
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci. , 10 November 2022
Sec. Animal Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1049731
This article is part of the Research Topic Nutrition and Management of Animals We Keep as Companions, volume II View all 21 articles
Raw meat-based diets (RMBDs) or sometimes described as biologically appropriate raw food (BARFs) are gaining in popularity amongst dog and cat owners. These pet guardians prefer their animals to eat minimally processed and more “natural” foods instead of highly heat-processed diets manufactured with synthetic preservatives. The market for RMBDs for dogs and cats is estimated at $33 million in the United States. This figure is likely underestimated because some pet owners feed their animals raw diets prepared at home. Despite their increasing demand, RMBDs have been plagued with numerous recalls because of contamination from foodborne pathogens like Salmonella, E. coli, or Campylobacter. Existing literature regarding mitigation strategies in RMBD's for dogs/cats are very limited. Thus, a comprehensive search for published research was conducted regarding technologies used in meat and poultry processing and raw materials tangential to this trade (e.g., meats and poultry). In this review paper, we explored multiple non-thermal processes and GRAS approved food additives that can be used as potential antimicrobials alone or in combinations to assert multiple stressors that impede microbial growth, ultimately leading to pathogen inactivation through hurdle technology. This review focuses on use of high-pressure pasteurization, organic acidulants, essential oils, and bacteriophages as possible approaches to commercially pasteurize RMBDs effectively at a relatively low cost. A summary of the different ways these technologies have been used in the past to control foodborne pathogens in meat and poultry related products and how they can be applied successfully to impede growth of enteric pathogens in commercially produced raw diets for companion animals is provided.
The domestication of the modern dog (Canis lupus familiaris) has resulted in a remarkable shift from the diet that its ancestor the wolf derived sustenance through scavenging and hunting small prey (1). The 2021–2022 American Pet Products Association (APPA) national pet owners survey reported that 70% of United States households owned a pet, which equates to 90.5 million homes (2). Because of anthropomorphism, there is an increasing number of pet owners who consider their animal a family member (3–6). Thus, the shift in human dietary choices due to increasing health consciousness are reflected in the ingredients pet owners prefer their animal consumed, thus there has been an increase in demand in foods that are considered “raw” and/or minimally processed (1, 7–9).
Raw meat-based diets (RMBDs) are a subset of minimally processed commercial diets (MPCD) or minimally processed home diets (MPHD) for companion animals and consist of raw or uncooked proteins sourced from animals such as lamb, pork, poultry, beef, venison, organ meats or offal, and supplemented with vegetables, tubers, dairy, or eggs (10, 11). However, the scope of this review will focus on RMBDs that are manufactured and marketed commercially in fresh and frozen forms. The market for MPCD diets is estimated at $120 million (11) of which RMBDs are a subcategory with estimates exceeding $33 million as of 2019. The feeding of companion animals with RMBDs is becoming increasingly popular because pet owners perceive these diets as “natural” and therefore presumed to provide additional health benefits to their animals, including but not limited to improved oral health, skin and coat compared to when these animals are fed conventionally heat sterilized foods manufactured through canning or extrusion (12–14). The shift toward RMBDs has resulted from concern that commercially available pet foods are over cooked when they are produced by extrusion (kibbles), baking (treats), or through canning (wet loaf and chunks and gravy styles). All undergo extremes in heat treatments during manufacturing to increase digestibility and assure microbial safety. However, these high temperatures are associated with increased degradation of some nutrients and formation of undesirable and potentially harmful compounds such as advanced glycation products (AGEs) (15–19). This tends to reinforce the argument for raw, and/or minimally processed pet food products (20–22). Presuming of course that safety can be assured by other means.
Typically, RMBDs are formulated with proteins from chicken, beef, lamb, duck, veal, and venison, organs like heart or liver, and are supplemented with bones, dairy products, fish, vegetables, fruits, and plant oils (10, 11). Vitamins and trace minerals may be added to these diets to adjust for any micronutrient shortcomings. Characteristically, these ingredients are ground and mixed into a batter and formed into patties, nuggets or placed into trays for commercial sale. Some pet owners prepare RMBDs from their homes because these diets are often expensive and not widely available in stores. Some pet owners opt to prepare these diets themselves because of the mistrust they have for “big” pet food companies due to numerous product recalls associated with aflatoxins and emerging research highlighting the ill health effects associated with animal consumption of AGEs, present in ultra-processed commercial diets (11). Regardless, the goal is that these diets meet the animal's nutrient requirements for amino acids, fatty acids, minerals, and vitamins.
Presuming nutrition can be met, the rest of the focus on these diets is safety and how to reduce microbial contamination by enteric foodborne pathogens such as non-typhoidal Salmonella spp. or Campylobacter that are inherently found in meat and poultry products. This is because raw diets cannot be heat processed, fermented, rendered, purified, extracted, or hydrolyzed by enzymolysis (23), thus leaving few avenues for efficient non-thermal antimicrobial interventions. Moreover, numerous studies have shown that RMBDs produced without adequate kill-steps are important vehicles for the transmission of pathogens to companion animals and to their human owners, during handling of food, or via cross-contamination with contact surfaces (24, 25). Foodborne enteric pathogens such as Salmonella spp., Campylobacter jejuni, Listeria spp., Yersinia spp., and Escherichia coli have been isolated from some commercial RMBDs globally (24, 25). Supplementary Table 1 provides a summary of pet food product recalls and withdrawals that were minimally processed due to contamination with foodborne pathogens reported by the Food and Drug Administration (FDA) from January 2017 to March 2021.
Raw meat-based diets, contaminated with foodborne pathogens have been linked to pathogenesis of certain diseases in pets for instance: Stiver et al. (25) prepared a case report of two cats that were diagnosed with Salmonella gastroenteritis and septicemia after necropsy, having been fed a home prepared RMBD. Morley et al. (24), observed cases of Salmonella enterica infections in a greyhound breeding facility that consumed raw diets and van Dijik et al. (26) reported that a dog fed with wild rabbit (hare) had tested positive for brucellosis. Although most healthy cats and dogs do not get ill from consuming contaminated RMBDs, some remain asymptomatic upon infection, and thus might shed the pathogen into the environment if animal excreta are not appropriately disposed (27, 28). Reports about the transmission of enteric foodborne pathogens from RMBDs to humans are still few with infections widely under-reported (24, 28). The CDC linked four outbreaks of multi-drug resistant Salmonella infections to raw turkey intended for feeding pets (29). Investigations by Public Health England (PHE) of the UK in (30) also linked an outbreak of Shiga toxin producing Escherichia coli (STEC) O157:H7 to contaminated raw pet food. Furthermore, antibiotic resistant strains of Enterobacteriaceae have been isolated from raw meats (beef, poultry, and fish) in retail shops by the World Health Organization (WHO). To corroborate WHO findings, Baede et al. (31); Jans et al. (32) reported that E. coli isolated from RMBDs exhibited similar resistance mechanisms as antimicrobial isolates that had been isolated from food production animals such as cattle, and pigs. However, it is worth noting that the transmission of enteric foodborne pathogens from RMBDs and companion animals fed these diets is a complex phenomenon to describe. This is because companion animals like dogs have a unique relationship with their environment and thus may nibble at objects, wild animal excreta or dead animal matter contaminated with any pathogen during normal daily activities not associated with the meal, thus complicating the process of tracking and analyzing the risk factors associated with RMBDs. Therefore, the purpose of this review is to investigate the different non-thermal methods of microbial control that have been successfully applied to meat and poultry while exploring alternative ways that these technologies can be employed to control and impede the proliferation of foodborne pathogens in RMBDs for companion animals.
The choice of ingredients and the process of manufacturing RMBDs results into products that are highly perishable because they have a relatively high pH (5.5–6.5) and water activity of >0.98 (33). Animal and poultry carcasses are natural reservoirs of enteric foodborne pathogens such as Salmonella and E. coli, although muscle from healthy animals is sterile. These pathogens find their way into RMBDs because upstream harvesting techniques do not preclude fecal pathogens completely (34) and most processes do not involve an efficient pasteurization process and rely on the microbial quality of their ingredients and freezing/refrigeration of the product to control microbial growth during transportation or storage (10, 33). Contamination of RMBDs by foodborne pathogens such as Salmonella, Campylobacter, and enterohemorrhagic E. coli is not only a public health threat, but it leads to multiple product recalls annually which are also a significant financial loss to pet food manufacturers. The U.S. Department of Agriculture, Food Safety Inspection Service (USDA-FSIS) considers foodborne pathogens as adulterants in human foods whereas the FDA, the regulatory body for pet foods effectively have a zero-tolerance policy for enteric pathogens such as Salmonella, Shiga toxin-producing E. coli (STEC) and Listeria monocytogenes in commercial pet food, making the manufacturing and commercialization of RMBDs a herculean task. Discussed below are some of the most feasible non-thermal antimicrobial interventions that can be implemented in commercial pet food manufacturing plants to enhance the microbial safety of RMBDs.
There are several non-thermal pasteurization technologies currently available such as irradiation and ultrasonication that could theoretically be used to pasteurize RMBDs. The pet food industry in the United States relies heavily on high-pressure pasteurization (HPP) as the main technology for microbial inactivation in RMBDs (35). High pressure pasteurization utilizes hydrostatic force derived from the compression of water (or any incompressible fluid) applied to a food product intended for pasteurization (36, 37). The pressure used during HPP ranges between 100 and 1,000 MPa and system temperatures ranges between 4 and 90°C for a short duration (a few seconds or minutes) depending on the microbiological quality of the product being pasteurized (38). Unlike thermal pasteurization, HPP has several benefits in that the pressure is transmitted uniformly across the product, has a low environmental impact (low energy consumption and gaseous emissions), preserves heat labile nutrients like vitamins, pigments, antioxidants, and flavor/volatile compounds (39–41). The demand for clean label, minimally processed human/animal food products is on the rise, and HPP offers an alternative to using extensive heat processing or synthetic food additives to ensure safety and prolong shelf life of a product (38, 41).
High pressure pasteurization technology is a promising antimicrobial intervention strategy currently being employed as a microbial inactivation step to address microbiological hazards and ensure compliance with federal food safety regulations (39). Raw meat-based diets for companion animals utilize raw meat as their main source of protein. This meat does not undergo any pasteurization or cooking step to kill pathogenic or spoilage bacteria which makes HPP a prime candidate for RMBDs. The biological composition of raw meat (high moisture, fat, and protein) makes it highly perishable and an important vehicle for pathogen transmission, thus safety and quality concerns are a high priority (38, 40). When spoilage bacteria contaminate meat, they metabolize low molecular weight compounds like glucose, amino acids, and lactate to produce off-odors, sliminess, and discolorations associated with putrefaction. This putrefaction affects the organoleptic, visual, and nutritional quality of raw pet food. Beyond food safety, it is imperative that the proliferation of these spoilage microbes be controlled.
High pressure pasteurization relies on the principles of Pascal's law which states that compression applied on one part of a liquid medium can be transmitted instantaneously through all parts of the mass being treated (37). The application of high pressure might lead to a slight increase in temperature, and thus the net effect of HPP might be a combination of heat, pH change, or other microbial stressors that could achieve cellular disruption and inactivation. Either way, it is an example of hurdle technology that involves increasing the number of barriers for microorganism growth and survival. In various applications, HPP has been used successfully to inactivate enzymes, and pathogenic and spoilage microorganisms (36, 38, 39). The effects observed on meats treated with high pressure are dependent on the amount of pressure applied, temperature, and the duration (time) of the process. Thus, components of meat sensitive to high pressures such as myosin and myoglobin may limit the application of HPP to fresh meats in favor of fermented, precooked, or restructured meats (38, 39) due to weeping and syneresis.
The mechanism in which high pressure processing kills or mitigates the growth of pathogenic and spoilage bacteria is via cellular injury. This leads to death or impedes the ability of the microbes to repair, resuscitate or grow. The events that lead to cell death by high pressure processing are not well-understood even though several bacterial species have been studied (39). High pressure processing carried out at ambient conditions and hydrostatic pressure held between 300 and 800 MPa showed significant inactivation of vegetative cells. The inactivation of vegetative cells was because of denaturation and unfolding of critical metabolic and physiological enzymes in the cytoplasm, happening simultaneously with cell membrane rapture resulting from phase transitions of the cytoplasmic fluids (36, 38, 39, 41). The method of inactivation is reliant on hydrostatic pressure and intrinsic and extrinsic factors associated with a given microorganism. For instance, synergism has been observed with increased pressure and increased adiabatic temperatures that potentiate the lethality process (39, 41, 42).
There is limited published research investigating the use of HPP to inactivate enteric foodborne pathogens in RMBDs. Thus, to understand applicable research, we conducted a systematic search of the literature. The search was conducted by selecting key words, which were input into selected databases, and then the inclusion/exclusion criterion was established. The key words included “pet food,” “dog,” “RMBD,” “raw meat-based diet,” “raw pet food,” “BARF,” “meat,” “poultry,” “high pressure pasteurization,” “high pressure processing,” “HPP,” “ground meat,” “ground poultry,” “minced,” and “filets.” These key words were applied to Google Scholar and Scopus with no limit to years or language. Original research and review articles investigating the use of HPP in microbial inactivation of RMBDs and comminuted meats were considered in this section. Comminuted meats have an increased surface area for pathogen attachment and proliferation, which decreases the antimicrobial efficacy of HPP treatments. Articles in book chapters, patents, trade publications, extension bulletins, and conference abstracts were excluded from this section.
Pasteurization of meat and poultry using HPP has been demonstrated as an effective process to control spoilage and pathogenic bacteria in meat and poultry products (38, 43–48). These studies demonstrated that whole chunks/cuts of meat were easier to pasteurize as the interior was sterile compared to when comminuted meats were used. Serra-Castelló et al. (49) reported that the antimicrobial efficacy of HPP (450–750 Mpa) against Salmonella inoculated in RMBDs formulated with lactic acid (0–7.2 g/kg) was enhanced as they observed log reductions ranging from 0.76 to 9.0 Log CFU/g depending on different combination of factors (time, pressure, and lactic acid concentrations). However, Simonin et al. (50) conceded that high-pressure treatments above 400 MPa resulted in significant reduction in microbial counts but induced adverse changes in the quality attributes of meat such as color, texture, and accelerated lipid oxidation.
The process of comminuting meat and poultry products increases surface area for microbial attachment and facilitates the redistribution of spoilage and pathogenic bacteria making pasteurization by HPP less effective. For instance, Sheen et al. (51) was able to achieve a 5 Log CFU/g reduction after treating 90 g of ground chicken using HPP at 500 Mpa for 10 min. The log reduction achieved by Sheen et al. (51) was notable but could not be feasibly applied industrially to pasteurize ground chicken. This is because high levels of pressure are required to inactivate pathogens, increasing energy costs which are exacerbated by the low throughput (90 g/10 min) that was reported in this study.
New studies indicate that the antimicrobial efficacy of HPP can be potentiated through combinations with food additives such as organic acids and essential oils to achieve higher log reductions while keeping the required pressure relatively low whilst increasing the shelf-life and safety of the meat (52, 53). Combination of HPP and organic acidulants or essential oils allows for the destruction of sub-lethally injured bacterial cells that often resuscitate and multiply, leading to product recalls. However, HPP operations may require that products be transported in chubs into a “clean room” for reformation, which might result in recontamination of the product during handling, packaging, or transit. Thus, the costs and contamination risks associated with HPP can be avoided through the utilization of generally recognized as safe (GRAS) food additives since they are relatively inexpensive, can be uniformly distributed in a product and have a residual antimicrobial effect which enhances safety of the RMBD products over prolonged periods of time compared to HPP.
There is limited research regarding organic acidulants to control foodborne pathogens in RMBDs. To understand the published work that might be applicable, we conducted a two-part systematic search of the literature. The search was organized by selecting key words, identifying the appropriate databases, and determining inclusion and/or exclusion criterion. Search one key words included “pet food,” “dog,” “RMBD,” “raw meat-based diet,” “raw pet food,” and “BARF,” applied to Google Scholar and Scopus with no limit to years or language. Original research, and review papers with synthesis of new findings were included, and book chapters, patents, trade publications, extension bulletins, and conference abstracts were excluded. Search two key words included “essential oils,” “organic acids,” “bacteriophages,” “ground,” “minced,” “cubed,” “trimmings,” “skin,” “filets,” “beef,” “chicken,” “lamb,” “pork,” and “turkey” was also applied to Google Scholar and Scopus with no limitations to years and language. Only research and review articles evaluating the antimicrobial efficacy of food additives in comminuted meats were considered for this section of the review paper. Cases where whole chunks and comminuted meats were analyzed concurrently were also considered and included in the summary tables. This is because comminuted meats have increased surface area for pathogen attachment, proliferation, and difficulty of decontamination as these mimicked the way RMBDs are manufactured and retailed. Articles in book chapters, patents, trade publications, extension bulletins, and conference abstracts were also excluded from the summary tables.
Organic acidulants are considered by the Code of Federal Regulations (CFR) as generally recognized as safe (GRAS) additives. They have been commonly applied to animal and poultry meats because these acids are relatively inexpensive and have been demonstrated to be efficient antimicrobials (54, 55). Examples of these acids are lactic, citric, succinic, propionic, malic, and acetic and their salts. Most GRAS organic acids do not have a daily (maximum) acceptable intake for humans or animals which increases their applicability. However, their dosage is limited by their negative impact on organoleptic and color attributes of meat and poultry products. Most organic acids are described as weak acids because they do not fully dissociate in water but rather dissociate in a pH-dependent manner (56). When organic acids are added to meats, the pH of the meat is lowered to that which is equal to or lower than the acid dissociation constant (pKa) of the acid, resulting in an increased concentration of protonated acid which is responsible for the antimicrobial activity of the organic acid (56).
There are two primary mechanisms by which organic acids elicit antimicrobial activity: first, by cytoplasmic acidification which impedes ATP production and regulation, and secondly through accumulation of dissociated anions from the organic acid to toxic levels affecting cell physiology and metabolism (56). A transmembrane gradient may be created if the cytoplasmic pH is higher than that of the surrounding membrane leading to diffusion of undissociated acid through the cell membrane. The more alkaline pH of the cytoplasm then encourages the dissociation of the acid yielding anions and protons (57, 58). Accumulation of undissociated acid in the cytoplasm was associated with shifting the cytoplasmic pH which affected enzymatic activity, protein, and nucleic acid synthesis (59). Lactic acid was reported to make the cell membrane more permeable in Gram-negative bacteria, causing a leakage of lipopolysaccharides (60). Alakomi et al. (61), further reported that the chelating properties of citric and malic acids caused an intercalation of the outer membrane of Salmonella. Additionally, mold inhibitors such as sorbic acids contain more hydrophobic compounds and have been reported to increase the permeability of the membranes while interfering with membrane proteins; thus, helping to inhibit mold (62). However, recent research shows that the mechanisms of cellular inhibition or death by organic acidulants are not unilateral as these acids interact with different bacterial membranes and structures creating crippling hurdles that lead to either growth inhibition or inactivation. This would suggest that one mechanism is inadequate to accurately describe the mode of action for a singular organic acid as a food additive for control of spoilage and (or) enteric pathogens in human and animal foods (63).
Generally recognized as safe (GRAS) organic acids such as acetic (21CFR184.1005), citric (21CFR184.1033), and lactic (21CFR184.1061) acids have been approved by the FDA for direct addition to manufactured foods as antimicrobial interventions on meat carcasses and derived cuts pre- and post-chilling at concentrations of <5% (54, 64). Studies regarding the antimicrobial efficacies of these organic acids in the meat industry have been widely conducted and reported. Lactic acid at 150 mM was vacuum infused into boneless/skinless chicken breast cubes that had been inoculated with 108 Log CFU/g of S. Typhimurium and stored at 4°C and a 2.5 log reduction was observed by the 6th day while there were no significant reductions on day 9 and 12 (65). Over et al. (65) further tested different organic acids, citric, malic, and tartaric acids at the same concentration of 150 mM using the same procedure described above. By day 6, the initial inoculum of S. Typhimurium had dropped from its initial concentration of 108 Log CFU/g to just 102 Log CFU/g and were undetectable by day 9. Citric acid was just as effective as acetic acid in the control of S. Typhimurium compared to lactic acid, but its application was limited by the negative impact on the quality attributes of the chicken.
Most studies have evaluated the antimicrobial efficacy of organic acids in ridding surfaces of pathogenic contaminants in both animal and poultry meats because the inside tissues of the meat are considered sterile. However, when these tissues/cuts/trimmings are ground, a new challenge arises when utilizing organic acids as antimicrobial interventions due to an increase in surface area available for microbial attachment and proliferation. For example, Harris et al. (66) inoculated beef trimmings with strains of Salmonella or E. coli O157:H7 at a concentration of 4.0 Log CFU/g and then ground the beef trimmings with two different levels of lactic acid and citric acid at 2.0 and 4.0%, respectively. Microbial analysis of the ground inoculated meats revealed a 2.5 log reduction in E. coli O157:H7 and a 1.5 log reduction of Salmonella after the ground meats were held frozen for a month.
Published studies (Table 1) that utilized organic acids at various doses to control enteric foodborne pathogens in meat/poultry products and the log reductions that occurred in the microbial challenge studies. Overall, the log reductions reported in Table 1 ranged from 0.3 to 4.3 Log CFU/g. When whole chunks/cuts of meat were treated with organic acids, and challenged against a foodborne pathogen, relatively higher log reductions were observed than when ground meats and sausages were used in a study. Also, the types of acidulant used to treat the meat or poultry may affect the log reductions observed, for instance, when Hamby et al. (67) treated beef cubes with both acetic and lactic acid at 1.0%, the latter resulted in a significant reduction of the aerobic plate counts (APC). However, Tamblyn and Conner (73) reported no differences in log reductions when they treated chicken breast skins with malic or tartaric acids at 1.0%. The log reductions were also dependent on the dose of acidulants used as higher concentrations of acid resulted in higher log reductions.
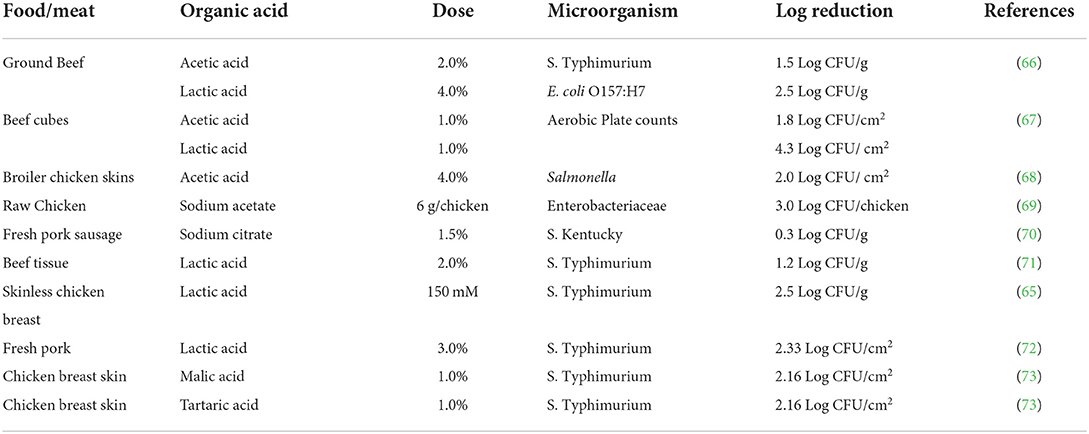
Table 1. Summary of antimicrobial efficacy for organic acids at various doses used to control spoilage and pathogenic bacteria in meat and poultry products.
The broad potential applicability of organic acids in food products to enhance safety and quality is complicated because the high acid and low pH usually alters the sensory properties of meats and poultry. Application of acids directly at higher concentrations alters the quality of meat products resulting in changes in meat color and syneresis perceived as negative by pet owners (82). Consequently, there needs to be a means to slowly deliver the acidulants into the meat product to ensure minimal changes in product quality. For instance, encapsulating organic acidulants with soluble and edible vegetable oil films allows for a “slow release” mechanism, melting and releasing the acid into the meat at a slow and controlled rate, avoiding the acid shock effect observed when direct/raw acids are applied to meat (82). Ultimately, one way to increase the utilization of organic acids in RMBDs as antimicrobial interventions would be through encapsulation.
Essential oils (EOs) are types of phytochemicals produced by aromatic plants primarily for defense against microbial invasion (83–85). These EOs consist of many components, such as terpenes, alcohols, acids, esters, aldehydes, and ketones (83, 86). Of these components, the volatile bioactive components are responsible for the antimicrobial activity of EOs (87). Examples of EOs are thyme, rosemary, cinnamon, eucalyptus oils, etc. Furthermore, certain components of these oils have been extracted and used as antimicrobials such as thymol, eugenol or cinnamaldehyde. As an example, the adaptation of the list of EOs by Bajpai et al. (88) (Figure 1) shows the different chemical structures of components that make up EOs.
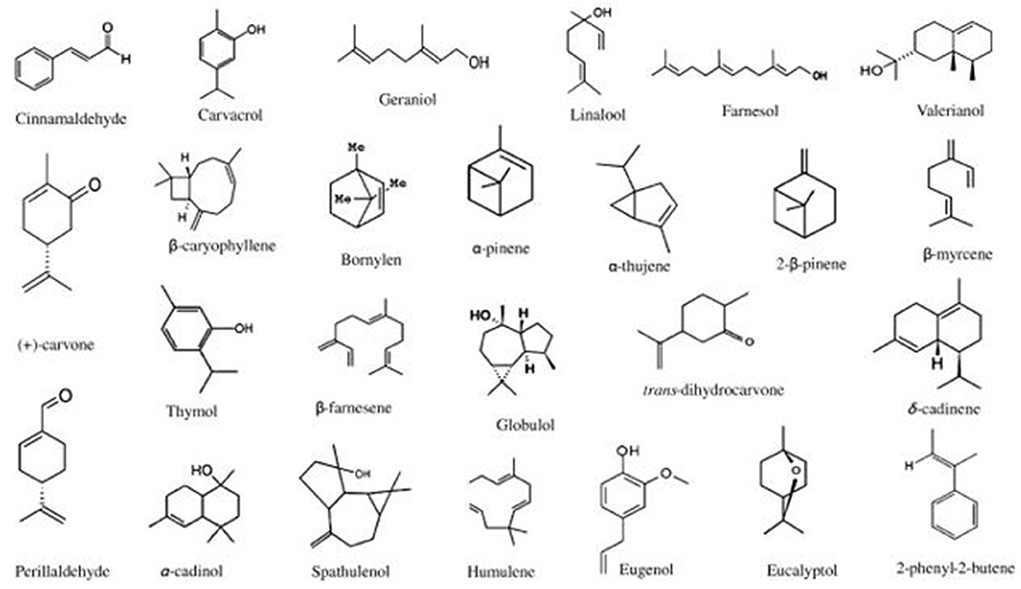
Figure 1. Chemical structures of components of essential oils [adopted from Bajpai et al. (88)].
To achieve microbial decontamination by these EOs, a few theories on their mechanism of action have been proposed. Many studies have demonstrated that components of EOs work synergistically to control the proliferation of microorganisms. Burt (89) reported that the hydrophobicity of the components of EOs increased cell permeability which allowed antimicrobial compounds to enter the cell cytoplasm. Essential oils contain different forms of phenols that disrupt cell membranes increasing permeability, leakage of cell contents, inhibition of ATPases which affects ATP production, and ultimately leading to cell death (88, 90, 91).
Biochemical reactions such as lipid oxidation, autolytic enzymatic spoilage, and microbial spoilage result in significant losses of meat and poultry products along the production chain and have substantial economic and environmental impacts (92, 93). Essential oils (EOs) and their components can be used as a natural alternative to synthetic preservatives and there are several studies that have explored their use in meat and poultry products (76, 84, 88). Spoilage microorganisms that lead to deterioration of meat quality include, Pseudomonas, Acinetobacter, Lactobacillus spp., Enterobacter etc., yeast, and mold (83). These microorganisms' metabolic activity results in the formation of off-flavors, odors, and changes in color which are associated with deterioration in meat products. In addition to spoilage organisms, meat potentially harbor pathogenic enteric microbes such as Salmonella spp., Staphylococcus aureus, Listeria monocytogenes, Clostridium perfringens, Clostridium botulinum, Enterohemorrhagic E. coli, and Campylobacter spp. that are inherent in meat and can be controlled using EOs (91).
Table 2 shows a summary of published studies in which different meat and poultry products were treated with EOs and challenged against enteric foodborne pathogens. The data in Table 2 show the type and dose of EO, the kind of pathogen or serovar challenged against the EO, and the log reduction observed after microbial analysis. The log reductions observed varied from 1 to 3 Log CFU/g when the EOs were challenged against different pathogens or their serovars. However, the difference in antimicrobial efficacies of the EOs observed in the various studies could be attributed to interaction of various factors such as type of meat, dose of EO, the strain/serovar of a pathogen or the duration of pathogen exposure to the EOs. The interaction of some of these factors resulted in varying log reductions across studies and among similar pathogens. For instance, Kiprotich et al. (76) reported a 3.48 Log CFU/mL reduction when Salmonella enterica was challenged against 0.5% (v/v) thyme oil. However, Boskovic et al. (79) applied 0.3% thyme oil and only observed a 1.69 Log CFU/g reduction. The difference between these two studies was that Kiprotich et al. (76) added thyme oil into lemon juice and supplemented the mixture with Yucca schidigera, a natural emulsifier, and allowed the mixture to stand at 23°C for 8 h when microbial analysis was performed; whereas Boskovic et al. (79) pulled a vacuum on the packaging of the minced meat and stored their samples at 3 ± 1°C for 15 days. The difference in the results obtained in these two studies can be attributed to the synergistic effects of the different conditions offered to the essential oils. The other avenue would be to combine different types of EOs because each may contain different concentrations and modes of action. For instance, Thannisery and Smith (77) combined thyme oil and orange essential oil at 0.5% (v/v) each and achieved a 2.6 Log CFU/mL of Salmonella Enteritidis and a 3.6 Log CFU/mL reduction of Campylobacter coli in chicken breast meat. Combinations of EOs and other antimicrobial strategies such as emulsifiers, modified atmospheric packaging or refrigeration might increase the applicability of EOs by fostering a synergistic, complimentary antimicrobial effect, which in turn circumvents the strong flavors and damage to sensory properties of food usually associated with application of higher concentrations of EOs (76, 94, 95).
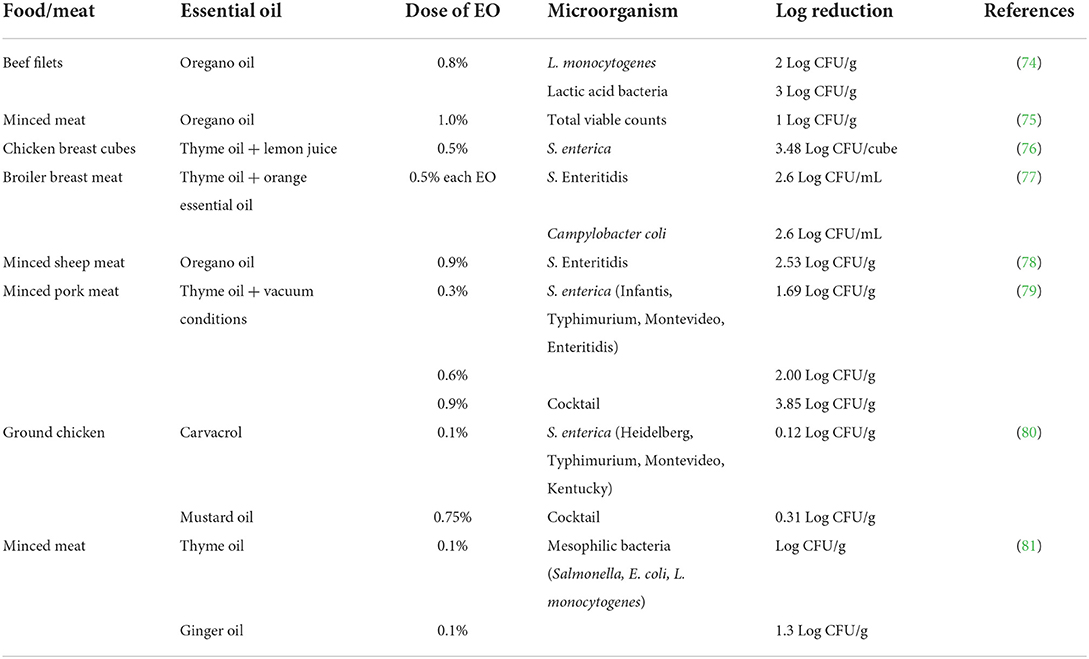
Table 2. Summary of antimicrobial efficacy for essential oils at various doses used to control spoilage and pathogenic bacteria in meat and poultry products.
To apply EOs in RMBDs, they might have to be added to the product during the grinding and mixing process. A formula for most commercially available pet foods consists of ground meats with bones, tubers, vegetables, and fruits. In this form, they present a challenge to decontamination since surface treatment alone is not sufficient. Unlike whole chunks of meat or poultry which have been successfully decontaminated with EOs, grinding reduces particle size while increasing surface area for pathogen attachment and distribution throughout the product. Supplementary measures such as modified atmospheric packaging (MAP), freezing or vacuumizing might synergize the antimicrobial processes discussed above.
Bacteriophages refer to host-specific viruses that parasitize bacteria by lysing, breaking, and penetrating through the cell membrane and multiplying inside the cell, causing its death (96, 97). Bacteriophages are ubiquitous in the environment, and highly specific making them ideal for the biocontrol of bacteria as they attack a wide range of spoilage and pathogenic microorganisms while maintaining their specificity (96, 98). These phages may belong to the Order Caudovirales, with their respective families including Myoviridae, Siphoviridae, and Podoviridae (99–101). Bacteriophages are increasingly being applied to liquid foods as an alternative to chlorine-based decontaminants which are associated with rising incidences of antimicrobial resistance (102).
The mechanism by which bacteriophages parasitize bacteria is based upon the specificity of the phage virus to a singular bacterial species or one very similar (98, 99, 103). Despite their ubiquity in the environment, a relatively small proportion of phage viruses possess the specificity required to bind with a target pathogen, thus their overall impact on the microbial ecosystem remains insignificant regarding negative effects (104, 105). As an example of bacteriophage specificity, Ricci and Piddock (106) demonstrated that ST27, ST29, and ST35 phages only bound to TolC receptors present on outer membranes of Salmonella serovars but were totally inactive against receptors found in the Enterobacteriaceae family. Whereas, some phages express a phenomenon described as “local adaptation,” that allows them to infect bacteria across several genera (105, 107, 108).
The phage attaches to specific receptors on the outer cell membrane and then injects itself by adsorption. Once in the cell, the phage will either follow a lytic or lysogenic lifecycle. The lytic or virulent cycle causes rapid cell death as the phage uses the cell to replicate (96). Daughter phages are released upon cell lysis to infect the next line of bacterium. For lysogenic phages they transfer their genome to bacterial cells and use the host replication which results in the transmission of phage genome through host daughter cells but does not result in cell death (101). Lytic phages minimize transduction of their genome into their host leading to cells resisting phage viruses (phage resistance) whereas lysogenic phages contribute to phage resistance as they transfer their genome through the host cells (99, 101). From the mechanisms of action discussed above, lytic phages would be appropriate for use in therapeutic and antimicrobial interventions in both animal and human food.
The relationship between bacteria and phages is expressed as ratio, described as “multiplicity of infection (MOI),” and multiplicity of adsorption (MOA) which is a ratio of the phage forming units to colony forming units (PFU/CFU) (96, 98). This ratio allows for phages to be applied as an antimicrobial intervention with the efficacies of different phage concentrations determined by the number (CFU) of bacterial cells inactivated by a specific concentration of phage viruses (PFU) (105, 109–111). However, the concentration of bacterial cells has been shown to have no effect on the antimicrobial potency of the phages as demonstrated by Bigwood et al. (112) who increased the concentrations of Salmonella while keeping constant Salmonella phages (P7) and observed no difference in inactivation efficiency. Likewise, Bigwood et al. (112) increased the phage concentration from 1.8 × 104 to over 5 × 108 PFU/mL and observed increased inactivation of Salmonella, and vice versa when the phage concentration was lowered. Bacteriophages have been mainly applied to liquid foodstuffs, but progress has been made for application to solid foods. The current challenges of phage application are the development of resistance to phages by bacteria which necessitates the use of phage cocktails to control mutating (adapting) cells. Secondarily not all phages are recognized by the FDA as GRAS.
Phages are ubiquitous which allows for flexibility when they come into contact against a serotype of a spoilage or pathogenic bacterium. They offer an alternative non-thermal method to treat minimally processed or raw foods or ingredients. Studies that employed bacteriophages in meat and poultry to control enteric pathogens is summarized in Table 3. There was a higher log reduction of the pathogens challenged against the phages in whole chunks of meat compared to ground meat. The phages' antimicrobial activity also appeared to depend on the type of serovar of pathogen they were exposed to. For instance, Spricigo et al. (113) challenged Salmonella enterica serovar Typhimurium and Enteritidis inoculated in poultry meat against a phage solution at 109 PFU/mL and observed a significant difference in log reduction (2.2 and 0.9 Log CFU/g, respectively). Also, when different types of phages were challenged against the same Salmonella serovar different log reductions were observed after treatment. Furthermore, Hungaro et al. (102) isolated bacteriophages from poultry feces and used them against Salmonella Enteritidis on chicken skin and reported a 1.0 Log CFU/cm2 reduction as an alternative to chlorine, a chemical disinfectant. Higgins et al. (114) sprayed carcasses of broilers and turkey inoculated with Salmonella Enteritidis with rinse water containing 109 PFU/mL of PHL4 bacteriophages and reported a 93.0% (on broilers) and 58% (on turkeys) reduction of the initial concentration of pathogens compared to the control carcasses that were sprayed with only water.
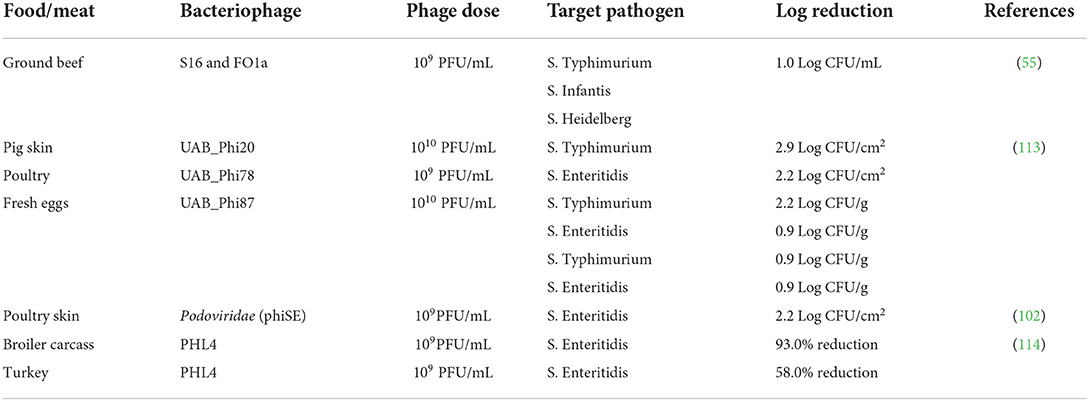
Table 3. Summary of antimicrobial efficacy for bacteriophages used to control enteric foodborne pathogens in meat and poultry products.
The application of bacteriophages is still limited by factors such as pH and temperature which affect their antimicrobial potency. For instance, Leverentz et al. (115) applied a specific phage cocktail to honeydew melon (pH 5.8) and apple slices (pH 4.2), stored at 5, 10, and 20°C. A 2.5–3.5 log reduction of Salmonella Enteritidis was observed on the honeydew slices that were stored at 5 and 10°C, whereas no significant log reduction was observed at 20°C. There was no significant reduction of Salmonella Enteritidis on the apple slices at any temperature level leaving the authors to hypothesize that the phages had been deactivated by low pH of the apple slices. The implication of their observation is that more acid-resistant phages need to be developed for application in low pH food systems or matrices if they are to be deployed as antimicrobial interventions.
The high-pressure pasteurization and food additives discussed as interventions in this review have exhibited antimicrobial efficacies of varying successes against spoilage and pathogenic bacteria in poultry and meat products. However, commercialization and adoption of these novel interventions by the animal and pet food industry has been slow because of the varying antimicrobial efficacies obtained from using these technologies when applied to control enteric foodborne pathogens in meat and poultry products. Variation in experimental design, microbial strains, equipment, and outcomes have made the adoption and scale-up of these interventions difficult due to inadequate reproducibility of the results from these studies. For instance, different studies that utilized the same intervention (i.e., essential oil, organic acidulant, or bacteriophage) against a similar pathogen resulted in different results under comparable conditions (Tables 1–3). The lack of consistency makes standardization of these antimicrobial interventions difficult given that they are mainly applicable to minimally processed foods which are at a higher risk of being contaminated. Furthermore, effective pasteurization requires that higher doses of these non-thermal interventions be applied which can have undesirable effects on the sensory and nutritional attributes of a given pet food, warranting additional research to address palatability concerns.
A path forward is rooted in hurdle technology on the premise that combining technologies will act synergistically. Harnessing this synergism could allow for lower doses to be applied to products, may lower the negative impact on quality and sensory attributes of the treated foods and has the potential to increase consistency in effective pathogen control. Combinations of essential oils, high-pressure processing, and low pH tolerant phages should be developed which would allow the combination of organic acids and bacteriophages to become a reality. Improving the safety of RMBDs for companion animals, given the biological hazards discussed in this review will require a holistic approach. First, utilization of food additives like organic acids or essential oils considered as GRAS and “natural” should be a first step. Secondly, these interventions should be evaluated in combination by taking advantage of their different mechanisms of antimicrobial action.
Also, strategies like modified or controlled atmospheric packaging should be researched in addition to these new emerging technologies because air composition affects microbial life and, thus, it might introduce a stressor, impeding the growth of pathogenic microbes. Kinetic mechanistic maps of bacteria from different genera can help scale up these proposed antimicrobial interventions by highlighting the more robust and resistant microbes in the matrices of a RMBD. In conclusion, as the demand for RMBDs increases, so will safety challenges associated with them. Innovative and holistic approaches will need to be developed and utilized to address microbial safety and hazards associated with commercial RMBDs. Therefore, the antimicrobial interventions discussed in this review may be a framework for future research aimed at controlling foodborne pathogens in commercially manufactured RMBDs for companion animals.
SK and CA came up with the idea for this review. SK wrote the manuscript. CA edited the final drafts of this review paper. All authors contributed to the article and approved the submitted version.
The authors would like to acknowledge the pet food program at Kansas State University for providing the platform and finances that has aided them to write this review paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1049731/full#supplementary-material
1. Buff PR, Carter RA, Bauer JE, Kersey JH. Natural pet food: a review of natural diets and their impact on canine and feline physiology. J Anim Sci. (2014) 92:3781–91. doi: 10.2527/jas.2014-7789
2. American Pet Products Association, Inc,. Pet Industry Market Size, Trends & Ownership Statistics. (2021). Available online at: https://www.americanpetproducts.org/press_industrytrends.asp (accessed October 19, 2022).
3. Acuff HL, Dainton AN, Dhakal J, Kiprotich S, Aldrich G. Sustainability and pet food: is there a role for veterinarians? Vet Clin. (2021) 51:563–81. doi: 10.1016/j.cvsm.2021.01.010
4. Stull JW, Peregrine AS, Sargeant JM, Weese JS. Household knowledge, attitudes and practices related to pet contact and associated zoonoses in Ontario, Canada. BMC Public Health. (2012) 12:1–5. doi: 10.1186/1471-2458-12-553
5. Mota-Rojas D, Mariti C, Zdeinert A, Riggio G, Mora-Medina P, del Mar Reyes A, et al. Anthropomorphism and its adverse effects on the distress and welfare of companion animals. Animals. (2021) 11:3263. doi: 10.3390/ani11113263
6. Coria-Avila GA, Pfaus JG, Orihuela A, Domínguez-Oliva A, José-Pérez N, Hernández LA, et al. The neurobiology of behavior and its applicability for animal welfare: a review. Animals. (2022) 12:928. doi: 10.3390/ani12070928
7. Lummis D. Natural, Organic and Eco-friendly Pet Products in the US. Rockville, MD: Packaged Facts (2012).
8. Santeramo FG, Carlucci D, De Devitiis B, Seccia A, Stasi A, Viscecchia R, et al. Emerging trends in European food, diets and food industry. Food Res Int. (2018) 104:39–47. doi: 10.1016/j.foodres.2017.10.039
9. Schlesinger DP, Joffe DJ. Raw food diets in companion animals: a critical review. Can Vet J. (2011) 52:50.
10. Nüesch-Inderbinen M, Treier A, Zurfluh K, Stephan R. Raw meat-based diets for companion animals: a potential source of transmission of pathogenic and antimicrobial-resistant Enterobacteriaceae. R Soc Open Sci. (2019) 6:191170. doi: 10.1098/rsos.191170
11. Raditic DM. Insights into commercial pet foods. Vet Clin. (2021) 51:551–62. doi: 10.1016/j.cvsm.2021.01.013
12. Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can Vet J. (2005) 46:513.
13. Fredriksson-Ahomaa M, Heikkilä T, Pernu N, Kovanen S, Hielm-Björkman A, Kivistö R. Raw meat-based diets in dogs and cats. Vet Sci. (2017) 4:33. doi: 10.3390/vetsci4030033
14. Davies RH, Lawes JR, Wales AD. Raw diets for dogs and cats: a review, with particular reference to microbiological hazards. J Small Anim Practice. (2019) 60:329–39. doi: 10.1111/jsap.13000
15. van Rooijen C, Bosch G, van der Poel AF, Wierenga PA, Alexander L, Hendriks WH. Quantitation of Maillard reaction products in commercially available pet foods. J Agric Food Chem. (2014) 62:8883–91. doi: 10.1021/jf502064h
16. Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, et al. Dietary advanced glycation end products and their role in health and disease. Adv Nutr. (2015) 6:461–73. doi: 10.3945/an.115.008433
17. Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, et al. Advanced glycation end products in food and their effects on health. Food Chem Toxicol. (2013) 60:10–37. doi: 10.1016/j.fct.2013.06.052
18. Delgado-Andrade C. Maillard reaction products: some considerations on their health effects. Clin Chem Lab Med. (2014) 52:53–60. doi: 10.1515/cclm-2012-0823
19. Teodorowicz M, Hendriks WH, Wichers HJ, Savelkoul HFJ. Immunomodulation by processed animal feed: the role of Maillard reaction products and advanced glycation end-products (AGEs). Front Immunol. (2018) 9:2088. doi: 10.3389/fimmu.2018.02088
20. Delgado-Andrade C, Tessier FJ, Niquet-Leridon C, Seiquer I, Pilar Navarro M. Study of the urinary and faecal excretion of N ε-carboxymethyllysine in young human volunteers. Amino Acids. (2012) 43:595–602. doi: 10.1007/s00726-011-1107-8
21. Förster A, Kühne Y, Henle TO. Studies on absorption and elimination of dietary maillard reaction products. Ann N Y Acad Sci. (2005) 1043:474–81. doi: 10.1196/annals.1333.054
22. Sandri M, Dal Monego S, Conte G, Sgorlon S, Stefanon B. Raw meat-based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet Res. (2016) 13:1–1. doi: 10.1186/s12917-017-0981-z
23. Association of American Feed Control Officials. Official Publication. Champaign, IL: Association of American Feed Control Officials (2020).
24. Morley PS, Strohmeyer RA, Tankson JD, Hyatt DR, Dargatz DA, Fedorka-Cray PJ. Evaluation of the association between feeding raw meat and Salmonella enterica infections at a Greyhound breeding facility. J Am Vet Med Assoc. (2006) 228:1524–32. doi: 10.2460/javma.228.10.1524
25. Stiver SL, Frazier KS, Mauel MJ, Styer EL. Septicemic salmonellosis in two cats fed a raw-meat diet. J Am Anim Hosp Assoc. (2003) 39:538–42. doi: 10.5326/0390538
26. van Dijk MA, Engelsma MY, Visser VX, Spierenburg MA, Holtslag ME, Willemsen PT, et al. Brucella suis infection in dog fed raw meat, the Netherlands. Emerg Infect Dis. (2018) 24:1127. doi: 10.3201/eid2406.171887
27. Greene CE. Enteric Bacterial Infections. In Infectious Diseases of the Dog and Cat. St Louis, MO: Elsevier Saunders (2012). p. 383–9.
28. Finley R, Reid-Smith R, Weese JS, Angulo FJ. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin Infect Dis. (2006) 42:686–91. doi: 10.1086/500211
29. Centers for Disease Control and Prevention. Outbreak of Multidrug-Resistant Salmonella Infections Linked to Raw Turkey Products. CDC Investigation Notice. (2019). Available online at: www.cdc.gov/salmonella/reading-07-18 (accessed July 18, 2022).
30. Public Health England. Investigation Into an Outbreak of Shiga Toxin Producing Escherichia coli (STEC) O157 PT 21/28 Stx2 in England, August 2017. London: PHE Publications (2018.
31. Baede VO, Broens EM, Spaninks MP, Timmerman AJ, Graveland H, Wagenaar JA, et al. Raw pet food as a risk factor for shedding of extended-spectrum beta-lactamase-producing Enterobacteriaceae in household cats. PLoS ONE. (2017) 12:e0187239. doi: 10.1371/journal.pone.0187239
32. Jans C, Sarno E, Collineau L, Meile L, Stärk KD, Stephan R. Consumer exposure to antimicrobial resistant bacteria from food at Swiss retail level. Front Microbiol. (2018) 9:362. doi: 10.3389/fmicb.2018.00362
33. Black JL, Jaczynski J. Effect of water activity on the inactivation kinetics of Escherichia coli O157: H7 by electron beam in ground beef, chicken breast meat, and trout fillets. Int J Food Sci Technol. (2008) 43:579–86. doi: 10.1111/j.1365-2621.2006.01480.x
34. Freeman LM, Chandler ML, Hamper BA, Weeth LP. Current knowledge about the risks and benefits of raw meat–based diets for dogs and cats. J Am Vet Med Assoc. (2013) 243:1549–58. doi: 10.2460/javma.243.11.1549
35. Pet Food Magazine,. Should You Use High-Pressure Processing for Your Pet Food? (2022). Available online at: https://www.petfoodindustry.com/articles/11284-should-you-use-high-pressure-processing-for-your-pet-food (accessed October 7, 2022).
36. Balamurugan S, Inmanee P, Souza JD, Strange P, Pirak T, Barbut S. Effects of high-pressure processing and hot water pasteurization of cooked sausages on inactivation of inoculated Listeria monocytogenes, natural populations of lactic acid bacteria, Pseudomonas spp, and coliforms and their recovery during storage at 4 and 10 C. J Food Protect. (2018) 81:1245–51. doi: 10.4315/0362-028X.JFP-18-024
37. Sukmanov V, Hanjun M, Li YP. Effect of high-pressure processing on meat and meat products. A review. Ukrainian Food J. (2019) 8:448–69. doi: 10.24263/2304-974X-2019-8-3-4
38. Argyri AA, Papadopoulou OS, Nisiotou A, Tassou CC, Chorianopoulos N. Effect of high-pressure processing on the survival of Salmonella Enteritidis and shelf-life of chicken fillets. Food Microbiol. (2018) 70:55–64. doi: 10.1016/j.fm.2017.08.019
39. Huang HW, Wu SJ, Lu JK, Shyu YT, Wang CY. Current status and future trends of high-pressure processing in food industry. Food Control. (2017) 72:1–8. doi: 10.1016/j.foodcont.2016.07.019
40. Argyri AA, Panagou EZ, Nychas GJ. Advances in vacuum and modified atmosphere packaging of poultry products. In:Kerry JP, , editor. Advances in Meat, Poultry and Seafood Packaging. Sawston: Woodhead Publishing. (2012). p. 205–47. doi: 10.1533/9780857095718.2.205
41. Georget E, Sevenich R, Reineke K, Mathys A, Heinz V, Callanan M, et al. Inactivation of microorganisms by high isostatic pressure processing in complex matrices: a review. Innov Food Sci Emerg Technolog. (2015) 27:1–4. doi: 10.1016/j.ifset.2014.10.015
42. Hygreeva D, Pandey MC. Novel approaches in improving the quality and safety aspects of processed meat products through high pressure processing technology-a review. Trends Food Sci Technol. (2016) 54:175–85. doi: 10.1016/j.tifs.2016.06.002
43. Escriu R, Mor-Mur M. Role of quantity and quality of fat in meat models inoculated with Listeria innocua or Salmonella Typhimurium treated by high pressure and refrigerated stored. Food Microbiol. (2009) 26:834–40. doi: 10.1016/j.fm.2009.05.011
44. Garriga M, Grebol N, Aymerich MT, Monfort JM, Hugas M. Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Innov Food Sci Emerg Technolog. (2004) 5:451–7. doi: 10.1016/j.ifset.2004.07.001
45. Hayman MM, Baxter I, O'riordan PJ, Stewart CM. Effects of high-pressure processing on the safety, quality, and shelf life of ready-to-eat meats. J Food Protect. (2004) 67:1709–18. doi: 10.4315/0362-028X-67.8.1709
46. Jofré A, Garriga M, Aymerich T. Inhibition of Salmonella sp. Listeria monocytogenes and Staphylococcus aureus in cooked ham by combining antimicrobials, high hydrostatic pressure and refrigeration. Meat Sci. (2008) 78:53–9. doi: 10.1016/j.meatsci.2007.06.015
47. McArdle R, Marcos B, Kerry JP, Mullen A. Monitoring the effects of high-pressure processing and temperature on selected beef quality attributes. Meat Sci. (2010) 86:629–34. doi: 10.1016/j.meatsci.2010.05.001
48. Morales P, Calzada J, Rodriguez B, De Paz M, Nunez M. Inactivation of Salmonella Enteritidis in chicken breast fillets by single-cycle and multiple-cycle high pressure treatments. Foodborne Pathog Dis. (2009) 6:577–81. doi: 10.1089/fpd.2008.0218
49. Serra-Castelló C, Possas A, Jofré A, Garriga M, Bover-Cid S. High pressure processing to control Salmonella in raw pet food without compromising the freshness appearance: the impact of acidulation and frozen storage. Food Microbiol. (2022) 9:104139. doi: 10.1016/j.fm.2022.104139
50. Simonin H, Duranton F, De Lamballerie M. New insights into the high-pressure processing of meat and meat products. Compr Rev Food Sci Food Saf. (2012) 11:285–306. doi: 10.1111/j.1541-4337.2012.00184.x
51. Sheen S, Cassidy J, Scullen B, Uknalis J, Sommers C. Inactivation of Salmonella spp. in ground chicken using high pressure processing. Food Control. (2015) 57:41–7. doi: 10.1016/j.foodcont.2015.04.005
52. Chen L, Jiao D, Liu H, Zhu C, Sun Y, Wu J, et al. Effects of water distribution and protein degradation on the texture of high pressure-treated shrimp (Penaeus monodon) during chilled storage. Food Control. (2022) 132:108555. doi: 10.1016/j.foodcont.2021.108555
53. Chien SY, Sheen S, Sommers C, Sheen LY. Combination effect of high-pressure processing and essential oil (Melissa officinalis extracts) or their constituents for the inactivation of Escherichia coli in ground beef. Food Bioprocess Technol. (2019) 12:359–70. doi: 10.1007/s11947-018-2211-5
54. Mani-López E, García HS, López-Malo A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res Int. (2012) 45:713–21. doi: 10.1016/j.foodres.2011.04.043
55. Yeh Y, De Moura FH, Van Den Broek K, De Mello AS. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. (2018) 139:44–8. doi: 10.1016/j.meatsci.2018.01.007
56. Taylor TM, RolfJoerger EP, Lopez-Malo A, Avila-Sosa R, Calix-Lara T. 13 alternatives to traditional antimicrobials for organically processed meat and poultry. Organic Meat Product Process. (2012) 53:ch13. doi: 10.1002/9781118229088.ch13
57. Eklund T. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J Appl Bacteriol. (1983) 54:383–9. doi: 10.1111/j.1365-2672.1983.tb02632.x
58. Gould GW. Mechanisms of Action of Food Preservation Procedures. Amsterdam: Elsevier Applied Science (1989).
59. Cherrington CA, Hinton M, Mead GC, Chopra I. Organic acids: chemistry, antibacterial activity and practical applications. Adv Microb Physiol. (1991) 32:87–108. doi: 10.1016/S0065-2911(08)60006-5
60. Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. (2000) 66:2001–5. doi: 10.1128/AEM.66.5.2001-2005.2000
61. Alakomi HL, Puupponen-Pimiä R, Aura AM, Helander IM, Nohynek L, Oksman-Caldentey KM, et al. Weakening of Salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J Agric Food Chem. (2007) 55:3905–12. doi: 10.1021/jf070190y
62. Stratford M, Plumridge A, Nebe-von-Caron G, Archer DB. Inhibition of spoilage mold conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory. Int J Food Microbiol. (2009) 136:37–43. doi: 10.1016/j.ijfoodmicro.2009.09.025
63. Koczoń P. Growth inhibition mode of action of selected benzoic acid derivatives against the yeast Pichia anomala. J Food Prot. (2009) 72:791–800. doi: 10.4315/0362-028X-72.4.791
64. U.S. Department of Agriculture, Food Safety and Inspection Service. Safe and suitable ingredients used in the production of meat and poultry products. Directive 7120.1, Rev. 55. (2010). Available online at: https://www.fsis.usda.gov/sites/default/files/media_file/2021-03/7120.1.pdf (accessed April 22, 2021).
65. Over KF, Hettiarachchy N, Johnson MG, Davis B. Effect of organic acids and plant extracts on Escherichia coli O157: H7, Listeria monocytogenes, and Salmonella Typhimurium in broth culture model and chicken meat systems. J Food Sci. (2009) 74:M515–21. doi: 10.1111/j.1750-3841.2009.01375.x
66. Harris K, Miller MF, Loneragan GH, Brashears MM. Validation of the use of organic acids and acidified sodium chlorite to reduce Escherichia coli O157 and Salmonella Typhimurium in beef trim and ground beef in a simulated processing environment. J Food Prot. (2006) 69:1802–7. doi: 10.4315/0362-028X-69.8.1802
67. Hamby PL, Savell JW, Acuff GR, Vanderzant C, Cross HR. Spray-chilling and carcass decontamination systems using lactic and acetic acid. Meat Sci. (1987) 21:1–4. doi: 10.1016/0309-1740(87)90038-6
68. Tamblyn KC, Conner DE. Bactericidal activity of organic acids in combination with transdermal compounds against Salmonella Typhimurium attached to broiler skin. Food Microbiol. (1997) 14:477–84. doi: 10.1006/fmic.1997.0112
69. Moye CJ, Chambers A. Poultry processing: an innovative technology for Salmonella control and shelf-life extension. Food Australia. (1991) 43:246–9.
70. Scannell AG, Hill C, Buckley DJ, Arendt EK. Determination of the influence of organic acids and nisin on shelf-life and microbiological safety aspects of fresh pork sausage. J Appl Microbiol. (1997) 83:407–12. doi: 10.1046/j.1365-2672.1997.00248.x
71. Özdemir H, Yildirim Y, Küplülü Ö, Koluman A, Göncüoglu M, Inat G. Effects of lactic acid and hot water treatments on Salmonella Typhimurium and Listeria monocytogenes on beef. Food Control. (2006) 17:299–303. doi: 10.1016/j.foodcont.2004.11.003
72. Choi YM, Kim OY, Kim KH, Kim BC, Rhee MS. Combined effect of organic acids and supercritical carbon dioxide treatments against non-pathogenic Escherichia coli, Listeria monocytogenes, Salmonella Typhimurium and E. coli O157: H7 in fresh pork. Lett Appl Microbiol. (2009) 49:510–5. doi: 10.1111/j.1472-765X.2009.02702.x
73. Tamblyn KC, Conner DE. Bactericidal activity of organic acids against Salmonella Typhimurium attached to broiler chicken skin. J Food Prot. (1997) 60:629–33. doi: 10.4315/0362-028X-60.6.629
74. Tsigarida E, Skandamis P, Nychas GJ. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5C. J Appl Microbiol. (2000) 89:901–9. doi: 10.1046/j.1365-2672.2000.01170.x
75. Skandamis PN, Nychas GJ. Effect of oregano essential oil on microbiological and physico-chemical attributes of minced meat stored in air and modified atmospheres. J Appl Microbiol. (2001) 91:1011–22. doi: 10.1046/j.1365-2672.2001.01467.x
76. Kiprotich S, Mendonça A, Dickson J, Shaw A, Thomas-Popo E, White S, et al. Thyme oil enhances the inactivation of Salmonella enterica on raw chicken breast meat during marination in lemon juice with added Yucca schidigera extract. Front Nutr. (2021) 7:619023. doi: 10.3389/fnut.2020.619023
77. Thanissery R, Smith DP. Marinade with thyme and orange oils reduces Salmonella Enteritidis and Campylobacter coli on inoculated broiler breast fillets and whole wings. Poult Sci. (2014) 93:1258–62. doi: 10.3382/ps.2013-03697
78. Govaris A, Solomakos N, Pexara A, Chatzopoulou PS. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int J Food Microbiol. (2010) 137:175–80. doi: 10.1016/j.ijfoodmicro.2009.12.017
79. Boskovic M, Djordjevic J, Ivanovic J, Janjic J, Zdravkovic N, Glisic M, et al. Inhibition of Salmonella by thyme essential oil and its effect on microbiological and sensory properties of minced pork meat packaged under vacuum and modified atmosphere. Int J Food Microbiol. (2017) 258:58–67. doi: 10.1016/j.ijfoodmicro.2017.07.011
80. Porter JA, Morey A, Monu EA. Antimicrobial efficacy of white mustard essential oil and carvacrol against Salmonella in refrigerated ground chicken. Poult Sci. (2020) 99:5091–5. doi: 10.1016/j.psj.2020.06.027
81. Barbosa LN, Rall VL, Fernandes AA, Ushimaru PI, da Silva Probst I, Fernandes Jr A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog Dis. (2009) 6:725–8. doi: 10.1089/fpd.2009.0282
82. Kiprotich S, Altom E, Mason R, Aldrich CG. Application of encapsulated lactic acid to control the growth and multiplication of Salmonella enterica in raw meat-based diets for dogs. Kansas Agri Exp Stat Res Rep. (2021) 7:15. doi: 10.4148/2378-5977.8153
83. Calo JR, Crandall PG, O'Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems–a review. Food Control. (2015) 54:111–9. doi: 10.1016/j.foodcont.2014.12.040
84. Dewi G, Manjankattil S, Peichel C, Jia S, Nair D, Vickers Z, et al. Effect of plant-derived antimicrobials against multidrug-resistant Salmonella Heidelberg in ground Turkey. Poult Sci. (2022) 101:101581. doi: 10.1016/j.psj.2021.101581
85. Sofos JN, Beuchat LR, Davidson PM, Johnson EA. Naturally occurring antimicrobials in food. Regulat Toxicol Pharmacol. (1998) 28:71–2. doi: 10.1006/rtph.1998.1246
86. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food Chemical Toxicol. (2008) 46:446–75. doi: 10.1016/j.fct.2007.09.106
87. Mahmoud SS, Croteau RB. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. (2002) 7:366–73. doi: 10.1016/S1360-1385(02)02303-8
88. Bajpai VK, Baek KH, Kang SC. Control of Salmonella in foods by using essential oils: a review. Food Res Int. (2012) 45:722–34. doi: 10.1016/j.foodres.2011.04.052
89. Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. (2004) 94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022
90. Li PH, Chiang BH. Process optimization and stability of D-limonene-in-water nano emulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem. (2012) 19:192–7. doi: 10.1016/j.ultsonch.2011.05.017
91. Sakkas H, Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J Microbiol Biotechnol. (2017) 27:429–38. doi: 10.4014/jmb.1608.08024
92. Dave D, Ghaly AE. Meat spoilage mechanisms and preservation techniques: a critical review. Am J Agri Biol Sci. (2011) 6:486–510. doi: 10.3844/ajabssp.2011.486.510
93. Jayasena DD, Jo C. Essential oils as potential antimicrobial agents in meat and meat products: a review. Trends Food Sci Technol. (2013) 34:96–108. doi: 10.1016/j.tifs.2013.09.002
94. Mendonca A, Jackson-Davis A, Moutiq R, Thomas-Popo E. Use of natural antimicrobials of plant origin to improve the microbiological safety of foods. In:Ricke SC, , editor. Food and Feed Safety Systems and Analysis. Cambridge, MA: Academic Press (2018). p. 249–72. doi: 10.1016/B978-0-12-811835-1.00014-2
95. Thomas-Popo E, Mendonca A, Dickson J, Shaw A, Coleman S, Daraba A, et al. Isoeugenol significantly inactivates Escherichia coli O157: H7, Salmonella enterica, and Listeria monocytogenes in refrigerated tyndallized pineapple juice with added Yucca schidigera extract. Food Control. (2019) 106:106727. doi: 10.1016/j.foodcont.2019.106727
96. Sharma CS, Dhakal J, Nannapaneni R. Efficacy of lytic bacteriophage preparation in reducing Salmonella in vitro, on turkey breast cutlets, and on ground turkey. J Food Prot. (2015) 78:1357–62. doi: 10.4315/0362-028X.JFP-14-585
97. Sukumaran AT, Nannapaneni R, Kiess A, Sharma CS. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFreshTM. Poult Sci. (2016) 95:668–75. doi: 10.3382/ps/pev332
98. Mukhopadhyay S, Ramaswamy R. Application of emerging technologies to control Salmonella in foods: a review. Food Res Int. (2012) 45:666–77. doi: 10.1016/j.foodres.2011.05.016
99. Hashem F, Parveen S. Salmonella and Campylobacter: Antimicrobial resistance and bacteriophage control in poultry. Food Microbiol. (2016) 53:104–9. doi: 10.1016/j.fm.2015.09.008
100. Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. (2014) 5:226–35. doi: 10.4161/viru.25991
101. Wright EE, Elliman JR, Owens L. Induction and characterization of lysogenic bacteriophages from Streptococcus iniae. J Appl Microbiol. (2013) 114:1616–24. doi: 10.1111/jam.12192
102. Hungaro HM, Mendonça RC, Gouvêa DM, Vanetti MC, de Oliveira Pinto CL. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res Int. (2013) 52:75–81. doi: 10.1016/j.foodres.2013.02.032
103. Kim M, Kim S, Park B, Ryu S. Core lipopolysaccharide-specific phage SSU5 as an auxiliary component of a phage cocktail for Salmonella biocontrol. Appl Environ Microbiol. (2014) 80:1026–34. doi: 10.1128/AEM.03494-13
104. Callaway TR, Edrington TS, Brabban A, Kutter E, Karriker L, Stahl C, et al. Occurrence of Salmonella-specific bacteriophages in swine feces collected from commercial farms. Foodborne Pathog Dis. (2010) 7:851–6. doi: 10.1089/fpd.2009.0512
105. Koskella B, Thompson JN, Preston GM, Buckling A. Local biotic environment shapes the spatial scale of bacteriophage adaptation to bacteria. Am Nat. (2011) 177:440–51. doi: 10.1086/658991
106. Ricci V, Piddock LJ. Exploiting the role of TolC in pathogenicity: identification of a bacteriophage for eradication of Salmonella serovars from poultry. Appl Environ Microbiol. (2010) 76:1704–6. doi: 10.1128/AEM.02681-09
107. Flores CO, Meyer JR, Valverde S, Farr L, Weitz JS. Statistical structure of host–phage interactions. Proc Nat Acad Sci USA. (2011) 108:E288–97. doi: 10.1073/pnas.1101595108
108. Hyman P, Abedon ST. Bacteriophage host range and bacterial resistance. Adv Appl Microbiol. (2010) 70:217–48. doi: 10.1016/S0065-2164(10)70007-1
109. Kasman LM, Kasman A, Westwater C, Dolan J, Schmidt MG, Norris JS. Overcoming the phage replication threshold: a mathematical model with implications for phage therapy. J Virol. (2002) 76:5557–64. doi: 10.1128/JVI.76.11.5557-5564.2002
110. Ross RP, Fitzgerald GF, Coffey A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157: H7. Appl Environ Microbiol. (2004) 70:3417–24. doi: 10.1128/AEM.70.6.3417-3424.2004
111. Whichard JM, Sriranganathan N, Pierson FW. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J Food Prot. (2003) 66:220–5. doi: 10.4315/0362-028X-66.2.220
112. Bigwood T, Hudson JA, Billington C. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol Lett. (2009) 291:59–64. doi: 10.1111/j.1574-6968.2008.01435.x
113. Spricigo DA, Bardina C, Cortés P, Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int J Food Microbiol. (2013) 165:169–74. doi: 10.1016/j.ijfoodmicro.2013.05.009
114. Higgins JP, Higgins SE, Guenther KL, Huff W, Donoghue AM, Donoghue DJ, et al. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult Sci. (2005) 84:1141–5. doi: 10.1093/ps/84.7.1141
Keywords: Salmonella, raw pet food, companion animals, raw meat-based diets (RMBDs), essential oils, organic acids
Citation: Kiprotich SS and Aldrich CG (2022) A review of food additives to control the proliferation and transmission of pathogenic microorganisms with emphasis on applications to raw meat-based diets for companion animals. Front. Vet. Sci. 9:1049731. doi: 10.3389/fvets.2022.1049731
Received: 21 September 2022; Accepted: 25 October 2022;
Published: 10 November 2022.
Edited by:
Anna Katharine Shoveller, University of Guelph, CanadaReviewed by:
Nicole Renee Cammack, University of Georgia, United StatesCopyright © 2022 Kiprotich and Aldrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles G. Aldrich, YWxkcmljaDRAa3N1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.