- Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University, Wuhan, China
Porcine epidemic diarrhea virus (PEDV) has become a challenging problem in pig industry all over the world, causing significant profit losses. Tannins and organic zinc have been shown to exert protective effects on the intestinal dysfunction caused by endotoxins. However, there is little information on tannic acid-chelated zinc (TAZ) supplementation in the diet of newborn piglets. This study was conducted to determine the effects of TAZ on the intestinal function of piglets infected with PEDV. Thirty-two 7-day-old piglets were randomly allocated to 1 of 4 treatments in a 2 × 2 factorial design consisting of 2 diets (0 or 50 mg/kg BW TAZ) and challenge (saline or PEDV). On day 9 of the trial, 8 pigs per treatment received either sterile saline or PEDV solution at 106 TCID50 (50% tissue culture infectious dose) per pig. Pigs infected with PEDV had greater diarrhea rate and lower average daily gain (ADG) (P < 0.05). PEDV infection decreased plasma D-xylose concentration, most antioxidative enzyme activities in plasma and intestine, as well as the small intestinal villus height (P < 0.05). Plasma diamine oxidase and blood parameters were also affected by PEDV infection. Dietary supplementation with TAZ could ameliorate the PEDV-induced changes in all measured variables (P < 0.05). Moreover, TAZ decreased the concentration of malondialdehyde in plasma, duodenum, jejunum, and colon (P < 0.05). Collectively, our results indicated that dietary TAZ could alleviate PEDV induced damage on intestinal mucosa and antioxidative capacity, and improve the absorptive function and growth in piglets. Therefore, our novel findings also suggest that TAZ, as a new feed additive for neonatal and weaning piglets, has the potential to be an alternative to ZnO.
Introduction
Porcine epidemic diarrhea virus (PEDV) spreads through feed and fecal oral route, which is a main pathogen that causes enteric diseases in swine industry (1). The virus induces apoptosis and necrosis of intestinal epithelium, mainly in the jejunum and ileum, which causes watery malabsorptive diarrhea, vomiting, and high mortality in pigs at all ages, especially during the neonatal and weanling periods (2–5). It has been demonstrated that feed additives, such as organic acids (6), organic trace minerals, medium-chain fatty acids (7), plant extracts (8), amino acid derivates (9) could ameliorate PEDV-infected intestinal injury. However, the mechanism of functional feed additives for prevention and treatment of PEDV-infected intestinal are still lacking.
In current practices, dietary supplementation with pharmacological zinc oxide (ZnO, 1600–2500 mg/kg zinc) in piglets during the first 2 weeks after weaning could prevent diarrhea (10, 11). A previous study conducted by our research team also reported that 100 mg/kg BW ZnO could improve growth performance, intestinal function, and antioxidant capacity in PEDV-infected piglets (12). However, medicinal ZnO in pig production will be disused by 2022 in Europe because of the environmental pollution and antibiotic-resistant issue (13). Therefore, hydrolyzed tannins have been widely used in piglet diets to decrease diarrhea rate, modulate intestinal health, and enhance growth performance (11, 14–16).
Studies have shown that tannic acid has various biological functions such as antioxidative, antibacterial, and antiviral property (17). However, there is still controversy on the effect of tannins in piglets (14, 18). Therefore, in the present study, we evaluated the effect of a new form of organic zinc, which is chelated with tannic acid, on the growth, antioxidative status, intestinal morphology in PEDV-infected piglets. Our findings are expected to explore an alternative to ZnO, and determine the mechanism of tannic acid-chelated zinc (TAZ) in alleviating the negative effects of PEDV in neonatal piglets.
Materials and methods
Animal care and diets
All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee of Wuhan Polytechnic University (Number: 20161121). A total of 32 healthy 7-day-old piglets (Duroc × Landrace × Yorkshire, BW = 2.46 ± 0.21 kg) were used in this experiment. Pigs were housed individually with strict control of cross-infection in two environment-controlled nursery rooms (30 ± 2°C) and given ad libitum access to water throughout the study. The TAZ was obtained from the Animal Nutrition and Intestinal Health Research Group of Wuhan Polytechnic University, which contained ≥80% tannin, 6–7% zinc, crude fiber <2.00%, ash <2.50%, and moisture <8.00%. Piglets were provided a basal diet (liquid milk replacer), which was formulated to meet or exceed the nutrient requirements of suckling piglets. The milk replacer was purchased from Wuhan Anyou Feed Co., Ltd (Wuhan, China). Before feeding, the milk replacer was dissolved in warm water (45–55°C) to form a liquid feed (dry matter content of 20%) (9). Pigs were fed the liquid feed every 3 h between 8:00 am and 8:00 pm.
Experiment design
Pigs were fed the control liquid diet or TAZ-supplemented liquid diet for 9 days before the PEDV challenge (16 pigs per group). Immediately after PEDV challenge, pigs were divided into four treatments in a 2 × 2 factorial design. The main factors consisted of diet (0 or 50 mg/kg BW TAZ supplementation in diet; +TAZ or –TAZ) and challenge (PEDV or saline administration; +PEDV or –PEDV). On day 9 of the experiment, eight pigs in each dietary treatment were orally administered with either PEDV at a dose of 106 TCID50 (50% tissue culture infectious dose) per pig or the same volume of sterile saline (Control). On day 12 of the trial, 10% D-xylose (1 mL/kg BW) was orally administrated to piglets to determine the intestinal absorption capacity and mucosal integrity (9). One hour later, all piglets were weighed and blood samples were collected from the anterior vena cava, and then all pigs were sacrificed under sodium pentobarbital anesthesia (50 mg/kg BW, iv) to obtain intestinal samples (12).
Collection of blood and intestinal samples
As mentioned previously, all blood samples were collected from anterior vena cava of piglets into heparinized vacuum tubes (Becton-Dickinson Vacutainer System, Franklin Lake, NJ, USA) at 1 h post D-xylose administration on day 12 of the trial (19). Blood samples were centrifuged at 3000 rpm for 15 min at 4°C to obtain plasma, which was then stored at −20°C until analysis.
After slaughtering, the pig abdomen was opened immediately and the whole gastrointestinal tract was exposed. The intestine was dissected free of the mesentery and placed on a chilled stainless steel tray. The 1- and 10-cm segments were obtained from the distal duodenum, mid-jejunum, mid-ileum and mid-colon, respectively (19, 20). The 5 cm intestinal segments were flushed gently with ice-cold phosphate buffered saline (PBS, pH = 7.4) and then placed in 4% fresh, chilled formalin solution for histological measurements. The 10-cm segments were opened longitudinally and the contents were flushed with ice-cold PBS. Mucosa was collected by scraping using a sterile glass microscope slide at 4°C, rapidly frozen in liquid nitrogen, and stored at −80°C until analysis. All samples were collected within 15 min after killing.
Growth performance and diarrhea rate
Piglets were weighted on d 0, 9, and 12 of the experiment to calculate the average daily gain (ADG). Health status and diarrhea score were recorded throughout the experimental period. The fecal score was classified into four levels: 0 = strip or granular feces, 1 = soft stool feces, 2 = thick and water feces, and 3 = water feces. Score ≥ 2 was considered diarrhea. The formula of diarrhea rate was given as follows: diarrhea rate (DR) = total number of pigs with diarrhea/(total number of test piglets × test days) × 100% (21).
Blood parameters
The concentrations of blood biochemical parameters, such as total protein (TP), albumin (ALB), aspartate transaminase (AST), alanine transaminase (ALT), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total cholesterol (TC), triacylglycerol (TG), glucose (GLU), calcium (Ca), phosphorus (P), creatinine (CREA), high density lipoprotein (HDL), and low density lipoprotein (LDL) were measured with Wako kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan) using a Hitachi 7060 Automatic Biochemical Analyzer (Hitachi, Tokyo, Japan).
Determination of D-xylose and diamine oxidase activity in plasma
Plasma D-xylose concentration and DAO activity were determined by colorimetric method using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All assays were performed according to the instructions of manufacturer.
Antioxidant capacity in plasma and intestinal mucosa
Plasma, mucosa of duodenum, jejunum, ileum, and colon were used for analysis of antioxidative enzymes and related products. The activities of glutathione peroxidase (GSH-Px), catalase (CAT), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), as well as the concentration of hydrogen peroxide (H2O2) and malondialdehyde (MDA) were determined by using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the protocols of manufacturer (22). Assays were performed in triplicate.
Intestinal histomorphology
Intestinal histomorphology were examined according to the method of Yi et al. (21). Briefly, the fixed intestinal segments were embedded in paraffin. Consecutive 5 μm sections were cut and then stained with haematoxylin and eosin. Intestinal morphology was determined using a light microscope (Leica Microsystems, Wetzlar, Germany) with Leica Application Suite image analysis software (Leica Microsystems, Wetzlar, Germany). The villus height, villus width at half-height, and crypt depth were measured from 10 randomly selected villi and associated crypts on each section at 40 × magnification. Villus height was measured from the tip of villus to the crypt opening and crypt depth was measured from the base of crypt to the level of crypt opening. The villus height/crypt depth ratio and villous surface area were then calculated from these measurements.
Statistical analyses
All data were analyzed by one-way ANOVA using the GLM procedure of SPSS 20.0 software appropriate for a 2 × 2 factorial design (SPSS Inc. Chicago, IL, USA). The statistical model consisted of the effects of diet (+TAZ vs. –TAZ) and challenge (saline vs. PEDV) and their interactions. Data were expressed as means and pooled SEMs. In cases where the differences were significant, the means were compared by Duncan's multiple range test. A value of P < 0.05 were considered significant, and 0.05 ≤ P < 0.10 as trends.
Results
Average daily gain and diarrhea rate
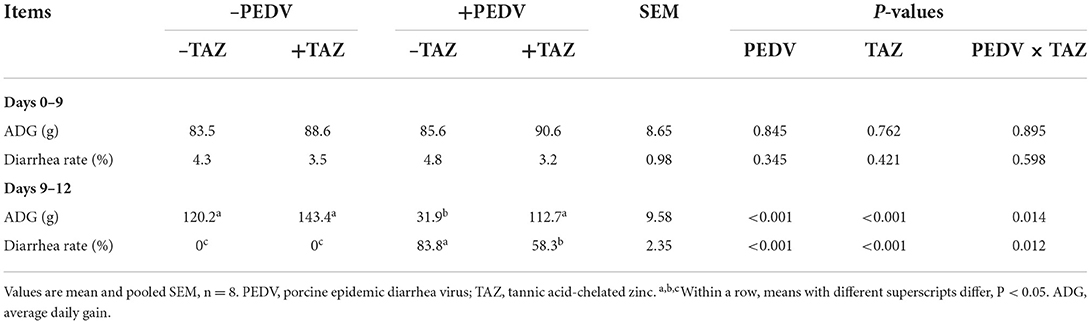
The effect of TAZ on ADG and diarrhea rate in PEDV-infected piglets is shown in Table 1. During days 0–9 (pre-infection), there was no difference in the ADG and DR of pigs fed the control and TAZ-supplemented diets (P > 0.05). During days 9–12 of the trial (post-infection), PEDV infection decreased the ADG, and increased the diarrhea rate (P < 0.05). There were interactive effects between TAZ and PEDV, the TAZ administration mitigated diarrhea and increased the ADG induced by PEDV infection (P < 0.05).
Blood parameters
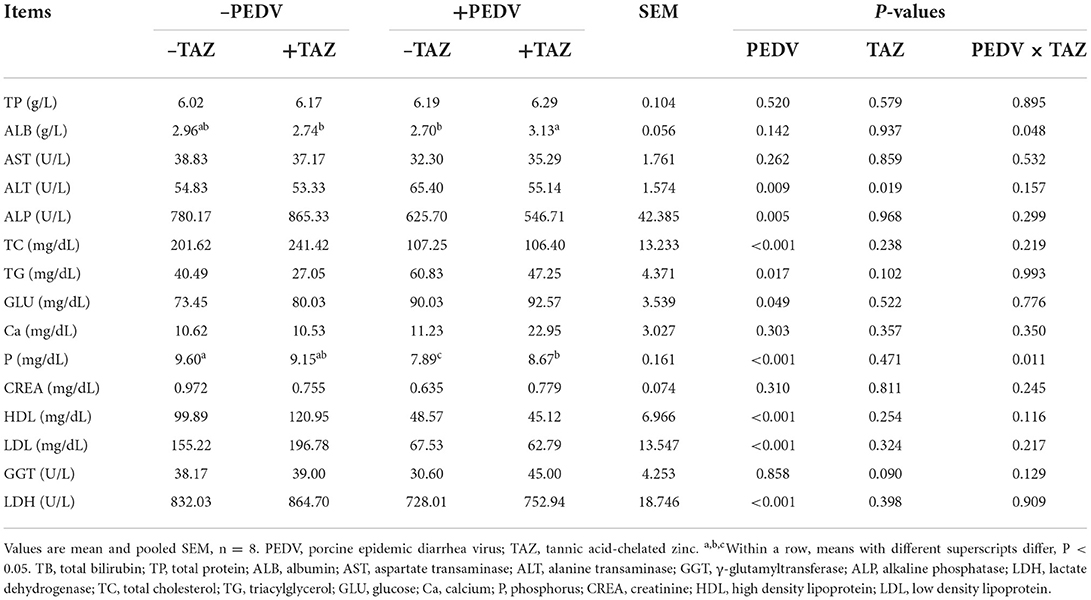
The effect of TAZ on blood parameters in PEDV-infected piglets is shown in Table 2. Compared with non-infected pigs, PEDV-infected piglets had lower concentrations of ALP, TC, P, HDL, LDL, and LDH in plasma, and had greater concentration of ALT, TG and GLU (P < 0.05). Pigs fed the TAZ diet had a lower plasma ALT level, and tended to have greater plasma GGT than the control pigs (P = 0.090). There were interactive effects between PEDV and TAZ on plasma ALB and P concentrations (P < 0.05). The concentrations of ALB and P was increased in pigs infected with PEDV fed the TAZ diet compared with the PEDV-infected pigs fed a diet without TAZ (–TAZ) (P < 0.05), whereas there was no difference in these parameters in saline (–PEDV) treatments (P > 0.05).
Diamine oxidase activity and D-xylose concentration
Data on plasma DAO activity and D-xylose concentration are summarized in Table 3. The PEDV-infected pigs had greater activity of DAO and lower D-xylose concentration in plasma than non-infected pigs (P < 0.05). Pigs fed the TAZ diet showed lower plasma DAO activity and greater D-xylose concentration than pigs in control group (P < 0.05).
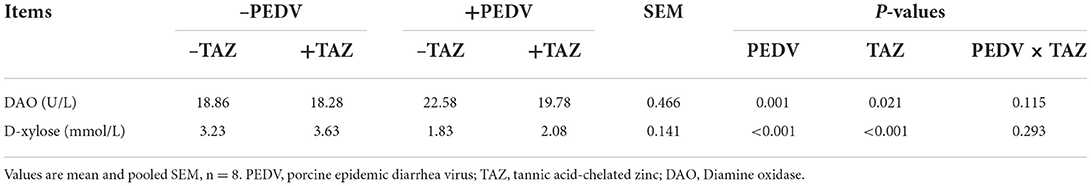
Table 3. The effect of tannic acid-chelated zinc on DAO activity and D-xylose concentration in PEDV-infected piglets.
Plasma antioxidant capacity
The effect of TAZ on plasma antioxidant capacity in PEDV-infected piglets is shown in Table 4. Compared with non-infected pigs, PEDV-infected pigs had lower GSH-Px and T-SOD activity in plasma, and greater H2O2 concentration than those in the control treatment (P < 0.05). The activity of CAT in plasma was increased in TAZ, and the plasma H2O2 and MDA concentration was decreased compared with the control group (P < 0.05). There was PEDV × TAZ interaction on the plasma CAT activity (P < 0.05). The data showed that TAZ supplementation was more effective to increase the activity of CAT in plasma of PEDV-infected pigs than non-infected pigs (P < 0.05). However, the T-AOC in plasma was not affected either by PEDV or dietary TAZ (P > 0.05).
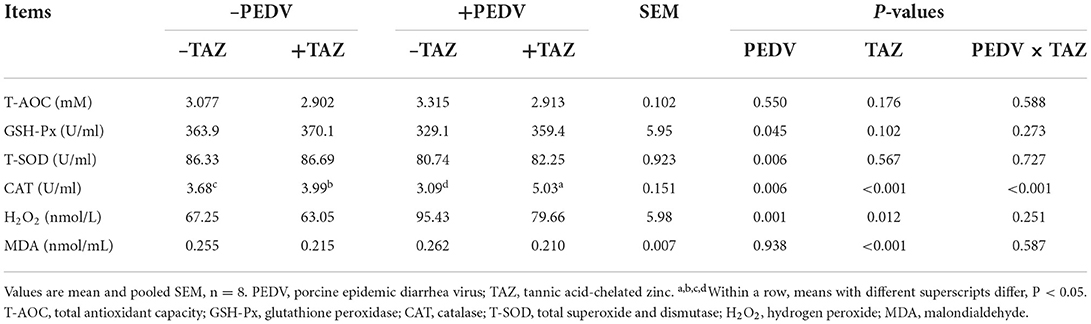
Table 4. The effect of tannic acid-chelated zinc on plasma antioxidant capacity in PEDV-infected piglets.
Intestinal antioxidant capacity
The effect of TAZ on the intestinal antioxidant capacity in PEDV-infected piglets is shown in Table 5. Compared with non-infected pigs, PEDV-infected pigs had lower GSH-Px and CAT activities in duodenum and jejunum, and greater MDA concentration in duodenum than those in the control treatment (P < 0.05). The concentration of H2O2 in colon was tended to increase in PEDV treatments compared with the non-infected pigs (P = 0.076). Pigs fed the TAZ diet had greater T-AOC and GSH-Px in duodenum, jejunum, and colon (P < 0.05), T-SOD in duodenum and jejunum (P < 0.05), tended to have higher CAT in jejunum (P = 0.075), and lower MDA concentration in duodenum, jejunum, and colon (P < 0.05). The colon H2O2 concentration was also decreased in the TAZ treatment compared with the control (P < 0.05). There were PEDV × TAZ interactions on the GSH-Px in duodenum and jejunum, jejunal T-SOD, as well as MDA and H2O2 concentration in colon (P < 0.05).
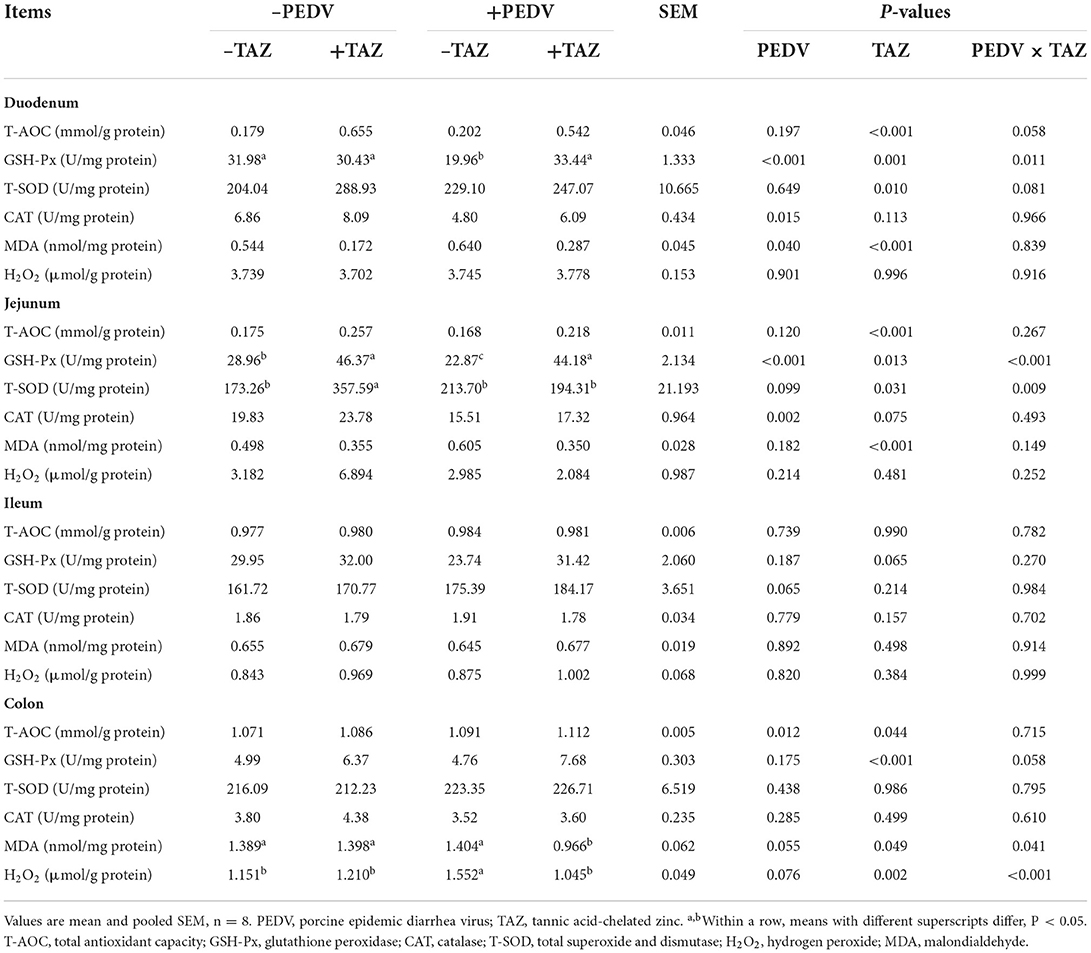
Table 5. The effect of tannic acid-chelated zinc on intestinal antioxidant capacity in PEDV-infected piglets.
Intestinal morphology
Data on the small intestinal histomorphology are summarized in Table 6. PEDV infection decreased villus height, villus height/crypt depth ratio, and villous surface area in all small intestinal segments (P < 0.05), and increased the crypt depth in small intestine and colon (P < 0.05). There were PEDV × TAZ interactions in villus height in the jejunum and villus height/crypt depth ratio in duodenum and jejunum, as well as the crypt depth in the small intestine and colon (P < 0.05). Data indicated that TAZ supplementation could increase the duodenal and jejunal villus height, villus height/crypt depth ratio and villous surface area, and decrease the crypt depth in duodenum, jejunum and colon (P < 0.05), as compared to the control (–TAZ).
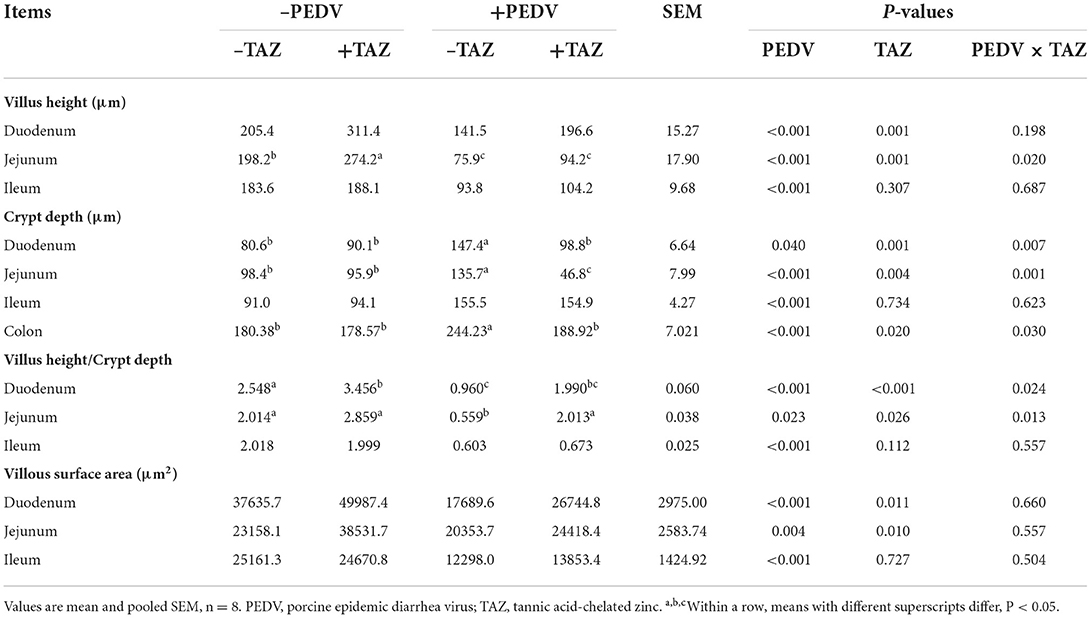
Table 6. The effect of tannic acid-chelated zinc on the intestinal morphology in PEDV-infected piglets.
Discussion
In the last decades, PEDV outbreaks all over the world induced huge economic loses in swine industry (23). Although a series of feed additive were evaluated to prevent PEDV, the results were inconsistent. As a potential alternative to inorganic ZnO to alleviate diarrhea, we set up a PEDV infection model to investigate the protective effect of TAZ on growth, antioxidant capacity and intestinal morphology in piglets.
In the present study, infected pigs exhibited the symptoms of PEDV, such as diarrhea, vomiting and thin intestinal wall. PEDV infection decreased the ADG, and increased the diarrhea rate of piglets, which was consistent with previous studies (24–26). Our previous studies also showed that oral administration of 104.5 TCID50 resulted in retarded growth and sever diarrhea in piglets (9, 12). Dietary TAZ alleviated the ADG reduction caused by infection. These results may be related to the interference effect on the integrity of enveloped structure of PEDV by tannin and zinc, and inhibition of the reproduction of pathogens (27). In agreement with our studies, lots of studies also showed that tannin and zinc (ZnO and organic zinc) improved the growth performance of piglets (11, 14–16). In addition, dietary administration of ZnO decreased the fecal score in a previous study, thus alleviating the ADG reduction caused by PEDV (12). These results indicated that TAZ could be a potential substitute of ZnO to prevent diarrhea and promote growth in neonatal and weaning piglets.
In our study, we found that PEDV increased the concentrations of ALT, TG and GLU in plasma, which indicated that PEDV infection already resulted in inflammatory reaction in piglets. It was reported that injury of gastrointestinal tract and liver could cause the increment of blood ALT and AST, which can sensitively reflect the function of the liver (28, 29). In this study, it was observed that the TAZ groups reduced plasma ALT, indicating that TAZ supplementation may have therapeutic effects on the hepatic architecture and function damage induced by PEDV infection (9). Moreover, the blood P concentration was increased in pigs infected with PEDV fed the TAZ diet, which may indicate that TAZ improve the integrity of intestinal epithelium, leading higher nutrient digestibility.
Plasma DAO activity and D-xylose can be used as indicators for the integrity of intestinal barrier, which is the basis for preventing pathogenic bacteria, virus, and other harmful substances (30, 31). Impaired intestine is a major cause of diarrhea, and the concentration of D-xylose in blood and urine will decrease because of malabsorption, and the activity of DAO will increase after the damage of the intestine mucosa (9, 12, 32). In consistent with previous studies, the plasma D-xylose content was decreased, and the DAO activity was increased after PEDV infection, indicating that PEDV induced intestinal epithelial cell apoptosis and impaired intestinal function. Interestingly, dietary supplementation of TAZ reduced the DAO activity and increased D-xylose concentration in plasma of piglets, indicating that TAZ is beneficial to reduce intestinal permeability, which also further explains the decreased diarrhea rate in the present study.
Oxidative damage of cell and tissues by weanling stress, mycotoxin, and virus is well documented (33–35). In the present study, PEDV challenge decreased plasma and intestinal mucosal GSH-Px, T-SOD, and CAT activities, while increased MDA and H2O2, indicating that PEDV successfully induced humoral and intestinal mucosal oxidative injury in piglets. Interestingly, supplementation with TAZ mitigated these series of oxidative damage. The hydroxyl groups of phenol rings are responsible for a strong antioxidant function of TAZ (36). A series of studies have reported that tannin rich diets could improve the antioxidative capacity in pigs. Furthermore, it was reported that polyphenols extracted from grape seeds, gallnut and chestnut improved the antioxidant status of pigs in challenge models (33, 34, 37). Different forms of zinc, especially the organic zinc sources were also reported to enhance the endogenous antioxidant defenses by acting on antioxidant enzymes and the synthesis of the metallothionein proteins, which are able to scavenge free radicals, such as hydroxyl radicals and reactive oxygen species (38, 39).
Intestinal health is the basis for meet the growth potential of piglets. Villus height, crypt depth and villous surface area are strongly related to the absorptive function, which are well accepted as indicators to reflect the morphological integrity of small intestine in animals (40, 41). In this study, PEDV infection decreased villus height and villus height/crypt depth ratios, and increased crypt depth in all segments of the small intestine, suggesting that PEDV induced intestinal structural damage and increased mucosal permeability, which was in agreement with our previous studies (9, 12). Notably, we found that TAZ supplementation increased villus height, villous surface area, and villus height/crypt depth ratios, as well as the crypt depth in colon. In consistent with our study, some studies on pigs also found that fruits and Chinese medicinal herb original tannins, and zinc could improve these intestinal morphological parameters (12, 33, 42–44). These results could also explain the better growth performance in +TAZ treatments in our study.
Conclusion
In conclusion, we provide significant evidence for the effect of TAZ on growth performance, antioxidant capacity, intestinal morphology in piglets. Supplementation with TAZ could alleviate PEDV-induced growth retardation, oxidative stress, intestinal integrity damage in the neonatal piglets model. Our novel findings also suggest that TAZ, as a new feed additive for neonatal and weaning piglets, has the potential to be an alternative to ZnO.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Wuhan Polytechnic University (Number: 20161121).
Author contributions
ZZ analyzed the data and wrote the manuscript. SW and LZheng conducted the animal experiment and analyzed the data. SG read and revised the manuscript. LZhu, CD, TW, and DY also performed the experiment work. YH and BD designed the study and acquired funding. All authors contributed to the article and approved the submitted version of the manuscript.
Funding
This research was financially supported by the Hubei Provincial Central Leading Local Science and Technology Development Fund Project (No. 2021BGE047).
Acknowledgments
We acknowledge the Wufeng Chicheng Biotechnology Company Limited (Yichang, China) for the Chinese gallnut tannins.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Phillips FC, Rubach JK, Poss MJ, Anam S, Goyal SM, Dee SA, et al. Monoglyceride reduces viability of porcine epidemic diarrhoea virus in feed and prevents disease transmission to post-weaned piglets. Transbound Emerg Dis. (2022) 69:121–7. doi: 10.1111/tbed.14353
2. Kim Y, Lee C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology. (2014) 460:180–93. doi: 10.1016/j.virol.2014.04.040
3. Madson DM, Magstadt DR, Arruda PHE, Hoang H, Sun D, Bower LP, et al. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet Microbiol. (2014) 174:60–8. doi: 10.1016/j.vetmic.2014.09.002
4. Madson DM, Arruda PHE, Magstadt DR, Burrough ER, Hoang H, Sun D, et al. Characterization of porcine epidemic diarrhea virus isolate US/Iowa/18984/2013 infection in 1-Day-Old cesarean-derived colostrum-deprived piglets. Vet Pathol. (2016) 53:44–52. doi: 10.1177/0300985815591080
5. Chen YM, Helm ET, Gabler N, Hostetter JM, Burrough ER. Alterations in intestinal innate mucosal immunity of weaned pigs during porcine epidemic diarrhea virus infection. Vet Pathol. (2020) 57:642–52. doi: 10.1177/0300985820932140
6. Trudeau MP, Verma H, Sampedro F, Urriola PE, Shurson GC, Mckelvey J, et al. Comparison of thermal and non-thermal processing of swine feed and the use of selected feed additives on inactivation of porcine epidemic diarrhea virus (PEDV). PLoS ONE. (2016) 11:1–14. doi: 10.1371/journal.pone.0158128
7. Cochrane RA, Dritz SS, Woodworth JC, Stark CR, Saensukjaroenphon M, Gebhardt JT, et al. Assessing the effects of medium-chain fatty acids and fat sources on PEDV infectivity. Transl Anim Sci. (2020) 4:txz179. doi: 10.1093/tas/txz179
8. Xu ZC, Liu Y, Peng P, Liu YF, Huang MY, Ma YH, et al. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet Microbiol. (2020) 249:108849. doi: 10.1016/j.vetmic.2020.108849
9. Wang L, Zhou J, Hou YQ, Yi D, Ding BY, Xie JQ, et al. N-Acetylcysteine supplementation alleviates intestinal injury in piglets infected by porcine epidemic diarrhea virus. Amino Acids. (2017) 49:1931–43. doi: 10.1007/s00726-017-2397-2
10. Walk CL, Wilcock P, Magowan E. Evaluation of the effects of pharmacological zinc oxide and phosphorus source on weaned piglet growth performance, plasma minerals and mineral digestibility. Animal. (2015) 9:1145–52. doi: 10.1017/S175173111500035X
11. Xu T, Ma X, Zhou X, Qian M, Yang Z, Cao P, et al. Coated tannin supplementation improves growth performance, nutrients digestibility, and intestinal function in weaned piglets. J Anim Sci. (2022) 100:1–12. doi: 10.1093/jas/skac088
12. Zhang Q, Wu T, Li S, Meng Y, Tan Z, Wu M, et al. Protective effect of zinc oxide and its association with neutrophil degranulation in piglets infected with porcine epidemic diarrhea virus. Oxid Med Cell Longev. (2021) 2021:3055810. doi: 10.1155/2021/3055810
13. Satessa GD, Kjeldsen NJ, Mansouryar M, Hansen HH, Bache JK, Nielsen MO. Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets. Animal. (2020) 14:1638–46. doi: 10.1017/S1751731120000154
14. Liu H, Hu J, Mahfuz S, Piao X. Effects of hydrolysable tannins as zinc oxide substitutes on antioxidant status, immune function, intestinal morphology, and digestive enzyme activities in weaned piglets. Animals. (2020) 10:757. doi: 10.3390/ani10050757
15. Dell'Anno M, Reggi S, Caprarulo V, Hejna M, Sgoifo RCA, Callegari ML, et al. Evaluation of tannin extracts, leonardite and tributyrin supplementation on diarrhoea incidence and gut microbiota of weaned piglets. Animals. (2021) 11:1693. doi: 10.3390/ani11061693
16. Song Y, Luo Y, Yu B, He J, Zhen P, Mao X, et al. Tannic acid extracted from gallnut prevents post-weaning diarrhea and improves intestinal health of weaned piglets. Anim Nutr. (2021) 7:1078–86. doi: 10.1016/j.aninu.2021.04.005
17. Myrie SB, Bertolo RF, Sauer WC, Ball RO. Effect of common antinutritive factors and fbrous feedstuffs in pig diets on amino acid digestibilities with special emphasis on threonine. J Anim Sci. (2008) 86:609–19. doi: 10.2527/jas.2006-793
18. Girard M, Thanner S, Pradervand N, Hu D, Ollagnier C, Bee G, et al. Hydrolysable chestnut tannins for reduction of postweaning diarrhea: effcacy on an experimental ETEC F4 model. PLoS ONE. (2018) 13:e0197878. doi: 10.1371/journal.pone.0197878
19. Hou YQ, Wang L, Ding BY, Liu YL, Zhu HL, Liu J, et al. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids. (2010) 39:555–64. doi: 10.1007/s00726-010-0473-y
20. Wang JJ, Chen LX, Li DF, Yin YL, Wang XQ, Li P, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver and skeletal muscle in newborn pigs. J Nutr. (2008) 138:60–6. doi: 10.1093/jn/138.1.60
21. Yi D, Li BC, Hou YQ, Wang L, Zhao D, Chen HB, et al. Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino Acids. (2018) 50:1089–100. doi: 10.1007/s00726-018-2586-7
22. Oskoueian E, Abdullah N, Idrus Z, Ebrahimi M, Goh YM, Shakeri M, et al. Palm kernel cake extract exerts hepatoprotective activity in heat-induced oxidative stress in chicken hepatocytes. BMC Complement Altern Med. (2014) 14:368. doi: 10.1186/1472-6882-14-368
23. Bertolini F, Harding JC, Mote B, Ladinig A, Plastow GS, Rothschild MF, et al. Genomic investigation of piglet resilience following porcine epidemic diarrhea outbreaks. Anim Genet. (2017) 48:228–32. doi: 10.1111/age.12522
24. Curry SM, Gibson KA, Burrough ER, Schwartz KJ, Yoon KJ, Gabler NK, et al. Nursery pig growth performance and tissue accretion modulation due to porcine epidemic diarrhea virus or porcine deltacoronavirus challenge. J Anim Sci. (2017) 95:173–81. doi: 10.2527/jas.2016.1000
25. Curry SM, Burrough ER, Schwartz KJ, Yoon KJ, Lonergan SM, Gabler NK, et al. Porcine epidemic diarrhea virus reduces feed efficiency in nursery pigs. J Anim Sci. (2018) 96:85–97. doi: 10.1093/jas/skx005
26. Wu M, Zhang Q, Yi D, Wu T, Chen H, Guo S, et al. Quantitative proteomic analysis reveals antiviral and anti-inflammatory effects of puerarin in piglets infected with porcine epidemic diarrhea virus. Front Immunol. (2020) 11:169. doi: 10.3389/fimmu.2020.00169
27. Dong G, Liu H, Yu X, Zhang X, Lu H, Zhou T, et al. Antimicrobial and anti-bioflm activity of tannic acid against Staphylococcus aureus. Nat Prod Res. (2018) 32:2225–8. doi: 10.1080/14786419.2017.1366485
28. Yi D, Hou YQ, Wang L, Ding BY, Yang ZG, Li J, et al. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br J Nutr. (2014) 111:46–54. doi: 10.1017/S0007114513002171
29. Jiao Z, Ma Y, Zhang Q, Wang Y, Liu T, Liu X, et al. The adipose-derived mesenchymal stem cell secretome promotes hepatic regeneration in miniature pigs after liver ischaemia-reperfusion combined with partial resection. Stem Cell Res Ther. (2021) 12:218. doi: 10.1186/s13287-021-02284-y
30. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Immunol. (2014) 14:141–53. doi: 10.1038/nri3608
31. Yu J, Song Y, Yu B, He J, Zheng P, Mao X, et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J Anim Sci Biotechnol. (2020) 11:87. doi: 10.1186/s40104-020-00496-5
32. Li JY, Yu Y, Hu S, Sun D, Yao YM. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J Gastroenterol. (2002) 8:168–71. doi: 10.3748/wjg.v8.i1.168
33. Xu X, Wei Y, Hua H, Jing X, Zhu H, Xiao K, et al. Polyphenols sourced from Ilex latifolia Thunb relieve intestinal injury via modulating ferroptosis in weanling piglets under oxidative stress. Antioxidants. (2022) 11:966. doi: 10.3390/antiox11050966
34. Zhang ZF, Xi Y, Wang ST, Zheng LY, Qi Y, Guo SS, et al. Effects of Chinese gallnut tannic acid on growth performance, blood parameters, antioxidative status, intestinal histomorphology, and cecal microbial shedding in broilers challenged with aflatoxin B1. J Anim Sci. (2022) 100:1–8. doi: 10.1093/jas/skac099
35. Yin J, Liu M, Ren W, Duan Y, Yang G, Zhao Y, et al. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE. (2015) 10:e0122893. doi: 10.1371/journal.pone.0122893
36. Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. (2008) 46:1271–8. doi: 10.1016/j.fct.2007.10.006
37. Jiang XR, Zhang HJ, Mantovani G, Alborali GL, Caputo JM, Savoini G, et al. The effect of plant polyphenols on the antioxidant defence system of weaned piglets subjected to an Escherichia coli challenge. J Anim Feed Sci. (2014) 23:324–30. doi: 10.22358/jafs/65668/2014
38. Prasad AS, Beck FWJ, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr Cancer. (2009) 61:879–87. doi: 10.1080/01635580903285122
39. Natalello A, Khelil-Arfa H, Luciano G, Zoon M, Menci R, Scerra M, et al. Effect of different levels of organic zinc supplementation on pork quality. Meat Sci. (2022) 186:108731. doi: 10.1016/j.meatsci.2021.108731
40. Liu Y, Chen F, Odle J, Lin X, Jacobi SK, Zhu H, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. (2012) 142:2017–24. doi: 10.3945/jn.112.164947
41. Giannenas I, Bonos E, Anestis V, Filioussis G, Papanastasiou DK, Bartzanas T, et al. Effects of protease addition and replacement of soybean meal by corn gluten meal on the growth of broilers and on the environmental performances of a broiler production system in Greece. PLoS ONE. (2017) 12:e0169511. doi: 10.1371/journal.pone.0169511
42. Fiesel A, Ehrmann M, Gessner DK, Most E, Eder K. Effects of polyphenol-rich plant products from grape or hop as feed supplements on iron, zinc and copper status in piglets. Arch Anim Nutr. (2015) 69:276–84. doi: 10.1080/1745039X.2015.1057065
43. Wang M, Huang H, Hu Y, Huang J, Yang H, Wang L, et al. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J Anim Sci. (2020) 98:1–12. doi: 10.1093/jas/skaa112
Keywords: antioxidant capacity, intestinal functions, piglets, porcine epidemic diarrhea virus, tannic acid-chelated zinc
Citation: Zhang Z, Wang S, Zheng L, Hou Y, Guo S, Wang L, Zhu L, Deng C, Wu T, Yi D and Ding B (2022) Tannic acid-chelated zinc supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Vet. Sci. 9:1033022. doi: 10.3389/fvets.2022.1033022
Received: 31 August 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Wen-Chao Liu, Guangdong Ocean University, ChinaReviewed by:
Yuxia Chen, Guangdong Ocean University, ChinaMarcin Barszcz, The Kielanowski Institute of Animal Physiology and Nutrition (PAN), Poland
Copyright © 2022 Zhang, Wang, Zheng, Hou, Guo, Wang, Zhu, Deng, Wu, Yi and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binying Ding, ZGJ5aW5nNzQ3MUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Zhengfan Zhang
Zhengfan Zhang Sitian Wang†
Sitian Wang† Yongqing Hou
Yongqing Hou