
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci., 05 January 2023
Sec. Veterinary Infectious Diseases
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1028880
This article is part of the Research TopicThe Role of the Bacteriome, Mycobiome, Archaeome and Virome in Animal Health and DiseaseView all 6 articles
 Maher Alsaaod1*
Maher Alsaaod1* Robin Michael Schmid1
Robin Michael Schmid1 Nathalie Zwahlen1
Nathalie Zwahlen1 Sara Soto2
Sara Soto2 Nicole Wildi3
Nicole Wildi3 Torsten Seuberlich3
Torsten Seuberlich3 Adrian Steiner1
Adrian Steiner1Interdigital hyperplasia (IH) is a fold of fibrous tissue protruding into the interdigital space that rarely occurs in sheep. Interdigital hyperplasia secondary infected with bovine digital dermatitis (BDD) treponemes has been reported in cattle in the course of the increasing spread of classical BDD lesions. In this report, we describe proliferative/ulcerative interdigital lesions associated with contagious ovine digital dermatitis (CODD) treponemes and clinically scored as (IH+CODD), occurring in both hind limbs of a ram and the left hindlimb of a ewe. Both cases exhibited epidermal hyperplasia, parakeratosis and focal-extensive areas of epidermal necrosis with numerous infiltrating neutrophils. Treponema PCR and fluorescence in situ hybridization (FISH) were positive for Treponema phylotype 1 (PT1). In addition, Dichelobacter (D.) nodosus and Porphyromonas (P.) levii were detected in the biopsy by PCR. In three slaughter sheep, without claw lesions, which were kept together with both affected sheep, Treponema spp. were detected neither with PCR nor FISH; the PCRs for D. nodosus and P. levii were also negative. Complete clinical healing occurred in the ewe within 6 weeks after three local applications of a chlortetracycline spray in 2 weeks intervals. This report is the first description of IH+CODD in sheep as demonstrated by a combination of histopathological and molecular analyses.
Interdigital hyperplasia (IH; interdigital fibroma; corn in cattle) is the outgrowth of skin folds from the junction of skin and horn in one or both claws and formation of hyperplastic interdigital skin of variable size located within the interdigital cleft (1–3). IH may have a heritable disposition in sheep and cattle, and predisposing factors in cattle may include stretching of the insertions of the distal interdigital ligaments, abnormally shaped hooves, animal overweight, bovine footrot, or slippery flooring (1, 4–6). IH is most commonly diagnosed in adult cattle and occasionally occurs in sheep and goats (2, 7). The degree of lameness depends on the size of the lesion and the presence of infection of the digital tissue. Ovine interdigital dermatitis (OID, foot scald) and bovine digital dermatitis (BDD) have been reported to occur concurrently with IH in sheep (3) and cattle (6, 8), respectively. OID is caused by Fusobacterium necrophorum (F. necrophorum) and often develops at both sides of the IH, causing further pain and lameness (3).
In cattle, several studies addressed the association between BDD and IH (9, 10). Since the wide spread of classical BDD lesions in the cattle population, superinfections of IH lesions with BDD-associated Treponema spp. (IH+BDD) have increased (11). Treponema spp., involved in BDD, are mainly of Treponema medium, Treponema phagedenis and Treponema pedis phylogroups (12, 13). These three phylogroups are also the major aetiological agents of contagious ovine digital dermatitis (CODD) (14–16). Other lameness-associated bacteria have also been isolated from CODD lesions; in particular, Dichelobacter nodosus (D. nodosus) and F. necrophorum (14, 17). D. nodosus is the primary etiological agent of ovine footrot and considered as a risk factor for CODD (14, 18).
This is the first report of IH+CODD in sheep, and it aimed at describing the clinical, histopathological and molecular findings in two clinical cases of IH+CODD.
In January and February 2022, a ram (Texel, 5 years and 9 months) and a ewe (crossbreed, 6 years and 10 months) were both clinically inspected in the course of clinical footrot control. Both sheep were fixed in a claw chute and all limbs scored for footrot according to the Swiss Health Service for Small Ruminants adapted from Egerton and Roberts (19) using a scale from 0 (clinically healthy) to 5 (complete loss of the horn capsule). All limbs of the ram were scored as 2 (extensive interdigital dermatitis with involvement of the axial horn), except for the left forelimb that was scored as 3 (severe interdigital dermatitis and under-running of the horn of the heel and sole). Both hind limbs of the ewe were scored as 3. In addition, ulcerative lesions in the dorso-axial coronary band area of both claws of the left forelimb of the ewe were noticed during the examination and scored as CODD grade 1 according to Angell et al. (20). Lameness was scored according to Angell et al. (21), where 0 = sound and 3 = severely lame.
Both hind limbs of the ram and the left hindlimb of the ewe additionally showed IH with proliferative and ulcerative lesions and were diagnosed clinically according to Fiedler et al. (6), Alsaaod et al. (8) and the International Committee for Animal Recording ICAR in cattle as IH+BDD (Figures 1A, B).
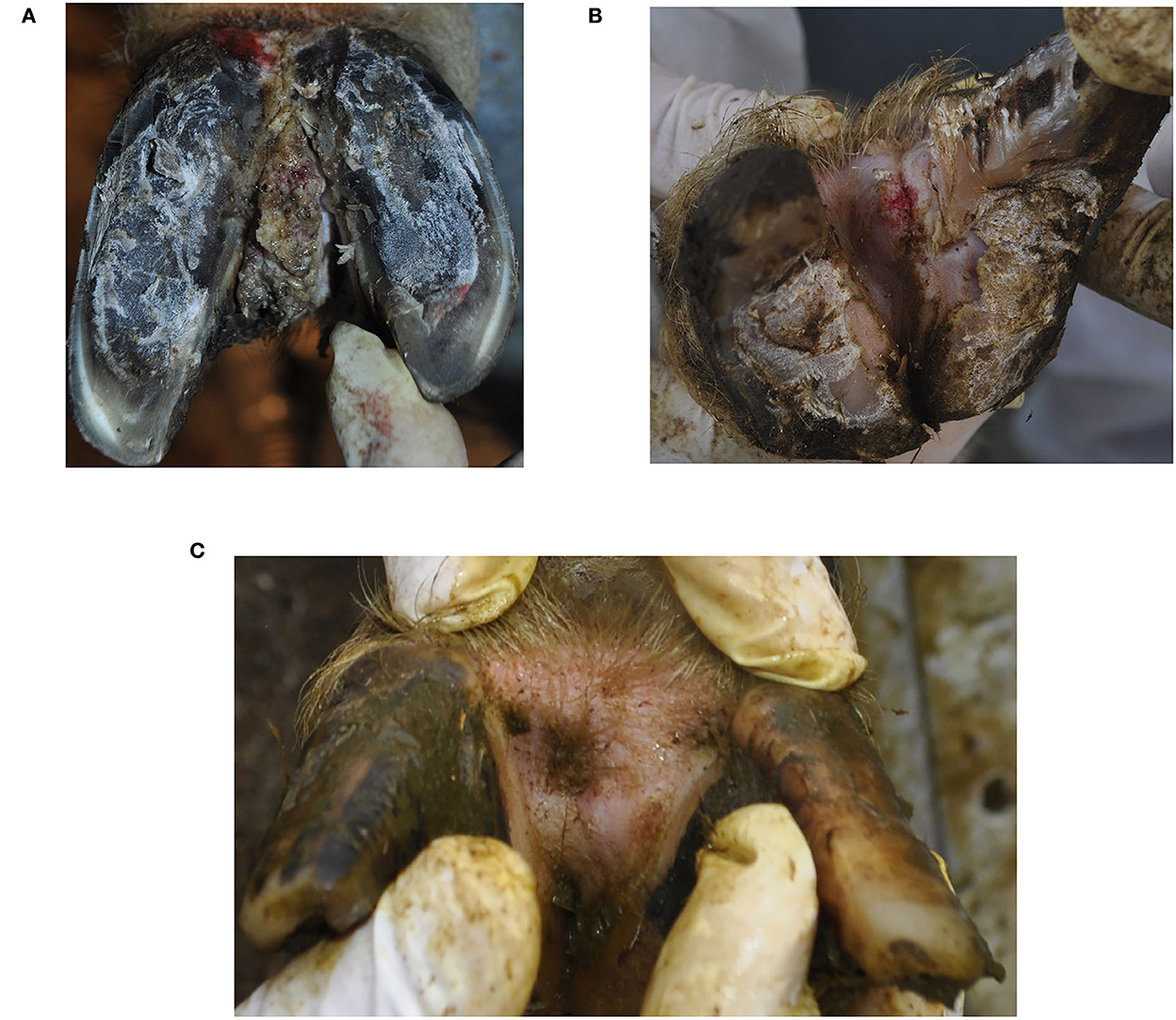
Figure 1. Clinical appearance of interdigital hyperplasia associated with contagious ovine digital dermatitis treponemes in a ram (A), ewe (B) and after the clinical healing in the ewe (C). The ewe (B) additionally showed signs of footrot (score 3).
After disinfection of the skin with active chlorine (AquaJet® Anolyte, Reichenburg SZ, Switzerland), an interdigital locoregional anesthesia at the level of the distal phalanx 1 was administered with 2% Lidocaine (Streuli Tiergesundheit AG, Uznach, Switzerland). After 10 min, one biopsy from the center of the lesion was collected using a sterile biopsy punch (4 mm in diameter with a maximum depth of 7 mm), and transferred directly to a sterile petri dish.
After biopsy sampling, chlortetracycline (Cyclospray, Dr. E. Graeub AG, Bern, Switzerland) was topically applied to the lesions without bandaging. The owner decided to slaughter the ram, and the ewe was further treated three times at intervals of 2 weeks with regular checks of the affected regions over a period of 2 months. The clinical healing of the CODD lesion occurred within 2 weeks and of IH+CODD within 6 weeks (Figure 1C).
Three slaughter sheep, which were kept together with both affected sheep but did not show any clinical signs of CODD or IH, served as negative controls. Two post-mortem biopsies were taken from each control sheep - one from the coronet and one from the skin of the interdigital cleft - and also transferred directly to a sterile petri dish. The control samples were pooled at animal level for PCR screening and separately evaluated by histopathology considering the most severe histopathological lesions.
Regular clinical inspections of the feet of both sheep were conducted within the framework of an ongoing footrot control program (animal experiment license no. SZ-34020).
Each individual biopsy was longitudinally sectioned. One half of each biopsy was used for PCR screening and the second half was fixed in 10% neutral buffered formalin for 24 h. These samples were then embedded in paraffin, cut into 4-μm sections and mounted on glass slides. The tissue sections were stained with hematoxylin and eosin (H&E) as well as silver stain (Warthin-Starry) for a better identification of spirochetes and were microscopically examined.
DNA was extracted from biopsy aliquots using a commercial DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufactures instructions. PCR compatible quality of DNA isolates was confirmed by standard β-actin PCR as previously described (22). Subsequently, DNA extracts were screened for the presence of treponemal DNA using total-treponema PCR (TT-PCR) and nested specific PCR assays for T. medium, T. phagedenis, and T. pedis, and performed according to Moe et al. (23) and Evans et al. (24), respectively. All PCR tests were performed with the GoTaq® Green Master Mix (Promega, Switzerland). All PCR products were separated by 1.5% agarose gel electrophoresis, with exception P. levii PCR with 2%.
Amplicon aliquots with positive TT-PCR and nested PCR (T. phagedenis) results were then gel-purified using NucleoSpin Gel and PCR Clean-up Kit (Marcherey-Nagel) according to the manufacturer's instructions and subjected to Sanger sequencing (Eurofins Genomics) using the same primer pairs as for the PCR reaction. The alignment of forward and reverse sequences and the identification of similar strains were performed using the NCBI BLAST webserver (https://blast.ncbi.nlm.nih.gov/Blast.cgi). To find the most similar strain, only the part of the sequence, matching in the alignment were used.
PCR for D. nodosus und F. necrophorum were performed according to Sullivan et al. (17). Primers for P. levii PCR were designed with Primer Blast (NCBI). The sequence of the forward primer (F-Primer 677) was 5′-AAGGCAGCTTACAAAAGTGTA-3′ and of the reverse primer (R-Primer 812) was 5′-TTTCGCTTGAGAGCATACAT-3′. The P. levii PCR parameters were as follows: 5 min at 95°C, 35 cycles (1 min, 95°C; 1 min, 54°C; 2 min, 72°C) and 5 min at 72°C. All primers are targeting 16S rRNA gene, with exception of hose for F. necrophorum, which leukotoxin (lktA).
Positive PCR results of D. nodosus were further analyzed by quantitative PCR (qPCR) according to Stauble et al. (25) with a cycle threshold (Ct) value of < 40 rated as positive. This qPCR distinguished between the protease genes aprV2 and aprB2, thereby allowing the direct detection and differentiation of virulent and benign strains of D. nodosus, respectively.
For fluorescent in situ hybridization (FISH), serial 4-μm sections were prepared and hybridized as previously described by Rasmussen et al. (26). The oligonucleotide probes included probes specific for the genus Treponema and Treponema phylotype 1 (PT1). Both probes were 5'-labeled with the isothiocyanate derivative Cy3 (Eurofins Genomics, Ebersberg, Germany). The hybridization signal was scored from 0 to 3 according to Klitgaard et al. (27): 0 = no hybridization, 1 = sparse hybridization, 2 = moderate hybridization, and 3 = strong hybridization.
Clinical examination revealed a large and bilateral IH with chronic proliferative lesions of the tissue covering the whole IH in the ram, while a small IH with an acute ulcerative lesion on the top of it was present in the ewe (Figures 1A, B). Both sheep showed uneven steps and were scored as mildly lame (score =1), as affected limbs were not clearly identifiable at locomotion (score = 1).
The histopathological findings were similar in the ewe and ram samples (Figures 2A, B). The epidermis was markedly hyperplastic and partly hyperkeratotic (mainly parakeratosis) with focal-extensive areas of epidermal necrosis with presence of numerous neutrophils. In the nearby epidermis, part of the keratinocytes displayed prominent cellular degeneration. Bacterial colonies of variable shape were present on the sample's surface. Rather numerous lymphocytes and plasma cells as well as some neutrophils and rarely eosinophils infiltrated the dermis. In both cases, helicoidal-shaped bacteria compatible with spirochetes were observed in the necrotic areas on the epidermal surface and between the keratinocytes, made visible with the Warthin-Starry-stain (Figure 2C). In the samples of the control animals, only mild to moderate epidermal hyperplasia and mild lymphoplasmacytic dermal inflammation were observed.
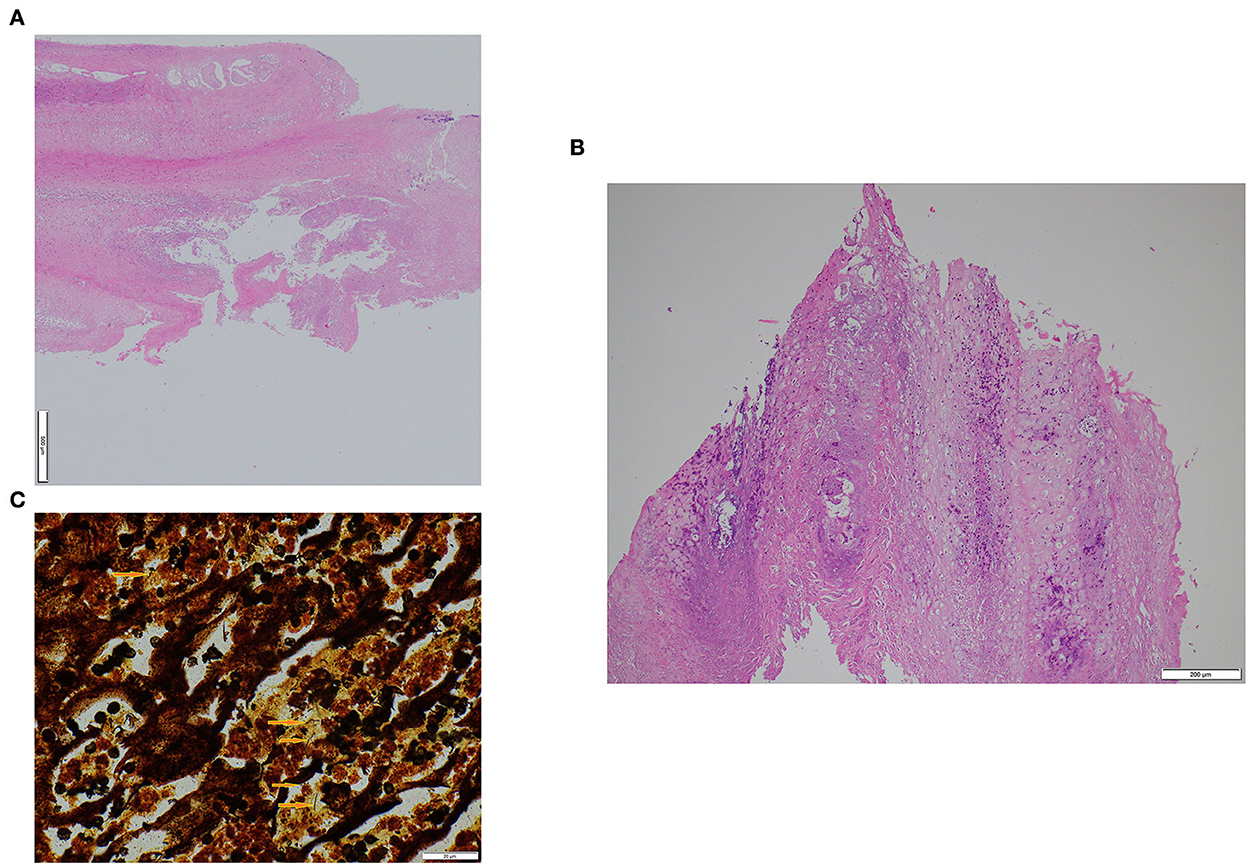
Figure 2. Histological appearance of interdigital hyperplasia associated with contagious ovine digital dermatitis treponemes in a ram (A) and a ewe (B). Warthin-Starry staining showing helicoidal-shaped bacteria (arrows) compatible with spirochetes in the ram (C). Magnification (100×).
The DNA samples of the two biopsies originating from the ram and the ewe tested positive by the TT-PCR and the nested PCR for T. phagedenis (T. medium and T. pedis were not detectable). The obtained nucleotide sequences of the two PCR products of each biopsy originating from the ram and ewe, respectively, showed an identity of 100 and 95.29% to Treponema PT1 (Accession number AM942445.1) for the TT-PCR product and 95.92 and 97.27% to Treponema PT1 (Accession number AM942445.1) for the nested PCR product.
In addition to Treponema PT1, D. nodosus and P. levii were detected in both biopsies; while the PCR for F. nechrophorum remained negative. The subsequent qPCR for D. nodosus was positive for the virulent strain of D. nodosus (Ct values = 24.5 and 24 for two biopsies originated from the ram and ewe, respectively).
The samples of the control biopsies of the three clinically healthy sheep were negative for Treponema spp. (TT-PCR and nested-PCR), D. nodosus, F. necrophorum, and P. levii.
By FISH, the general oligonucleotide probe for Treponema spp. revealed a severe, extensive treponemal epidermal infiltration (score 3) in both biopsies of affected ram and ewe (Figures 3A, B). Additional FISH analysis with the Treponema PT1 specific oligonucleotide probe revealed a strong hybridization (score 3) (Figures 3C, D), again in both biopsies of affected ram and ewe. All biopsies originating from three control sheep were tested negative for Treponema spp by FISH.
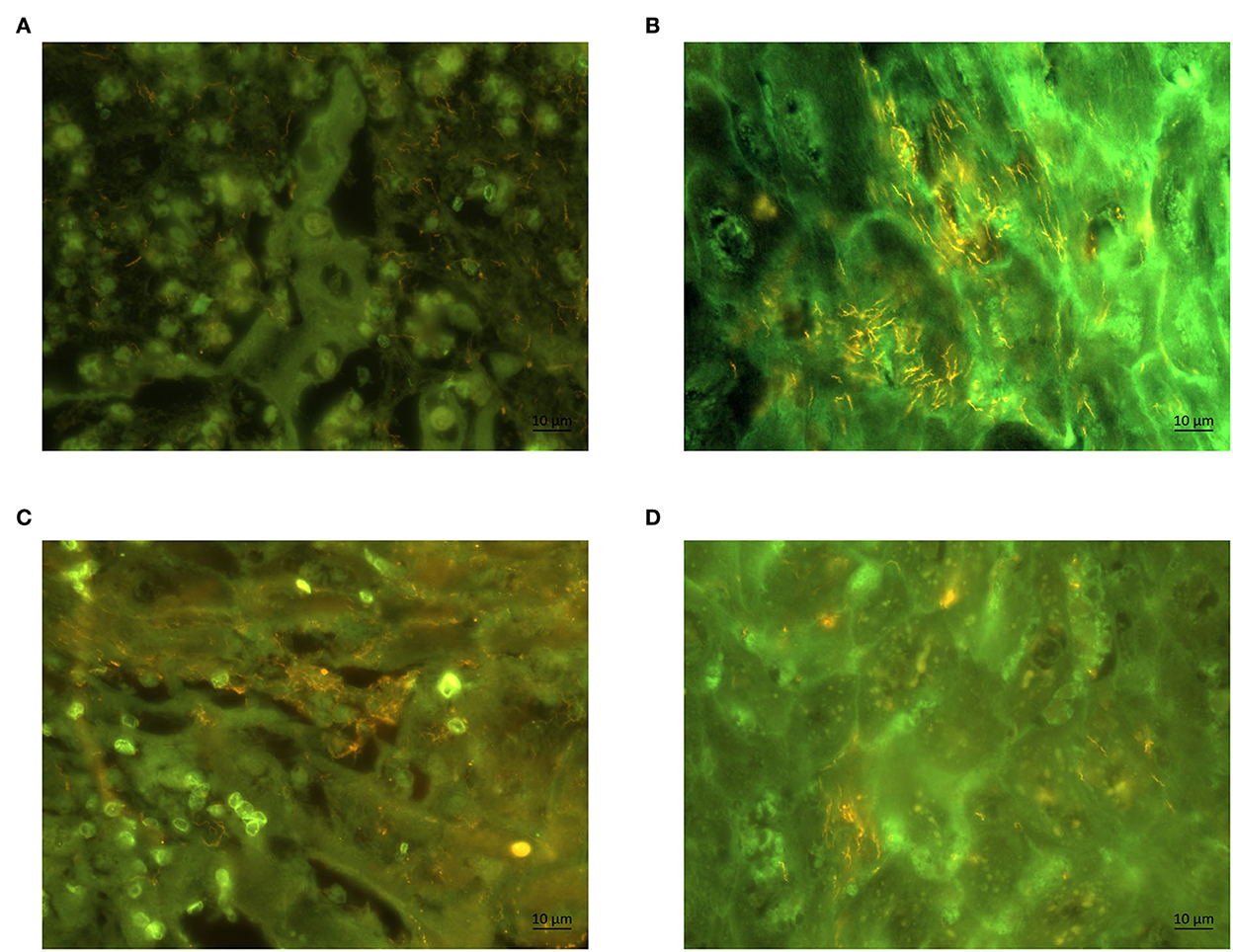
Figure 3. Fluorescent in situ hybridization of biopsies of a ram (A) and a ewe (B) with probes for genus Treponema (Cy3 labeled). Section of the same biopsy hybridized with (Cy3 labeled) species specific oligonucleotide probe for Treponema phylotype PT1 in a ram (C) and ewe (D). The Treponema organisms appear yellow; magnification (60×).
According to the best of the authors ' knowledge, this is the first report describing IH associated with CODD in sheep. Treponema spp. were found in the proliferative/ulcerative lesions of the hyperplastic interdigital tissue samples, and this was confirmed by histopathological and molecular analyses. In contrast, neither PCR nor FISH allowed to detect Treponema spp. in the control animals.
OID (foot scald) is caused by F. necrophorum (3) and its absence in both cases excluded the etiology of OID in our report. Both sheep were affected with ovine footrot caused by D. nodosus. Footrot is considered as a risk factor for CODD (14, 28). Several studies have investigated the association of Treponema spp. with CODD lesions (14–16) and hypothesized that CODD was derived from BDD lesions and may have crossed species barrier, primarily by co-grazing with cattle (29). CODD is a progressive infectious foot disease, which presents as erosion/ulceration at the coronary band and terminates in avulsion of the entire hoof capsule (20). Infection and transmission routes of Treponema spp. in both described cases are not known, as co-grazing was not practiced in this farm. The introduction of CODD treponemes by purchase of carriers is suspected.
Parakeratotic hyperkeratosis and spirochetal colonization in the necrotic areas on the epidermal surface and between the keratinocytes were the most prominent histopathological features in both cases. These findings are consistent with the typical histological features of BDD (30, 31).
The nested PCR for Treponema species according to Evans et al. (24) was positive for T. phagedenis with bands of the respective size (400 bp) and negative for T. medium and T. pedis. Sequence analysis of both PCR products (T. phagedenis) from two biopsies showed that these were not species-specific. Indeed, they matched sequences derived from the yet uncultured Treponema PT1. This indicates, that Treponema species other than T. phagedenis are present in both biopsy cases of IH+CODD and the applied nested PCR is not specific enough for Treponema spp. in sheep. Similar observations were found by sequencing Treponema spp. associated DD lesions, positive for T. phagedenis of captive European bison (32). The 16S rRNA gene used as target is highly conserved and present in all bacteria. This might explain this phenomenon.
Treponema PT1 is the most prevalent Treponema spp. in BDD lesions (95%), invading deep into the stratum spinosum (27). As the control biopsy samples tested negative for the known foot-infection associated bacteria, it is suggested that Treponema spp. are involved as primary bacterial pathogens in the described IH+CODD lesions. P. levii is a well-known opportunistic pathogen and has been already isolated from ID and BDD lesions in cattle (33, 34). In a previous study, the role of P. levii in influencing the overall metabolic processes of the BDD microbiota was demonstrated (35).
IH is characterized by a fold of fibrous tissue protruding into the interdigital space. It represents an uncommon foot disease in sheep. Just under 1% of the Swedish slaughter lambs showed IH in the footrot prevalence study by Konig et al. (7). IH may be hereditary; particularly affected rams should not be bred (3). In our report, only the ewe with the less severe lesions was treated with topical administration of chlortetracycline. Treatment with chlortetracycline revealed effective to treat CODD (36). Large lesions of IH causing chronic lameness can be surgically removed under local anesthesia (37) or may be treated with salicylic acid as successfully performed in cattle (8).
This report is the first description of IH+CODD lesions in sheep characterized by the presence of Treponema spp. in proliferative and ulcerative tissue covering IH lesions. It was confirmed by clinical, histopathological and by molecular biological analyses. Treponema (PT1), D. nodosus and P. levii and typical histological changes including parakeratotic hyperkeratosis and spirochetal colonization were identified. IH+CODD should be considered for the differential diagnosis of interdigital lesions in sheep.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by animal experiment license no. SZ-34020. Written informed consent was obtained from the owners for the participation of their animals in this study.
MA was responsible for data collection, molecular analyses, and writing the first draft of the manuscript. RS and NZ supported the data collection. SS performed the histological analyses. NW and TS supported the data analyses. AS edited the manuscript and supervised the study. All authors contributed to the manuscript and approved the final version.
This work was supported by the Heard Health Management Initiator Grant, Institute of Animal Pathology and Clinic for Ruminants, Vetsuisse Faculty, University of Bern, Switzerland.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Greenough. Interdigital Hyperplasia in Cattle (Corns). MSD Veterinary Manual. Rahway, NJ (2015). Available online at: https://www.msdvetmanual.com/musculoskeletal-system/lameness-in-cattle/interdigital-hyperplasia-in-cattle
2. Phythian CJ, Cripps PJ, Grove-White D, Michalopoulou E, Duncan JS. Inter-observer agreement for clinical examinations of foot lesions of sheep. Veterinary Journal. (2016) 216:189–95. doi: 10.1016/j.tvjl.2016.08.005
3. Winter A. Lameness in sheep 1. Diagnosis In Practice. (2004) 26:58–63. doi: 10.1136/inpract.26.2.58
4. van der Spek D, van Arendonk JAM, Bovenhuis H. Genetic relationships between claw health traits of dairy cows in different parities, lactation stages, and herds with different claw disorder frequencies. J Dairy Sci. (2015) 98:6564–71. doi: 10.3168/jds.2015-9561
5. Desrochers A, Anderson DE, Jean GS. Surgical diseases and techniques of the digit. Vet Clin North Am Food Animal Pract. (2008) 24:535–50. doi: 10.1016/j.cvfa.2008.07.005
6. Fiedler A, Maierl J, Nuss K. Erkrankungen der klauen und zehen des rindes. Deutschland Georg Thieme Verlag KG. (2019) 3:1627. doi: 10.1055/b-006-161627
7. Konig U, Nyman AKJ, de Verdier K. Prevalence of footrot in Swedish slaughter lambs. Acta Veterinaria Scandinavica. (2011) 53:27. doi: 10.1186/1751-0147-53-27
8. Alsaaod M, Plüss J, Studer E, Steiner A. Nicht-antibiotische Behandlung von Dermatitis digitalis infizierter Hyperplasia interdigitalis beim Milchvieh. Schweizer Archiv für Tierheilkunde. (2021) 12:17236. doi: 10.17236/sat00333
9. Holzhauer M, Hardenberg C, Bartels CJ, Frankena K. Herd- and cow-level prevalence of digital dermatitis in the Netherlands and associated risk factors. J Dairy Sci. (2006) 89:580–8. doi: 10.3168/jds.S0022-0302(06)72121-X
10. Solano L, Barkema HW, Mason S, Pajor EA, LeBlanc SJ, Orsel K. Prevalence and distribution of foot lesions in dairy cattle in Alberta, Canada. J Dairy Sci. (2016) 99:6828–41. doi: 10.3168/jds.2016-10941
11. Kofler. Nicht-heilende Klauenhorndefekte heilen - Therapie einer neuen Form der Mortellaro-Krankheit. in Klauentierpraxis. Österreich: Österreichische Buiatrische Gesellschaft (2016), pp. 57-65.
12. Evans NJ, Brown JM, Demirkan I, Murray RD, Vink WD, Blowey RW, et al. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet Microbiol. (2008) 130:141–50. doi: 10.1016/j.vetmic.2007.12.019
13. Alsaaod M, Locher I, Jores J, Grimm P, Brodard I, Steiner A, et al. Detection of specific Treponema species and Dichelobacter nodosus from digital dermatitis (Mortellaro's disease) lesions in Swiss cattle. Schweiz Arch Tierheilkd. (2019) 161:207–15. doi: 10.17236/sat00201
14. Staton GJ, Angell JW, Grove-White D, Clegg SR, Carter SD, Evans NJ, Duncan JS. Contagious ovine digital dermatitis. A novel bacterial etiology and lesion pathogenesis. Front Vet Sci. (2021) 8:2461. doi: 10.3389/fvets.2021.722461
15. Angell JW, Crosby-Durrani HE, Duncan JS, Carter SD, Blundell R. Histopathological characterization of the lesions of contagious ovine digital dermatitis and immunolabelling of treponema-like organisms. J Comp Pathol. (2015) 153:212–26. doi: 10.1016/j.jcpa.2015.10.178
16. Tegtmeyer PC, Staton GJ, Evans NJ, Rohde J, Punsmann TM, Ganter M. First cases of contagious ovine digital dermatitis in Germany. Acta Vet Scand. (2020) 62:46. doi: 10.1186/s13028-020-00544-0
17. Sullivan LE, Clegg SR, Angell JW, Newbrook K, Blowey RW, Carter SD, et al. High-level association of bovine digital dermatitis Treponema spp. with contagious ovine digital dermatitis lesions and presence of Fusobacterium necrophorum and Dichelobacter nodosus. J Clin Microbiol. (2015) 53:1628–38. doi: 10.1128/JCM.00180-15
18. Zanolari P, Durr S, Jores J, Steiner A, Kuhnert P. Ovine footrot. A review of current knowledge. Vet J. (2021) 271. doi: 10.1016/j.tvjl.2021.105647
19. Egerton JR, Roberts DS. Vaccination against ovine foot-rot. J Comparat Pathol. (1971) 81:179. doi: 10.1016/0021-9975(71)90091-0
20. Angell JW, Blundell R, Grove-White DH, Duncan JS. Clinical and radiographic features of contagious ovine digital dermatitis and a novel lesion grading system. Vet Rec. (2015) 176:544. doi: 10.1136/vr.102978
21. Angell JW, Cripps PJ, Grove-White DH, Duncan JS, A. practical tool for locomotion scoring in sheep. reliability when used by veterinary surgeons and sheep farmers. Vet Rec. (2015) 176:521. doi: 10.1136/vr.102882
22. Brandt S, Haralambus R, Schoster A, Kirnbauer R, Stanek C. Peripheral blood mononuclear cells represent a reservoir of bovine papillomavirus DNA in sarcoid-affected equines. J General Virol. (2008) 89:1390–5. doi: 10.1099/vir.0.83568-0
23. Moe KK, Yano T, Kuwano A, Sasaki S, Misawa N. Detection of treponemes in canker lesions of horses by 16S rRNA clonal sequencing analysis. J Vet Med Sci. (2010) 72:235–9. doi: 10.1292/jvms.09-0404
24. Evans NJ, Brown JM, Demirkan I, Singh P, Getty B, Timofte D, et al. Association of unique, isolated treponemes with bovine digital dermatitis lesions. J Clin Microbiol. (2009) 47:689–96. doi: 10.1128/JCM.01914-08
25. Stauble A, Steiner A, Frey J, Kuhnert P. Simultaneous detection and discrimination of virulent and benign dichelobacter nodosus in sheep of flocks affected by foot rot and in clinically healthy flocks by competitive real-time PCR. J Clin Microbiol. (2014) 52:1228–31. doi: 10.1128/JCM.03485-13
26. Rasmussen M, Capion N, Klitgaard K, Rogdo T, Fjeldaas T, Boye M, et al. Bovine digital dermatitis. possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet Microbiol. (2012) 160:151–61. doi: 10.1016/j.vetmic.2012.05.018
27. Klitgaard K, Boye M, Capion N, Jensen TK. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J Clin Microbiol. (2008) 46:3012–20. doi: 10.1128/JCM.00670-08
28. Angell JW, Grove-White DH, Duncan JS. Sheep and farm level factors associated with contagious ovine digital dermatitis. A longitudinal repeated cross-sectional study of sheep on six farms. Prev Vet Med. (2015) 122:107–20. doi: 10.1016/j.prevetmed.2015.09.016
29. Dhawi A, Hart CA, Demirkan I, Davies IH, Carter SD. Bovine digital dermatitis and severe virulent ovine foot rot. A common spirochaetal pathogenesis. Vet J. (2005) 169:232–41. doi: 10.1016/j.tvjl.2004.01.029
30. Berry SL, Read DH, Famula TR, Mongini A, Dopfer D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet J. (2012) 193:654–8. doi: 10.1016/j.tvjl.2012.06.048
31. Read DH, Walker RL. Papillomatous digital dermatitis (footwarts) in California dairy cattle. Clinical and gross pathologic findings. J Vet Diagn Invest. (1998) 10:67–76. doi: 10.1177/104063879801000112
32. Hoby S, Jensen TK, Brodard I, Gurtner C, Eicher R, Steiner A, et al. Detection of treponemes in digital dermatitis lesions of captive European bison (Bison bonasus). PLoS ONE. (2021) 16:e0255921. doi: 10.1371/journal.pone.0255921
33. Bay V, Griffiths B, Carter S, Evans NJ, Lenzi L, Bicalho RC, et al. 16S rRNA amplicon sequencing reveals a polymicrobial nature of complicated claw horn disruption lesions and interdigital phlegmon in dairy cattle. Sci Reports. (2018) 8:3993. doi: 10.1038/s41598-018-33993-9
34. Moe KK, Yano T, Misumi K, Kubota C, Nibe K, Yamazaki W, et al. Detection of antibodies against Fusobacterium necrophorum and Porphyromonas levii-like species in dairy cattle with papillomatous digital dermatitis. Microbiol Immunol. (2010) 54:338–46. doi: 10.1111/j.1348-0421.2010.00220.x
35. Caddey B, Orsel K, Naushad S, Derakhshani H, De Buck J. Identification and quantification of bovine digital dermatitis-associated microbiota across lesion stages in feedlot beef cattle. Msystems. (2021) 6:21. doi: 10.1128/mSystems.00708-21
36. Bernhard M, Frosth S, Konig U. First report on outbreaks of contagious ovine digital dermatitis in Sweden. Acta Vet Scand. (2021) 63:29. doi: 10.1186/s13028-021-00595-x
Keywords: interdigital hyperplasia, sheep, contagious ovine digital dermatitis (CODD), Treponema spp., lameness
Citation: Alsaaod M, Schmid RM, Zwahlen N, Soto S, Wildi N, Seuberlich T and Steiner A (2023) First description of interdigital hyperplasia associated with contagious ovine digital dermatitis in two sheep. Front. Vet. Sci. 9:1028880. doi: 10.3389/fvets.2022.1028880
Received: 26 August 2022; Accepted: 12 December 2022;
Published: 05 January 2023.
Edited by:
Mohamed Zeineldin, Animal and Plant Health Inspection Service (USDA), United StatesReviewed by:
Siti Zubaidah Ramanoon, Universiti Putra Malaysia, MalaysiaCopyright © 2023 Alsaaod, Schmid, Zwahlen, Soto, Wildi, Seuberlich and Steiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maher Alsaaod,  bWFoZXIuYWxzYWFvZEB2ZXRzdWlzc2UudW5pYmUuY2g=
bWFoZXIuYWxzYWFvZEB2ZXRzdWlzc2UudW5pYmUuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.