- 1Guangdong Provincial Key Laboratory of Veterinary Pharmaceutics Development and Safety Evaluation, National Risk Assessment Laboratory for Antimicrobial Resistance of Animal Original Bacteria, College of Veterinary Medicine, South China Agricultural University, Guangzhou, China
- 2Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China
- 3Guangzhou Insighter Biotechnology Co., Ltd., Guangzhou, China
This study aimed to evaluate the antibacterial activity of isopropoxy benzene guanidine (IBG) against C. perfringens based on pharmacokinetics/pharmacodynamics (PK/PD) modeling in broilers. The PK parameters of IBG in the plasma and ileal content of C. perfringens-infected broilers following oral administration at 2, 30, and 60 mg/kg body weight were investigated. in vivo PD studies were conducted over oral administration ranging from 2 to 60 mg/kg and repeated every 12 h for 3 days. The inhibitory Imax model was used for PK/PD modeling. Results showed that the MIC of IBG against C. perfringens was 0.5–32 mg/L. After oral administration of IBG, the peak concentration (Cmax), maximum concentration time (Tmax), and area under the concentration-time curve (AUC) in ileal content of broilers were 10.97–1,036.64 mg/L, 2.39–4.27 h, and 38.31–4,266.77 mg·h/L, respectively. After integrating the PK and PD data, the AUC0 − 24h/MIC ratios needed for the bacteriostasis, bactericidal activity, and bacterial eradication were 4.00, 240.74, and 476.98 h, respectively. For dosage calculation, a dosage regimen of 12.98 mg/kg repeated every 12 h for 3 days was be therapeutically effective in broilers against C. perfringens with MIC ≤ 2 mg/L. In addition, IBG showed potent activity against C. perfringens, which may be responsible for cell membrane destruction. These results can facilitate the evaluation of the use of IBG in the treatment of intestinal diseases in broilers caused by C. perfringens.
Introduction
Necrotizing enteritis (NE) is widely spread in broilers, which poses a major economic burden on poultry industry worldwide (1). NE was first described by Parish (2), with high morbidity and mortality (3). The pathogen of NE is Clostridium perfringens (C. perfringens), a Gram-positive spore-forming anaerobic bacteria (4). Toxins produced by C. perfringens can cause gastroenteritis, enterocolitis or enterotoxaemia in humans and animals (5). The use of antibiotic growth promoters in livestock industry must be decreased worldwide to delay the spread of antibiotic resistance (6, 7). However, these measures lead to the high prevalence of NE (8). Broilers are prone to NE at 2–6 weeks of age (9). Antibiotic therapy can effectively control NE. Drug resistance of Clostridium perfringens clinical isolates is becoming common because of the frequent use of antibiotics (10). Thus, developing new drugs different from existing drugs is an effective method to overcome antibiotic resistance.
Guanidine compounds have been widely used in the treatment of various diseases because of their biological activities, and they are potential candidates for structural modification of new drugs (11–13). Liu et al. reported that metformin, an antidiabetic drug, promotes intracellular accumulation of doxycycline to restore antibiotic activity against multidrug-resistant bacteria (14). Pi et al. reported that robenidine analog NCL195 alone or in combination with EDTA, polymyxin B non-apeptide, and polymyxin B has good antibacterial activity against various bacteria including Staphylococcus aureus (15). As a new candidate for substituted guanidine compounds, isopropoxy benzene guanidine (IBG) has been proven to be effective against Gram-positive bacteria (16, 17). IBG disrupts the cell membranes of drug-resistant Enterococci and Staphylococcus aureus. In addition, IBG can affect colistin against colistin-resistant Salmonella (18). IBG supplementation effectively improves the average daily gain and reduces diarrhea rate of broilers without adverse reactions (19).
The present study sought to determine the pharmacokinetic (PK) data of IBG in plasma and ileal content. The PK/pharmacodynamics (PD) indexes required for different levels of antibacterial effectiveness by using the inhibitory Imax model were also analyzed. Furthermore, the formulation of the dosage regimen of IBG in broilers could be used to formulate a reasonable dosage for treating NE.
Materials and methods
Antibiotic and bacteria
Isopropoxy benzene guanidine (99.9%) was provided by Guangzhou Insighter Biotechnology (Guangzhou, China). Mueller–Hinton broth and Mueller–Hinton agar were obtained from Qingdao Hope Bio-Technology Co., Ltd. (Qingdao, China). Tryptone-sulfite-cycloserine agar was obtained from Guangdong Huankai Microbial Technology (Guangdong, China). Twenty-four isolates of C. perfringens were used, including a standard strain (ATCC13124) was purchased from the Chinese Veterinary Culture Collection Center and 23 strains isolated from broilers in five cities in Guangdong province from March to November in 2021.
Animals
Two-week-old healthy Sanhuang broilers with weights 100 ± 10 g were used in this study. Broilers were allowed 7-day acclimation prior to experiments. All broilers were allowed with antibiotic-free food and water supply ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of South China Agricultural University (Approval Number: 2022A001).
Determination of MIC, MBC, MPC, and PAE
The susceptibility of the selected C. perfringens isolates to IBG in MH broth was evaluated in accordance with the micro-dilution method recommended by the CLSI (20). Minimal inhibitory concentration (MIC) was defined as the lowest concentration of IBG that inhibited the visible bacterial growth after 24 h of incubation. The MIC in ileal content was also evaluated in using the micro-dilution method (21). The mutant prevention concentration (MPC) of IBG was determined using the agar method (22). The 1010 CFU/mL C. perfringens strains were inoculated on the agar plates containing serial concentration of IBG (1 MIC, 2 MIC, 4 MIC, 8 MIC, 16 MIC, and 32 MIC) and cultured at 37°C for 72 h. The MPC was defined as the lowest concentration of IBG on agar plates without bacterial growth.
For the post-antibiotic effect (PAE) determination, the bacterial was exposed to three different concentrations (1 MIC, 2 MIC, and 4 MIC) of IBG for 1 or 2 h. The media containing IBG was removed by centrifuge at 12,000 × g for 5 min. The bacterial was re-grew in fresh media without IBG for another 24 h. The bacterial numbers were determined at different time points. The PAE was the time difference (in hours) for antimicrobial-treated bacterial to increase in number by 1 log10 minus the same determination for non-treated cultures of the same test bacterial (23).
In vitro time-killing curves
Different concentrations of IBG: 1/4MIC, 1/2MIC, 1MIC, 2MIC, and 4MIC were prepared in MH broth, the tubes were then inoculated with C. perfringens (106 CFU/mL) and incubated at 37°C. The bacterial count (CFU/mL) was determined for each tube after 0, 1, 2, 4, 6, 8, 12, and 24 h of incubation. In brief, 100 μL of culture was obtained for each time point, and serially diluted, and the colonies were counted the next morning. The limit of detection (LOD) was 10 CFU/mL. All experiments were performed in triplicate.
Establishing C. perfringens infection model
Based on references and proper modification (24, 25), broilers were infested by oral challenging with coccidial sporulated oocysts propagated from field isolates (30,000/in 1 mL/bird). After 4 days, broilers oral gauge with 1 mL of culture containing 109 CFU/mL of C. perfringens ATCC13124 for 3 days. Broilers were observed after inoculation for clinical symptoms and pathological changes.
Pharmacokinetics of IBG in a C. perfringens infection model
A total of 132 broilers were randomly divided into three groups and a single dose of 2, 30, or 60 mg/kg body weight (b.w.) IBG following oral gavage. At 0.08, 0.25, 0.50, 0.75, 1, 2, 4, 6, 8, 12, and 24 h after oral administration of IBG, four broilers in each group were euthanized to collect ileal contents and blood samples. The concentration of IBG in plasma and ileal content was determined by validated high-performance liquid chromatography (HPLC). In brief, ileal contents (0.5 g) were extracted with 1.5 mL of 1% formic acid acetonitrile, homogenized for 1 min, and centrifuged (13,000 g, 10 min) to obtain supernatant. Subsequently, 0.5 mL of supernatant was added to 1 mL 1% formic acid acetonitrile. After being vortexed (1 min) and centrifuged (13,000 g, 10 min), the supernatant was filtered through a 0.22 μm membrane for concentration analysis. The calibration range was 0.20–20 μg/g. Intraday and interday precision levels varied from 1.1 to 7.2% and from 1.7 to 6.5%, respectively. The LOD and limit of quantification (LOQ) were 0.10 and 0.20 μg/g, respectively. A 0.20 mL aliquot of plasma sample mixed with 0.80 mL of 1% formic acid acetonitrile. After being vortexed (1 min) and centrifuged (13,000 g, 10 min), the supernatant was filtered through a 0.22 μm membrane for concentration analysis. The calibration range was 0.02–1 μg/mL. Intraday and interday precision levels varied from 1.9 to 9.1% and from 2.4 to 8.1%, respectively. The LOD and LOQ were 0.005 and 0.010 μg/mL, respectively. The concentration data of IBG in the plasma and intestinal content were submitted to a non-compartmental analysis in Phoenix WinNonlin® 8.2 (Certara, L.P., Princeton, NJ, USA). The corresponding intestinal content concentration-time profiles after multiple dosage regimens were predicted using Phoenix's non-parametric superposition function based on the single-dose intestinal content PK concentration-time profile.
Pharmacodynamics of IBG in an intestinal infection model
Infected broilers were treated gavage two times a day for three successive days with 0, 2, 5, 10, 20, 30, 40, and 60 mg/kg b.w. of IBG (n = 4) to evaluate the in vivo effectiveness of IBG. Treatment started at 12 h post-infection. At 24 h after the last dose, the intestinal content was sampled sterilely and homogenized for CFU determination (26). Broilers in the control group were sacrificed before and 24 h after IBG treatment.
Analysis of the PK/PD relationship
The in vivo PK/PD relationships of IBG in intestinal were simulated using the Imax model in the WinNonlin® 8.2 (Certara, L.P., Princeton, NJ, USA) using the following equation (27):
where E0 is the difference in bacterial count of (log10CFU/g) control samples. Imax is the maximum antimicrobial growth inhibition determined as the change in log10CFU/g after treatment with IBG. X is the predictive variable (AUC0 − 24h/MIC), and IC50 is the X value producing 50% of the maximum antibacterial effect.
The potential optimal dosage can be calculated using the following equation (28, 29):
where dose (per day) is at a steady state; CL is the clearance per day; AUC/MIC is the targeted endpoint for optimal efficacy in hours; MIC is the target pathogen; F is the bioavailability factor, and fu is the free fraction of the drug.
Cell membrane integrity assay
Cell membrane integrity assay was performed as a previous report (30). Clostridium perfringens ATCC13124 were grown overnight at 37°C in an anaerobic system. Then culture cells were washed and resuspended in PBS (pH 7.4) to obtain OD600 of 0.5, followed by the addition of 0.5 μmol/L of propidium iodide (PI; Beyotime, Catalog No. ST511) in the presence of IBG (final concentrations ranging from 0 to 16 mg/mL). After incubation for 30 min, fluorescence was measured by using a Hitachi F-7000 Fluorescence Spectrometer with an excitation and emission wavelengths of 535 and 615 nm, respectively.
Membrane depolarization assay
The membrane potential of cells was using a fluorescent probe DiSC3(5) as described previously (14). Then bacterial cells were washed and suspended with 5 mmol/L of HEPES (pH 7.0, plus 5 mM glucose). OD600 of bacterial suspension was standardized to 0.5 in the same buffer, and 0.5 μmol/L of 3,3-dipropylthiadicarbocyanine iodide DiSC3(5) (Aladdin, Catalog No. D131315) was added. After incubation at 37°C for 30 min, 190 μL of probe-labeled bacterial cells was added to a 96-well plate and 10 μL of IBG (final concentrations ranging from 0 to 16 mg/mL) was added. After incubation at 37°C for 30 min, fluorescence was measured with an excitation wavelength at 622 nm and an emission wavelength at 670 nm.
Proton motive force assay
The PMF of C. perfringens ATCC13124 treated with IBG was measured with pH-sensitive fluorescence probe BCECF-AM (20 × 10−6 M, UElandy Catalog No. B3016). After the fluorescence was stabilized, varying IBG were added. The excitation and emission wavelengths on the fluorescence spectrometer were set to 488 and 525 nm, respectively.
ATP determination
Intracellular ATP levels of C. perfringens ATCC13124 were determined using an Enhanced ATP Assay Kit (Beyotime, Catalog No. 50027). C. perfringens ATCC13124 grown overnight at 37°C in an anaerobic system was washed and resuspended to obtain OD600 of 0.5 with PBS (pH 7.4). After treating with different concentrations (0–16 mg/L) of IBG for 30 min, bacterial cultures were centrifuged and the supernatant was removed. Bacterial precipitates were lysed with lysozyme and centrifuged, and the supernatant was prepared for measurement at intracellular ATP levels. Recording in the luminescence model using the Hitachi F-7000 Fluorescence Spectrometer.
Results
In vitro susceptibility testing and time-killing assays
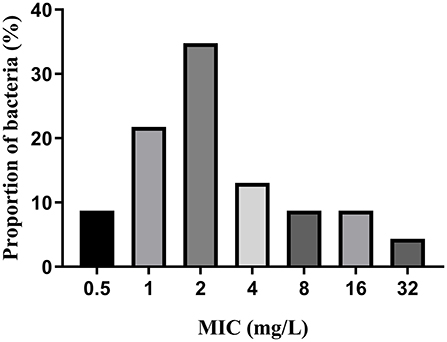
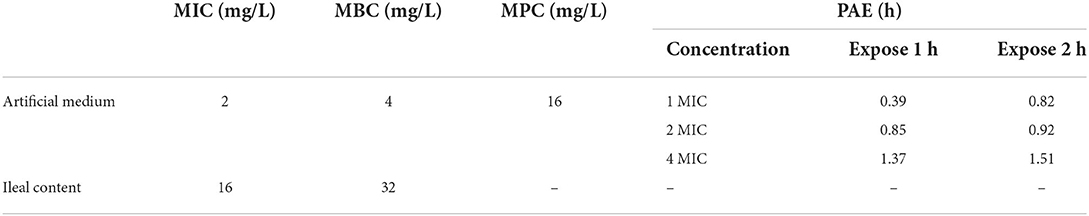
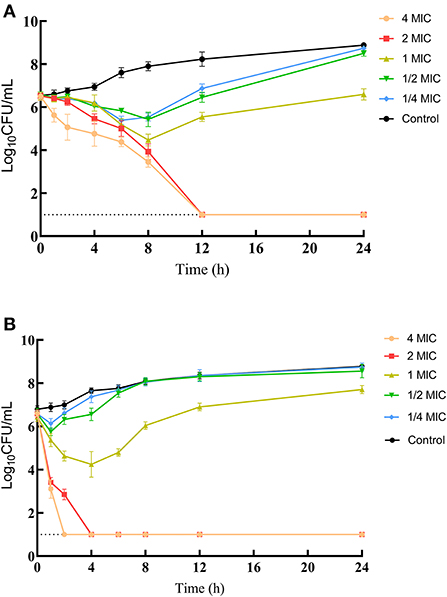
MICs of IBG against 23 C. perfringens strains varied, ranging from 0.5 to 32 mg/L. The percentage of each MIC (0.5, 1, 2, 4, 8, 16, and 32 mg/L) was 8.70, 21.74, 34.78, 13.04, 8.70, 8.70, and 4.35%, respectively. The MIC distribution is shown in Figure 1. The MIC and MBC of IBG against C. perfringens ATCC13124 in MH broth were 2 and 4 mg/L, whereas those in ileal content were eight times higher at 16 and 32 mg/L, respectively. The MPC in the medium was eight times higher than the MIC, with a value of 16 mg/L. The PAE of C. perfringens exposed to IBG for 1 and 2 h ranged from 0.39 to 1.37 h and from 0.82 to 1.51 h, respectively (Table 1). The in vitro time-killing curves of IBG against C. perfringens ATCC13124 and GDZ21C59W in the MH broth are illustrated in Figure 2. The time-killing curves imply a concentration–dependent killing characteristic of IBG. When C. perfringens was exposed to IBG with a concentration >2 mg/L, the continuous inhibitory effect on bacterial growth could be observed.
Pharmacokinetics analysis
The concentration–time profiles of plasma and intestinal content in C. perfringens-infected broilers following single oral gavage at 2, 30, and 60 mg/kg is shown in Figure 3. The PK parameters of IBG in plasma and intestinal content are illustrated in Table 2. After oral administration, IBG had a significantly lower AUClast and Cmax in plasma vs. in intestinal content (P < 0.01). In plasma, AUClast and Cmax ranged from 0.38 to 2.18 mg·h/L and from 0.08 to 0.27 mg/L, respectively. In intestinal content, AUClast and Cmax ranged from 38.31 to 4,266.77 mg·h/L and from 10.97 to 1,036.64 mg/L, respectively. A good linearity of IBG was observed in the intestine (R2 ≥ 0.988 for Cmax and AUClast).
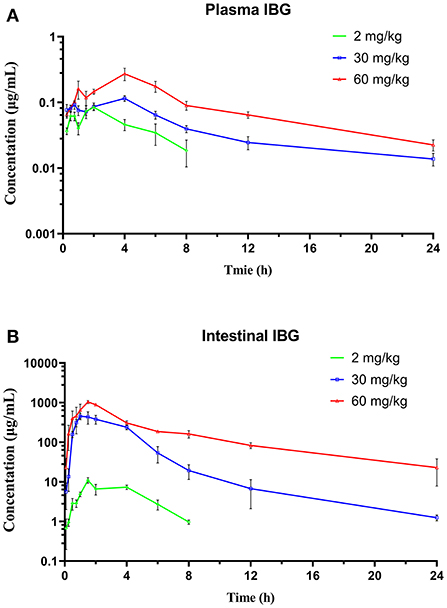
Figure 3. The time–concentration profile of IBG in plasma (A) and intestinal contents (B) of broilers following a single oral administration of 2, 30, and 60 mg/kg (n = 4).
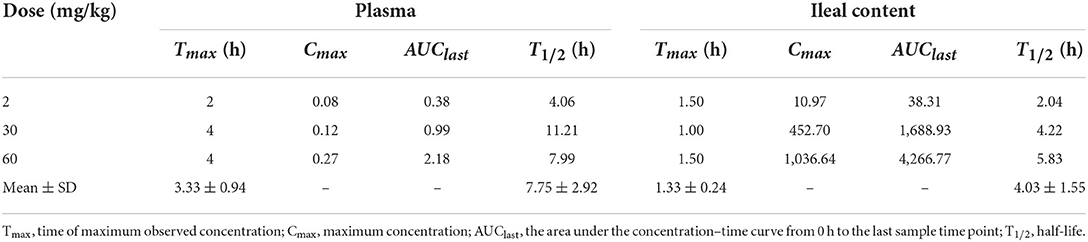
Table 2. Pharmacokinetic parameters of IBG in plasma and ileal content following single gavage in C. perfringens-infected broilers.
PK/PD analysis
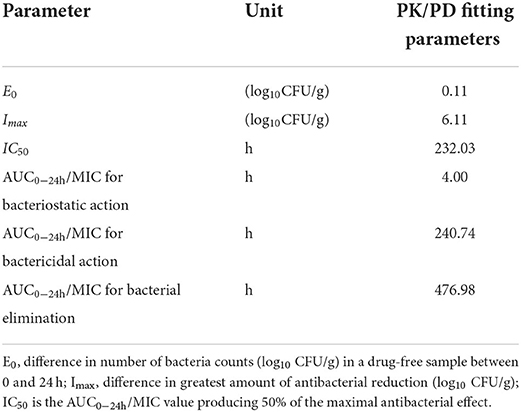
At the start of IBG therapy, bacterial burdens were 8.05 ± 0.25 log10CFU/g. The most effective IBG dosage regimens result in the reduction of bacterial number at the start of treatment (4.06 ± 0.19 log10CFU/g). The relationship between the effect of IBG against C. perfringens and each of the PK/PD indices in the intestinal infection model is shown in Figure 4. The PK/PD index of AUC0 − 24h/MIC (R2 > 0.9542) had a strong correlation with antibacterial activity in the intestinal infection model. The AUC0 − 24h/MIC ratios required for various efficacy targets are shown in Table 3.

Figure 4. Relationships between the effect of IBG against C. perfringens and PK/PD indices AUC0 − 24h/MIC in the intestinal infection model. R2 is the coefficient of determination.
IBG disrupted cell membrane in multiple ways
Based on IBG killing against C. perfringens in vitro and in vivo, we try to elucidate its potential mechanisms. In addition, IBG killing against Staphylococcus aureus and Enterococcus by damaging cell membrane (16, 17), may exert antibacterial activity against C. perfringens in a similar manner. Thus, we first tested the effect of IBG on the permeability of the cytoplasmic membrane. We used a fluorescent probe PI to assess the effect of IBG on the inner membrane of the bacteria as previously described (31). The results showed that IBG increased the permeability of C. perfringens ATCC13124 (Figure 5A). The fluorescence value clearly increased with IBG treatment compared with that of untreated cells., which indicated that IBG might cause dysfunctions in the cytoplasmic membrane. Hence, DiSC3(5) was used to evaluate the bacterial membrane potential (32). When the concentration of IBG was more than four times that of MIC, the fluorescence was significantly reduced, suggesting that IBG disrupted the electric potential of C. perfringens (Figure 5B). Existing research has shown that membrane depolarization is related to the production of ROS and PMF (33, 34). Dyes DCFH-DA (35) and BCECF-AM (36) were used to analyze the effects of ROS and PMF, respectively. There was no effect on ROS accumulation in C. perfringens treated with IBG. A large reduction in the magnitude of PMF accumulation was observed in the IBG-treated group compared with untreated cells (Figure 5C). Considering that PMF is the driving force for ATP synthesis (37), the intracellular ATP levels of C. perfringens treated with IBG was also significantly decreased (Figure 5D). Collectively, IBG stimulates a membrane-dependent mechanism to exert an antibacterial effect.
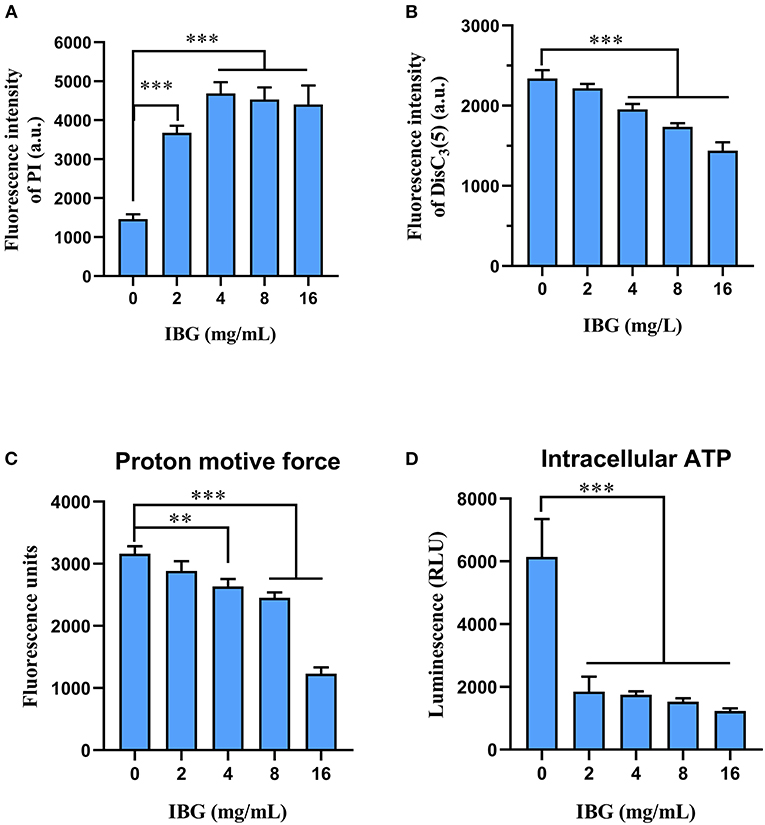
Figure 5. Mechanism of IBG against C. perfringens. (A) Increased permeability of the inner membrane of C. perfringens ATCC13124 treated with IBG (0–16 mg/L) for 30 min. (B) IBG dissipates membrane potential of C. perfringens ATCC13124. (C) Disruption of PMF with increased IBG by monitoring the fluorescence intensity of BCECF-AM-probed C. perfringens cells. (D) Decreased levels of intracellular ATP in C. perfringens ATCC13124 after treatment with IBG. All data are presented as mean ± SD, and the significant difference was determined by non-parametric one-way ANOVA (**p < 0.01, ***p < 0.001).
Discussion
Due to overuse and misuse, and resistance to commonly used antibiotics, the control of C. perfringens is very difficult (38). Therefore, new antimicrobial drugs are needed for the effective management of necrotizing enteritis. IBG, as a novel guanidine substituted compound, has been shown to be effective against Gram-positive bacteria by disrupting cell membrane (16, 17). In this study, the MIC range of IBG to clinical C. perfringens strains was 0.5–32 mg/L. Given the differences in bacterial growth under in vitro and in vivo conditions, the MIC in MH broth and ileal content were detected in this study. The MIC of IBG against C. perfringens ATCC13124 in an MH broth and ileal content was 2 and 16 mg/L, respectively. In vitro antibacterial effects of IBG under different conditions were quite different. Therefore, using the MIC in ileal content is more appropriate when calculating the PK/PD index of AUC0 − 24h/MIC.
Based on the PK results, the absorption and distribution of IBG in the intestine of broilers were rapid following oral gavage. Therefore, IBG can be used to treat intestinal bacterial infections. Previous PK/PD studies mostly focused on the integration of PK and the serum or plasma of PD parameters to indicate the relationship between drug time-course and curative effect (39, 40). Taking the intestine as the target organ, the plasma concentration of oral non-absorbable drugs such as colistin is negligible, which cannot provide an effective quantification of the gastrointestinal antibacterial effect (41). In this investigation, we described the PK of IBG in C. perfringens-infected broiler ileal content for PK/PD investigations. The concentration of IBG in ileal content increased gradually after gavage, and then decreased rapidly with the transportation of chyme, which was similar to the pharmacokinetic characteristics of other oral non-absorbable drugs (24). Thus, we used intragastric administration once every 12 h for in vivo PD study.
PK-PD analysis has become an important tool for formulating rational dosage regimens and preventing the emergence of antimicrobial drug resistance (42). In this study, the PK/PD index of AUC0 − 24h/MIC (R2 > 0.9542) had a strong correlation with antibacterial activity in the intestinal infection model. The AUC0 − 24h/MIC targets required to achieve bacteriostatic, bactericidal, and virtual eradication effect were 4.00, 240.74, and 476.98 h, respectively. According to the dosage equation, the dose regimen could be calculated. In this study, the MIC of C. perfringens isolate was 2 mg/L. For dosage calculation, bioavailability considered because of the extravascular route of administration, and Cl/F in ileal content was 0.03 ± 0.02 L/kg·h. fu was not required for using PD data generated in the small intestine (20). For the treatment of C. perfringens with MIC ≤ 2 mg/L, the dose of IBG for therapeutic and elimination of C. perfringens was 12.98 and 25.71 mg/kg repeated every 12 h, respectively.
This study was the first to demonstrate the antibacterial activity of IBG against C. perfringens in vitro and in vivo and then determine the AUC0 − 24h/MIC targets in the intestine of broilers, which were simulated using an Imax model. In addition, IBG displayed a potent antimicrobial activity against C. perfringens by targeting the cell membrane. The results demonstrate that IBG has the promising potential to become a new class of antimicrobials for the treatment of C. perfringens infections in broilers.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of South China Agricultural University.
Author contributions
DZ and ZZ conceived this study and participated in its design and coordination. YL and LY designed the experiments and drafted the manuscript. YL, LY, and WZ carried out the in vivo animal experiments. YL, LY, and JL carried out the mechanism experiments. XP and ZQ worked on the synthesis of compound IBG. DZ, ZZ, and YL conducted the PK/PD analysis. All authors read and approved the final manuscript.
Funding
This work was supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 32121004).
Conflict of interest
Authors XP and ZQ were employed by Guangzhou Insighter Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang Q, Liu J, Robinson KJ, Whitmore MA, Stewart SN, Zhang G. Perturbations of the ileal mycobiota by necrotic enteritis in broiler chickens. J Anim Sci Biotechnol. (2021) 12:107. doi: 10.1186/s40104-021-00628-5
2. Parish WE. Necrotic enteritis in the fowl (Gallus gallus domesticus). I Histopathology of the disease and isolation of a strain of Clostridium welchii. J Comp Pathol. (1961) 71:377–93.
3. Lovland A, Kaldhusdal M, Redhead K, Skjerve E, Lillehaug A. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. (2004) 33:83–92. doi: 10.1080/0379450310001636255
4. Lepp D, Zhou Y, Ojha S, Mehdizadeh GI, Carere J, Yang C, et al. Clostridium perfringens produces an adhesive pilus required for the pathogenesis of necrotic enteritis in poultry. J Bacteriol. (2021) 203:e00578–20. doi: 10.1128/JB.00578-20
5. Shrestha A, Uzal FA, McClane BA. Enterotoxic Clostridia: Clostridium perfringens enteric diseases. Microbiol Spectr. (2018) 6:1128. doi: 10.1128/microbiolspec.GPP3-0003-2017
6. Singer RS, Finch R, Wegener HC, Bywater R, Walters J, Lipsitch M. Antibiotic resistance–the interplay between antibiotic use in animals and human beings. Lancet Infect Dis. (2003) 3:47–51. doi: 10.1016/S1473-3099(03)00490-0
7. Ungemach FR, Muller-Bahrdt D, Abraham G. Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int J Med Microbiol. (2006) 41:33–8. doi: 10.1016/j.ijmm.2006.01.059
8. Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. (2004) 33:537–49. doi: 10.1080/03079450400013162
9. Engstrom BE, Fermer C, Lindberg A, Saarinen E, Baverud V, Gunnarsson A. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet Microbiol. (2003) 94:225–35. doi: 10.1016/S0378-1135(03)00106-8
10. Martel A, Devriese LA, Cauwerts K, De Gussem K, Decostere A, Haesebrouck F. Susceptibility of Clostridium perfringens strains from broiler chickens to antibiotics and anticoccidials. Avian Pathol. (2004) 33:3–7. doi: 10.1080/0307945031000163291
11. Rauf MK. Imtiaz-ud-Din, Badshah A. Novel approaches to screening guanidine derivatives. Expert Opin Drug Discov. (2014) 9:39–53. doi: 10.1517/17460441.2013.857308
12. Saczewski F, Balewski L. Biological activities of guanidine compounds, 2008 - 2012 update. Expert Opin Ther Pat. (2013) 23:965–95. doi: 10.1517/13543776.2013.788645
13. Liu J, Li X, Guo Y. Recent advances in the isolation, synthesis and biological activity of marine guanidine alkaloids. Mar Drugs. (2017) 15:324. doi: 10.3390/md15100324
14. Liu Y, Jia Y, Yang K, Li R, Xiao X, Zhu K, et al. Metformin restores tetracyclines susceptibility against multidrug resistant bacteria. Adv Sci. (2020) 7:1902227. doi: 10.1002/advs.201902227
15. Pi H, Nguyen HT, Venter H, Boileau AR, Woolford L, Garg S, et al. In vitro Activity of robenidine analog NCL195 in combination with outer membrane permeabilizers against Gram-negative bacterial pathogens and impact on systemic Gram-positive bacterial infection in mice. Front Microbiol. (2020) 11:1556. doi: 10.3389/fmicb.2020.01556
16. Zhang X, Han D, Pei P, Hao J, Lu Y, Wan P, et al. In vitro antibacterial activity of isopropoxy benzene guanidine against multidrug-resistant Enterococci. Infect Drug Resist. (2019) 12:3943–53. doi: 10.2147/IDR.S234509
17. Zhang X, Xiong W, Peng X, Lu Y, Hao J, Qin Z, et al. Isopropoxy benzene guanidine kills Staphylococcus aureus without detectable resistance. Front Microbiol. (2021) 12:633467. doi: 10.3389/fmicb.2021.633467
18. Kong L, Lu Y, Yang L, Zhang W, Zuo B, Peng X, et al. Pharmacokinetics and pharmacodynamics of colistin combined with isopropoxy benzene guanidine against mcr-1-positive Salmonella in an intestinal infection model. Front Microbiol. (2022) 13:907116. doi: 10.3389/fmicb.2022.907116
19. Xiao QX, Qiang MH, Zhang SM, Ou YR, Peng XF. Effects of isopropoxy benzene guanidine as a substitute for antibiotics on the performance and immune organ index of broilers. Anim Sci Abroad. (2018) 38:14–8.
20. CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Approved Standard. 5th ed. Wayne: CLSI Supplement VET01S (2020).
21. Zhou YF, Yu Y, Sun J, Tao MT, Zhou WJ, Li X, et al. Ex vivo pharmacokinetic/pharmacodynamic relationship of valnemulin against Clostridium perfringens in plasma, the small intestinal and caecal contents of rabbits. Anaerobe. (2016) 39:150–7. doi: 10.1016/j.anaerobe.2016.04.005
22. Gebru E, Damte D, Choi MJ, Lee SJ, Kim YH, Park SC. Mutant prevention concentration and phenotypic and molecular basis of fluoroquinolone resistance in clinical isolates and in vitro-selected mutants of Escherichia coli from dogs. Vet Microbiol. (2012) 154:384–94. doi: 10.1016/j.vetmic.2011.07.033
23. Sang K, Hao H, Huang L, Wang X, Yuan Z. Pharmacokinetic-pharmacodynamic modeling of enrofloxacin against Escherichia coli in broilers. Front Vet Sci. (2015) 2:80. doi: 10.3389/fvets.2015.00080
24. Wang F, Mi K, Ahmad I, Xie S, Hussain HI, Yuan Z, et al. Antibacterial activity of cyadox against Clostridium perfringens in broilers and a dosage regimen design based on pharmacokinetic-pharmacodynamic modeling. Microb Pathog. (2020) 141:103981. doi: 10.1016/j.micpath.2020.103981
25. Srinivasu B, Preetam VC, Gurram S, Reddy AR. Comparative evaluation of herbal coccidiostat with chemotherapeutic coccidiostats on performance of broilers to control coccidiosis. Trop Anim Health Prod. (2020) 52:1985–9. doi: 10.1007/s11250-020-02220-x
26. Xiao X, Pei L, Jiang LJ, Lan WX, Xiao JY, Jiang YJ, et al. In vivo pharmacokinetic/pharmacodynamic profiles of danofloxacin in rabbits infected with Salmonella typhimurium after oral administration. Front Pharmacol. (2018) 9:391. doi: 10.3389/fphar.2018.00391
27. Riviere JE, Toutain PL. Chapter 13: Simultaneous pharmacokinetic-pharmacodynamic modeling. In:Riviere JE, , editor. Comparative Pharmacokinetics: Principles, Techniques & Applications. 2nd ed. Ames, IA: Wiley-Blackwell, John Wiley & Sons, Ltd. (2011). p. 255–94.
28. Toutain PL, Del CJ, Bousquet-Melou A. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Res Vet Sci. (2002) 73:105–14. doi: 10.1016/S0034-5288(02)00039-5
29. Toutain PL, Bousquet-Melou A, Martinez M. AUC/MIC: a PK/PD index for antibiotics with a time dimension or simply a dimensionless scoring factor? J Antimicrob Chemoth. (2007) 60:1185–8. doi: 10.1093/jac/dkm360
30. Song M, Liu Y, Huang X, Ding S, Wang Y, Shen J, et al. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat Microbiol. (2020) 5:1040–50. doi: 10.1038/s41564-020-0723-z
31. Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. (1991) 139:271–9. doi: 10.1016/0022-1759(91)90198-O
32. Hamamoto H, Urai M, Ishii K, Yasukawa J, Paudel A, Murai M, et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat Chem Biol. (2015) 11:127–33. doi: 10.1038/nchembio.1710
33. Glasser NR, Kern SE, Newman DK. Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol Microbiol. (2014) 92:399–412. doi: 10.1111/mmi.12566
34. Van Acker H, Coenye T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. (2017) 25:456–66. doi: 10.1016/j.tim.2016.12.008
35. Antognelli C, Gambelunghe A, Talesa VN, Muzi G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: involvement of superoxide anion, hydrogen peroxide and NF-kappaB. Apoptosis. (2014) 19:102–16. doi: 10.1007/s10495-013-0902-y
36. Cochrane SA, Findlay B, Bakhtiary A, Acedo JZ, Rodriguez-Lopez EM, Mercier P, et al. Antimicrobial lipopeptide tridecaptin A1 selectively binds to Gram-negative lipid II. Proc Natl Acad Sci U S A. (2016) 113:11561–6. doi: 10.1073/pnas.1608623113
37. Maloney PC, Kashket ER, Wilson TH. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. (1974) 71:3896–900. doi: 10.1073/pnas.71.10.3896
38. Burgess DS. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest. (1999) 115:19S−23S. doi: 10.1378/chest.115.suppl_1.19S
39. Toutain PL, Pelligand L, Lees P, Bousquet-Melou A, Ferran AA, Turnidge JD. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: recent advances and critical appraisal. J Vet Pharmacol Ther. (2021) 44:172–200. doi: 10.1111/jvp.12917
40. Rodriguez-Gascon A, Solinis MA, Isla A. The Role of PK/PD Analysis in the development and evaluation of antimicrobials. Pharmaceutics. (2021) 13:833. doi: 10.3390/pharmaceutics13060833
41. Guyonnet J, Manco B, Baduel L, Kaltsatos V, Aliabadi MHFS, Lees P. Determination of a dosage regimen of colistin by pharmacokinetic/pharmacodynamic integration and modeling for treatment of GIT disease in pigs. Res Vet Sci. (2010) 88:307–14. doi: 10.1016/j.rvsc.2009.09.001
Keywords: isopropoxy benzene guanidine, pharmacokinetic/pharmacodynamic (PK/PD), intestinal infection model, Clostridium perfringens, broiler
Citation: Lu Y, Yang L, Zhang W, Li J, Peng X, Qin Z, Zeng Z and Zeng D (2022) Pharmacokinetics and pharmacodynamics of isopropoxy benzene guanidine against Clostridium perfringens in an intestinal infection model. Front. Vet. Sci. 9:1004248. doi: 10.3389/fvets.2022.1004248
Received: 27 July 2022; Accepted: 15 September 2022;
Published: 29 September 2022.
Edited by:
Nora Mestorino, National University of La Plata, ArgentinaReviewed by:
Fan Yang, Henan University of Science and Technology, ChinaZigang Qu, Lanzhou Veterinary Research Institute (CAAS), China
Copyright © 2022 Lu, Yang, Zhang, Li, Peng, Qin, Zeng and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenling Zeng, emx6ZW5nQHNjYXUuZWR1LmNu; Dongping Zeng, ZG9ueXRzYW5nQHNjYXUuZWR1LmNu
†These authors have contributed equally to this work
 Yixing Lu
Yixing Lu Liuye Yang
Liuye Yang Wanying Zhang
Wanying Zhang Jie Li1,2
Jie Li1,2 Xianfeng Peng
Xianfeng Peng Zhenling Zeng
Zhenling Zeng Dongping Zeng
Dongping Zeng