- 1Centre for Integrative Ecology, School of Life and Environmental Science, Deakin University, Melbourne, VA, Australia
- 2EcoHealth Alliance, New York, NY, United States
- 3Institute of Epidemiology, Disease Control and Research (IEDCR), Dhaka, Bangladesh
- 4One Health Laboratory, International Center for Diarrheal Diseases Research, Bangladesh (icddr, b), Dhaka, Bangladesh
- 5Queensland Alliance for One Health Sciences, School of Veterinary Science, University of Queensland, Brisbane, QLD, Australia
- 6National Reference Laboratory for Avian Influenza, Bangladesh Livestock Research Institute (BLRI), Savar, Bangladesh
The avian influenza virus (AIV) impacts poultry production, food security, livelihoods, and the risk of transmission to humans. Poultry, like pigeons and quail farming, is a growing sector in Bangladesh. However, the role of pigeons and quails in AIV transmission is not fully understood. Hence, we conducted this study to investigate the prevalence and risk factors of AIV subtypes in pigeons and quails at live bird markets (LBMs) in Bangladesh. We collected oropharyngeal and cloacal swab samples from 626 birds in 8 districts of Bangladesh from 2017 to 2021. We tested the swab samples for the matrix gene (M gene) followed by H5, H7, and H9 subtypes using real-time reverse transcriptase-polymerase chain reaction (rRT-PCR). We then used exploratory analysis to investigate the seasonal and temporal patterns of AIV and a mixed effect logistic model to identify the variable that influences the presence of AIV in pigeons and quails. The overall prevalence of AIV was 25.56%. We found that the prevalence of AIV in pigeons is 17.36%, and in quail is 38.75%. The prevalence of A/H5, A/H9, and A/H5/H9 in quail is 4.17, 17.92, and 1.67%, respectively. Furthermore, the prevalence of A/H5, A/H9, and A/H5/H9 in pigeons is 2.85, 2.59, and 0.26%. We also found that the prevalence of AIV was higher in the dry season than in the wet season in both pigeons and quail. In pigeons, the prevalence of A/untyped (40%) increased considerably in 2020. In quail, however, the prevalence of A/H9 (56%) significantly increased in 2020. The mixed-effect logistic regression model showed that the vendors having waterfowl (AOR: 2.13; 95% CI: 1.04–4.33), purchasing birds from the wholesale market (AOR: 2.96; 95% CI: 1.48–5.92) instead of farms, mixing sick birds with the healthy ones (AOR: 1.60; 95% CI: 1.04–2.45) and mingling unsold birds with new birds (AOR: 3.07; 95% CI: 2.01–4.70) were significantly more likely to be positive for AIV compared with vendors that did not have these characteristics. We also found that the odds of AIV were more than twice as high in quail (AOR: 2.57; 95% CI: 1.61–4.11) as in pigeons. Furthermore, the likelihood of AIV detection was 4.19 times higher in sick and dead birds (95% CI: 2.38–7.35) than in healthy birds. Our study revealed that proper hygienic practices at the vendors in LBM are not maintained. We recommend improving biosecurity practices at the vendor level in LBM to limit the risk of AIV infection in pigeons and quail in Bangladesh.
Introduction
The avian influenza virus (AIV) is a severe threat to Bangladesh's poultry sector, with substantial consequences for the economy and public health. It has also been identified in wild birds in Bangladesh, mainly crows (1, 2). Since the initial outbreak in poultry in 2007, H5N1 has posed public health and economic danger in Bangladesh. The virus has become enzootic in poultry, with 585 outbreaks documented in 54 of the 64 districts, making the country one with the most significant outbreaks globally (3, 4). On the other hand, since 2006, the H9N2 subtype has been the most common, followed by the highly pathogenic avian influenza (HPAI) H5N1 subtype, which has been detected from domestic land-based poultry in Bangladesh (5–8). H5N1 and H9N2 AIV have become endemic in poultry and have been observed sporadically infecting humans (9). Eight cases of H5N1 with one mortality and three cases of H9N2 have also been reported in Bangladesh (4, 9, 10). There is also a significant cause for concern regarding the co-infection of HPAI H5N1 and LPAI viruses, particularly H9N2.
In Bangladesh couple of steps have been taken to control the AIV, including the development of the national avian influenza and human pandemic influenza preparedness and response plan 2006–2008, which facilitate a co-coordinated and effective national response in the event of an incursion of HPAI/H5N1 in domestic poultry, and to minimize the risk of human pandemic influenza (HPI) (9). Isolation of HPAIV-infected flocks has relatively lower mortality and stamping out when mortality is higher (9). Official reporting to the Department of Livestock Services (DLS) is practiced in the latter case. Vaccination of poultry reduces the shedding of viruses, thereby decreasing the amount of viruses in the environment and at the poultry-human interface (11, 12). Multiple sporadic and discontinued AIV surveillance. Adopting essential biosecurity measures at the farm level and nationwide public awareness-raising efforts.
Most of the studies on AIV in Bangladesh have focused on chicken and ducks, but pigeons and quail are now being farmed in Bangladesh and are in danger of contracting the virus. The poultry industry is the most efficient and cost-effective source of animal protein, but rising future demand prohibits it from addressing the supply-demand gap for animal protein (10). Along with Contemporary broiler and layer, customers continually seek other safe meat options such as pigeons and quail (13). As a result, pigeons and quail raising has emerged to meet public demand and become economically prosperous.
Pigeons and quail farming is now a thriving industry in Bangladesh. Domestic pigeons have been raised for meat in Bangladesh for many years. According to a 2020 study, Bangladesh has a pigeon population of 10.8 million (14). As one of the most promising species for future income-earning options for many people, pigeons rearing is being investigated further to reduce Bangladesh's unemployment rate. Pigeons reproduce prolifically, and squab meat is in high demand in the market because of its delicacy and flavor (10).
On the other hand, quails are now utilized to produce commercial eggs and meat (13). They are the most suitable and effective birds for economic and nutritional purposes because they achieve sexual maturity quickly, have a shorter incubation time, and produce up to four generations yearly (15). Rearing quails can also boost nutritional value because quail meat has more protein than chicken (16). Quail farming began in Bangladesh in 1990, but it is now a thriving industry due to its economic importance as a commercially farmable species that produces excellent meats with delicate flavors (17). In recent years, native chickens and ducks, domestic pigeons, and quails have emerged as small-scale commercial ventures and demonstrated viability as a source of revenue for rural farmers under conventional management structures. The supply of pigeons and quail has expanded in the LBMs of Bangladesh due to the significant and rising demand for pigeons and quail meats and eggs. They are also kept as pet birds. Many vendors nowadays keep pigeons and quails in their stores; however, these vendors often lack the knowledge of biosecurity protocols necessary to maintain a healthy population of pigeons and quails, which puts the birds at risk of contracting AIV.
Pigeons and quails sold at live bird markets in countries such as Egypt, China, Indonesia, and Vietnam have previously been infected with the AIVs (18–20). Pigeons and quails in Bangladeshi LBMs have also been infected with the AIV virus (21). We should take appropriate measures to control the infection of the virus in these birds in the LBMs. However, to the best of our knowledge, there have not been many comprehensive studies that have explicitly targeted the circulation of AIV in pigeons and quails in the LBMs of Bangladesh, nor has there been an investigation into the risk factors that lead to the infection. As a result, we conducted this study to determine the prevalence of AIV and its subtypes in pigeons and quails in the LBMs in Bangladesh and determine the factors associated with AIV infection.
Materials and methods
Study sites, period, and design
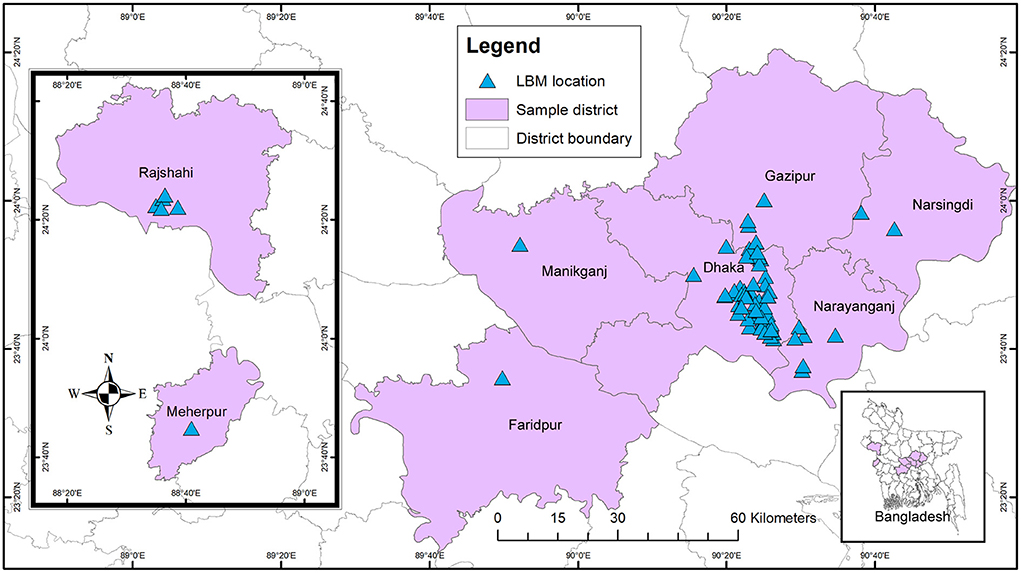
We conducted a cross-sectional study on pigeons and quail in LBMs and the bio-security practices maintained in the vendor shop level and LBMs in 8 districts (Figure 1) from February 2017 to September 2021. We purposively selected the study areas based on the poultry and LBM density.
Ethical approval
The study protocol was approved by the Animal Experimentation Ethics Committee of [protocol: CVASU/Dir (R&E) AEEC/2015/751] and Ethics Committee (EC) (Protocol: CVASU/Dir (R&E) EC/2015/1011) of the Chattogram Veterinary and Animal Sciences University.
Biological samples and data collection
We collected pooled cloacal and oropharyngeal swab samples from each bird using two sterile cotton swabs and placed them in a 1.8 ml sterile cryovial containing 1 ml of viral transport media (VTM) sample as previously described (22). However, we are aware that some pet birds are very costly, and the owner is reluctant to provide cloacal or oropharyngeal swabs. In that case, we collected swabs from freshly laid feces. The number of collected samples from pigeons and quail during each year and season from LBMs in our studied locations are presented in Supplementary Figure S1.
Immediately after collection, samples were stored in liquid nitrogen in the field and at −80°C in the lab until laboratory testing.
We collected the necessary information for individual vendors, bird health conditions, and selling practices related to hygiene and sanitation using a structured multiple-choice questionnaire.
Lab testing
We tested the swab samples to detect the viral M gene for the presence of AIV. The MagMAXTM-96 AI/ND Viral RNA Isolation Kit (Applied BiosystemsTM, San Francisco, CA) extracted RNA from collected samples in a KingFisherTM Flex 96-well robot (Thermo ScientificTM, Waltham, MA) according to the manufacturer's instructions. The samples were first tested for the presence of the M gene using real-time reverse transcription-polymerase chain reaction (rRT-PCR) with reference primers and probes, followed by the procedure as reported by Spackman (23). The H5, H9, and H7 sub-typing of M gene-positive samples were then determined utilizing primers and probes in an rRT-PCR test (24), followed by Spackman and Suarez (25). An example was considered positive if the cycle threshold value was <40 (26). Among M gene-positive samples, those negative for H5, H9, and H7 were considered Influenza A HA/untyped.
Statistical analysis
We summarized the characteristics of biosecurity practices by using descriptive analyses. We then estimated the prevalence of influenza A viruses in different species and seasons, along with 95% CIs and visualized them using graphical analysis. We used a time plot of the prevalence of AIV subtypes to show the temporal trend of AIV subtypes. We then performed Pearson's chi-square test (27) to find the bio-security practices significantly associated with AIV. Factors associated with AIV with p < 0.05 in univariate analysis were selected for multivariable analyses. We then calculated Cramer's V to identify the relationship between the predictor variables. We then used a mixed-effect logistic regression model (28), accounting for clustering by district and live bird market, to estimate adjusted odds ratios. We calculated model χ2 to measure model fitness for the mixed-effect logistic regression model by Wald's test. We performed all statistical analyses using Stata version 16 software (StataCorp LLC, https://www.stata.com) and RStudio version 4.1.2 (29). We used “lme4” and “tidyverse” packages for the analysis in R software. For graphical presentation, GraphPad Prism (https://www.graphpad.com) and for mapping, ArcGIS (https://www.arcgis.com) were used. The shape file was collected from freely available DIVA-GIS (https://www.diva-gis.org/gdata).
Results
Assessment of the hygienic status of vendor level and live bird market level
The hygienic and sanitation status of the vendor and LBM are presented in Table 1. Sixty-four percent of the LBM had closing days, and more than eighty-six percent of the LBMs had more than one bird species (Chicken, duck, pigeon, quail). The presence of wild birds (93.29%) and waterfowl (59.27%) was noticed in most LBM and vendor stalls, respectively. On the other hand, pigeons were found traded at more vendor stalls than quails (38.34%). Over eighty percent of the vendors buy birds from the wholesale market and get them through inter-district trading. Most (62.94%) of the vendors do not separate the sick birds from the healthy birds, but they (72.36%) do not usually mix the unsold birds with the new birds. When it comes to the disposal of offal and dead birds, most vendors (68.89%) throw them away.

Table 1. Proportion and 95% CI of biosecurity and hygienic status of pigeons and quail vendor stall level and live bird markets level.
In contrast, only 13.42% of shops reported having dead birds within the past week. Retail and wholesale businesses made up the majority (71.73%) of the stalls. The preponderance of shops (88.50%) had healthy rather than sick birds.
Prevalence of AIV and subtypes at the vendor level in pigeons and quail
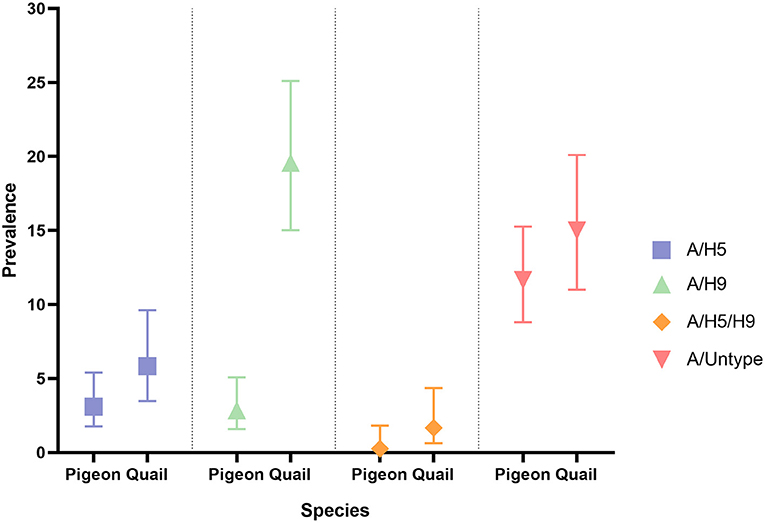
Prevalence and 95% CI of Influenza A subtypes in pigeons and quail during 2017–2021 is presented in Figure 2.
The overall prevalence of AIV was 25.56%, A/H5 was 3.35%, and A/H9 was 8.47% in our sample. Co-circulation of H5 and H9 were also found in our sample (8%). We did not detect any H7 in our sample. Prevalence of AIV was higher in quail (38.75%) than in pigeons (17.36%). Similar results were also found for A/H5 (quail: 4.17% and pigeon: 2.85%), A/H9 (quail: 17.92% and pigeon: 2.59%) and A/H5/H9 (quail: 1.67% and pigeon: 0.26%).
Seasonal pattern and temporal trend of prevalence of AIV and subtypes
From Figures 3A,B, we can observe that for both pigeons and quail, there is a higher prevalence of AIV in the dry season every year than in the wet season. We also noticed the same seasonal pattern in the overall sample (Supplementary Figure S3). Figures 3C,D show the temporal trend of AIV subtypes in pigeons and quail from 2017 to 2021. In pigeons, we can see that, while the prevalence of A/H5, A/H9, and A/H5/H9 did not vary considerably between 2017 and 2021, the prevalence of A/untyped (40%) increased greatly in 2020. In quail, however, we detected no substantial shift in the prevalence of A/H5, A/untyped, and A/H5/H9 over the years, although the prevalence of A/H9 (56%) significantly increased in 2020. From Supplementary Figure S2, we can also see that in the dry season, the overall prevalence of A/H5 and A/H9 across all the years was higher than the overall prevalence of A/H5 and A/H9 in the wet season.
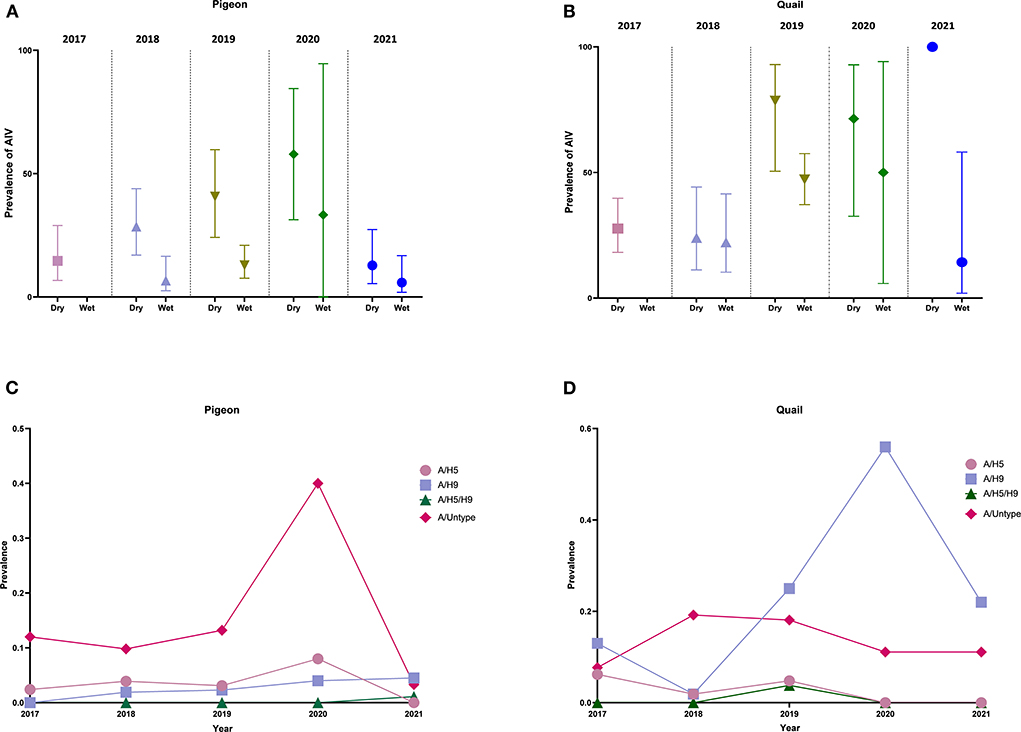
Figure 3. Seasonal pattern and temporal trend of prevalence of AIV and subtypes (2017–2021). (A) Prevalence and 95% Confidence Interval of AIV in pigeons in dry and wet seasons. (B) Prevalence and 95% Confidence Interval of AIV in quail in dry and wet seasons. (C) Temporal trend of AIV subtypes in pigeons from 2017 to 2021. (D) Temporal trend of AIV subtypes in quail from 2017 to 2021.
Association between bio-security practices and AIV circulation in pigeons and quail
Univariable analysis to identify predictor variables using chi-square test
We used Pearson's chi-square test to determine the bio-security practices that influence the prevalence of AIV (Table 2). We only considered vendor-level bio-security practices for univariable analysis. In the univariate analysis, significant associations (p < 0.05) with AIV were found: the presence of waterfowl; type of species; source of birds of the vendor; source area of birds of the vendor; separate sick birds at the vendor; mixing unsold birds with new birds; history of any birds died in the last seven days at the shop; selling the number of species of birds; type of business of the vendor; and health conditions of birds.
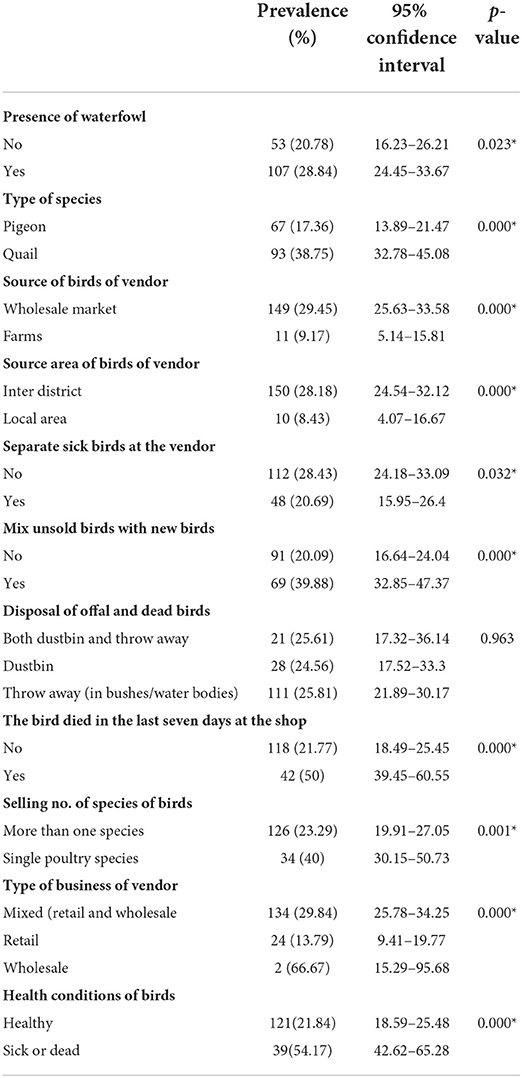
Table 2. Factors associated with AIV circulation (results from Pearson's chi-square test *p-value < 0.05, statistically significant).
Exploring correlation between predictor variables to identify potential multicollinearity
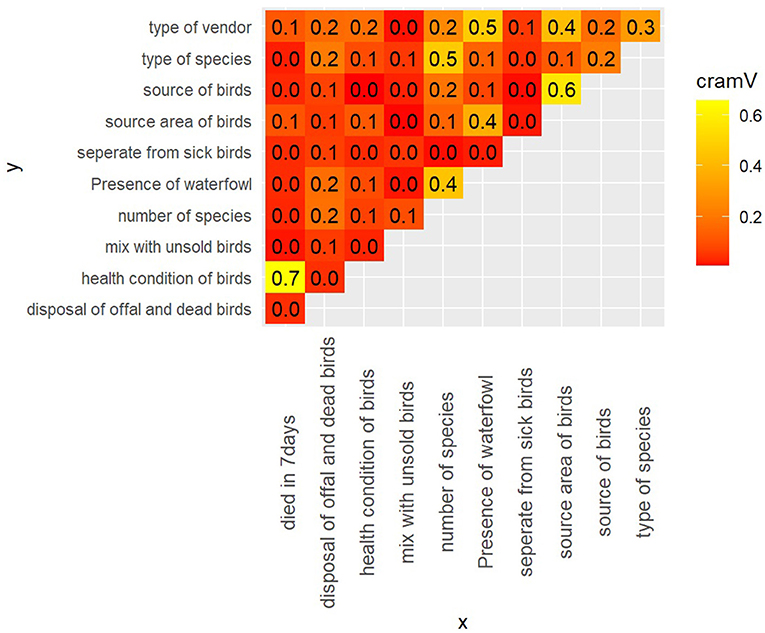
The value of Cramer's V between the predictor variables is shown in Figure 4. Greater Cramer's V value suggests a stronger association. We chose 0.60 as the cutoff value (28). We can see a strong correlation between the health condition of the birds and the birds that died in the vendor shop in the last seven days (Cramer's V = 0.70). Similarly, the source of the birds of the vendors and the source area of the birds of the vendors were strongly correlated (Cramer's V = 0.60). To eliminate potential multi-collinearity, the source area of birds of vendors and birds that died in the last seven days at the shop will be omitted from the multivariable model.
Multivariable modeling using mixed effect logistic regression model
We used a mixed-effect logistic regression model, accounting for clustering by district and live birth market, to estimate the adjusted odds ratio (Table 3). In the multivariable analysis, we found that the presence of waterfowl, type of species (pigeons or quail), source of birds of the vendor, separate sick birds at the vendor, mixing of unsold birds with new birds, and health conditions of birds are the factors that have a significant association (p < 0.05) with AIV. Having waterfowl (AOR: 2.13; 95% CI: 1.04–4.33), Purchasing birds from the wholesale market (AOR: 2.96; 95% CI: 1.48–5.92) instead of farms, mixing sick birds with the healthy ones (AOR: 1.60; 95% CI: 1.04–2.45) and mixing unsold birds with new birds (AOR: 3.07; 95% CI: 2.01–4.70) were significantly more likely to be positive for influenza A viruses compared with vendors that did not have these characteristics. We also found that the odds of AIV were more than twice as high in quail (AOR: 2.57; 95% CI: 1.61–4.11) as in pigeons. Conversely, sick and dead birds had 4.19 times higher chance of AIV detection (AOR: 4.19; 95% CI: 2.38–7.35) compared to healthy birds. The mixed-effect logistic model fit our data well, with a chi-square value: of 87.68 and a p-value < 0.001.
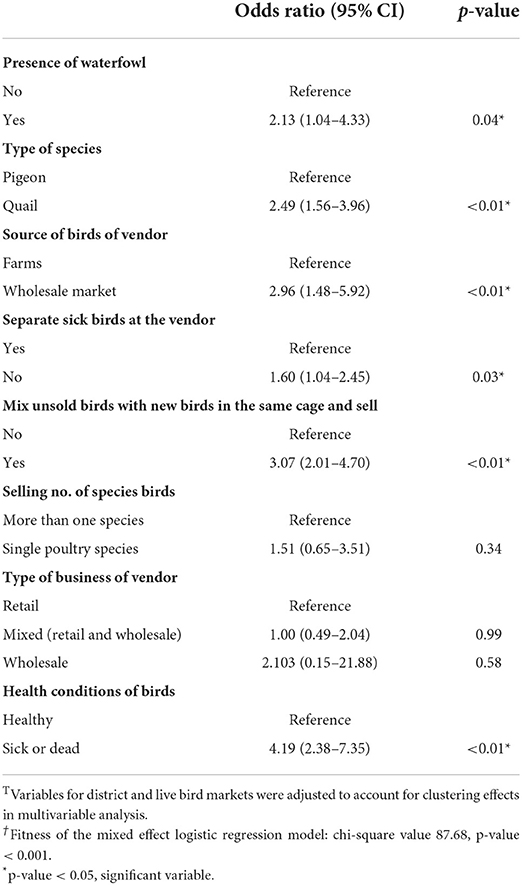
Table 3. Bio-security practices associated with AIV circulation (results from mixed-effect logistic regression model)T, †.
Discussion
Avian influenza viruses have caused devastating epidemics in domestic poultry and human infections in Bangladesh. AIV has also been detected in pet birds such as pigeons and quail in Bangladesh (19), but there have not been many studies identifying the variables that increase the risk of AIV in pigeons and quail. Our analysis depicts existing biosecurity practices in Bangladesh's vendor shops in selected LBMs. We studied the trends in AIV infections in pigeons and quail from 2017 to 2021. We identified certain biosecurity practices associated with the circulation of AIVs in pigeons and quail.
Prevalence of AIV and subtypes in pigeon and quail
In the present study, the prevalence of AIV RNA in pigeons was 17.36%, higher than the prevalence previously observed in Bangladesh (21, 30). It may be because we collected extensive data from pigeons through intensive sampling, where they collected data from other birds along with pigeons and quail. In pigeons, we detected A/H5, A/H9, and co-circulation of A/H5/H9; similarly, prior investigations detected A/H5 and A/H9 (21). The prevalence of A/untyped was higher than A/H5, A/H9, and A/H5/H9 in pigeons suggesting other LPAIV subtypes are circulating among pigeons in the LBMs of Bangladesh. There is evidence that pigeons could be infected by LPAIV subtypes like H3N6 (31).
We detected that the prevalence of AIV in quails is 38.75%, which is comparable to the prevalence of AIV in the LBM (32) but higher than the prevalence identified in the Pet bird market (PBM) (21) in earlier studies on quails in Bangladesh. LBMs are considered hotspots for the occurrence and contamination of AIV (30). Therefore, our study's prevalence of quail was higher as we obtained data from LBMs. Similar to our research, AIV subtype H5, H9, and co-circulation of H5 and H9 were also detected in quail in the previous studies (4, 6). We found that the prevalence of A/H9 was higher in quail than in other subtypes. It has been found that quails are highly susceptible to H9N2 viruses, with the HA gene requiring few modifications for efficient replication and transmission in the quail (33). Unless other pathogens complicate the infection, birds infected with H9N2 AIV often exhibit no clinical symptoms or minor respiratory symptoms and a decline in egg production; hence, the virus remains unreported and spreads more rapidly than other subtypes (34).
We found that the prevalence of AIV in quail was more significant than in pigeons. AIVs do not replicate well in pigeons, which only shed a trace amount of the virus (33) with little or no clinical symptoms (33, 35). On the other hand, gallinaceous poultry, such as quail, is thought to be highly susceptible to AIV infection, resulting in significant morbidity, mortality, and gross and histological lesions (35). Also, quails are smaller in size, allowing more birds to be caged together; they are more vulnerable to influenza infection and have been linked to the land-based transmission and adaptation of H5 and H9 viruses in other hosts (33).
Seasonal pattern and temporal trend of prevalence of AIV subtypes in pigeons and quail
According to the findings of our study, the incidence of AIV in both pigeons and quails is higher during the dry season. We observed this pattern to be consistent every year from 2017 to 2021. It is possible because of the dry season's low temperatures and low humidity (36). During the dry season, the average temperature in Bangladesh stays between 18 and 22°C, and during the wet season, the average temperature stays between 23 and 30°C (36). Hassan, Hoque (30) showed that the prevalence of AIV increased in colder winter months. A study examined how long the Indian H5N1 HPAI virus could survive in dry and wet poultry feces at 42, 37, 24, and 4 °C. They discovered that the virus could survive for long periods in the feces at low temperatures and could potentially act as a long-term source of influenza virus in the environment (37).
We then showed that the circulation of A/untyped increased in pigeons in 2020, followed by a reduction in 2021, and the circulation of A/H9 in quail increased during 2019–2020, followed by a decrease in 2021. The increase in the prevalence of A/untyped and A/H9 during 2019 and 2020 in pigeons and quail, respectively, can be due to the unexpected emergence of the COVID-19 pandemic; LBMs in several cities throughout the country were forced to shut immediately; highways were also blocked, logistics were hindered; socially concentrated activities were canceled; consumer demand decreased, and poultries in LBM specifically birds like pigeons and quail could not be sold. Because of the large number of live birds kept together, the virus spread rapidly among the birds. Similar results were also found by Guo, Song (38) in LBMs in China. On the other hand, in 2020, the Government of Bangladesh approved an H9N2 vaccination (CEVAC NEW FLU H9K) (39). However, pet birds such as pigeons and quails are seldom vaccinated, and vaccination programs typically target layer and breeder chickens (40).
Nevertheless, pigeons and quail are kept close to chickens and ducks in LBMs. Consequently, the virus spread from poultry to pigeons and quails might have decreased. Vaccination of poultry may thus have a beneficial impact on the spread of AIV in pigeons and quails. Therefore, the decline in the prevalence of A/H9 in 2021 in quails may be attributable to the vaccination campaign.
AIV virus and associated factors
Our study reveals a lack of hygienic and biosecurity practices in the majority of vendor shops and LBMs, such as the presence of wild birds and waterfowl, purchase of birds from wholesale markets rather than farms, acquisition of birds through inter-district trading, failure to separate sick birds from healthy birds and to throw away dead birds and offal instead of disposing of in the dustbin (Table 2). These practices have increased AIV in other countries (20, 41).
From multivariable analysis, we found that the presence of waterfowl in the vendor shop increases the risk of AIV in pigeons and quail. There is ample evidence that influenza viruses were spread from waterfowl to commercial poultry and pet birds (42). Ducks are regarded as the AIV virus's 'Trojan horse' (43–45). Infection in ducks may go unnoticed, even with HPAI, and it often manifests itself clinically only after the infection has spread or until quick and aggressive monitoring is performed (46). Furthermore, the LBM's vendors are congested and lack space (47). Different birds are kept together in a small space and mixed (47). If waterfowl, pigeons, and quail are sold in the same shop, the virus can easily spread from waterfowl to pigeons and quail.
We identified that purchasing pigeons and quail from a wholesale market raises the risk of AIV rather than buying from a farm. Wu, et al. (48) also showed that the transmission risk of AIVs gradually increases along the poultry supply chain from farms to wholesale markets in China. Most farms breed one or two species of birds, and different species of birds are rarely mingled when transported from the farm to the vendor shops because farms usually trade nearby (49). In wholesale marketplaces, on the other hand, different types of poultry are kept together. Furthermore, several species of birds are mingled together and maintained in crowded spaces while transported from wholesale markets to vendor shops, significantly increasing the danger of AIV infection (50).
Based on the findings of our study, we determined that mixing unsold birds with new birds considerably raises the probability of AIV infection. There is evidence that Poultry that has not been sold but is infected with the influenza virus could potentially infect new poultry. (41). Conversely, after overnight poultry keeping was declared illegal in China, the percentage of AIV virus isolates found in chickens dropped by 84% (51).
Our study revealed that vendors who do not separate sick from healthy birds have a higher risk of AIV. We also found that the chance of AIV detection is higher in sick or dead birds. Both the highly pathogenic avian influenza H5N1 and the low-pathogenic avian influenza H9N2 have been linked to cases of illness in birds (52, 53). Furthermore, it is possible that the HPAI AIV was the cause of the birds' deaths (54). Therefore, the presence of sick and dead birds may indicate that the birds are infected with AIV, and not separating them from healthy birds causes the virus to disseminate to healthy birds, thereby increasing the risk of infection with AIV.
Conclusion and recommendation
We found that circulation of AIV in pigeons and quail in LBM of Bangladesh was positively associated with the presence of waterfowl, purchasing a bird from the wholesale market, mixing sick birds with healthy ones, mixing unsold with new birds, keeping the sick or dead bird, having quails in the shop. It is possible that improving these biosecurity practices could prevent the spread of AIV. Our study revealed that purchasing pigeons and quail directly from farms rather than wholesale markets was associated with a lower risk of AIV. Therefore, to understand the stages of the AIV subtypes infection in pigeons and quails, we should conduct a comprehensive investigation to determine what stage of the birds' distribution chain becomes infected and what factors contribute to this concern. Our research findings showed that proper biosecurity practices and hygiene standards are not maintained in the LBM in Bangladesh. Training for poultry workers on effective biosecurity practices to reduce the risk of AIV contamination should be implemented. Suitable vaccination programs targeting pigeons and quails must be carried out precisely to lessen the likelihood of AIV.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
We performed all procedures in studies in accordance with the Ethical Standards of the Institutional and National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. As part of standard Ethics approval procedures, participants were informed about the purpose, methods, risks, and benefits of participating in the study and were allowed to ask questions before providing voluntary consent. We obtained informed consent from all study participants before conducting interviews. The study protocol was approved by the Ethics Committee (EC) (Protocol: CVASU/Dir(R&E) EC/2015/1011) of the Chattogram Veterinary and Animal Sciences University. The animal study was reviewed and approved by the Chattogram Veterinary and Animal Sciences University-Animal Experimentation Ethics Committee (Protocol: CVASU/Dir (R&E) AEEC/2015/751).
Author contributions
AI: conceptualization. AI and SI: field investigation. AI, MEH, and SI: data curation. RH, MS, MEH, and MR: laboratory analysis. AI and EA: formal analysis and wrote initial draft. AI and MR: funding. MMH and MR: supervision. SI and MMH: review and edited manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
We acknowledge the Institute of Epidemiology, Disease Control and Research (IEDCR) Bangladesh and EcoHealth Alliance, and the Center for Integrative Ecology at Deakin University, Australia, for their support in conducting this research. The sample collection was partially supported by the Emerging Pandemic Threats PREDICT project (cooperative agreement number GHN-A-OO-09-00010-001) through Eco Health Alliance. We also acknowledge Bangladesh Livestock Research Institute (BLRI), and International Center for Diarrhoeal Disease Research, Bangladesh (icddr,b), for their support in testing samples for avian influenza. We thank the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support to icddr, b. The team was partially supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institutes of Health (NIH) (U01AI153420).
Conflict of interest
AI was employed by EcoHealth Alliance.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1016970/full#supplementary-material
References
1. Hassan MM, Hoque M, Debnath NC, Yamage M, Klaassen M. Are poultry or wild birds the main reservoirs for avian influenza in Bangladesh? Ecohealth. (2017) 14:490–500. doi: 10.1007/s10393-017-1257-6
2. Islam A, Islam S, Samad M, Hossain M, Hassan M, Alexandersen S, et al. Epidemiology and molecular characterization of multiple avian influenza A/H5 subtypes circulating in house crow (corvus splendens) and poultry in Bangladesh. Int J Infec Dis. (2022) 116:S92–S3.
3. World Health Organization. Avian Influenza. World Health Organization. (2011) .Available online at: https://www.who.int/mediacentre/factsheets/avian_influenza/en. (accessed May 02, 2022).
4. OIE. World Organization for Animal Health, Update on avian influenza in animals. (2022) Available online at: https://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2020/ (accessed on 20 March 2022).
5. Biswas PK, Christensen JP, Ahmed SS, Barua H, Das A, Rahman MH, et al. Avian influenza outbreaks in chickens, Bangladesh. Emerg Infect Dis. (2008) 14:1909. doi: 10.3201/eid1412.071567
6. Monne I, Yamage M, Dauphin G, Claes F, Ahmed G, Giasuddin M, et al. Reassortant avian influenza A (H5N1) viruses with H9N2-PB1 gene in poultry, Bangladesh. Emerg Infect Dis. (2013) 19:1630. doi: 10.3201/eid1910.130534
7. Negovetich NJ, Feeroz MM, Jones-Engel L, Walker D, Alam SR, Hasan K, et al. Live bird markets of Bangladesh: H9N2 viruses and the near absence of highly pathogenic H5N1 influenza. PLoS ONE. (2011) 6:e19311. doi: 10.1371/journal.pone.0019311
8. Parvin R, Heenemann K, Halami MY, Chowdhury EH, Islam M, Vahlenkamp TW. Full-genome analysis of avian influenza virus H9N2 from Bangladesh reveals internal gene reassortments with two distinct highly pathogenic avian influenza viruses. Arch Virol. (2014) 159:1651–61. doi: 10.1007/s00705-014-1976-8
9. Parvin R, Nooruzzaman M, Kabiraj CK, Begum JA, Chowdhury EH, Islam MR, et al. Controlling avian influenza virus in Bangladesh: challenges and recommendations. Viruses. (2020) 12:751. doi: 10.3390/v12070751
10. Asaduzzaman M, Mahiuddin M, Howlider M, Hossain M, Yeasmin T. Pigeon farming in Gouripur upazilla of Mymensingh district. Bangl J Anim Sci. (2009) 38:142–50. doi: 10.3329/bjas.v38i1-2.9923
11. Domenech J, Dauphin G, Rushton J, McGrane J, Lubroth J, Tripodi A, et al. Experiences with vaccination in countries endemically infected with highly pathogenic avian influenza: the Food and Agriculture Organization perspective. Revue Sci et Techn. (2009) 28:293. doi: 10.20506/rst.28.1.1865
12. Sims LD. Intervention strategies to reduce the risk of zoonotic infection with avian influenza viruses: scientific basis, challenges and knowledge gaps. Influenza Other Respi Viruses. (2013) 7:15–25. doi: 10.1111/irv.12076
13. Onyewuchi U, Offor I, Okoli C. Profitability of quail bird and egg production in IMO state. Nig J Agric Food Environ. (2013) 9:40–4.
15. Saidu S, Afanasyev G, Popova L, Komarchev A, Ibrahim U, editors. Dynamic of reproductive qualities of Japanese quails. in International conference on earth, environment and life sciences,(eels-2014) December, (2014).
16. Redoy M, Shuvo A, Al-Mamun M, A. review on present status, problems and prospects of quail farming in Bangladesh. Bangl J Anim Sci. (2017) 46:109–20. doi: 10.3329/bjas.v46i2.34439
17. Islam M, Akter S, Sultana S. Quail Farming and its prospect at Chattogram in Bangladesh. Bangl J Veter Anim Sci. (2018) 6.
18. Nguyen DC, Uyeki TM, Jadhao S, Maines T, Shaw M, Matsuoka Y, et al. Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol. (2005) 79:4201–12. doi: 10.1128/JVI.79.7.4201-4212.2005
19. Zhao K, Gu M, Zhong L, Duan Z, Zhang Y, Zhu Y, et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol. (2013) 163:351–7. doi: 10.1016/j.vetmic.2012.12.025
20. Indriani R, Samaan G, Gultom A, Loth L, Indryani S, Adjid R, et al. Environmental sampling for avian influenza virus A (H5N1) in live-bird markets, Indonesia. Emerg Infect Dis. (2010) 16:1889. doi: 10.3201/eid1612.100402
21. Hossain M, Haider N, Sturm-Ramirez K, Hasan R, Hossain M, Rahman M, et al. Identification of avian influenza viruses among birds in pet bird markets. Int J Infect Dis. (2020) 101:349. doi: 10.1016/j.ijid.2020.09.916
22. Druce J, Garcia K, Tran T, Papadakis G, Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J Clin Microbiol. (2012) 50:1064–5. doi: 10.1128/JCM.06551-11
23. Spackman E. Avian influenza virus detection and quantitation by real-time RT-PCR. Methods Mol Biol. (2014) 1161:105–18. doi: 10.1007/978-1-4939-0758-8_10
24. Ali MZ, Hasan M, Giasuddin M. Potential risk factors of avian influenza virus infection in asymptomatic commercial chicken flocks in selected areas of Bangladesh during 2019. J Adv Vet Anim Res. (2021) 8:51–7. doi: 10.5455/javar.2021.h484
25. Spackman E, Suarez DL. Detection and identification of the H5 hemagglutinin subtype by real-time RT-PCR. Methods Mol Biol. (2008) 436:27–33. doi: 10.1007/978-1-59745-279-3_5
26. Heine HG, Trinidad L, Selleck P, Lowther S. Rapid detection of highly pathogenic avian influenza H5N1 virus by TaqMan reverse transcriptase-polymerase chain reaction. Avian Dis. (2007) 51:370–2. doi: 10.1637/7587-040206R.1
27. Plackett RL. Karl Pearson and the chi-squared test. International statistical review/revue. Int de Statist. (1983):59–72. doi: 10.2307/1402731
28. Hox JJ, Moerbeek M, Van de. Schoot R. Multilevel analysis. Routledge: Techniques and application. (2017). doi: 10.4324/9781315650982
29. Team RC. R: A language and environment for statistical computing. in R Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org/. (2013).
30. Hassan MM, Hoque MA, Ujvari B, Klaassen M. Live bird markets in Bangladesh as a potentially important source for Avian Influenza Virus transmission. Prev Vet Med. (2018) 156:22–7. doi: 10.1016/j.prevetmed.2018.05.003
31. Liu T, Xie Z, Wang G, Song D, Huang L, Xie Z, et al. Avian influenza virus with hemagglutinin-neuraminidase combination H3N6, isolated from a domestic pigeon in Guangxi, southern China. Genome Announc. (2015) 3:e01537–14. doi: 10.1128/genomeA.01537-14
32. Turner JC, Feeroz MM, Hasan MK, Akhtar S, Walker D, Seiler P, et al. Insight into live bird markets of Bangladesh: an overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg Microbes Infect. (2017) 6:1–8. doi: 10.1038/emi.2016.142
33. Perez DR, Lim W, Seiler JP Yi G, Peiris M, Shortridge KF, et al. Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J Virol. (2003) 77:3148–56. doi: 10.1128/JVI.77.5.3148-3156.2003
34. El-Zoghby EF, Arafa A-S, Hassan MK, Aly MM, Selim A, Kilany WH, et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch Virol. (2012) 157:1167–72. doi: 10.1007/s00705-012-1269-z
35. Kraidi Q, Langeroudi A, Madadgar O, Karimi V. Prevalence of AIV subtype H9 among poultry with respiratory signs in Iraq. Bulg J Veten Med. (2017) 20. doi: 10.15547/bjvm.1022
36. Mossammat Ayesha Khatun MBR, Hans Olav Hygen. Climate of Bangladesh. Norwegian Meterological Institute 31.05.2016.
37. Kurmi B, Murugkar H, Nagarajan S, Tosh C, Dubey S, Kumar M. Survivability of highly pathogenic avian influenza H5N1 virus in poultry faeces at different temperatures. Ind J Virol. (2013) 24:272–7. doi: 10.1007/s13337-013-0135-2
38. Guo J, Song W, Ni X, Zhou K, Wu J, Liu W, et al. The impact of the closure of the live poultry market due to COVID-19 on the avian influenza virus in Nanchang, Jiangxi Province, China. Am J Trop Med Hyg. (2022) 106:127. doi: 10.4269/ajtmh.21-0732
39. Rahman MM, Nooruzzaman M, Kabiraj CK, Mumu TT, Das PM, Chowdhury EH, et al. Surveillance on respiratory diseases reveals enzootic circulation of both H5 and H9 avian influenza viruses in small-scale commercial layer farms of Bangladesh. Zoonoses Public Health. (2021) 68:896–907. doi: 10.1111/zph.12879
40. Rimi NA, Hassan MZ, Chowdhury S, Rahman M, Sultana R, Biswas PK, et al. A decade of avian influenza in bangladesh: where are we now? Trop Med Infect Dis. (2019) 4. doi: 10.3390/tropicalmed4030119
41. Offeddu V, Cowling BJ, Peiris JM. Interventions in live poultry markets for the control of avian influenza: a systematic review. One Health. (2016) 2:55–64. doi: 10.1016/j.onehlt.2016.03.002
42. Hassan MM, Islam A, Hasan RB, Rahman MK, Webby RJ, Hoque MA, et al. Prevalence and distribution of avian influenza viruses in domestic ducks at the waterfowl-chicken interface in wetlands. Pathogens. (2020) 9:953. doi: 10.3390/pathogens9110953
43. Hulse-Post D, Sturm-Ramirez K, Humberd J, Seiler P, Govorkova E, Krauss S, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Nat Acad Sci. (2005) 102:10682–7. doi: 10.1073/pnas.0504662102
44. Sturm-Ramirez K, Hulse-Post D, Govorkova E, Humberd J, Seiler P, Puthavathana P, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. (2005) 79:11269–79. doi: 10.1128/JVI.79.17.11269-11279.2005
45. Songserm T. Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, et al. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg Infect Dis. (2006) 12:575. doi: 10.3201/eid1204.051614
46. Koch G, Elbers AR. Outdoor ranging of poultry: a major risk factor for the introduction and development of High-Pathogenicity Avian Influenza. NJAS Wagen J Life Sci. (2006) 54:179–94. doi: 10.1016/S1573-5214(06)80021-7
47. Irin N, Dilshad SM, Al Sattar A, Chisty NN, Sultana A, Hasan M, et al. Live bird market in Bangladesh: Regulatory systems and operations. J Adv Veter and Anim Res. (2021) 8:671–8. doi: 10.5455/javar.2021.h559
48. Wu J-Y, Lau EH, Yuan J, Lu M-L, Xie C-J, Li K-B, et al. Transmission risk of avian influenza virus along poultry supply chains in Guangdong, China. J Infect. (2019) 79:43–8. doi: 10.1016/j.jinf.2019.05.006
49. Das S, Chowdhury S, Khatun M, Nishibori M, Isobe N, Yoshimura Y. Poultry production profile and expected future projection in Bangladesh. World's Poultry Sci J. (2008) 64:99–118. doi: 10.1017/S0043933907001754
50. Wei J, Zhou J, Cheng K, Wu J, Zhong Z, Song Y, et al. Assessing the risk of downwind spread of avian influenza virus via airborne particles from an urban wholesale poultry market. Build Environ. (2018) 127:120–6. doi: 10.1016/j.buildenv.2017.10.037
51. Leung YC, Lau EH, Zhang LJ, Guan Y, Cowling BJ, Peiris JM. Avian influenza and ban on overnight poultry storage in live poultry markets, Hong Kong. Emerg Infect Dis. (2012) 18:1339. doi: 10.3201/eid1808.111879
52. Yuan R, Cui J, Zhang S, Cao L, Liu X, Kang Y, et al. Pathogenicity and transmission of H5N1 avian influenza viruses in different birds. Vet Microbiol. (2014) 168:50–9. doi: 10.1016/j.vetmic.2013.10.013
53. Arafa A-S, Hagag NM, Yehia N, Zanaty AM, Naguib MM, Nasef SA. Effect of cocirculation of highly pathogenic avian influenza H5N1 subtype with low pathogenic H9N2 subtype on the spread of infections. Avian Dis. (2012) 56:849–57. doi: 10.1637/10152-040812-Reg.1
Keywords: avian influenza, seasonality, pigeons, quail, live bird market, biosecurity practices
Citation: Islam A, Islam S, Amin E, Hasan R, Hassan MM, Miah M, Samad MA, Shirin T, Hossain ME and Rahman MZ (2022) Patterns and risk factors of avian influenza A(H5) and A(H9) virus infection in pigeons and quail at live bird markets in Bangladesh, 2017–2021. Front. Vet. Sci. 9:1016970. doi: 10.3389/fvets.2022.1016970
Received: 11 August 2022; Accepted: 06 October 2022;
Published: 26 October 2022.
Edited by:
Shawn Babiuk, National Center for Foreign Animal Disease (NCFAD), CanadaReviewed by:
Jiewen Guan, Canadian Food Inspection Agency (CFIA), CanadaLisanework Ayalew, University of Prince Edward Island, Canada
Copyright © 2022 Islam, Islam, Amin, Hasan, Hassan, Miah, Samad, Shirin, Hossain and Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ariful Islam, YXJpZkBlY29oZWFsdGhhbGxpYW5jZS5vcmc=
 Ariful Islam
Ariful Islam Shariful Islam3
Shariful Islam3 Mohammad Mahmudul Hassan
Mohammad Mahmudul Hassan Mojnu Miah
Mojnu Miah Mohammed Abdus Samad
Mohammed Abdus Samad Mohammad Enayet Hossain
Mohammad Enayet Hossain Mohammed Ziaur Rahman
Mohammed Ziaur Rahman