- 1Clinic for Ruminants, Vetsuisse Faculty, University of Bern, Bern, Switzerland
- 2Center for Diagnostic, Technical University of Denmark, Kongens Lyngby, Denmark
- 3Section for Pathobiological Sciences, University of Copenhagen, Copenhagen, Denmark
- 4Research Group Oncology, Equine Clinic of Surgery, Department of Companion Animals and Horses, University of Veterinary Medicine, Vienna, Austria
- 5Department of Infectious Diseases and Pathobiology, Vetsuisse Faculty, Institute of Animal Pathology, University of Bern, Bern, Switzerland
- 6Department of Farm Animals, Vetsuisse Faculty, University of Zurich, Zürich, Switzerland
The increasing prevalence of bovine digital dermatitis (BDD) contributes to a higher occurrence of secondary infections of exposed corium with Treponema spp. in bovine claws. “Non-healing” claw horn lesions (NHL) clinically resemble BDD lesions. They are severe, cause chronic lameness, and may persist for several months. They poorly respond to standard treatments of BDD and represent a serious welfare issue. In this study, four cases of NHL were classified clinically either as BDD-associated axial horn fissures (BDD-HFA; n = 3) or BDD-associated sole ulcer (BDD-SU; n = 1). In all four cases, pronounced multifocal keratinolysis of the stratum corneum, ulceration, and severe chronic lymphoplasmacytic perivascular to interstitial dermatitis were observed. All lesional samples tested positive for Treponema spp., Fusobacterium (F.) necrophorum, and Porphyromonas (P.) levii by PCRs. BDD-HFA lesions contained Treponema pedis as revealed by genetic identities of 93, 99, and 100%. Treponemes in the BDD-SU lesion were 94% homologous to Treponema phylotype PT3. Fluorescent in situ hybridization (FISH) revealed extensive epidermal infiltration by treponemes that made up > 90% of the total bacterial population in all four lesions. FISH also tested positive for P. levii and negative for F. necrophorum in all four cases, whilst only one BDD-HFA contained Dichelobacter nodosus. Our data point to BDD-associated treponemes and P. levii constituting potential etiological agents in the development of “non-healing” claw horn lesions in cattle.
Introduction
Bovine digital dermatitis (BDD) was first described by Cheli and Mortellaro in Italy in 1974 (1). Since then, the prevalence of the disease has continuously increased in many countries (2, 3). The etiology of BDD is polymicrobial (4), with specific Treponema spp. being most commonly associated with disease development and progression (5–7). BDD typically involves the digital skin and is often classified using the M-stage scoring system (8). An increase in the prevalence of BDD also contributes to a higher occurrence of secondary infections of the exposed corium by Treponema spp. (9, 10). Clinically, “non-healing” claw horn lesions (NHL) are similar to BDD lesions and characterized by a moist, topical granular appearance and pungent fetid smell (11, 12). In contrast to the classical forms of non-infectious claw horn diseases, Evans et al. (11) reported that NHL are typically more severe, cause chronic lameness, and poorly respond to state-of-art BDD treatment strategies. NHL may persist for many months, and thus, can seriously compromise the welfare of affected animals (13). NHL is also known as “BDD-associated claw horn lesion”. Today, a curative treatment strategy for NHL is available (13–15). The latter consists of extensive debridement of loose horn and infected corium under local anesthesia, followed by topical application of copper sulfate, antibiotics (e.g., oxy-/ chlortetracycline), or salicylic acid under a bandage, and application of a block on the sound partner claw (14).
Molecular studies provide evidence of a strong association of three distinct phylogroups of BDD-associated Treponema spp. with NHL (11, 16, 17). The objective of the herein-reported study was to characterize four NHL cases in Swiss dairy cows with respect to their clinical appearance, histopathological features, and bacterial infection by a combination of histopathological and molecular biological techniques.
Materials and methods
Case history
Two NHL-affected cows from the same farm were presented at the Clinic of Ruminants, Vetsuisse Faculty, University Zurich, one NHL case was diagnosed at the Clinic of Ruminants, Vetsuisse Faculty, University of Bern, and one NHL case was detected during a herd health visit at a dairy farm located in the canton of Fribourg, Switzerland.
Respiratory rate (30, 32, and 32) was assessed by observation of thoracic movements, heart rate (92, 72, and 60) was assessed by cardiac auscultation in cows 1, 2, and 3, respectively. The rectal body temperature (38.4, 38.2, 38.4, and 38.8°C) was measured with a rectal thermometer in cows 1, 2, 3, and 4, respectively.
All cows showed mild to moderate lameness for several weeks (locomotion score ranged between 2 and 3; Table 1) according to Sprecher et al. (19), with 1 = non-lame to 5 = severely lame. After applying a wooden block on the partner claw and cleansing of the affected area with 1%-povidone-iodine or Octenidine solution (Octenisan® Schülke und Mayr AG, Frauenfeld, Switzerland), all lesions were treated by extensive surgical debridement including the removal of loose horn and removal of granulation tissue under three-point local anesthesia using lidocaine hydrochloride (Lidocaine 2%, Streuli Tiergesundheit AG, Uznach, Switzerland). Thereafter, a wound dressing with diluted oxytetracycline (Engemycin® 10% injectable solution, MSD Animal Health GmbH, Luzern, Switzerland) or an octenidine ointment (Octenisept® Gel, Schülke und Mayr Ag, Frauenfeld, Switzerland) was administered topically onto all BDD-HFA, and bandages were applied. Treatment was repeated every 2–3 days until the wound healed, and then at weekly intervals until defects were covered by a sufficient and stable new horn.
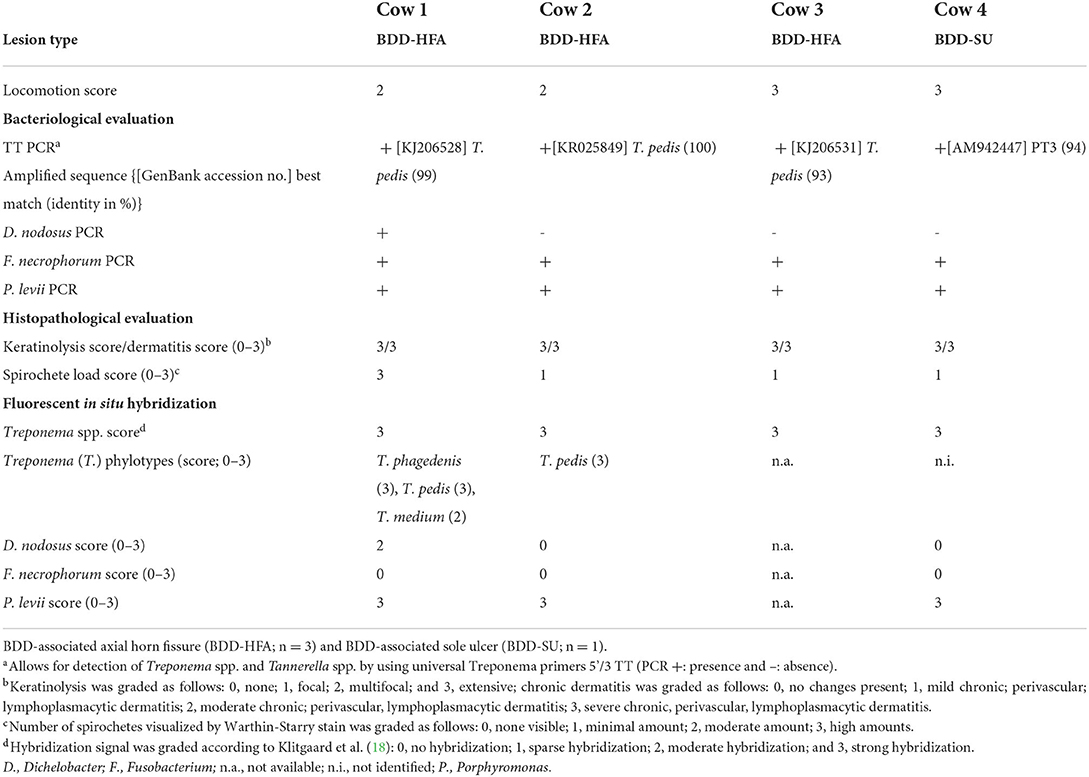
Table 1. Locomotion score and results of bacteriological and histopathological investigations as well as fluorescent in situ hybridization of biopsies collected from four non-healing lesions associated bovine digital dermatitis (BDD).
Management of the BDD-SU consisted of removal of granulation tissue under regional intravenous anesthesia using 20 mL of lidocaine hydrochloride, application of povidone-iodine ointment (Betadine®, Covetrus AG, Lyssach, Switzerland) under the bandage and weekly bandage changes until defects were covered by new horn.
Sample collection, processing, and DNA extraction
Affected feet were washed with 1%-povidone-iodine or octenidine solution. Subsequently, lesional tissue was obtained in the course of therapeutic debridement. Each tissue sample was longitudinally bidissected on a sterile petri dish using sterile forceps and #11 scalpel blades.
Sample processing for both molecular biological and histopathological analyses was done as described previously by Alsaaod et al. (20). For histopathological evaluation, one sample section was fixed in 10% neutral buffered formalin, embedded in paraffin, cut, and stained with hematoxylin and eosin or with the Warthin-Starry stain.
From the other sample section, DNA was extracted using a DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's protocols. Purified DNA samples were stored at −20°C until further use.
Histopathological evaluation
Histopathological evaluation was performed according to Read and Walker (21) modified by Klitgaard (18) to classify epidermal and dermal changes. Characteristic BDD-associated changes commonly include (i) a focally circumscribed hyperplastic epidermis with or without parakeratotic papillomatous proliferation, (ii) loss of the stratum granulosum, and/or iii) dermal inflammation. Histopathological scoring also took degrees of keratinolysis and chronic, perivascular, lymphoplasmacytic dermatitis into account. Keratinolysis was graded as follows: 0 = none, 1 = focal, 2 = multifocal, and 3 = extensive. Chronic dermatitis was classified as follows: 0 = no changes present, 1 = mild chronic, perivascular, lymphoplasmacytic dermatitis, 2 = moderate chronic, perivascular, lymphoplasmacytic dermatitis, and 3 = severe chronic, perivascular, lymphoplasmacytic dermatitis. The number of spirochetes visualized by Warthin-Starry stain was semi-quantitatively categorized as 0 = non-visible, 1 = minimal amount, 2 = moderate amount, and 3 = high amount of spirochetes.
PCR assays and sequencing
DNA extracts were screened for the presence of treponemal DNA using consensus “total” Treponema (TT) primers 5′/3′ (TT-PCR) designed by Moe et al. (22) according to an optimized protocol (23), which is described in detail by Alsaaod et al. (20). For each PCR, sterile water, confirmed Treponema-free equine skin DNA, and Treponema DNA-positive BDD DNA were co-analyzed as no-template, negative, and positive control, respectively. Amplification products (corresponding to the most abundant spirochetal DNA per sample) were analyzed by gel electrophoresis and visualized by ethidium bromide staining, with a GeneRuler 100 bp DNA ladder (ThermoScientific, Vienna, Austria) serving as molecular weight marker. Amplicon aliquots of anticipated size were gel-purified using a QIAex II gel extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and then subjected to direct bidirectional sequencing (Eurofins Genomics) using Treponema primers 5′/3′ TT (10 pmol/μl). After alignment of positive and negative strand sequences, only the 5'/3'matching DNA sequence was subjected to BLAST alignment to search for highly homologous bacterial sequences (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Furthermore, NHL-derived DNA aliquots were subjected to specific Dichelobacter (D.) nodosus, and Fusobacterium (F.) necrophorum PCRs as described by Sullivan et al. (24). For detection of Porphyromonas (P.) levii, primers for amplification of a 16s rRNA gene fragment were designed according to P. levii reference strain GU454798. The 5' primer sequence (F-Primer 677) was 5'-AAGGCAGCTTACAAAAGTGTA-3' and the 3' primer sequence (R-Primer 812) was 5'-TTTCGCTTGAGAGCATACAT-3'. Each PCR contained 10 μl GoTaq® Green Master Mix (Promega AG, Switzerland), 0.1 μl of each primer, and 1 μl of PCR template. The reaction mixtures were heated to 95 °C for 5 min, then cycled 35 times at 95 °C for 1 min, 54 °C for 1 min, and 72 °C for 2 min, followed by incubation at 72 °C for 5 min. As a positive control, skin samples from sheep (D. nodosus) and cattle (F. necrophorum and P. levii) were included, and sterile water was used as no-template control.
Fluorescent in situ hybridization
For FISH analysis, serial 4-μm sections were prepared from formalin-fixed, paraffin-embedded biopsies and hybridized as described previously (25). In brief, hybridization was carried out at 45 °C for 16 h and a final probe concentration of 5 ng/μL. After hybridization, the slides were washed three times in pre-warmed (45 °C) hybridization buffer for 3 minutes and subsequently with washing buffer at the same time intervals. The sections were rinsed in water, air dried, and mounted in Vectashield (Vector Laboratories Inc., Burlingame, CA, USA) for fluorescence microscopy. The probe for domain bacteria was 5' labeled with fluorescein isothiocyanate (FITC) and all other bacteria probes were 5' labeled with the isothiocyanate derivative Cy3 (Eurofins MWG Operon, Ebersberg, Germany).
The oligonucleotide probes used in this study are listed in Supplementary Table S1 and included probes specific for the domain Bacteria, F. necrophorum, D. nodosus, P. levii, the genus Treponema, and four different Treponema phylotypes (i.e., T. pedis, T. phagedenis, T. medium, and T. refringens). The hybridization signal was scored from 0 to 3 according to Klitgaard et al. (18), where 0 = no hybridization, 1 = sparse hybridization, 2 = moderate hybridization, and 3 = strong hybridization.
Results
Clinical findings
BDD-HFA and BDD-SU lesions were severe, penetrating the horn capsule, and involving the corium. Lesions exhibited hypergranulation tissue covering the axial horn fissure (n = 3) or the plantar sole area (n = 1) and were classified according to the International Committee for Animal Recording Claw Health Atlas Kofler et al., (13) as BDD-associated axial horn fissures (BDD-HFA; Figure 1A), and BDD-associated sole ulcer (BDD-SU; Figure 1B), respectively.
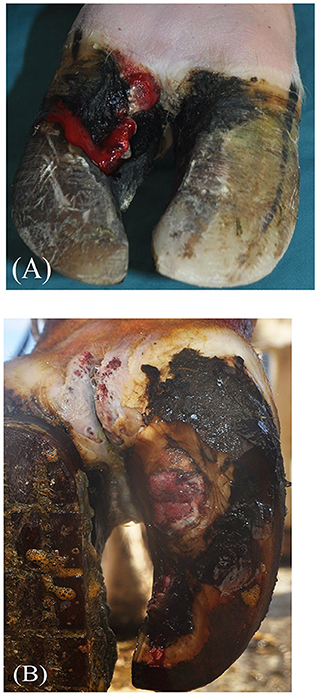
Figure 1. Gross pathology of bovine digital dermatitis (BDD)-associated axial horn fissure [BDD-HFA; (A)] and BDD-associated sole ulcer (BDD-SU) before treatment (B).
Figure 1 exemplarily depicts the gross pathological features of one case of BDD-HFA (A) and BDD-SU (B). The treatment duration ranged between 2 and 4 weeks until defects were covered by a sufficient and stable new horn.
Histopathological findings
All four lesions exhibited severe multifocal keratinolysis of the stratum corneum with ulceration (Figures 2A,B) as well as loss of the stratum granulosum. In three cases, the epidermis was acanthotic. Warthin-Starry stain pointed to the presence of spirochetes on the surface and within the epidermis in all samples, with spiral bacteria being observed (Table 1). All lesions also displayed severe, chronic lymphoplasmacytic perivascular to interstitial dermatitis.
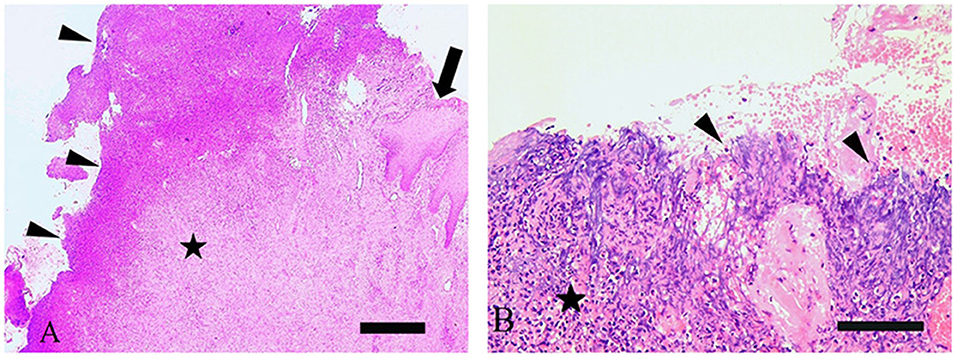
Figure 2. (A) Histological investigation of bovine digital dermatitis (BDD)-associated axial horn fissure revealed severe fibrosis (star) of dermis, focal areas with nests of epidermis (arrow) and large areas with ulceration covered by cellular debris (arrowheads). H&E, bar 500 μm. (B) BDD-associated sole ulcer covered by cellular debris (arrowheads), superficial bleeding and severe fibrosis (stars) of dermis. H&E, bar 200 μm.
PCR detection and sequencing results
All lesions tested positive by total Treponema PCR. Amplicon sequencing and subsequent BLAST alignment resulted in the identification of three T. pedis sequences from BDD-HFA lesions, showing 99% identity with T. pedis strain G2JD, 100% identity with T. pedis strain DD3F, and 93% identity with T. pedis strain G9JD, respectively. The amplicon detected from the BDD-SU lesion was 94% homologous to Treponema phylotype PT3 AM942447 (18). All lesions also tested positive for F. necrophorum and P. levii, whilst only one BDD-HFA scored positive for D. nodosus (Table 1). TT PCR from no-template, negative, and positive control yielded the expected results, thus confirming the authenticity of obtained results.
FISH results
FISH analysis revealed severe, extensive epidermal infiltration (score 3) by treponemes that made up 90% of the total bacterial population in all four cases (Figure 3A). Additional FISH analysis of Treponema spp. positive biopsy samples revealed a mixed infection with T. phagedenis, T. pedis, and T. medium in one BDD-HFA, whereas another BDD-HFA was only infected by T. pedis (Figure 3B). BDD-SU tissue tested FISH positive for the genus Treponema but was negative for all four Treponema phylotypes assessed. Moderate hybridization for D. nodosus was only noted in one case of BDD-HFA. All three BDD-HFA lesions exhibited a strong hybridization signal for P. levii (Figure 3C). F. necrophorum could not be detected in any of the lesions (Table 1).
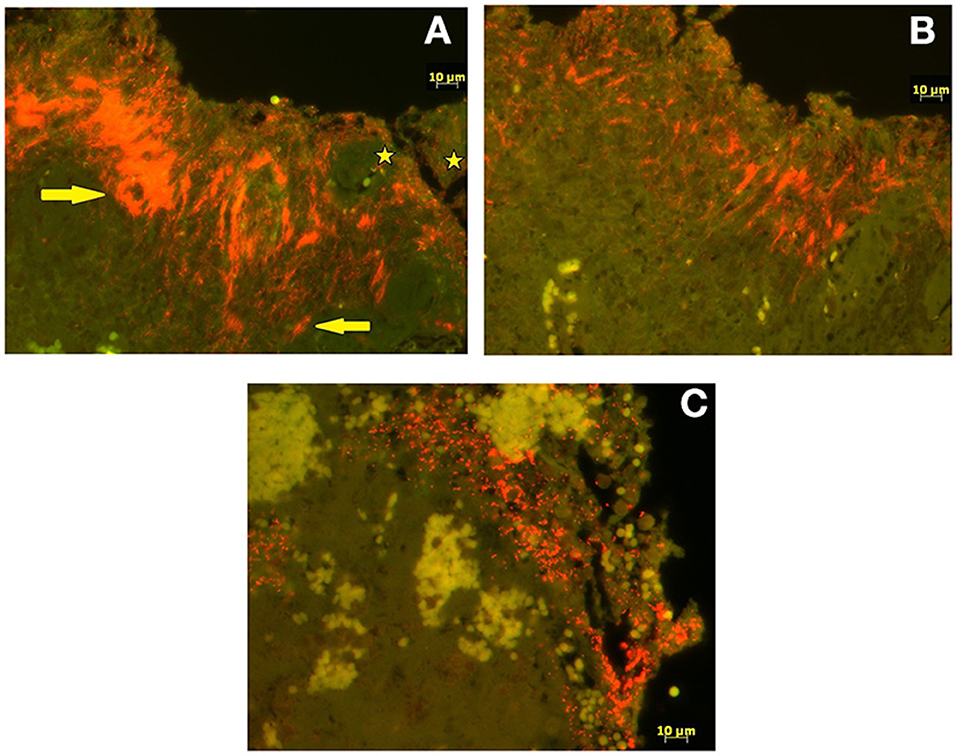
Figure 3. Fluorescent in situ hybridization. (A) Double fluorescent in situ hybridization with probe for genus Treponema (Cy3 labeled; orange) and for domain Bacterium (fluorescein labeled; bright green). Treponema organisms (arrows) infiltrating deep into the epidermis whereas other bacteria (stars) are seen superficially. (B) Single fluorescent in situ hybridization (Cy3 labeled; orange) for species specific oligonucleotide probes for Treponema pedis. (C) Single fluorescent in situ hybridization (Cy3 labeled; orange) for species specific oligonucleotide probes for Porphyromonas levii.
Discussion
Reports on the prevalence and biopathological features of bovine NHL have increased over the past years. Yet, many aspects of the etiopathogenesis of the disease remain unclear. To our knowledge, to date, NHL has not been histopathologically analyzed in combination with molecular biological techniques.
All four BDD-NHL-affected dairy cows originated from herds with a history of BDD and were kept in a freestall systems. Endemically BDD-affected herds are described to be at higher risk of developing NHL (9, 10, 26).
Severe multifocal keratinolysis of the stratum corneum, ulceration and severe, chronic lymphoplasmacytic perivascular to interstitial dermatitis in concert with spirochetal colonization were the predominant histological features noted in all four cases. These findings are consistent with the typical pathological features of BDD (18, 27).
Treponema spp. have been reported as a cause of contagious ovine digital dermatitis (CODD) in sheep (28, 29). In small ruminants and wildlife elk, treponemal infections typically lead to dermatitis along the sole, which can result in complete hoof avulsion in severe cases (29, 30). More recently, BDD-associated Treponema spp. were detected in captive European bison (Bison bonasus) in Switzerland (31). In agreement with Evans et al. (11) and Sykora et al. (17), treponemal DNA was detected in all cases of NHL in our study. In the study by Sykora et al. (17), T. medium was almost exclusively found in BDD-WLD and BDD-SU, whereas T. pedis was equally detected in common BDD lesions and NHL. As a result, it was proposed that T. medium might play a key role in the pathogenesis of BDD-WLD and BDD-SU. In the present study, T. medium was only identified in one case of BDD-HFA. Two of the four cases were positive for T. pedis as confirmed by FISH and PCR followed by amplicon sequencing. Therefore, further research is needed to clarify the role of different Treponema spp. by screening different types of NHL vs. common BDD lesions using Treponema spp.-specific detection methods.
P. levii is a pleomorphic, gram-negative, anaerobic, rod-shaped bacterium possibly associated with bovine metritis (32), bovine necrotic vulvovaginitis (33), BDD (34) and bovine interdigital phlegmon (35). Whilst there is a lack of information regarding the presence of P. levii in bovine NHL, Staton et al. (36) identified P. endodontalis [which is highly associated with chronic oral infections in humans, (37)] by PCR in the vast majority of BDD-WLD and BDD-SU lesions. Thus, it was suggested that P. endodontalis could chiefly contribute to the development of NHL. Since P. levii was detected in three out of four NHL lesions as revealed by a strong hybridization signal, the assumption is supported that P. levii might indeed have a pathobiological role in BDD onset and progression. Further studies on higher numbers of NHL cases are needed to understand the role of P. levii in this and other types of claw diseases.
F. necrophorum was frequently found in BDD-WLD, BDD-SU, and BDD-associated toe necrosis in a previous study (36). These findings contrast with our results, as we did not detect F. necrophorum in the investigated NHL lesions. We hence question the proposed theory on a causative role of F. necrophorum in the pathogenesis of NHL.
D. nodosus was only detected in one case of BDD-HFA, and the hybridization signal was moderate. This finding is in line with the observations by Staton et al. (36) who detected these bacteria in only 2/10 NHL lesions screened. While sheep are the primary hosts for benign and virulent strains of D. nodosus involved in the multifactorial pathogenesis of ovine footrot (38), cattle typically host benign D. nodosus strains (39). Therefore, a relevant role in the development of NHL seems unlikely.
One main advantage of FISH as a culture-independent molecular biological method compared to PCR techniques is that it can localize bacterial infection. Using FISH, a distinction can be made between superficial colonization and invasion of deeper epidermal layers, and well-established infection scoring methods can be used (18, 25). Therefore, FISH constitutes a valuable tool to assess the involvement of different pathogens in the development of NHL, especially in combination with highly sensitive amplification techniques.
In conclusion, P. levii was detected in three of four cases of NHL, suggesting a potential synergistic activity of P. levii and BDD-associated treponemes in the development of bovine NHL. In contrast, present results do not allow a central role to be assigned to F. necrophorum or D. nodosus in the pathogenesis of NHL. The synergistic activity of P. levii and BDD-associated treponemes in the development of bovine NHL should be further investigated by the inclusion of a higher number of cases to show a consolidated association. Still, the results presented in this report provide new insights into the etiopathogenesis of NHL and encourage further research on this relevant claw disease.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the animal study because lesional tissue was obtained in the course of therapeutic debridement. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MA was responsible for data collection, molecular analyses, and writing the first draft of the manuscript. ES and DD supported the data collection. CG performed the histological analyses. TJ and SB supported the data analyses. AS, SB, and JW edited the manuscript. AS supervised the study. All authors contributed to the manuscript and approved the final version.
Funding
This work was supported by the Heard Health Management Initiator Grant, Institute of Animal Pathology and Clinic for Ruminants (Vetsuisse Faculty, University of Bern, Switzerland).
Acknowledgments
We would like to thank the staff of the Department of Farm Animal Clinic in Zürich and the Clinic for Ruminants in Bern for their support in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1041215/full#supplementary-material
References
1. Cheli R, Mortellaro C. La dermatite digitale del bovino. In: The 8th International Conference on Diseases of Cattle. Piacenza, Milan (1974).
2. Holzhauer M, Hardenberg C, Bartels CJ, Frankena K. Herd- and cow-level prevalence of digital dermatitis in the netherlands and associated risk factors. J Dairy Sci. (2006) 89:580–8.
3. Solano L, Barkema HW, Mason S, Pajor EA, LeBlanc SJ, Orsel K. Prevalence and distribution of foot lesions in dairy cattle in Alberta, Canada. J Dairy Sci. (2016) 99:6828–41. doi: 10.3168/jds.2016-10941
4. Krull AC, Shearer JK, Gorden PJ, Scott HM, Plummer PJ. Digital dermatitis: Natural lesion progression and regression in Holstein dairy cattle over 3 years. J Dairy Sci. (2016) 99:3718–31. doi: 10.3168/jds.2015-10535
5. Nordhoff M, Moter A, Schrank K, Wieler LH. High prevalence of treponemes in bovine digital dermatitis-a molecular epidemiology. Vet Microbiol. (2008) 131:293–300. doi: 10.1016/j.vetmic.2008.04.019
6. Evans NJ, Brown JM, Demirkan I, Murray RD, Birtles RJ, Hart CA, et al. Treponema pedis sp.nov, a spirochaete isolated from bovine digital dermatitis lesions. Internat J Syst Evul Microbiol. (2009) 59:987–91. doi: 10.1099/ijs.0.002287-0
7. Alsaaod M, Locher I, Jores J, Grimm P, Brodard I, Steiner A, et al. Detection of specific Treponema species and Dichelobacter nodosus from digital dermatitis (Mortellaro's disease) lesions in Swiss cattle. Schweiz Arch Tierheilkd. (2019) 161:207–15. doi: 10.17236/sat00201
8. Döpfer D, Koopmans A, Meijer FA, Szakáll I, Schukken YH, Klee W, et al. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet Rec. (1997) 140:620–3. doi: 10.1136/vr.140.24.620
9. Kofler J. Nicht-heilende Klauenhorndefekte heilen – Therapie einer neuen Form der Mortellaro-Krankheit. Klauentierpraxis. (2016) 57–65.
10. Blowey RW. Non-healing hoof lesions in dairy cows. Vet Rec. (2011) 169:534. doi: 10.1136/vr.d7267
11. Evans NJ, Blowey RW, Timofte D, Isherwood DR, Brown JM, Murray R, et al. Association between bovine digital dermatitis treponemes and a range of ‘non-healing' bovine hoof disorders. Vet Rec. (2011) 168:214–7. doi: 10.1136/vr.c5487
12. Blowey RW. Non-healing hoof lesions in dairy cows. Vet Rec. (2012) 170:26–7. doi: 10.1136/vr.e32
13. Kofler J, Fiedler A, Charfeddine N, Capion N, Fjeldaas T, Cramer G. ICAR Claw Health Atlas – Appendix 2: Digital Dermatitis-associated Claw Horn Lesions. (2020). Available online at: http://www.icar.org/Documents/ICAR-Claw-Health-Atlas-Appendix-2-DD-associated-Claw-Horn-Lesions.pdfs (accessed September 30, 2022)
14. Kofler J, Glonegger-Reichert J, Dietrich J, Sykora S, Tichy A, Brandt S. A simple surgical treatment for bovine digital dermatitis-associated white line lesions and sole ulcers. Vet J. (2015) 204:229–31. doi: 10.1016/j.tvjl.2015.03.016
15. Nouri M, Helan JA. Clinical and gross pathologic findings of complicated vertical fissures with digital dermatitis in a dairy herd. Vet Res Forum. (2012) 3:291–5.
16. Evans NJ, Brown JM, Demirkan I, Murray RD, Vink WD, Blowey RW, et al. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet Microbiol. (2008) 130:141–50. doi: 10.1016/j.vetmic.2007.12.019
17. Sykora S, Kofler J, Glonegger-reichert J, Dietrich J, Auersperg G, Brandt S. Treponema DNA in bovine ‘non-healing' versus common sole ulcers and white line disease. Vet J. (2015) 205:417–20. doi: 10.1016/j.tvjl.2015.05.023
18. Klitgaard K, Boye M, Capion N, Jensen TK. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J Clin Microbiol. (2008) 46:3012–20. doi: 10.1128/JCM.00670-08
19. Sprecher DJ, Hostetler DE, Kaneene JB. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology. (1997) 47:1179–87. doi: 10.1016/S0093-691X(97)00098-8
20. Alsaaod M, Jensen TK, Miglinci L, Gurtner C, Brandt S, Plüss J, et al. Proof of an optimized salicylic acid paste-based treatment concept of ulcerative M2-stage digital dermatitis lesions in 21 dairy cows. PLoS ONE. (2022) 17:e0269521. doi: 10.1371/journal.pone.0269521
21. Read DH, Walker RL. Papillomatous digital dermatitis (footwarts) in California dairy cattle: clinical and gross pathologic findings. J Vet Diagn Invest. (1998) 10:67–76. doi: 10.1177/104063879801000112
22. Moe KK, Yano T, Kuwano A, Sasaki S, Misawa N. Detection of treponemes in canker lesions of horses by 16S rRNA clonal sequencing analysis. J Vet Med Sci. (2010) 72:235–9. doi: 10.1292/jvms.09-0404
23. Sykora S, Brandt S. Occurrence of Treponema DNA in equine hoof canker and normal hoof tissue. Equine Vet J. (2014) 47:627–30. doi: 10.1111/evj.12327
24. Sullivan LE, Evans NJ, Blowey RW, Grove-White DH, Clegg SR, Duncan JS, et al. A molecular epidemiology of treponemes in beef cattle digital dermatitis lesions and comparative analyses with sheep contagious ovine digital dermatitis and dairy cattle digital dermatitis lesions. Vet Microbiol. (2015) 178:77–87. doi: 10.1016/j.vetmic.2015.04.011
25. Rasmussen M, Capion N, Klitgaard K, Rogdo T, Fjeldaas T, Boye M, et al. Bovine digital dermatitis: possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet Microbiol. (2012) 160:151–61. doi: 10.1016/j.vetmic.2012.05.018
26. Gomez A, Döpfer D, Cook NB, Burgi K, Socha M. Non-healing hoof lesions in dairy cows. Vet Rec. (2011) 169:642. doi: 10.1136/vr.d7969
27. Moreira TF, Facury Filho EJ, Carvalho AU, Strube ML, Nielsen MW, Klitgaard K, et al. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. PLoS One. (2018) 13:e0193870. doi: 10.1371/journal.pone.0193870
28. Sayers G, Marques PX, Evans NJ, O'Grady L, Doherty ML, Carter SD, et al. Identification of spirochetes associated with contagious ovine digital dermatitis. J Clin Microbiol. (2009) 47:1199–201. doi: 10.1128/JCM.01934-08
29. Tegtmeyer PC, Staton GJ, Evans NJ, Rohde J, Punsmann TM, Ganter M. First cases of contagious ovine digital dermatitis in Germany. Acta Vet Scand. (2020) 62:46. doi: 10.1186/s13028-020-00544-0
30. Han S, Mansfield KG. Severe hoof disease in free-ranging Roosevelt elk (Cervus elaphus roosevelti) in southwestern Washington, USA. J Wildl Dis. (2014) 50:259–70. doi: 10.7589/2013-07-163
31. Hoby S, Jensen TK, Brodard I, Gurtner C, Eicher R, Steiner A, et al. Detection of treponemes in digital dermatitis lesions of captive European bison (Bison bonasus). PLoS ONE. (2021) 16:e0255921. doi: 10.1371/journal.pone.0255921
32. Santos TMA, Gilbert RO, Bicalho RC. Metagenomic analysis of the uterine bacterial microbiota in healthy and metritic postpartum dairy cows. J Dairy Sci. (2011) 94:291–302. doi: 10.3168/jds.2010-3668
33. Elad D, Friedgut O, Alpert N, Stram Y, Lahav D, Tiomkin D, et al. Bovine necrotic vulvovaginitis associated with porphyromonas levii. Emerg Infect Dis. (2004) 10:505–7. doi: 10.3201/eid1003.020592
34. Nielsen MW, Strube ML, Isbrand A, Al-Medrasi WDHM, Boye M, Jensen TK, et al. Potential bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet Microbiol. (2016) 186:139–49. doi: 10.1016/j.vetmic.2016.03.003
35. Sweeney M, Watts J, Portis E, Lucas M, Nutsch R, Meeuwse D, et al. Identification of Porphyromonas levii isolated from clinical cases of bovine interdigital necrobacillosis by 16S rRNA sequencing. Vet Ther. (2009) 10:E1–E10.
36. Staton GJ, Sullivan LE, Blowey RW, Carter SD, Evans NJ. Surveying bovine digital dermatitis and non-healing bovine foot lesions for the presence of Fusobacterium necrophorum, Porphyromonas endodontalis and Treponema pallidum. Vet Rec. (2020) 186:450. doi: 10.1136/vr.105628
37. Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. (2003) 82:338–44. doi: 10.1177/154405910308200503
38. Kennan RM, Han X, Porter CJ, Rood JI. The pathogenesis of ovine footrot. Vet Microbiol. (2011) 153:59–66. doi: 10.1016/j.vetmic.2011.04.005
39. Arduser F, Moore-Jones G, Gobeli Brawand S, Durr S, Steiner A, Ryser-Degiorgis MP, et al. Dichelobacter nodosus in sheep, cattle, goats and South American camelids in Switzerland-Assessing prevalence in potential hosts in order to design targeted disease control measures. Prev Vet Med. (2020) 178:104688. doi: 10.1016/j.prevetmed.2019.05.001
Keywords: bovine digital dermatitis, dairy cow, fluorescent in situ hybridization, treponemes, PCR
Citation: Alsaaod M, Weber J, Jensen T, Brandt S, Gurtner C, Devaux D, Studer E and Steiner A (2022) “Non-healing” claw horn lesions in dairy cows: Clinical, histopathological and molecular biological characterization of four cases. Front. Vet. Sci. 9:1041215. doi: 10.3389/fvets.2022.1041215
Received: 10 September 2022; Accepted: 26 September 2022;
Published: 19 October 2022.
Edited by:
Mohamed Zeineldin, Animal and Plant Health Inspection Service (USDA), United StatesReviewed by:
Hussein M. El-Husseiny, Tokyo University of Agriculture and Technology, JapanMohamed Ghanem, Benha University, Egypt
Copyright © 2022 Alsaaod, Weber, Jensen, Brandt, Gurtner, Devaux, Studer and Steiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maher Alsaaod, bWFoZXIuYWxzYWFvZEB2ZXRzdWlzc2UudW5pYmUuY2g=
 Maher Alsaaod
Maher Alsaaod Jim Weber1
Jim Weber1 Sabine Brandt
Sabine Brandt Adrian Steiner
Adrian Steiner