- 1National Animal Protozoa Laboratory, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2Key Laboratory of Animal Epidemiology of the Ministry of Agriculture, College of Veterinary Medicine, China Agricultural University, Beijing, China
Sarcocystis spp., Neospora caninum and Toxoplasma gondii are globally ubiquitous pathogens, and domestic sheep are considered to be one of the intermediate hosts. 83 myocardial samples of sheep were collected from 12 retail stores in Beijing, China. Sarcocystis spp., N. caninum and T. gondii were identified by PCR amplification of the 18S rRNA gene, Nc-5 gene and 529bp DNA fragment with a prevalence of 86.7% (95% CI: 77.5–93.2) and 43.4% (95% CI: 32.5–54.7) for Sarcocystis spp. and N. caninum infections, respectively, and no T. gondii was detected. The co-infection prevalence of Sarcocystis and N. caninum was 38.6% (95% CI: 28.1–49.9). Two Sarcocystis species were subtyped by analyzing 18SrRNA sequences and were identified as Sarcocystis tenella and Sarcocystis arieticanis. The prevalence of S. tenella and S. arieticanis infections was 84.3% (95% CI: 74.7–91.4) and 56.6% (95% CI: 45.3–67.5), respectively. This study shows that sheep have a high risk of infection with Sarcocystis and N. caninum, suggests that effective prevention measures are needed to avoid the spread of these parasites in sheep. Toxoplasmosis in sheep poses a threat to human and animal health and requires monitoring and preventing continuously.
Introduction
Toxoplasmosis, neosporosis and sarcocystosis are parasitic diseases caused by Toxoplasma gondii, Neospora caninum and Sarcocystis spp., respectively (1–3). These diseases have a wide geographic distribution and the ability to infect warm-blooded animals. Toxoplasmosis is an important zoonotic disease that not only causes severe reproductive and economic losses, but also poses a great threat to public health (2). Humans are primarily infected through ingestion of raw or undercooked meat containing tissue cysts or food contaminated with oocysts that have been disseminated by cats or other felids (4). A series of investigations of T. gondii infections in farm animals have confirmed that the meat of sheep is one of the most important sources of infection, with seroprevalence of T. gondii ranging from 1.2 to 39.1% in sheep in China (5).
N. caninum has been identified as an important cause of reproductive failure in cattle and small ruminants (1, 6). Although antibodies against N. caninum have been reported in human serum samples, its zoonotic potential has not been confirmed (7). In China, molecular survey on N. caninum in Chinese sheep is rare and focuses mainly on serological investigations, with seroprevalence of N. caninum infection among Chinese sheep ranging from 7.32 to 57.25% (8–11).
In China, three validated species of Sarcocystis have been described in sheep: S. tenella, S. arieticanis, and S. gigantea (12). S. tenella and S. arieticanis are pathogenic and can lead to abortion, neurological symptoms, and even death in the early stages of infection and chronic disease in the late stages of infection (3). Sarcocystis infection in sheep has been reported in several studies with a prevalence of 7.74–100% (12).
Under natural conditions, sheep may be infected with these protozoa at any time (13). However, no studies have investigated the co-infection prevalence of these three protozoa among sheep in the Beijing area. Therefore, the aims of the present study were to investigate the prevalence of Sarcocystis spp., N. caninum and T. gondii in retail sheep hearts from Beijing, China.
Materials and methods
Sample collection and processing
A total of 83 sheep hearts (all sheep were about 2 years old) were collected between August 2020 to January 2021 from 12 meat retail stores in Beijing, China. A minimum of five to a maximum of ten hearts were collected each week. Samples collected were marked with date and store location and transferred to the laboratory in cool conditions. Samples were analyzed for the presence of Sarcocystis cysts by gross inspection, examination of unstained squash preparations, and tissue digestion to detect the release of bradyzoites from tissue cysts. All operations were completed within 3 days to ensure freshness of the samples. Digestive fluids or muscle tissues were submitted to PCR for DNA of Sarcocystis spp., N. caninum and T. gondii detection.
Detection of tissue cysts and bradyzoites
Muscle connective tissue and fat were removed from the sample, and three rice-sized portions of muscle tissue were randomly selected from each sample along the direction of the muscle fibers and pressed between two glass slides to make them thin. Then, the tissue was observed under a 10× magnification microscope. The remaining tissue was digested with HCl-pepsin solution (14).
Each sample (50 g) was digested in HCl-pepsin solution, and then 100 μL of the digestive fluid was taken for detection of bradyzoites under light microscopy (14). Protozoan bradyzoites were purified using the Percoll density gradient centrifugation method (14). The purified bradyzoites were preserved at −20°C for molecular identification.
DNA extraction and PCR amplification
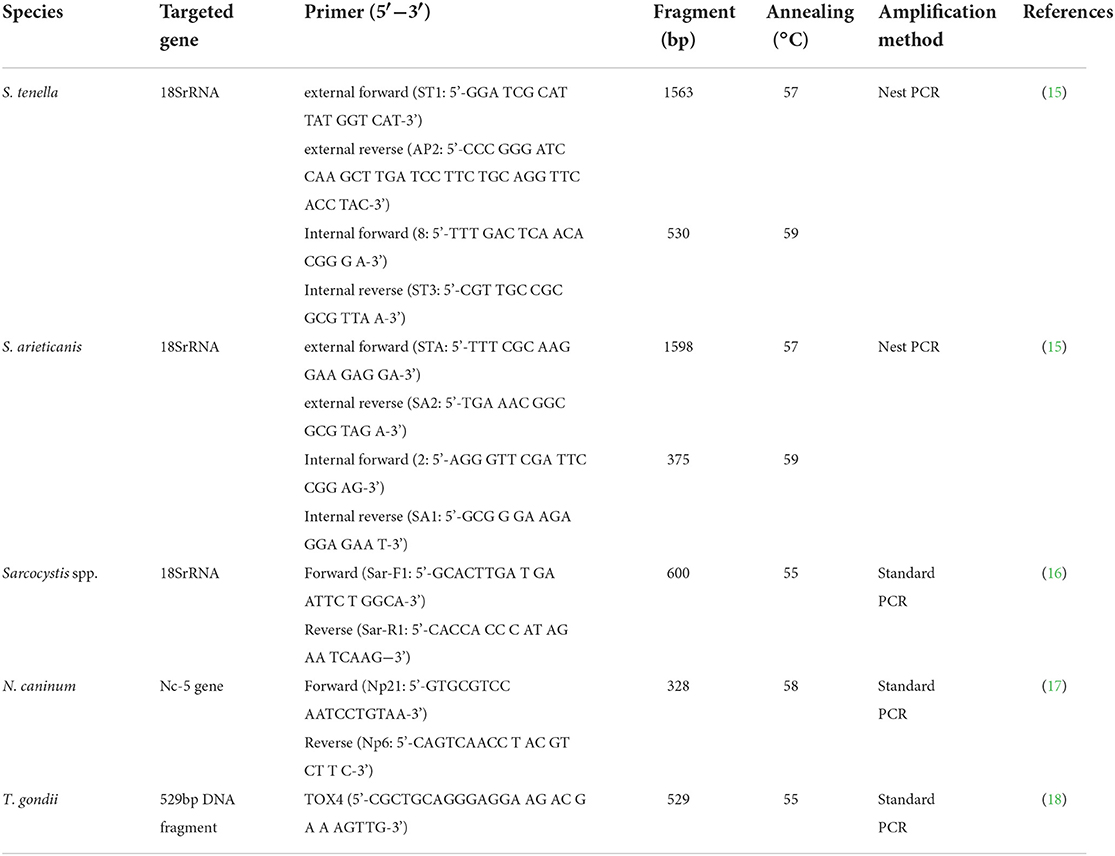
Genomic DNA was extracted from purified bradyzoites and/or tissue homogenate of each sample following the instructions of the Genomic DNA Extraction Kit (Aidlab Biotech, Beijing, China). The extracted DNA was stored at −20°C until PCR amplification. Identification of T. gondii, N. caninum and Sarcocystis spp. was performed by PCR using primers for species-specific genes. The primer sequences are shown in Table 1. 20 μL of PCR mix was used for each amplification: 10 μL of 2 × M5 HiPer plus Taq HiFi PCR mix (Mei5 Biotechnology, Co., Ltd, Beijing, China), 1 μM of each primer (forward and reverse), 6 μL deionized water, 2 μL of genomic DNA for the primary PCR template, or 2 μL of diluted primary amplification products used for the secondary PCR templates. PCR primers are shown in Table 1. PCR was performed using the T100TM Thermal Cycler (Bio-Rad). Each PCR assay had a negative control (deionized water) and a positive control (T. gondii RH standard strain, N. caninum Nc-1 standard strain, previously characterized as a positive control for S. tenella or S. arieticanis DNA, respectively). The PCR products were visualized by 1.5 g/L agarose gel electrophoresis. Each sample was subjected under three PCR replications.
Nucleotide sequencing and analysis
The positive PCR products of the target genes were direct sequenced bidirectionally by a sanger sequencing in a commercial sequencing company (Ruibiotech, Beijing, China). The obtained nucleotide sequences were aligned with reference sequences on the National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis
A Chi-square test was applied to compare the prevalence of Sarcocystis spp., N. caninum and T. gondii in sheep using GraphPad Prism 8.0 software. Statistical significance was set at p < 0.05.
Results
Prevalence rates of three intracellular parasites in retail sheep
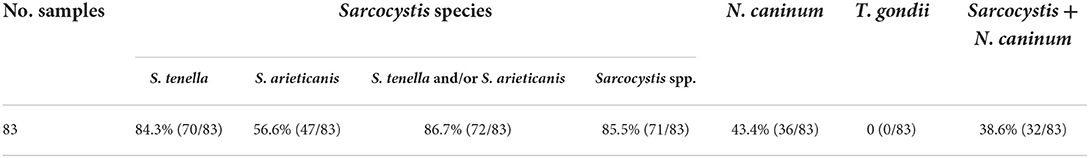
The sample were considered positive if at least one typical cyst or bradyzoite was observed. In this study, no visible cysts were observed in 83 sheep heart samples. Light microscopy revealed Sarcocystis cysts that were spindle-shaped or oval in shape, parallel to the muscle fibers, and contained large numbers of bradyzoites (Figure 1A). Besides, all samples were digested in HCl-pepsin solution to detect bradyzoites released from muscle tissue (Figure 1B). All samples confirmed by microscopy were tested positive for Sarcocystis based on the 18SrRNA gene. In summary, the prevalence of muscle squashing microscopic observation, pepsin digestion examination and PCR detection was 26.5% (95% CI: 17.4–37.3), 77.1% (95% CI: 66.6–85.6) and 86.7% (95% CI: 77.5–93.2), respectively (Table 2).
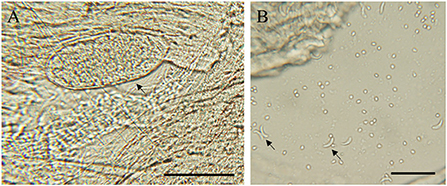
Figure 1. Morphological characteristics of S. tenella in the myocardium of sheep. (A) light micrograph of a sarcocyst (arrow), unstained; (B) light micrograph of bradyzoites (arrow) in pepsin-digestion liquid; unstained. Bar = 50 μm.
Of the 83 muscle samples collected from Beijing, 36 (43.4%, 95% CI: 32.5–54.7) and 0 (0.0%, 95% CI: 0.0–4.3) tested positive for N. caninum and T. gondii based on the Nc-5 gene sequence and the T. gondii 529 bp DNA fragment, respectively. The co-infection prevalence of Sarcocystis and N. caninum was 38.6% (95% CI: 28.1–49.9; Table 3).
Molecular identification of Sarcocystis spp. and N. caninum
From the 18SrRNA gene of S. tenella and S. arieticanis, 70 (84.3%, 95% CI: 74.7–91.4) out of 83 muscle specimens tested positive for S. tenella and 47 (56.6%, 95% CI: 45.3–67.5) tested positive for S. arieticanis. S. tenella was the most prevalent identified Sarcocystis species. The co-infection prevalence of S. tenella and S. arieticanis was 54.2% (95% CI: 42.9–65.2; Table 3). After sequencing of PCR products, the nucleotide sequence of the 18SrRNA gene fragment of S. tenella (n = 67) from the Beijing sheep samples was identical to isolates from the Chinese domestic sheep (MF039329), and the three S. tenella sequences showed 99.81% homology with MF039329 and one substitution at nucleotide position 1309 (A→ G). The S. arieticanis sequence (n = 47) was identical to isolates from the Chinese domestic sheep (MF039331). The N. caninum sequence (n = 36) was identical to isolates from aborted bovine fetuses in China (EF581827), followed by N. caninum (EF463099) from Polish cattle (98.51% identity), and N. caninum (MT340527) from pigs in China (97.01% identity).
Discussion
The sheep industry is an important part of China's animal husbandry, with mutton being a major ingredient in hot pots and barbecues, and consumption of undercooked meat containing T. gondii tissue cysts poses a health risk. Sheep is also the intermediate host of N. caninum and Sarcocystis spp., and large numbers of parasitic tissue cysts in the muscle can reduce meat quality and compromise food hygiene (13). Mutton fed to dogs and cats can also lead to infection and parasite transmission in pets and economic animals. However, molecular survey on sheep infected with T. gondii, N. caninum and Sarcocystis spp. are limited. Here, we investigated the molecular prevalence of Sarcocystis, N. caninum and T. gondii in sheep intended for human consumption in Beijing, China. The molecular prevalence of Sarcocystis spp. and N. caninum was 86.7 and 43.4%, respectively. T. gondii was not observed. These results highlight the public health risks of Sarcocystis spp. and N. caninum as well as the prevalence of sarcocystosis and neosporosis in sheep farms in Beijing, China.
Sarcocystis is usually detected by muscle squashing microscopic observation and histological examination. Since the occurrence of sarcocysts detection is random, the detection rate examination of unstained squash preparations will be slightly lower than the true value. Digestion of host tissue with HCl-pepsin solution has been reported to be the most sensitive method for detecting mild infections of Sarcocystis (3). However, these methods do not identify Sarcocystis species. Heckeroth et al. established species-specific nested PCR assays based on the unique 18SrRNA gene sequences of S. tenella and S. arieticanis to diagnose and differentiate between S. tenella and S. arieticanis infections in sheep (15). In comparison with other methods, the HCl-pepsin digestion method combined with nested PCR assays showed a higher sensitivity for the detection of Sarcocystis. Hence, the 86.7% prevalence of Sarcocystis infection in sheep found in this study may be close to the true value, and the identification of S. tenella and S. arieticanis confirms that this experimental design can accurately identify multiple Sarcocystis infections in actual samples.
The prevalence of Sarcocystis found in this study was higher than that in Henan, Xinjiang and Qinghai, but lower than that in Yunnan (19–21). Combined with these studies on Sarcocystis infection, it is implied that Sarcocystis spp. may be widely spread among sheep in China. The prevalence of Sarcocystis spp. in sheep varies in different regions of the world. The prevalence rate of Sarcocystis in other countries was reported to be 63.83% (95% CI: 45.84–80.01) in Iran (22), 96.9% in Mongolia (23), 95.8% in Brazil (24). Sarcocystis can cause weight loss, abortion, premature birth, and even death in sheep (3). Animals usually infected by ingesting food or drinking water contaminated with Sarcocystis sporocysts (3). Although sheep sarcocystiosis is not zoonotic, Sarcocystis spp. possess the powerful sar-cocystin neurotoxin, Sarcocystis spp. infection is a cause of condemnation of adult sheep meat (25). Therefore, it is necessary to enhance the husbandry management of sheep to prevent and control Sarcocystis infections.
In this study, the detection rate of N. caninum was 43.4% (95% CI: 32.5–54.7), which is significantly higher than that of Central China (7.32%) (26) and Southwest China (8.55%) (11). Compared to other studies in the world, it was higher than the rates in Iran (6.7%) (27), North Africa (10.6 ± 4.3%) (28), but lower than those observed in São Paulo, Brazil (59.23%) (29) and the state of Pernambuco, Brazil (64.2%) (30). Although the economic, clinical, and epidemiological importance of N. caninum infection in sheep remains uncertain, studies have reported that N. caninum can cause abortion, birth of weak lambs, suggested an association between N. caninum infection and reproductive losses in sheep (31, 32). In addition, antibodies to N. caninum have been reported in human serum, but the parasite has not been detected in human tissues, the zoonotic potential is uncertain (7).
Variations in the prevalence of N. caninum in different regions may be related to sheep breeds, age of test samples, detection methods, sample sizes, as well as farm hygiene management and animal health status. Furthermore, these sheep are free-ranging in rural areas, where most farmers usually keep dogs to protect the sheep sheds. Dogs are the final hosts of S. tenella, S. arieticanis and N. caninum, and sheep can be infected by ingesting sporulated oocysts found in the feces of infected dogs in contaminated food or drinking water. Therefore, the presence of dogs is also a potential factor in the high prevalence of S. tenella and N. caninum.
In this study, the prevalence of T. gondii infection was 0.0% (95% CI: 0.0–4.3), which was significantly lower than the prevalence of T. gondii in sheep worldwide (14.7%, 95% CI: 0–57) (33). T. gondii tissue cysts have been usually reported to parasitize the muscles and CNS. In sheep, samples pooled within the animal resulted in a significantly higher prevalence compared to single organ samples (33). Therefore, the low detection rate in this study may be due to insufficient sample size, low level of infection, and sample types. T. gondii is an important zoonotic species that is usually found in the muscles, viscera, and blood of warm-blooded animals (2). Carnivorous animals and humans can be infected with T. gondii via the consumption of rarely cooked meat (mutton, beef, or viscera) containing tissue cysts, with livestock considered to be the major source of human infection (2). Mutton has very rich nutritional value and is popular in China. In this case, humans are at high risk for T. gondii infection through ingestion of undercooked or raw meat of infected sheep, which may contain T. gondii cysts. Although no T. gondii infection was detected in this study, the pooled prevalence of T. gondii in China is 8.5% (95% CI: 6.5–10.9) (34), it is necessary to monitor regularly T. gondii infection in sheep.
In conclusion, this is the first molecular survey and characterization of Sarcocystis spp., N. caninum and T. gondii infections among sheep in Beijing, China. This study shows that S. tenella, S. arieticanis and N. caninum are highly prevalent in Beijing sheeps. The high prevalence of Sarcocystis spp. and N. caninum in sheep indicates the need for effective prevention measures, as improve breeding management in sheep, cats and dogs, to avoid the spread of these parasites in the sheep industry and in public health. These results will provide a reference basis for further research and control of the three intracellular parasites among sheep in China.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of China Agricultural University (Approval No. AW71211202-2-1).
Author contributions
ZZ: data curation, formal analysis, investigation, project administration, and writing-original draft. YC: investigation and methodology. XY: investigation and software. LW: methodology. QL: data curation, methodology, conceptualization, and supervision. JL: conceptualization, funding acquisition, resources, supervision, validation, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31972700) and the Beijing Municipal Natural Science Foundation (62732622).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dubey JP, Schares G. Neosporosis in animals—the last 5 years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
2. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
3. Rosenthal BM. Zoonotic Sarcocystis. Res Vet Sci. (2021) 136:151–7. doi: 10.1016/j.rvsc.2021.02.008
4. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/S0020-7519(00)00124-7
5. Pan M, Lyu C, Zhao J, Shen B. Sixty years (1957–2017) of research on toxoplasmosis in China-an overview. Front Microbiol. (2017) 8:1825. doi: 10.3389/fmicb.2017.01825
6. Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. (2003) 41:1–16. doi: 10.3347/kjp.2003.41.1.1
7. Calero-Bernal R, Horcajo P, Hernández M, Ortega-Mora LM, Fuentes I. Absence of Neospora caninum DNA in human clinical samples, Spain. Emerg Infect Dis. (2019) 25:1226–7. doi: 10.3201/eid2506.181431
8. Chen YJ, Zhu ZF, Yang YR, Liu Q, Liu J. Epidemiological investigations of three intracellular protozoan parasites of sheep. Chinese J Vet Med. (2020) 56:10–14.
9. Liu ZK Li JY, Pan H. Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infections in small ruminants in China. Prev Vet Med. (2015) 118:488–92. doi: 10.1016/j.prevetmed.2014.12.017
10. Nie LB, Cong W, Zou Y, Zhou DH, Liang QL, Zheng WB, et al. First report of seroprevalence and risk factors of Neospora caninum infection in Tibetan sheep in China. Biomed Res Int. (2018) 2018:2098908. doi: 10.1155/2018/2098908
11. Sun LX, Liang QL, Nie LB, Hu XH Li Z, Yang JF, Zou FC, et al. Serological evidence of Toxoplasma gondii and Neospora caninum infection in black-boned sheep and goats in southwest China. Parasitol Int. (2020) 75:102041. doi: 10.1016/j.parint.2019.102041
12. Dong H, Lu YY, Yang YR. Epidemiology and classification of Sarcocystis in sheep and goat. Chinese J Zoonoses. (2017) 33:828–836.
13. Tenter AM. Current research on Sarsosystis species of domestic animals. Int J Parasitol. (1995) 25:1311–30. doi: 10.1016/0020-7519(95)00068-D
14. Verma SK, Lindsay DS, Grigg ME, Dubey JP. Isolation, culture and cryopreservation of Sarcocystis species. Curr Protoc Microbiol. (2017) 45:20D 1 1–27. doi: 10.1002/cpmc.32
15. Heckeroth AR, Tenter AM. Development and validation of species-specific nested PCRs for diagnosis of acute Sarcocystosis in sheep. Int J Parasitol. (1999) 29:1331–49. doi: 10.1016/S0020-7519(99)00111-3
16. Bahari P, Salehi M, Seyedabadi M, Mohammadi A. Molecular identification of macroscopic and microscopic cysts of Sarcocystis in sheep in North Khorasan Province, Iran. Int J Mol Cell Med. (2014) 3:51–6.
17. Yamage M, Flechtner O, Gottstein B. Neospora caninum: specific oligonucleotide primers for the detection of brain “cyst” DNA of experimentally infected nude mice by the polymerase chain reaction (PCR). J Parasitol. (1996) 82:272–9. doi: 10.2307/3284160
18. Homan WL, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. (2000) 30:69–75. doi: 10.1016/S0020-7519(99)00170-8
19. Dong H, Su RJ, Wang YH, Tong ZX, Zhang LX, Yang YY, et al. Sarcocystis species in wild and domestic sheep (Ovis ammon and Ovis aries) from China. BMC Vet Res. (2018) 14:377. doi: 10.1186/s12917-018-1712-9
20. Hu JJ, Huang S, Wen T, Esch GW, Liang Y, Li HL. Sarcocystis spp. in domestic sheep in Kunming City, China: prevalence, morphology, and molecular characteristics. Parasite. (2017) 24:30. doi: 10.1051/parasite/2017025
21. Sun YL, Ju JL, Su XX, Xie CY Li Y, Kang M. Infection survey and morphological characteristics of Sarcocystis spp. in naturally infected Tibetan sheep from Qinghai in northwestern. China Parasitol Int. (2021) 80:102219. doi: 10.1016/j.parint.2020.102219
22. Anvari D, Narouei E, Hosseini M, Narouei MR, Daryani A, Shariatzadeh SA, et al. Sarcocystosis in ruminants of Iran, as neglected food-borne disease: a systematic review and Meta-analysis. Acta Parasitol. (2020) 65:555–68. doi: 10.2478/s11686-020-00210-5
23. Fukuyo M, Battsetseg G, Byambaa B. Prevalence of Sarcocystis infection in meat-producing animals in Mongolia. Southeast Asian J Trop Med Public Health. (2022) 33:490–5.
24. Bittencourt MV, Meneses ID, Ribeiro-Andrade M, de Jesus RF, de Araújo FR, Gondim LF. Sarcocystis spp. in sheep and goats: frequency of infection and species identification by morphological, ultrastructural, and molecular tests in Bahia, Brazil. Parasitol Res. (2016) 115:1683–9. doi: 10.1007/s00436-016-4909-5
25. Cano-Fructuoso M, Sanchez-Martínez P, Llopis-Morant A, Perez-Castarlenas B, Goyena E, Berriatua Fernández de Larrea E. Short communication Sarcocystis infection: a major cause of carcass condemnation in adult sheep in Spain. Span J Agric Res. (2012) 10:388–92. doi: 10.5424/sjar/2012102-523-11
26. Wang S, Li LJ, Lu Y, Zhang HZ, Xie Q, Zhang ZC. Seroprevalence and risk factors of Neospora caninum infection among domestic sheep in Henan province, central China. Parasite. (2018) 25:15. doi: 10.1051/parasite/2018019
27. Arbabi M, Abdoli A, Dalimi A, Pirestani M. Identification of latent neosporosis in sheep in Tehran, Iran by polymerase chain reaction using primers specific for the Nc-5 gene. Onderstepoort J Vet Res. (2016) 83:e1–7. doi: 10.4102/ojvr.v83i1.1058
28. Amdouni Y, Rjeibi MR, Awadi S, Rekik M, Gharbi M. First detection and molecular identification of Neospora caninum from naturally infected cattle and sheep in North Africa. Transbound Emerg Dis. (2018) 65:976–82. doi: 10.1111/tbed.12828
29. Paiz LM, da Silva RC, Menozzi BD, Langoni H. Antibodies to Neospora caninum in sheep from slaughterhouses in the state of Sa'o Paulo, Brazil. Rev Bras Parasitol Vet. (2015) 24:95–100. doi: 10.1590/S1984-29612015009
30. Tembue AA, Ramos RA, de Sousa TR, Albuquerque AR, da Costa AJ, Meunier IM, et al. Serological survey of Neospora caninum in small ruminants from Pernambuco State, Brazil. Rev Bras Parasitol Vet. (2011) 20:246–8. doi: 10.1590/S1984-29612011000300013
31. González-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, Benavides J, Álvarez-García G, Fuertes M, et al. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet Res. (2014) 45:88. doi: 10.1186/PREACCEPT-1141386470129662
32. Al-Shaeli SJJ, Ethaeb AM, Gharban HAJ. Molecular and histopathological identification of ovine neosporosis (Neospora caninum) in aborted ewes in Iraq. Vet World. (2020) 13:597–603. doi: 10.14202/vetworld.2020.597-603
33. Belluco S, Mancin M, Conficoni D, Simonato G, Pietrobelli M, Ricci A. Investigating the determinants of Toxoplasma gondii prevalence in meat: a systematic review and meta-regression. PLoS ONE. (2016) 11:e0153856. doi: 10.1371/journal.pone.0153856
Keywords: sheep, Sarcocystis spp., N. caninum, T. gondii, prevalence
Citation: Zhu Z, Chen Y, Yang X, Wang L, Liu Q and Liu J (2022) Molecular detection and identification of three intracellular parasites of retail mutton products in Beijing, China. Front. Vet. Sci. 9:1018788. doi: 10.3389/fvets.2022.1018788
Received: 13 August 2022; Accepted: 14 September 2022;
Published: 30 September 2022.
Edited by:
Haroon Ahmed, COMSATS University, PakistanReviewed by:
Patrícia Bräunig, Federal University of Santa Maria, BrazilHamidreza Majidiani, Neyshabur University of Medical Sciences, Iran
Copyright © 2022 Zhu, Chen, Yang, Wang, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, bGl1amluZ3ZldEBjYXUuZWR1LmNu
 Zifu Zhu1
Zifu Zhu1 Qun Liu
Qun Liu Jing Liu
Jing Liu