- 1Clinic for Small Animals, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany
- 2Institute for Biometry, Epidemiology and Information Processing, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany
Brachycephalic Obstructive Airway Syndrome (BOAS) is a pathologic condition of the upper airways, frequently occurring in dogs of brachycephalic breeds including pugs. It has been suspected that BOAS may be associated with cardiovascular changes and an increased risk for hypertension. The cardiac biomarker NT-proBNP can help to differentiate cardiac from non-cardiac respiratory distress. A possible influence of BOAS on NT-proBNP values has not been investigated, however. The aim of the current study was to examine blood pressure and NT-proBNP levels in pugs with and without clinical signs of BOAS and compare them to values of mesocephalic dogs. For this purpose, NT-proBNP values of 42 pugs and six mesocephalic dogs and blood pressure measurements of 34 pugs and four mesocephalic dogs were explored in the present study. Pugs were examined for clinical signs of BOAS at rest and after a submaximal fitness test, and a functional BOAS grading was applied. Blood pressure (BP) was measured at the beginning and end of the study day and NT-proBNP values were obtained before and after exercise. Measured values of pugs with different degrees of clinical impairment due to BOAS were compared among each other as well as to the CG. In terms of systolic, mean, diastolic BP, and NT-pro BNP, there were no relevant differences between pugs and the CG and no obvious connection between the severity of BOAS symptoms and measured values. BP values of all groups were lower at the second measurement at the end of the study day. NT-proBNP measurements were higher after exercise. BP and NT-proBNP values in all groups were in agreement with commonly used reference ranges. In conclusion, the study adds evidence, that BP and NT-proBNP values did not differ between mesocephalic dogs and pugs with different levels of severity of BOAS but between the measurement times. Thus, in the present study, excitement and exercise seemed to have a greater influence on BP and NT-proBNP values than presence of BOAS symptoms or breed. Discovered values show that the commonly used reference ranges for BP and NT-proBNP are applicable in pugs. This indicates that NT-proBNP can be used to differentiate between cardiac and non-cardiac respiratory distress even in pugs with clinical symptoms of BOAS.
1. Introduction
Due to their specific anatomy, brachycephalic breeds, such as the pug, have an increased risk of developing various diseases and often suffer from the so-called Brachycephalic Obstructive Airway Syndrome (BOAS) (1–5). There is a paucity of literature regarding the impact of brachycephaly and BOAS on the cardiovascular system. Studies have investigated differences between brachycephalic and mesocephalic dogs in echocardiography (6, 7), blood pressure (8, 9), and cardiac biomarkers (10). However, studies concerned with measuring blood pressure (BP) and the cardiac biomarker N-terminal pro-B-Type natriuretic peptide (NT-proBNP) in pugs with different severity of BOAS symptoms are lacking.
Regarding BP, there are two studies in which dogs of different brachycephalic breeds had significantly higher BP values than mesocephalic dogs (8, 9). In two other studies, no significant differences between brachy- and mesocephalic dogs could be found (6, 11). A mechanism similarity to obstructive sleep apnoea (OSA) in humans is presumed as a possible reason for higher BP in brachycephalic dogs (8, 9). OSA in humans is a sleep-related breathing disorder associated with disruptions in sleep, hypoxemia, hypercapnia, and an increased risk for hypertension (12–14). A sleep-related breathing disorder showing similarities to OSA in humans was found in English Bulldogs, which therefore were described as a natural model for OSA (15). Brachycephaly is also listed as a risk factor for the development of hypertension in dogs in the current American College of Veterinary Internal Medicine (ACVIM) consensus statement on the identification, evaluation, and management of systemic hypertension in dogs and cats (16).
Cardiac biomarkers are a subject of growing interest in veterinary medicine and can assist in the diagnosis and prognosis of heart diseases (17–21). The cardiac biomarker NT-proBNP is one of the most important cardiac natriuretic peptides in dogs and a marker of cardiac wall stress (22, 23). Various studies showed that NT-proBNP could be used to differentiate between cardiac and non-cardiac respiratory distress (17, 22, 24–28). In one of these studies, BOAS is mentioned as a non-cardiac cause of respiratory distress (22), although the connection between BOAS and NT-proBNP levels has not been the subject of further investigation.
Breed-related influence on cardiac biomarkers, such as NT-proBNP, has already been demonstrated (29), but a breed-specific examination for pugs is still lacking. Investigations into breed-specific NT-proBNP levels in pugs might be helpful due to the prevalence of BOAS-related respiratory disorders in this breed, which might influence NT-proBNP levels.
NT-proBNP has already been shown to be increased after exercise (21) and was therefore measured at rest and after a submaximal fitness test (FT) to point out possible differences. Since clinical signs of BOAS increase under exercise conditions as well (30–32), investigations into the impact of exercise on NT-proBNP may clarify its efficiency in detecting relations to BOAS. In brachycephalic dogs with clinical symptoms of BOAS, the cardiac biomarker cardiac troponin (cTnI) was investigated and found to be increased in 47.8% of the study population (10). However, this was not the case for any of the 11 pugs, which had been included in the study (10).
The objective of the present study was to explore BP and NT-proBNP in pugs from a regular population of patients and to compare values with mesocephalic dogs. To differentiate between BOAS-related increases in BP and NT-proBNP levels, pugs were allocated to groups with different levels of severity of BOAS depending on their clinical presentation.
2. Materials and methods
The study protocol was approved by an institutional Ethics Commission (Lower Saxony State Office for Consumer Protection and Food Safety (LAVES), Oldenburg, Germany, 33.19-42502-05-19A424) and every dog owner had to sign a consent form.
2.1. Study animals and examinations
This prospective study was performed at the Clinic for Small Animals at the University of Veterinary Medicine, Foundation, Hannover, Germany between July 2019 and August 2020. All dogs participating in the study were privately owned. Pugs were eligible for study inclusion if they were at least 2 years old, had no previous upper airway surgery or relevant systemic diseases apart from possible BOAS. Mesocephalic dogs of different breeds served as control group (CG), with the same inclusion criteria applying to them. For a good comparability between the study groups, dogs of a similar age and weight range were chosen as CG.
Physical examination, echocardiography, blood count, and serum biochemical analysis were performed in each dog. Cardiac ultrasound was performed by an experienced investigator (JPB) using ultrasonic devices (Vivid E7 or E9, General Electrics, Inc., Boston, MA, USA). Dogs were excluded from the evaluation of BP or NT-proBNP if they showed a pathologic heart murmur in the physical examination, non-sinus arrhythmia or significant cardiac abnormalities identified on two dimensional (2D), M-Mode, and/or Doppler echocardiography. LA/Ao (ratio of diameters of left atrium and aortic root) and LVIDd (diastolic diameter of left ventricle) were assessed as described previously (33, 34). Blood count and serum biochemical analysis were performed to rule out systemic diseases, which could affect the results of BP or NT-proBNP measurements.
In total, 62 pugs and ten mesocephalic dogs serving as CG were included in the study. Of these, 11 pugs and three dogs in the CG were excluded from the evaluation of BP and NT-proBNP because they did not meet the inclusion criteria in the echocardiographic examination. Due to unwillingness to run on the treadmill, another eight pugs had to be excluded. Since dogs were required to have values for BP and NT-proBNP at both examination points for comparison, another nine pugs and three dogs in the CG were excluded from final calculations of BP because adequate values were only available in one of two measurement times. For NT-proBNP, this was the case in one pug and one dog in the CG. A flow chart giving an overview of the exclusion can be found in the (Supplementary Figure S1).
2.2. Blood pressure
BP was indirectly measured using an automated High Definition Oscillometry device (HDO, S+B med Vet GmbH, Babenhausen, Germany). Measurements were directly visualised on a tablet with the MDSWIN Software Analyses (S+B med Vet GmbH) and thus examined for possible errors like movement artifacts. Regarding the recommendations of the ACVIM, the first measurement was discarded and once reliable, consistent readings had been obtained, five to seven consecutive, consistent measurements were taken (16). A detailed protocol, based on the recommendations of the ACVIM, was completed with additional information like size of the cuff, owner presence, position, and stress level of each dog. Systolic, mean, and diastolic arterial blood pressure values (SBP, MBP, DBP, respectively) as well as pulse frequency were obtained. BP measurements were taken at two different times. The first BP measurement was performed during echocardiographic examination, which was performed as one of the earliest examinations. The second BP measurement was performed at the end of the study day in a quiet environment after the dogs had had a sufficient amount of time to recover from the previous examinations. Only dogs (n = 38) for which reliable measurement results had been obtained at both examination times were included in the final evaluation.
2.3. NT-proBNP
Blood was collected in EDTA-tubes from the vena saphena or vena cephalica antebrachii. Samples were taken before and after an FT. Plasma was obtained by centrifugation and the samples were stored at −80°C until shipment on dry ice to the laboratory (IDEXX Laboratories, Ludwigsburg, Germany). NT-proBNP was measured from 0.3 ml EDTA-Plasma by canine Cardiopet® proBNP ELISA (IDEXX Laboratories).
2.4. Submaximal fitness test
The FT was performed on a motorised treadmill (“quasar,” h/p/cosmos sports and medical GmbH, Nussdorf-Traunstein, Germany). During the FT, dogs trotted 15 min at their individual comfort speed (four to eight kilometres per hour) on the treadmill with a measurement break of 1 min after 5–10 min. With the measurement breaks, the FT lasted 17 min in total.
2.5. Functional BOAS grading
A functional grading system, originally designed and validated by Liu et al. (35), was modified and applied to the dogs based on the findings after 15 min of exercise (35). It has been previously used in various studies investigating BOAS (30, 31, 36, 37). The dogs were classified as having no (grade 0), mild (grade 1), moderate (grade 2) or severe (grade 3) signs of BOAS by evaluating respiratory noises and inspiratory effort before and after exercise and possible signs of dyspnoea or cyanosis (Supplementary Table S1).
2.6. Statistical analysis
The study was analysed within an exploratory approach and only descriptive statistics were presented. For statistical analyses, SAS 9.4 and SAS Enterprise Guide 7.1 (SAS Institute, Inc., Cary., NC, USA) were used. Graphics were created with GraphPad Prism (GraphPad, San Diego, CA, USA).
3. Results
3.1. Study animals
A total of 62 pugs and 10 mesocephalic dogs serving as CG were examined. For final evaluation of BP, measurements of 34 pugs and four dogs in the CG could be included. For evaluation of NT-proBNP, values of 42 pugs and six dogs in the CG were taken for analysis. A detailed overview of baseline characteristics of included dogs can be found in Table 1.
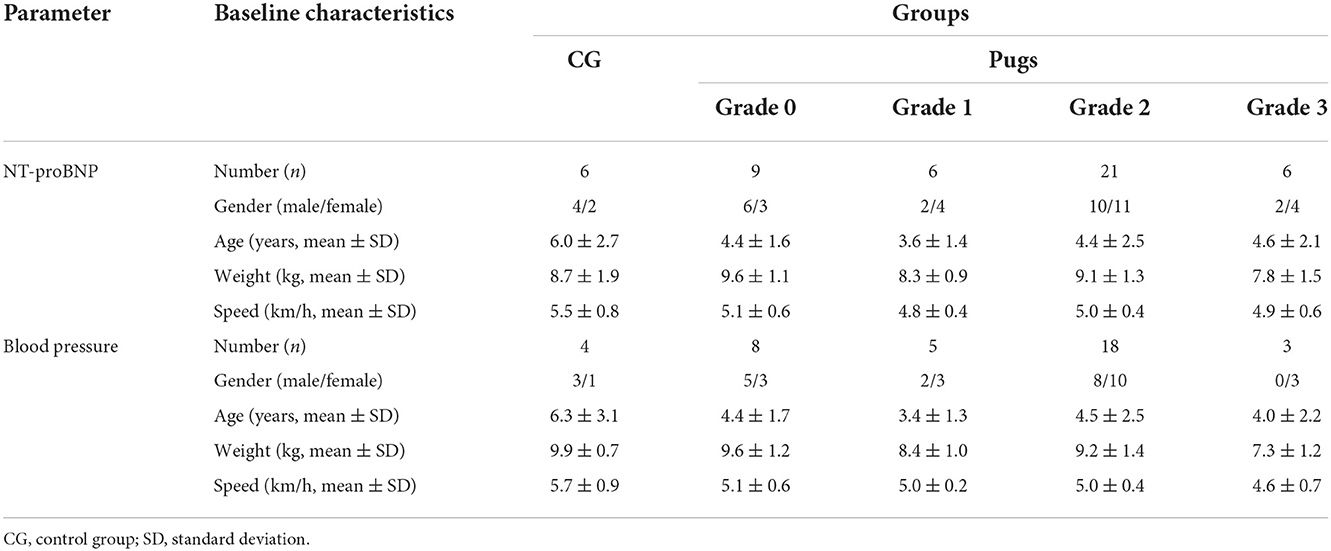
Table 1. Baseline characteristics and running speed of the control group (CG) and pugs subdivided into BOAS grades 0–3 included in the evaluation of NT-proBNP and blood pressure.
In the blood count and serum biochemical analysis, only few dogs showed minimal deviations from the reference values, which were therefore assumed to have no influence on BP and NT-proBNP (Supplementary Table S2).
3.2. Blood pressure
An overview of BP values of the CG and BOAS grades 0–3 pugs can be found in Table 2. Eleven dogs had SBP values between 140 and 160 mmHg (one in the CG, four in the BOAS grade 0 group, three in the BOAS grade 1 group, and three in the BOAS grade 2 group). None of the dogs had values above 160 mmHg. In all but three BP measurements, at least five readings were available. Due to the reason that for these three measurements at least three consecutive, consistent readings were available, they were also taken for evaluation.
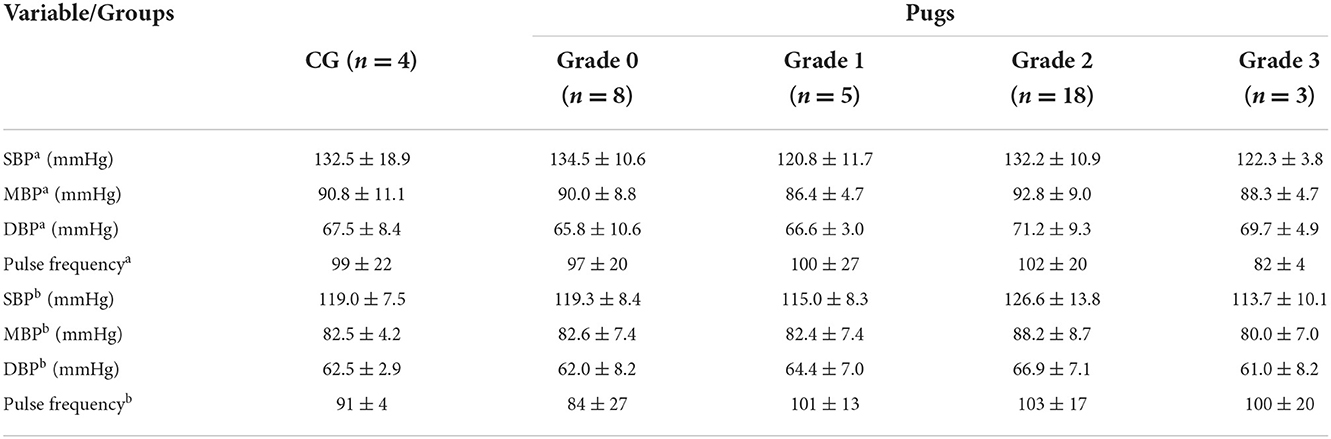
Table 2. Mean (± SD) systolic (SBP), mean (MBP), diastolic blood pressure (DBP), and pulse frequency of dogs in the control group (CG) and pugs subdivided into BOAS grades 0–3 at first measurement time point during echocardiographya and the second one at the end of the study dayb.
Since the first measurement was performed during echocardiography, all dogs (100%) lay in lateral recumbency. Thirteen percent of the dogs were relaxed, most patients (66%) slightly nervous, 18% were nervous, and 3% very nervous. During the second measurement, 16% lay in lateral recumbency, 37% in sternal recumbency, 31% were sitting and 16% standing. At that time, most dogs (84%) were relaxed and some (16%) were slightly nervous. No correlation between BP values with age, body weight, owner presence, position or stress level of the dogs was found.
3.3. NT-proBNP
NT-proBNP values of the CG and BOAS grades 0–3 pugs before and after exercise are shown in Figure 1. Three dogs had values above 900 pmol/l (two dogs in the BOAS grade 2 group and one dog in the CG). A small correlation between NT-proBNP values at rest with body weight was observed (correlation coefficient = −0.29). There was no correlation between NT-proBNP measurements with age, LA/Ao or LVIDd.
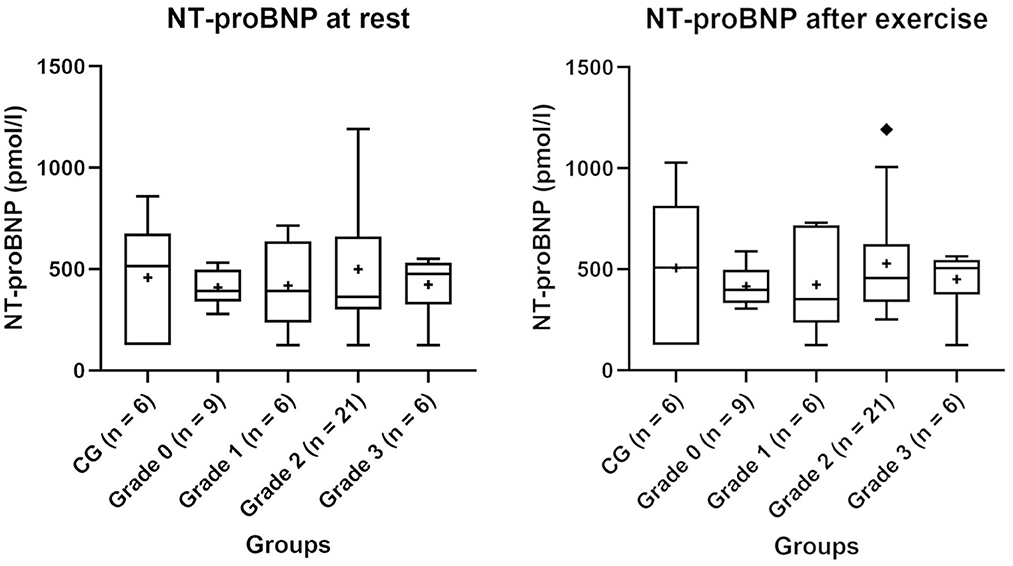
Figure 1. NT-proBNP values of the control group (CG) and groups of pugs subdivided into BOAS grades 0–3 at rest and after exercise. Boxes contain values from 1st to 3rd quartile, lines inside boxes indicate median values, + inside the boxes represents the mean, endpoints of vertical lines are limited to a maximum of 1.5 times the interquartile range, and ♦ represents the outliers.
4. Discussion
The aim of this study was to explore BP and NT-proBNP levels in pugs with different degrees of BOAS and to compare them to levels in mesocephalic dogs. However, no differences between BP and NT-proBNP measurements of mesocephalic dogs and pugs of different grades of BOAS were identified. Therefore, in the present study, the severity of BOAS symptoms did not seem to have an impact on the measured values.
Regarding BP, some studies have observed significant differences between brachy- and mesocephalic dogs (8, 9), while others have not (6, 11). It has been shown that BP can differ among breeds (38), and use of different measurement methods can have a significant influence on the measurement results, which limits the comparability between studies including the comparison of the present study with previous studies (39, 40). The mean SBP of pugs obtained during the first measurement in the present study was comparable to mean values of the two mentioned studies in which no difference between meso- and brachycephalic dogs could have been detected (6, 11). In one of these studies, pugs were included in the brachycephalic group (11).
In one of the former studies, which detected a significant difference between BP of brachy- and mesocephalic dogs (8), mesocephalic dogs had lower mean (±SD) (153.5 ± 21.7) values than brachycephalic dogs (177.6 ± 25.0), but values of the mesocephalic dogs were still higher than the range considered normotensive (<140 mmHg) (16). These findings were also higher than values of meso- and brachycephalic dogs found in the present study. As a possible explanation for the high BP values in the brachycephalic group in this former study, a mechanism similar to OSA in humans was presumed (8).
In studies concerning OSA in humans, intermittent hypoxia led to an activation of the renin-angiotensin system, sympathetic nervous system, endothelial dysfunction and thus an increased risk for hypertension (41, 42). Since a breathing pattern similar to OSA-affected humans has been shown in English Bulldogs (15), a similar mechanism is hypothesised causing an increased risk for hypertension (8, 9). However, studies indicated that there are breed-related differences in symptoms of BOAS (2, 37, 43, 44). As results in previous studies, which found differences between brachy- and mesocephalic dogs were not divided by breed, breed-related variations among the different brachycephalic breeds cannot be ruled out. This would be a possible explanation for the different results in the studies by de Melo Dias et al. (9) and Hoareau et al. (8) and the results presented here.
In the present study, BP values in all groups were lower during the second measurement. Since this measurement was performed at the end of the examination day, it is likely that the dogs were more familiar with the environment and investigators at that time. A familiarisation also affected the behaviour and stress level of the dogs, since most dogs (84%) were relaxed during the second measurement, while the majority of dogs (66%) were slightly nervous during the first measurement. The influence of different settings and stress level on BP values has been shown in several studies (45–48). The results of the present study emphasise the need to consider the settings when interpreting BP values, whereby this factor seemed to have a greater impact on BP levels than breed or clinical signs of BOAS in the present study.
The mean values of each group were below 900 pmol/l, which is the recommended cut-off value for the differentiation between dyspnoea related to cardiac or non-cardiac disease (49). Two pugs in the BOAS grade 2 group and one dog in the CG had slightly higher values than 900 pmol/l but with a mean (range) of 1071.60 (942.0–1192.0) pmol/l just slightly above the cut-off value. In these dogs, no explanations for the higher values were found in the physical examination, echocardiography or blood test. When comparing NT-proBNP values with those from other studies in which the same test (canine Cardiopet® proBNP ELISA, IDEXX Laboratories, Ludwigsburg, Germany) was used, the results were in the same range as the healthy control groups (21, 50).
In the present study, NT-proBNP values in all groups were higher after exercise. Exercise-induced increases in NT-pro BNP levels have been shown in humans (51, 52) and also in dogs after submaximal exercise (21). Despite the increase in NT-proBNP levels through exercise in this study, the mean values were below the laboratory recommended cut-off value. Since no relevant differences between the groups occurred after exercise and the increase within the individual groups was small, the measurement after exercise did not seem to provide additional information for dogs in the CG and pugs in this study.
Another cardiac biomarker that was investigated in one study in the context of brachycephaly and BOAS is cTnI (10). In the study by Planellas et al. (10), 47.8% of the brachycephalic dogs had increased cTnI levels. The authors of this previous study further hypothesised that BOAS may lead to myocardial damage, whereas no significant association between cTnI levels and severity of respiratory signs could be detected. It is noticeable that increased levels only occurred in English and French Bulldogs but in none of the examined pugs (10). Both, the abovementioned study as well as findings in the present study in pugs may indicate that cardiac biomarkers may be more susceptible to breed-specific BOAS characteristics than an overall estimation of clinical BOAS severity.
Limitations of the present study include the small sample size of the groups, especially of the CG and the BOAS grade 3 group. However, the sample size of 34 pugs exceeds sample sizes of previous studies in brachycephalic dogs with regard to BP (6, 8, 9, 11). Additionally, a sample size of 42 pugs for analysing NT-proBNP is comparable to the existing study on brachycephalic dogs and cTni levels, which included 50 brachycephalic dogs, of which 29 dogs were French Bulldogs (10). Moreover, severity of BOAS symptoms was evaluated using a functional BOAS grading based on physical examination, but there was no assessment of anatomic structures under general anaesthesia. Furthermore, since the first BP measurement took place during echocardiography and the dogs therefore could not choose their position, this could also have contributed to higher values in addition to the unfamiliar environment.
In conclusion, BP and NT-pro BNP values did not differ between mesocephalic dogs and pugs with different degrees of severity of BOAS in the present study. Moreover, results of the current study indicate that the commonly used reference ranges for BP and NT-proBNP are applicable in pugs. In the present study, different levels of severity of BOAS do not seem to affect BP and NT-proBNP values. Rather, clinical settings and stress level of the dogs seem important when interpreting BP values. A clinical implication of this regarding NT-proBNP is that the cardiac biomarker may be used to differentiate between cardiac and non-cardiac respiratory distress even in pugs with clinical symptoms of BOAS. In the present study population, no association was found between BOAS symptoms in pugs and hypertension.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Lower Saxony State Office for Consumer Protection and Food Safety (LAVES). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
IN, J-PB, PW, and RM conceived the study and participated in its design and coordination. PW and RM performed and assisted in all examinations. J-PB performed all echocardiographic examinations. RM made substantial contributions to acquiring, analysing, and interpreting the data and wrote the original draught. IN was involved in critically revising the manuscript for important intellectual content and made substantial contributions to its conception and design. MB made substantial contributions to statistical analysis and was involved in revising the manuscript for important intellectual content. J-PB, PW, and CL made substantial contributions to conception and design, analysis, and data interpretation and were involved in critically revising the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Funding
The study was financially supported by the Gesellschaft zur Förderung Kynologischer Forschung e.V. https://www.gkf-bonn.de/index.php/startseite.html and the Hannoversche Gesellschaft zur Förderung der Kleintiermedizin e.V. https://www.hgfk.eu. This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)−491094227 Open Access Publication Funding and the University of Veterinary Medicine Hannover, Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors would like to thank all owners and their dogs for their participation, Frances Sherwood-Brock for proofreading the English language, and Prof. Kreienbrock for the help with the statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1015157/full#supplementary-material
References
1. Asher L, Diesel G, Summers JF, McGreevy PD, Collins LM. Inherited defects in pedigree dogs. Part 1: disorders related to breed standards. Vet J. (2009) 182:402–11. doi: 10.1016/j.tvjl.2009.08.033
2. O'Neill DG, Jackson C, Guy JH, Church DB, McGreevy PD, Thomson PC, et al. Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genet Epidemiol. (2015) 2:1–10. doi: 10.1186/s40575-015-0023-8
3. O'Neill DG, Pegram C, Crocker P, Brodbelt DC, Church DB, Packer RMA. Unravelling the health status of brachycephalic dogs in the UK using multivariable analysis. Sci Rep. (2020) 10:1–13. doi: 10.1038/s41598-020-73088-y
4. Poncet CM, Dupre GP, Freiche VG, Estrada MM, Poubanne YA, Bouvy BM. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract. (2005) 46:273–9. doi: 10.1111/j.1748-5827.2005.tb00320.x
5. Packer RM, Hendricks A, Burn CC. Impact of facial conformation on canine health: corneal ulceration. PLoS One. (2015) 10:e0123827. doi: 10.1371/journal.pone.0123827
6. Canola RAM, Sousa MG, Braz JB, Restan WAZ, Yamada DI, Silva JC, et al. Cardiorespiratory evaluation of brachycephalic syndrome in dogs. Pesqui Vet Brasil. (2018) 38:1130–6. doi: 10.1590/1678-5150-pvb-5376
7. Vurucu M, Ekinci G, Gunes V. An echocardiographic study of breed-specific reference ranges in healthy French Bulldogs. Vet Radiol Ultrasound. (2021) 62:573–82. doi: 10.1111/vru.12997
8. Hoareau GL, Jourdan G, Mellema M, Verwaerde P. Evaluation of arterial blood gases and arterial blood pressures in brachycephalic dogs. J Vet Intern Med. (2012) 26:897–904. doi: 10.1111/j.1939-1676.2012.00941.x
9. de Melo Dias ML, Morris CFM, Moreti BM, do Espírito Santos AV, McManus CM, de Almeida RM, et al. Anatomical, cardiovascular, and blood gas parameters in dogs with brachycephalic syndrome. Acta Sci Vet. (2016) 44:6. doi: 10.22456/1679-9216.80932
10. Planellas M, Cuenca R, Tabar MD, Bertolani C, Poncet C, Closa JM, et al. Evaluation of C-reactive protein, haptoglobin and cardiac troponin 1 levels in brachycephalic dogs with upper airway obstructive syndrome. BMC Vet Res. (2012) 8:152. doi: 10.1186/1746-6148-8-152
11. Arulpagasam S, Lux C, Odunayo A, Biskup J, Sun X. Evaluation of pulse oximetry in healthy brachycephalic dogs. J Am Anim Hosp Assoc. (2018) 54:344–50. doi: 10.5326/JAAHA-MS-6654
12. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. (2000) 342:1378–84. doi: 10.1056/NEJM200005113421901
13. Seravalle G, Grassi G. Sleep apnea and hypertension. High Blood Press Cardiovasc Prev. (2022) 29:23–31. doi: 10.1007/s40292-021-00484-4
14. Durán J, Esnaola S, Rubio R, Iztueta Á. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. (2001) 163:685–9. doi: 10.1164/ajrccm.163.3.2005065
15. Hendricks JC, Kline LR, Kovalski RJ, O'Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol. (1987) 63:1344–50. doi: 10.1152/jappl.1987.63.4.1344
16. Acierno MJ, Brown S, Coleman AE, Jepson RE, Papich M, Stepien RL, et al. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. (2018) 32:1803–22. doi: 10.1111/jvim.15331
17. Ettinger SJ, Farace G, Forney SD, Frye M, Beardow A. Evaluation of plasma N-terminal pro-B-type natriuretic peptide concentrations in dogs with and without cardiac disease. J Am Vet Med Assoc. (2012) 240:171–80. doi: 10.2460/javma.240.2.171
18. Oyama MA. Using Cardiac Biomarkers in Veterinary Practice. Clin Lab Med. (2015) 35:555. doi: 10.1016/j.cll.2015.05.005
19. de Lima GV, Ferreira FDS. N-terminal-pro brain natriuretic peptides in dogs and cats: a technical and clinical review. Vet World. (2017) 10:1072–82. doi: 10.14202/vetworld.2017.1072-1082
20. Iwanuk N, Nolte I, Wall L, Sehn M, Raue J, Pilgram A, et al. Effect of Pimobendan on NT-proBNP and c troponin I before and after a submaximal exercise test in dogs with preclinical mitral valve disease without cardiomegaly - a randomised, double-blinded trial. BMC Vet Res. (2019) 15:1–11. doi: 10.1186/s12917-019-1980-z
21. Wall L, Mohr A, Ripoli FL, Schulze N, Penter CD, Hungerbuehler S, et al. Clinical use of submaximal treadmill exercise testing and assessments of cardiac biomarkers NT-proBNP and cTnI in dogs with presymptomatic mitral regurgitation. PLoS ONE. (2018) 13:e0199023. doi: 10.1371/journal.pone.0199023
22. Oyama MA, Rush JE, Rozanski EA, Fox PR, Reynolds CA, Gordon SG, et al. Assessment of serum N-terminal pro-B-type natriuretic peptide concentration for differentiation of congestive heart failure from primary respiratory tract disease as the cause of respiratory signs in dogs. J Am Vet Med Assoc. (2009) 235:1319–25. doi: 10.2460/javma.235.11.1319
23. Van Kimmenade RRJ, Januzzi JL Jr. The evolution of the natriuretic peptides–Current applications in human and animal medicine. J Vet Cardiol. (2009) 11:S9–S21. doi: 10.1016/j.jvc.2009.01.001
24. Prošek R, Sisson DD, Oyama MA, Solter PF. Distinguishing cardiac and noncardiac dyspnea in 48 dogs using plasma atrial natriuretic factor, B-type natriuretic factor, endothelin, and cardiac troponin-I. J Vet Intern Med. (2007) 21:238–42. doi: 10.1111/j.1939-1676.2007.tb02955.x
25. Boswood A, Dukes-McEwan J, Loureiro J, James RA, Martin M, Stafford-Johnson M, et al. The diagnostic accuracy of different natriuretic peptides in the investigation of canine cardiac disease. J Small Anim Pract. (2008) 49:26–32. doi: 10.1111/j.1748-5827.2007.00510.x
26. Fine DM, DeClue AE, Reinero CR. Evaluation of circulating amino terminal-pro-B-type natriuretic peptide concentration in dogs with respiratory distress attributable to congestive heart failure or primary pulmonary disease. J Am Vet Med Assoc. (2008) 232:1674–9. doi: 10.2460/javma.232.11.1674
27. Fox PR, Oyama MA, Hezzell MJ, Rush JE, Nguyenba TP, DeFrancesco TC, et al. Relationship of plasma N- terminal pro- brain natriuretic peptide concentrations to heart failure classification and cause of respiratory distress in dogs using a 2nd generation ELISA Assay. J Vet Intern Med. (2015) 29:171–9. doi: 10.1111/jvim.12472
28. Haßdenteufel E, Kresken JG, Henrich E, Hildebrandt N, Schneider C, Stosic A, et al. NT-proBNP in der Diagnostik bei Hunden mit Dyspnoe und asymptomatischen Hunden mit Herzgeräusch. Tierarztl Prax Ausgabe K: Kleintiere/Heimtiere. (2012) 40:171–9. doi: 10.1055/s-0038-1623639
29. Sjostrand K, Wess G, Ljungvall I, Haggstrom J, Merveille AC, Wiberg M, et al. Breed differences in natriuretic peptides in healthy dogs. J Vet Intern Med. (2014) 28:451–7. doi: 10.1111/jvim.12310
30. Aromaa M, Lilja-Maula L, Rajamaki MM. Assessment of welfare and brachycephalic obstructive airway syndrome signs in young, breeding age French Bulldogs and Pugs, using owner questionnaire, physical examination and walk tests. Anim Welf. (2019) 28:287–98. doi: 10.7120/09627286.28.3.287
31. Lilja-Maula L, Lappalainen AK, Hyytiainen HK, Kuusela E, Kaimio M, Schildt K, et al. Comparison of submaximal exercise test results and severity of brachycephalic obstructive airway syndrome in English bulldogs. Vet J. (2017) 219:22–6. doi: 10.1016/j.tvjl.2016.11.019
32. Liu NC, Adams VJ, Kalmar L, Ladlow JF, Sargan DR. Whole-Body barometric plethysmography characterizes upper airway obstruction in 3 brachycephalic breeds of dogs. J Vet Intern Med. (2016) 30:853–65. doi: 10.1111/jvim.13933
33. Hansson K, Haggstrom J, Kvart C, Lord P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. (2002) 43:568. doi: 10.1111/j.1740-8261.2002.tb01051.x
34. Cornell CC, Kittleson MD, Della Torre P, Haggstrom J, Lombard CW, Pedersen HD, et al. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med. (2004) 18:311–21. doi: 10.1111/j.1939-1676.2004.tb02551.x
35. Liu NC, Sargan DR, Adams VJ, Ladlow JF. Characterisation of Brachycephalic Obstructive Airway Syndrome in French Bulldogs Using Whole-Body Barometric Plethysmography. PLoS ONE. (2015) 10:e0130741. doi: 10.1371/journal.pone.0130741
36. Liu NC, Oechtering GU, Adams VJ, Kalmar L, Sargan DR, Ladlow JF. Outcomes and prognostic factors of surgical treatments for brachycephalic obstructive airway syndrome in 3 breeds. Vet Surg: VS. (2017) 46:271–80. doi: 10.1111/vsu.12608
37. Liu NC, Troconis EL, Kalmar L, Price DJ, Wright HE, Adams VJ, et al. Conformational risk factors of brachycephalic obstructive airway syndrome (BOAS) in pugs, French bulldogs, and bulldogs. PLoS ONE. (2017) 12:e0181928. doi: 10.1371/journal.pone.0181928
38. Bodey AR, Michell AR. Epidemiological study of blood pressure in domestic dogs. J Small Anim Pract. (1996) 37:116–25. doi: 10.1111/j.1748-5827.1996.tb02358.x
39. Seliškar A, Zrimšek P, Sredenšek J, Petrič AD. Comparison of high definition oscillometric and Doppler ultrasound devices with invasive blood pressure in anaesthetized dogs. Vet Anaesth Analg. (2013) 40:21–7. doi: 10.1111/j.1467-2995.2012.00774.x
40. Vachon C, Belanger MC, Burns PM. Evaluation of oscillometric and Doppler ultrasonic devices for blood pressure measurements in anesthetized and conscious dogs. Res Vet Sci. (2014) 97:111–7. doi: 10.1016/j.rvsc.2014.05.003
41. Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension. (2010) 56:369–77. doi: 10.1161/HYPERTENSIONAHA.110.152108
42. Salman LA, Shulman R, Cohen JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. (2020) 22:1–9. doi: 10.1007/s11886-020-1257-y
43. Oechtering TH, Oechtering GU, Nöller C. Strukturelle besonderheiten der nase brachyzephaler hunderassen in der computertomographie. Tierärztl Prax. (2007) 35:177–87. doi: 10.1055/s-0038-1622615
44. Ginn JA, Kumar MSA, McKiernan BC, Powers BE. Nasopharyngeal turbinates in brachycephalic dogs and cats. J Am Anim Hosp Assoc. (2008) 44:243–9. doi: 10.5326/0440243
45. Hoglund K, Hanas S, Carnabuci C, Ljungvall I, Tidholm A, Haggstrom J. Blood pressure, heart rate, and urinary catecholamines in healthy dogs subjected to different clinical settings. J Vet Intern Med. (2012) 26:1300–8. doi: 10.1111/j.1939-1676.2012.00999.x
46. Marino CL, Cober RE, Iazbik MC, Couto CG. White-coat effect on systemic blood pressure in retired racing greyhounds. J Vet Intern Med. (2011) 25:861–5. doi: 10.1111/j.1939-1676.2011.00735.x
47. Kallet AJ, Cowgill LD, Kass PH. Comparison of blood pressure measurements obtained in dogs by use of indirect oscillometry in a veterinary clinic versus at home. J Am Vet Med Assoc. (1997) 210:651–4.
48. Remillard RL, Ross JN, Eddy JB. Variance of indirect blood pressure measurements and prevalence of hypertension in clinically normal dogs. Am J Vet Res. (1991) 52:561–5.
49. IDEXX, Laboratories. Interpretive Criteria for the Canine Cardiopet®proBNP Test. Available online at: https://www.idexx.com/files/cardiopet-interpretive-criteria-canine.pdf (accessed March 01 2020).
50. Winter RL, Saunders AB, Gordon SG, Buch JS, Miller MW. Biologic variability of N-terminal pro-brain natriuretic peptide in healthy dogs and dogs with myxomatous mitral valve disease. J Vet Cardiol. (2017) 19:124–31. doi: 10.1016/j.jvc.2016.11.001
51. Sinha SK, Garg S, Thakur R, Krishna V, Singh K, Sachan M, et al. Prognostic importance of exercise brain natriuretic peptide in asymptomatic chronic organic severe mitral regurgitation: an observational study. J Clin Med Res. (2016) 8:797. doi: 10.14740/jocmr2680w
Keywords: NT-proBNP, blood pressure, pug, Brachycephalic Obstructive Airway Syndrome (BOAS), cardiac biomarker
Citation: Mach R, Wiegel PS, Bach J-P, Beyerbach M, Levicar C and Nolte I (2022) Evaluation of blood pressure and NT-proBNP in pugs with and without clinical signs of Brachycephalic Obstructive Airway Syndrome. Front. Vet. Sci. 9:1015157. doi: 10.3389/fvets.2022.1015157
Received: 09 August 2022; Accepted: 29 November 2022;
Published: 22 December 2022.
Edited by:
J. Alberto Montoya-Alonso, University of Las Palmas de Gran Canaria, SpainReviewed by:
Josep Pastor, Universitat Autònoma de Barcelona, SpainZeki Yilmaz, Faculty of Veterinary Medicine, Turkey
Vladimira Erjavec, University of Ljubljana, Slovenia
Copyright © 2022 Mach, Wiegel, Bach, Beyerbach, Levicar and Nolte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebekka Mach, UmViZWtrYS5NYWNoQHRpaG8taGFubm92ZXIuZGU=; Ingo Nolte, SW5nby5Ob2x0ZS5pckB0aWhvLWhhbm5vdmVyLmRl
 Rebekka Mach1*
Rebekka Mach1* Ingo Nolte
Ingo Nolte