:81–4. doi: 10.1016/j.vetpar.2017.07.022
30. Ma L, Li S, Zhang Y, Wen Z. Seroprevalence of Toxoplasma gondii and Neospora caninum in dairy cows in Hebei province, China. Anim Biotechnol. (2021) 32:451–3. doi: 10.1080/10495398.2020.1714636
31. Ghalmi F, China B, Ghalmi A, Hammitouche D, Losson B. Study of the risk factors associated with Neospora caninum seroprevalence in Algerian cattle populations. Res Vet Sci. (2012) 93:655–61. doi: 10.1016/j.rvsc.2011.12.015
32. Corbellini LG, Smith DR, Pescador CA, Schmitz M, Correa A, Steffen DJ, et al. Herd-level risk factors for Neospora caninum seroprevalence in dairy farms in southern Brazil. Prev Vet Med. (2006) 74:130–41. doi: 10.1016/j.prevetmed.2005.11.004
Keywords: Neospora caninum, seroprevalence, cattle, ELISA, Shanxi Province, China
Citation: Cao H, Zheng W-B, Wang Y, Gao W-W, Liu Q, Zhu X-Q, Lei Y-P, Tumen B and Song H-Y (2022) Seroprevalence of Neospora caninum infection and associated risk factors in cattle in Shanxi Province, north China. Front. Vet. Sci. 9:1053270. doi: 10.3389/fvets.2022.1053270
Received: 25 September 2022; Accepted: 14 November 2022;
Published: 29 November 2022.
Edited by:
Ragab Makhlouf Mahmoud Fereig, South Valley University, EgyptReviewed by:
Mosaab Omar, Qassim University, Saudi ArabiaCopyright © 2022 Cao, Zheng, Wang, Gao, Liu, Zhu, Lei, Tumen and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bayaer Tumen, dHVtZW5nYmF5YWVyQDE2My5jb20=; Hong-Yu Song, c29uZ2hvbmd5dTk1QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.